Abstract
The growth factor granulocyte/macrophage-colony stimulating factor (GM-CSF) has an important role in pulmonary surfactant metabolism and the regulation of antibacterial activities of lung sentinel cells. However, the potential of intra-alveolar GM-CSF to augment lung protective immunity against inhaled bacterial pathogens has not been defined in preclinical infection models. We hypothesized that transient overexpression of GM-CSF in the lungs of mice by adenoviral gene transfer (Ad-GM-CSF) would protect mice from subsequent lethal pneumococcal pneumonia. Our data show that intra-alveolar delivery of Ad-GM-CSF led to sustained increased pSTAT5 expression and PU.1 protein expression in alveolar macrophages during a 28 day observation period. Pulmonary Ad-GM-CSF delivery two or four weeks prior to infection of mice with S. pneumoniae significantly reduced mortality rates relative to control vector treated mice. This increased survival was accompanied by increased iNOS expression, antibacterial activity and a significant reduction in caspase 3 dependent apoptosis and secondary necrosis of lung sentinel cells. Importantly, therapeutic treatment of mice with recombinant GM-CSF improved lung protective immunity and accelerated bacterial clearance after pneumococcal challenge. We conclude that prophylactic delivery of GM-CSF triggers long-lasting immunostimulatory effects in the lung in vivo and rescues mice from lethal pneumococcal pneumonia by improving antibacterial immunity. These data support use of novel antibiotic-independent immunostimulatory therapies to protect patients against bacterial pneumonias.
Keywords: GM-CSF, S. pneumoniae, PU.1, pneumonia, therapy, infection
Introduction
Streptococcus pneumoniae (the pneumococcus) is the most prevalent pathogen causing community-acquired pneumonia, septic meningitis, and otitis media and is known to frequently progress to invasive pneumococcal disease associated with high morbidity and mortality rates worldwide. The increase in incidence and global dissemination of multidrug-resistant clones of S. pneumoniae necessitates the development of novel lung-active antibiotic substances. It also requires the development of adjunctive antibiotic-independent strategies, including prophylactic, immunostimulatory approaches to improve lung innate immunity against S. pneumoniae infections in both immuno-competent and immuno-compromized patients (1-3). Our group has shown that experimental maneuvers leading to increased intra-alveolar accumulation of mononuclear phagocytes significantly increased survival of mice challenged with S. pneumoniae (4-5). However, there are only a few inconclusive reports that have directly addressed the issue of enhanced immunostimulation to improve lung innate immunity against bacterial challenge (6).
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a hematopoietic growth factor and cytokine that exerts pleiotropic effects on survival, proliferation, differentiation, priming, and activation of myeloid and non-myeloid cells (7). In the lung, GM-CSF is primarily released by epithelial cells and macrophages and regulates both pulmonary surfactant homeostasis and macrophage-mediated protective immunity (8-9). Binding of GM-CSF to its receptor triggers activation of the JAK/STAT5 signaling pathway and increased gene expression of the myeloid specific transcription factor PU.1, which in turn mediates terminal differentiation and is necessary for the function of resident alveolar macrophages (8-10). In humans, disruption of the GM-CSF signaling pathway through GM-CSF-neutralizing autoantibodies or mutations in the GM-CSFR results in the development of pulmonary alveolar proteinosis (PAP), which is characterized by progressive accumulation of surfactant lipids and proteins within alveoli and in alveolar macrophages and abnormalities of host defense (8, 11-13). Absence of GM-CSF in gene-targeted mice results in the development of a PAP-like disorder similar to the human disease (14-15). At the same time, deactivation of the GM-CSF in mice increases their susceptibility to pulmonary and systemic infections to a wide range of pathogens, including Gram-negative and - positive bacteria, mycobacteria, fungi, parasites, and viruses (14-24). Previous reports have demonstrated that recombinant GM-CSF delivered to the airways of GM-CSF KO mice can improve host defense against group B streptococci, while aerosolized GM-CSF results in faster clearance of group B streptococci even in wild-type mice (19). Together, these data support a critical role for endogenous cytokines such as GM-CSF in the regulation of lung protective immunity against inhaled pathogens.
However, whether or not GM-CSF is able to prophylactically enhance lung protective immunity against clinically relevant pathogens such as Streptococcus pneumoniae when prophylactically given into the airways has not been addressed in the literature. Development of adjunctive antibiotic-independent therapies for patients with risk of developing drug resistant pneumococcal pneumonia would have important clinical applications with improvement of outcomes and reduction of costs.
Materials and Methods
Animals
C57BL/6N mice were purchased from Charles River Laboratories (Sulzfeld, Germany). All animal experiments were approved by our local government authorities.
Reagents
Recombinant murine GM-CSF was purchased from PeproTec (London, UK). Rabbit anti-PU.1 polyclonal antibody, rabbit anti-phosphorylated STAT5 antibody, rabbit anti-STAT5 antibody, rabbit anti-cleaved caspase-3 antibody, rabbit anti-iNOS antibody, and rabbit anti GAPDH antibody were obtained from Cell Signaling Technologies (Danvers, USA). Mouse anti-β-actin antibody was from Sigma (Deisenhofen, Germany) and Peroxidase-conjugated donkey anti-rabbit polyclonal IgG (H+L) was from Jackson ImmunoResearch (Suffolk, UK). All antibodies used in the current study for flow cytometry analysis including anti-CD11c PE-Cy5.5, anti-CD11b PE-Cy7, anti-MHC II PE, and anti-Ly-6G/Ly-6C (GR-1) PE-Cy7 were purchased from BD Biosciences (Heidelberg, Germany). Anti-F4/80 FITC and anti-F4/80 APC were from Serotec (Düsseldorf, Germany). The magnetic cell separation (MACS) kit and CD11c beads for purification of CD11c-positive cells from lung tissue were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany).
Culture and quantification of Streptococcus pneumoniae
For infection experiments, we used the capsular group 19 Streptococcus pneumoniae strain EF3030, which is characterized by a relatively low virulence in mice (5, 25-26). The bacteria were grown in Todd-Hewitt broth (Oxoid, Wesel, Germany) supplemented with 20 % FCS to mid-log phase and aliquots were snap frozen in liquid nitrogen and stored at −80°C until use (5, 27-28). Quantification of pneumococci was done by plating thawed aliquots in ten-fold serial dilutions on sheep blood agar plates (BD Biosciences, Heidelberg, Germany), followed by incubation of the plates at 37°C/5 % CO2 for 18 hours and subsequent determination of colony-forming units (CFU). We routinely checked the viability of thawed bacteria and bacterial stocks older than 4 weeks were not used for infection experiments.
Infection of mice with S. pneumoniae
Intratracheal infection of C57BL/6N mice with serotype 19 S. pneumoniae was done using thawed aliquots adjusted to the given infection dose, essentially as recently described (26, 29). After instillation, mice were kept in individually ventilated cages (IVC) with free access to autoclaved food and water and were monitored twice daily for disease symptoms and survival during the entire observation period.
Delivery of adenoviral vectors and recombinant murine GM-CSF into the lung
Ad-5E1GM-CSF (Ad-GM-CSF) or control vector Ad5dl70-3 (Addl-70-3) were diluted in PBS to a concentration of 1×108 PFU/50 μl and were instilled orotracheally into C57BL/6N mice (1×108 PFU/mouse) using the same method as described above for infection with S. pneumoniae (26, 29). For prophylactic treatment regimen, 10 μg of recombinant murine GM-CSF in PBS/0.1 % HSA or PBS/0.1 % HSA only were instilled orotracheally into the lungs of C57BL/6N mice followed by infection of the mice with S. pneumoniae (26, 29). Therapeutic treatment of mice with 10 or 20 μg of recombinant murine GM-CSF in PBS/0,1 % HSA was done under desfluran anaesthesia (Baxter, Unterschleissheim, Germany) as described recently (30).
Treatment groups
The effect of GM-CSF on lung innate immunity against S. pneumoniae was evaluated in the following treatment groups: (i) groups of mice received Ad-GM-CSF or control vector (108 pfu/mouse) to characterize baseline effects of GM-CSF transgene expression in the lungs of mice; (ii) mice were prophylactically treated for 3, 14, or 28 days with Ad-GM-CSF or control vector (108 pfu/mouse) followed by infection with serotype 19 S. pneumoniae. For determination of lung bacterial loads, mice were infected with 107 CFU/mouse. For assessment of mortality rates in groups of mice pre-treated with vectors (control and Ad-GM-CSF) for 14 or 28 days, mice were infected with 1.3×107 CFU/mouse (14 days vector pretreatment) or 1.1×107 CFU/mouse (28 days vector pretreatment), as indicated; (iii) groups of mice were pretreated with vehicle or recombinant mGM-CSF (10 μg per mouse) at 3, 6, or 12 hours prior to infection with serotype 19 S. pneumoniae (5×106 CFU/mouse), and (iv) groups of mice were infected with serotype 19 S. pneumoniae (5×106 CFU/mouse) followed by therapeutic application of vehicle or recombinant mGM-CSF (20 μg) at 1.5, 3, 6, or 12 hours after infection with S. pneumoniae.
Determination of bacterial loads in bronchoalveolar lavage fluid and lung tissue
Bacterial loads in the lungs of S. pneumoniae infected mice of the various experimental groups were determined both in BAL fluids (lung washes) and lung tissue homogenates (washed lungs) from the same mice. Briefly, mice were euthanized with an overdose of isoflurane (Baxter, Unterschleissheim, Germany) and bronchoalveolar lavage was performed by repeated intratracheal instillation of 300 μl aliquots of cold PBS supplemented with EDTA (Versen; Biochrom, Berlin, Germany) into the lungs and careful aspiration, until a BAL volume of 1.5 ml was collected as describedrecently (27-28, 31-32). Subsequently, BAL was continued until an additional volume of 4.5 ml was collected. Colony-forming units (CFU) in the respective 1.5 ml and 4.5 ml BAL fluid aliquots collected from S. pneumoniae infected mice were quantified by plating ten-fold serial dilutions on sheep blood agar plates (BD Biosciences, Heidelberg, Germany), followed by incubation of the plates at 37°C, 5 % CO2 for 18 hours. Whole lung washes were centrifuged (1,400 rpm, 4°C, 9 min) and cell pellets were pooled and re-suspended in RPMI/10 % FCS and total numbers of BAL fluid leukocytes were determined. In addition, cell-free BAL fluid supernatants of the individual 1.5 ml BAL fluid aliquots were collected and subsequently subjected to ELISA for determination of cytokines.
After collection of BAL, individual lobes from the same previously lavaged lungs were removed, dissected and homogenized in 2 ml Hank’s balanced salt solution without supplements (HBSS, PAA, Cölbe, Germany) using a tissue homogenizer (IKA, Staufen, Germany). Subsequently, CFU was determined by plating ten-fold serial dilutions on sheep blood agar plates followed by incubation of the plates at 37°C/5 % CO2 for 18 hours.
BAL and preparation of lung parenchymal tissue
Mice were euthanized with an overdose of isoflurane (Baxter, Unterschleissheim, Germany). Collection of BAL for the isolation of resident alveolar macrophages and alveolar recruited leukocytes from mice of the various treatment groups was done as described in detail recently (27-28, 31-32). Quantification of BAL fluid neutrophils, eosinophils, and lymphocytes was done on differential cell counts of Pappenheim-stained cytocentrifuge preparations, using overall morphological criteria, including cell size and shape of nuclei and subsequent multiplication of those values by the respective absolute BAL cell counts (27-28, 31-32). Quantification of resident and recruited mononuclear phagocyte subsets (alveolar macrophages, alveolar dendritic cells, and exudate macrophages) recovered by BAL from the lungs of mice from the various treatment groups was done using FACS-based differences in immunophenotypic profiles as characterized by differences in their cell surface antigen expression profiles of F4/80, CD11b, CD11c, and MHC class II (MHC-II), as outlined below in detail and elsewhere (27).
To quantify CD11c+ mononuclear phagocyte subsets in lung parenchymal tissue of mice of the various treatment groups, animals were subjected to BAL as described, followed by careful perfusion of the lungs via the right ventricle with HBSS until they were visually free of blood. Lung lobes were carefully removed and digested with collagenase A and DNase I. Leukocyte subsets contained in lung homogenates were further purified using a CD11c MACS kit following the instructions of the manufacturer (Militenyi Biotech, Bergisch Gladbach, Germany) and as previously described (28, 33-34). The purity of isolated CD11c+ cells was always ~90%. CD11c+ cells were then subjected to FACS analysis of differential cell surface antigen expression profiles, as outlined below.
Immunophenotypic analysis of mononuclear phagocyte subsets in BAL and lung parenychmal tissue
CD11c+ mononuclear phagocyte subsets isolated from lung parenchyma and BAL fluid were immunophenotypically analyzed according to their cell surface antigen expression profiles. Briefly, after pre-incubation with Octagam, 2-5×105 cells were stained with various combinations of fluorochrome-conjugated monoclonal antibodies directed against the corresponding cell surface markers for 20 min at 4°C. Subsequently, cells were washed twice with FACS buffer at 1,200 rpm, 3 min at 4°C and cell acquisition was performed on a FACSCanto flow cytometer (BD Biosciences, Heidelberg, Germany), equipped with an argon ion laser operating at 488 nm excitation wavelength and a helium neon laser operating at 633 nm excitation wavelength. First, the respective CD11c+ mononuclear phagocyte subsets purified from lung homogenates and BAL fluids of mice of the various treatment groups were gated according to their FSC-A versus SSC-A characteristics and FSC-A versus autofluorescence properties. Highly autofluorescent cells were further characterized as lung macrophages (F4/80pos/CD11bneg/CD11cpos/MHCIIneg), or as autofluorescent exudate macrophages characterized by their increased CD11b expression profile when compared to resident alveolar macrophages (F4/80pos/CD11bpos/CD11cpos/ MHCIIneg-low). Data analysis and careful post-acquisition compensation of spectral overlaps between the various fluorescence channels was performed using BD FACSDiva software.
Analysis of apoptosis/necrosis
Analysis of apoptosis and necrosis induction in alveolar macrophages and neutrophils was done by incubation of BAL cells with APC-labeled annexin V (for determination of apoptosis) in the presence of propidium iodide (for the determination of necrosis) for 15 min at room temperature, according to the manufacturer’s instructions (BD Biosciences, Heidelberg, Germany). Subsequently, macrophages were gated according to their FSC/SSC and FSC/F4/80 cell surface expression and neutrophils were gated according to their FSC/SSC and GR-1 cell surface expression followed by determination of the percentage of apoptotic or secondary necrotic macrophages and neutrophils (28, 33).
Flow sorting of alveolar macrophages and neutrophils
A high-speed FACSAria II flow cytometer (BD Biosciences, Heidelberg, Germany) equipped with an argon ion laser operating at 488 nm excitation wavelength and an aerosol management system was used for sorting of alveolar macrophages and neutrophils from S. pneumoniae infected mice therapeutically treated with GM-CSF. Briefly, BAL fluids were spun at 1,400 rpm for 10 min at 4°C, and cell pellets were resuspended in FACS buffer. After pre-incubation with octagam, 2–5×105 cells were stained with various combinations of fluorochrome-conjugated monoclonal antibodies directed against the corresponding cell surface markers for 20 min at 4°C. Subsequently, cells were washed two times with FACS buffer at 1,200 rpm, 3 min at 4°C and were resuspended for sorting in RPMI. A BD FACSAria II high-speed cell sorter was prepared for aseptic sorting according to the manufacturer’s instructions. Alveolar macrophages and neutrophils were gated according to their FSC-A versus SSC-A characteristics and FSC-A versus autofluorescence properties. Alveolar macrophages were further gated according to their characteristic expression of the following cell surface markers: F4/80pos/CD11cpos/CD11bneg. Neutrophils were gated according to their GR-1pos/CD11bpos cell surface expression profile. After appropriate gating and compensation setting, cells were sorted at a flow rate of approximately ~10,000 particles per second with an 85 μm nozzle and sample agitation turned on. The complete sorting process (pre-sort BALF samples and post-sort macrophages or neutrophils) was performed at a constant temperature of 4°C and was finished with re-sort analysis of sorted cells to verify sort purities, which were found to always exceed 98 % (34).
Total cellular RNA isolation, cDNA synthesis and real-time RT-PCR
Total cellular RNA was isolated from flow-sorted, highly purified (> 98 %) alveolar macrophages and neutrophils from S. pneumoniae infected and GM-CSF-treated mice 6 hours post-infection or from control alveolar macrophages sorted from vehicle or GM-CSF treated mice using a commercially available RNeasy Micro-kit (QIAGEN, Hilden, Germany), following the instructions of the manufacturer. 150,000 sorted alveolar macrophages or neutrophils were lysed and from the resultant RNA preparations, 100 ng total RNA were used for cDNA synthesis. Real-time RT-PCR analysis was performed as described recently (34-35). For normalization, beta-actin (forward primer: 5′-CCACAGCTGAGAGGGAAATC-3′, reverse primer: 5′-TCTCCAGGGAG GAAGAGGAT-3′) was used as the housekeeping gene and mean fold-changes were calculated using the 2−ΔΔCt method (34, 36). Primers used for determination of iNOS expression in sorted alveolar macrophages and neutrophils were: forward primer: 5′-GCCACCAACAATGGCAACA-3′, reverse primer: 5′-CGTACCGGATGACTGTGAA TT-3′.
Western blot analysis
For western blot analysis of PU.1, STAT5, phospho-STAT5, iNOS, cleaved caspase 3, caspase 3, and GAPDH protein expression in lung tissue, lungs were removed and homogenized on dry-ice. The resulting lung tissue homogenate was passed through a cell strainer and transferred to ice-cold lysis buffer containing protease inhibitors. Similar to lung homogenates, CD11c+ lung mononuclear phagocytes, and alveolar macrophages were lysed in ice-cold lysis buffer (100 μl/1–2×106 cells) as described previously (37). Expression analysis of phosphorylated STAT5, STAT5, PU.1, iNOS, cleaved caspase 3, caspase 3, β-actin, and GAPDH was performed using specific antibodies against these proteins as specified above. Expression of immunogenic proteins was determined by evaluation of enhanced chemiluminescence signals (ECL plus™; GE Healthcare, Buckinghamshire, UK) using a Vilber/Lourmat Chemismart 5000 and STAT5 phosphorylation and iNOS protein expression was further quantified using the Bio1D software package (Vilber/Lourmat Deutschland GmbH, Eberhardzell, Germany).
Phagocytosis and bacterial killing assay
Alveolar macrophages (2×105 per well) were infected with serotype 19 S. pneumoniae at an MOI of 50 for 30 min in RPMI/10 % FCS + 1 % glutamine at 37°C/5 % CO2. Subsequently, non-phagocytized pneumococci were removed by four washing steps with HBSS and either vehicle (PBS) or 100 ng/ml recombinant murine GM-CSF dissolved in RPMI were added to each well followed by incubation at 37°C/5 % CO2. Sixty and 120 min later, alveolar macrophages were washed and lysed with 0.1 % saponin in HBSS to release intracellular pneumococci. Subsequently, CFU were quantified by plating of ten-fold serial dilutions of cell lysates on sheep blood agar plates followed by incubation of the plates at 37°C/5 % CO2 for 18 hours. In control experiments, S. pneumoniae infected alveolar macrophages were incubated in the presence of gentamicin (20 μg/ml) to exclude CFU determinations to be affected by extracellular pneumococci.
Lung histopathology
Mice were euthanized and non-lavaged, whole lungs were harvested and immediately submitted to immersion fixation in buffered formalin. After routine embedding, two whole lung-sections from each lung lobe were cut (3 μm sections), stained with hematoxylin/eosin (H/E) and analyzed by an experienced lung pathologist (F.L.) (5) blinded to the respective pre-treatment regimens. For all specimens, the composition and distribution of the inflammatory infiltrate, if present, was analyzed. The presence of lymphocytes, granulocytes and macrophages was semi-quantitatively scored in 10 % increments and their allocation in alveolar spaces, alveolar walls, peribronchial or perivascular tissue was noted. The percentage of bronchovascular bundles with significant inflammatory infiltrates was recorded as well as the density of the infiltrate using a 4-tier score (0=no infiltrate; 1=lymphocytic cuffs less than 5 cell layers thick; 2=thicker lymphocytic cuffs than 5 cell layers; 3=spill over of lymphocytes into the alveolar walls). Furthermore, the presence of bronchopneumonia (florid intra-alveolar granulocytic infiltrate) or a pattern of organizing pneumonia was noted.
ELISA
Cytokine concentrations (GM-CSF, IL-6, TNF-α, KC, MIP-2) were determined in BAL fluids of S. pneumoniae infected or control mice by using commercially available Duo-Set enzyme-linked immunosorbent assays (ELISAs) according to the manufacturer’s instructions (R&D Systems, Wiesbaden. Germany).
Statistics
All data are given as mean ± SEM. Differences between controls and respective treatment groups were analyzed by ANOVA followed by post hoc Dunnett test. Significant differences between groups were analyzed by Levene’s test for equality of variances followed by Student’s t test using SPSS for Windows software package. CFU determinations were analyzed using Mann-Whitney-U test. Survival curves were compared by log-rank test. Statistically significant differences between treatment groups were assumed when P values were < 0.05.
Results
Overexpression of GM-CSF triggers transient leukocyte recruitment into the lungs of mice
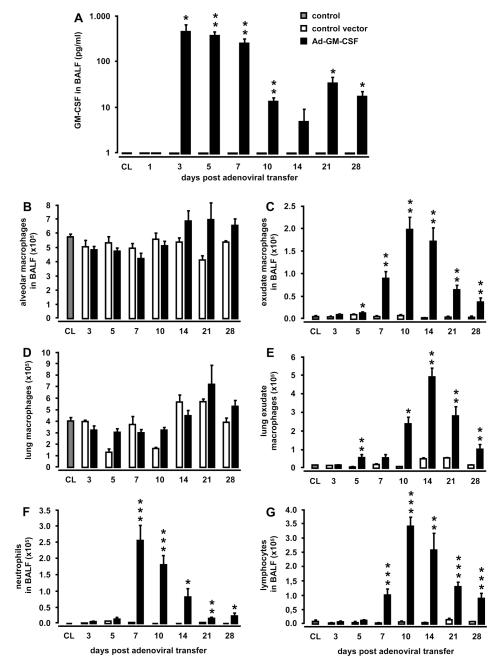
First, we characterized GM-CSF protein levels in BAL fluid in response to adenoviral gene transfer of murine GM-CSF or empty control vector. As shown in Figure 1A, adenoviral gene transfer of GM-CSF rapidly increased GM-CSF protein levels in BAL fluids with peak by day 3, and significantly increased GM-CSF protein levels were still detectable in BAL fluids until day 28. No GM-CSF was detectable in BAL fluids of control vector treated animals (Fig. 1A).
Figure 1. Effect of lung-specific overexpression of GM-CSF on pulmonary leukocyte recruitment.
Mice were treated with either control vector or Ad-GM-CSF (1×108 pfu/mouse) for 1, 3, 5, 7, 10, 14, 21, or 28 days. At indicated time points, mice were subjected to bronchoalveolar lavage and lungs were removed for isolation of lung mononuclear phagocyte subsets. GM-CSF levels were determined in BAL fluids by ELISA (A). Recruitment profiles of resident alveolar macrophages (B) or newly recruited alveolar exudate macrophages in BAL fluids (C), or resident or recruited lung macrophages (D, E), as well as BAL fluid neutrophils (F), and lymphocytes (G) were determined. Values are shown as mean ± SEM for n=5-9 mice per time point and treatment group. * (**, ***) indicates significant difference of p<0.05 (p<0.01, p<0.001) relative to control vector treated mice.
Next, we analyzed the impact of transient GM-CSF overexpression on pulmonary leukocyte recruitment by determining changes in the leukocyte population in BAL fluid. As shown in Fig. 1B-E, recruitment of exudate macrophages into the bronchoalveolar and lung parenchymal compartment was substantially increased in mice receiving Ad-GM-CSF compared to control vector treated mice, reaching significantly increased numbers by day 5 until day 28 post-treatment (Fig. 1C, E). Moreover, intraalveolar GM-CSF release also led to increased numbers of neutrophils (Fig. 1F) and lymphocytes (Fig. 1G) accumulating in the alveoli, with peak cell numbers noted by days 7-10 (Fig. 1F,G), whereas numbers of eosinophils in BAL fluid of Ad-GM-CSF treated mice were only marginally elevated (data not shown). These changes in the alveolar leukocyte recruitment profile were accompanied by increased expression of IL-6 and KC in BAL fluids of mice receiving Ad-GM-CSF relative to control vector treated mice (Fig. S1A, B, online supplement), whereas Ad-GM-CSF did not lead to increased expression of TNFα, CCL2, IFNγ, and IL-12 in BAL fluids of mice (data not shown). No GM-CSF was detectable in the serum of Ad-GM-CSF treated mice and no significant differences were observed in blood neutrophil and lymphocyte counts or numbers of resident or inflammatory monocyte subsets between Ad-GM-CSF treated and control vector treated mice (data not shown).
Transient intra-alveolar overexpression of GM-CSF triggers sustained STAT5 phosphorylation and increased PU.1 protein levels in lung tissue and alveolar macrophages
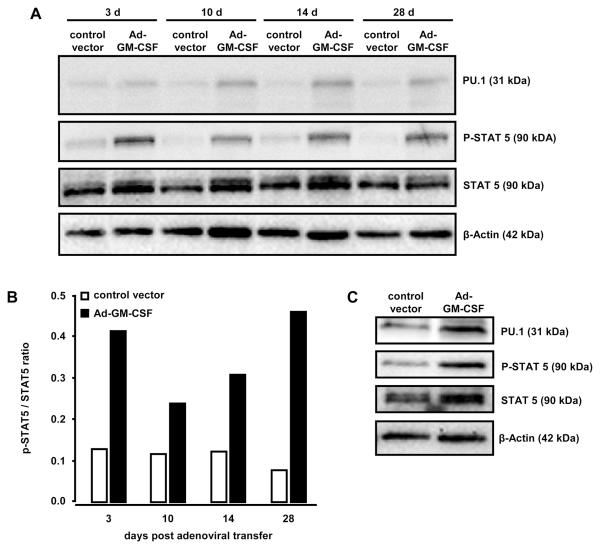
Western blot analysis of whole lung tissue demonstrated increased PU.1 protein expression and increased STAT5 phosphorylation starting as early as day 3 up to day 28 following GM-CSF gene transfer (Fig. 2A, B). To determine the role of alveolar macrophages in this process, alveolar macrophages were flow-sorted from BAL fluid of Ad-GM-CSF or control vector treated mice and their STAT5 phosphorylation and PU.1 protein levels were determined. As shown in Fig. 2C, GM-CSF overexpression resulted in increased PU.1 expression and STAT5 phosphorylation in alveolar macrophages by day 14. FACS analysis of alveolar macrophages from Ad-GM-CSF treated mice demonstrated upregulation of TLR2 and CD11b cell surface expression, both of which are known to be dependent on functional PU.1 (data not shown). Collectively, these data show that intratracheal Ad-GM-CSF delivery leads to sustained increased PU.1 expression and STAT5 signaling in lung tissue of mice (up to 28 days), which is associated with increased GM-CSF-dependent accumulation and activation of alveolar and lung macrophages (Fig. 2A, B).
Figure 2. Pulmonary PU.1 expression and STAT 5 phosphorylation in the lungs of mice transiently overexpressing GM-CSF.
Mice were treated with either control vector or Ad-GM-CSF (1×108pfu/mouse) for 3, 10, 14, or 28 days, as indicated. Subsequently, mice were sacrificed and immunoblot analysis of PU.1 expression or STAT5 phosphorylation was performed in whole lung lysates (A, B) or in lysates of alveolar macrophages 14 days after adenoviral transfer of GM-CSF (C). β-actin was used to control for similar protein loading of the gel (A, C). (B) Fold change of STAT5 phosphorylation relative to unphosphorylated STAT5 in Ad-GM-CSF versus control vector pre-treated mice. The western blots shown are representative of 3 independently performed experiments.
Effect of transient pulmonary overexpression of GM-CSF on lung protective immunity against focal pneumonia-inducing S. pneumoniae strain
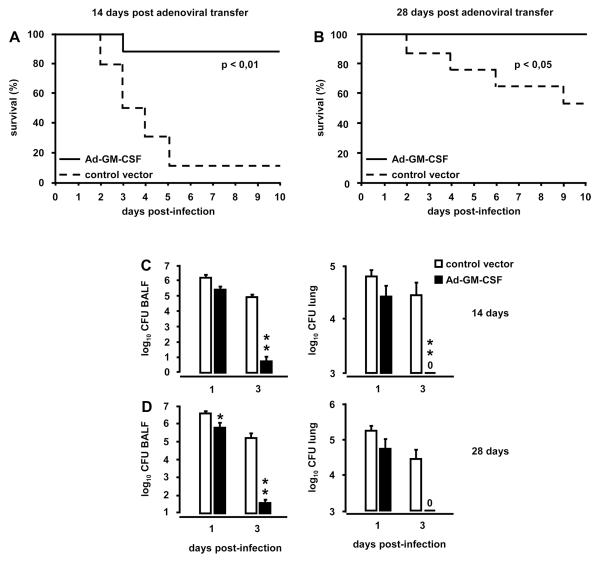
To determine whether prophylactic administration of Ad-GM-CSF is able to enhance protective immunity against S. pneumoniae infections, mice were treated with Ad-GM-CSF or control vector followed by infection with focal pneumonia-inducing serotype 19 S. pneumoniae on days 3, 14, or 28 post gene delivery. Mice that were infected with S. pneumoniae on day 3 after Ad-GM-CSF exhibited significantly reduced bacterial loads in BAL fluids and lungs at day 3 post-infection (CFU in BAL fluids of control vector-treated mice: 7.3×105 ± 3.7×105 CFU versus Ad-GM-CSF treated mice: 1.4×104 ± 9.5×103 CFU; n=6; p=0.03; CFU in lung tissue of control vector-treated mice: 1.2×105 ± 6.0×104 CFU versus Ad-GM-CSF treated mice: 2.0×103 ± 1.7×103 CFU; n=6; p=0.02). Importantly, mice infected with S. pneumoniae at day 14 after Ad-GM-CSF treatment had an overall survival of 90 %, whereas the survival of control vector treated mice was just 10 % (Fig. 3A, p<0.01). Importantly, infection of mice with serotype 19 S. pneumoniae at day 28 post GM-CSF delivery resulted in 100 % survival relative to ~ 50 % survival noted in control vector treated mice (Fig. 3B, p<0.05).
Figure 3. Survival and bacterial pathogen elimination in Ad-GM-CSF treated mice challenged with S. pneumoniae.
Mice were pretreated with either control vector or Ad-GM-CSF (1×108 pfu/mouse). At day 14 mice were infected with serotype 19 S. pneumoniae (~ 1.3×107 CFU/mouse; n=10 mice per group) (A) or were infected with S. pneumoniae (~1.1×107 CFU/mouse; n=10 mice per group) at day 28 (B). The time point of pneumococcal infection is indicated as day 0 (A, B) and corresponds to day 14 (A) or 28 (B) post control vector or Ad-GM-CSF treatment. Survival was monitored during an observation period of 10 days. In separate experiments, bacterial loads were determined in bronchoalveolar lavage fluids and lung parenchymal tissue of mice pretreated for 14 or 28 days with Ad-GM-CSF or control vector prior to infection with serotype 19 S. pneumoniae (~1×107 CFU/mouse), as indicated (C, D). Values are shown as mean ± SEM for n=5-9 mice per time point and treatment group. * (**, ***) indicates significant difference of p<0.05 (p<0.01, p<0.001) relative to control vector treated mice.
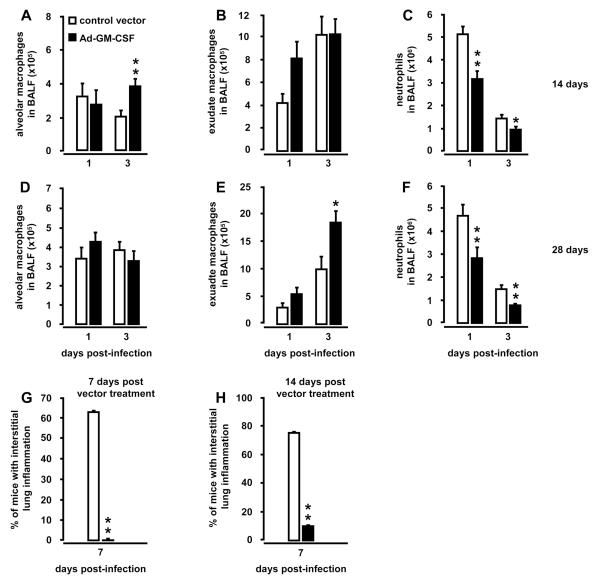
Increased survival in serotype 19 S. pneumoniae-infected mice was accompanied by significantly improved pneumococcal clearance on days 1 and 3 post-infection in BAL fluids and lungs of mice pretreated with Ad-GM-CSF for 14 days or 28 days (Fig. 3 C, D). Closer examination of leukocytic responses revealed that mice infected with S. pneumoniae at day 14 after Ad-GM-CSF had significantly increased numbers of alveolar macrophages and exudate macrophages on day 1 and 3 post-infection, respectively (Fig. 4A, B), whereas neutrophil recruitment profiles at the same time were strongly reduced on days 1 and 3 post-infection (Fig. 4C). Mice infected with S. pneumoniae on day 28 after Ad-GM-CSF showed overall similar numbers of alveolar macrophages on days 1 and 3 post-infection (Fig. 4D). However, numbers of exudate macrophages were significantly increased on day 3 (Fig. 4E), while recruited neutrophils were significantly reduced in the alveolar lumen by days 1 and 3 post-infection (Fig. 4F). Expression of proinflammatory CXC cytokines KC and MIP-2 as well as IL-6 and TNF-α were significantly reduced in S. pneumoniae-infected mice pretreated with Ad-GM-CSF at day 14 or 28 prior to infection (Fig. S2, online supplement). Notably, further histopathological examination of lung tissue revealed substantially reduced interstitial inflammation in mice pretreated with Ad-GM-CSF for 7 or 14 days followed by infection with S. pneumoniae for seven days (Fig. 4G, H). Collectively, these data demonstrate that pulmonary overexpression of GM-CSF improves lung protective immunity against non-invasive S. pneumoniae, suggesting a major role for intra-alveolar GM-CSF in enhancing local rather than systemic host defense mechanisms.
Figure 4. Alveolar leukocyte recruitment profiles in Ad-GM-CSF pretreated mice subsequently infected with S. pneumoniae.
Mice were pretreated with either control vector or Ad-GM-CSF (1×108 pfu/mouse) for 14 days (A-C) or 28 days (D-F) prior to infection with serotype 19 S. pneumoniae (1×107 CFU/mouse). At indicated time points, mice of the respective treatment groups were subjected to bronchoalveolar lavage for determination of resident alveolar macrophages (A, D), or newly recruited exudate macrophages (B, E), or neutrophils (C, F). (G, H) Histopathological assessment of interstitial lung inflammation in hematoxylin/eosin (HE)-stained lung tissue sections of mice either pretreated with control vector or Ad-GM-CSF vector for 7 days (G) or 14 days (H) followed by infection with serotype 19 S. pneumoniae (1×107 CFU/mouse) for 7 days. Values are shown as mean ± SEM for n=5-9 mice per time point and treatment group. * (**, ***) indicates significant differences of p<0.05 (p<0.01, p<0.001) relative to control vector treated mice.
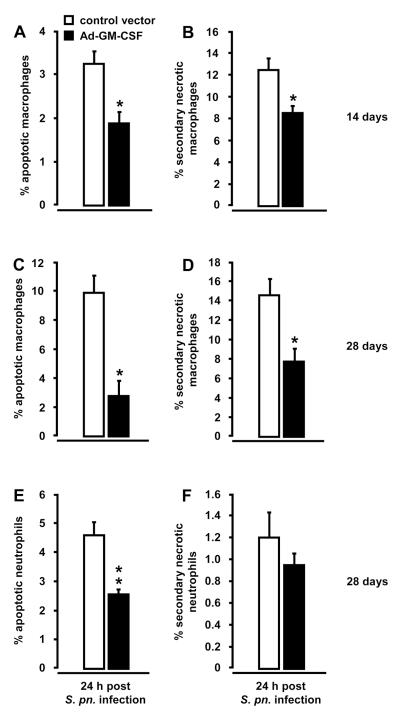
Lung-specific overexpression of GM-CSF protects pulmonary macrophages and neutrophils from S. pneumoniae-induced apoptosis
Infections of the lung with S. pneumoniae are known to rapidly induce apoptosis in resident alveolar macrophages within 24 hours, thereby transiently depleting alveolar macrophages from distal airspaces (5, 27-28, 30, 38-40). Therefore, we examined whether pretreatment of mice with Ad-GM-CSF would protect lung macrophages from S. pneumoniae-induced apoptosis. As shown in Fig. 5, transfer of the GM-CSF gene 14 or 28 days prior to infection with S. pneumoniae significantly reduced the percentage of primary apoptotic macrophages (annexin V positive) as well as secondary necrotic macrophages (annexin V positive and propidium iodide positive) in BAL fluids 24 h post-infection (Fig. 5A, B, C, and D). No significant differences were observed in the percentage of necrotic macrophages between both groups (data not shown). In addition, the percentage of apoptotic neutrophils, but not secondary necrotic neutrophils, was significantly reduced in BAL fluids of mice pretreated with Ad-GM-CSF for 28 days followed by 24 hours of pneumococcal challenge (Fig. 5E, F). No differences were observed in the percentage of apoptotic and secondary necrotic neutrophils in S. pneumoniae challenged mice pretreated with Ad-GM-CSF for 14 days (data not shown).
Figure 5. Apoptosis and necrosis induction in Ad-GM-CSF treated mice subsequently infected with S. pneumoniae.
Mice were pretreated with either control vector or Ad-GM-CSF (1×108 pfu/mouse) for 14 days (A, B) or 28 days (C, D, E, F) prior to infection with serotype 19 S. pneumoniae (1×107 CFU/mouse). At 24 hours post-infection, mice of the respective treatment groups were subjected to bronchoalveolar lavage and percentage of apoptosis (annexin V-positive; A, C, E) and secondary necrosis (annexin V-positive and propidium iodide-positive; B, D, F) was determined by flow cytometry. Values are shown as mean ± SEM for n=6 mice per time point and treatment group. * indicates significant difference (p<0.05) relative to control vector treated mice. The shown experiment is representative of three independent determinations.
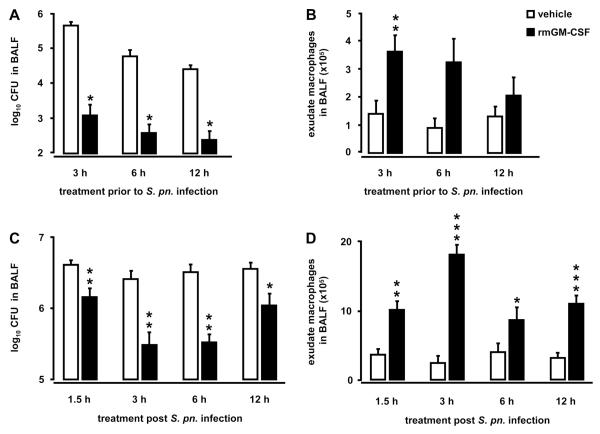
Prophylactic and therapeutic delivery of mice with recombinant GM-CSF improves lung innate immunity against S. pneumoniae
After having demonstrated a protective role for Ad-GM-CSF against pulmonary infections with non-invasive S. pneumoniae, we examined whether intratracheal application of recombinant murine GM-CSF would also improve antibacterial responses in the lungs of mice. We first treated mice orotracheally with GM-CSF (10 μg/mouse) at 3, 6, and 12 hours prior to infection with non-invasive serotype 19 S. pneumoniae. As shown in Fig. 6, we found significantly decreased bacterial loads in BAL fluids of GM-CSF pretreated mice compared to vehicle treated mice at 24 h post-infection with S. pneumoniae (Fig. 6A). At the same time, numbers of exudate macrophages were increased in GM-CSF pretreated mice compared to vehicle treated mice 24 h post-infection (Fig. 6B), whereas no significant differences in numbers of alveolar neutrophils were observed (Fig. S3A, online supplement). Notably, subcutaneous pretreatment of mice for 6 days with GM-CSF (10 μg/mouse/d) followed by pneumococcal challenge did not show any differences in bacterial clearance and pulmonary leukocyte recruitment relative to vehicle treated mice (data not shown), suggesting that intratracheal rather than systemic delivery of GM-CSF is important to enhance lung protective immunity against inhaled bacterial pathogens.
Figure 6. Prophylactic and therapeutic treatment of S. pneumoniae-infected mice with recombinant murine GM-CSF.
For prophylactic GM-CSF treatment, mice were treated orotracheally with either vehicle (white bars) or recombinant murine GM-CSF (10 μg/mouse, black bars) for 3, 6, or 12 hours prior to infection with serotype 19 S. pneumoniae (5×106 CFU/mouse) (A, B). For therapeutic GM-CSF treatment, mice were infected with serotype 19 S. pneumoniae (5×106 CFU/mouse) followed by therapeutic application of either vehicle or recombinant murine GM-CSF (20 μg/mouse) applied at 1.5, 3, 6, or 12 hours post-infection (C, D). At 24 hours post-infection, mice were subjected to bronchoalveolar lavage and bacterial loads (A, C), or newly recruited exudate macrophages (B, D) were quantified in BAL fluids. Values are shown as mean ± SEM for n=5-9 mice per time point and treatment group. * (**) indicates significant difference of p<0.05 (p<0.01) relative to vehicle treated mice.
Next, we examined the therapeutic potential of intratracheally applied GM-CSF to improve lung innate immunity against S. pneumoniae infections in mice. Therapeutic treatment of mice with recombinant murine GM-CSF (20 μg/mouse) applied at 1.5, 3, 6 or 12 hours after pneumococcal infection led to significantly increased pneumococcal clearance at 24 h (Fig. 6C) and 48 h post-infection (data not shown). Therapeutic treatment of pneumococcal pneumonia with GM-CSF was most effective when initiated at 3-6 hours post-infection with S. pneumoniae (Fig. 6C), resulting in significantly reduced bacterial loads in BAL fluids of mice analyzed at 24 h post pneumococcal infection (Fig. 6C) and even at 48 h post pneumococcal infection (vehicle application at 3 h post-infection: 2.7×104 ± 2.6×103 CFU in BALF; GM-CSF application at 3 h post-infection: 9.3×102 ± 5.7×102 CFU in BALF; p<0.05). Under these experimental conditions, we observed significantly increased GM-CSF protein levels in BAL fluids of mice after 24 h and even 48 h post pneumococcal infection (24 h post-infection: vehicle treated mice: 0.01 ± 0.006 ng/ml; GM-CSF treated mice: 59.8 ± 17.0 ng/ml; 48 h post-infection: vehicle treated mice: 0 ng/ml; GM-CSF treated mice: 1.2 ± 0.5 ng/ml). At the same time, numbers of exudate macrophages were significantly increased in mice treated with GM-CSF at all investigated time points post pneumococcal infection (Fig. 6D), whereas GM-CSF therapy of pneumococcal pneumonia did not induce increased neutrophilic alveolitis in mice (Fig, S3B, online supplement).
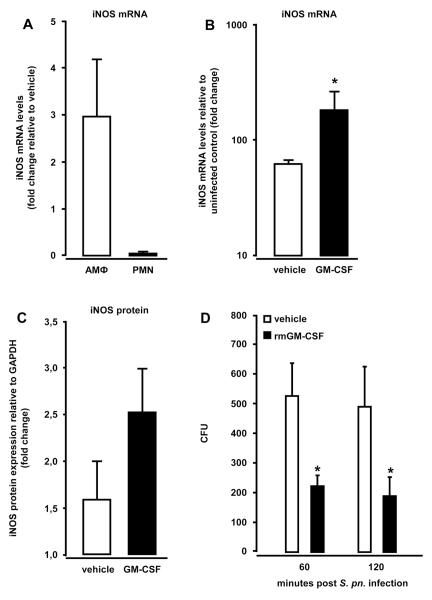
Treatment of mice with GM-CSF triggers increased iNOS expression and antibacterial activity in alveolar macrophages
To elucidate whether GM-CSF-induced activation of alveolar macrophages directly improved their bactericidal functions, we analyzed iNOS gene expression in flow-sorted macrophages and neutrophils collected from the lungs of S. pneumoniae-infected mice therapeutically treated with GM-CSF. As shown in Fig. 7, iNOS mRNA levels (Fig. 7B) and protein expression (Fig. 7C) were significantly increased in sorted macrophages of S. pneumoniae-infected mice treated with GM-CSF at 3 h post-infection. Notably, treatment of mice with GM-CSF increased iNOS mRNA levels in sorted macrophages by six-fold relative to alveolar macrophages of vehicle treated, S. pneumoniae-infected mice (Fig. 7B). Moreover, sorted macrophages showed a strongly increased upregulation of iNOS mRNA relative to sorted neutrophils from GM-CSF treated mice (Fig. 7A). In vitro pneumococcal killing assays with primary alveolar macrophages revealed a significantly improved bacterial killing at 60 and 120 min post-infection in the presence of recombinant GM-CSF (100 ng/ml) (Fig.7D). Collectively, these data show that therapeutic treatment of S. pneumoniae-infected mice with GM-CSF particularly triggers increased iNOS expression in alveolar macrophages but not neutrophils, and induces increased bactericidal activities in alveolar macrophages, thereby contributing to GM-CSF-induced improved lung innate immunity to this prototypic lung bacterial pathogen.
Figure 7. Effect of intra-alveolar therapeutic GM-CSF application on iNOS expression in flow-sorted alveolar macrophages after S. pneumoniae infection.
Mice were infected with serotype 19 S. pneumoniae (5×106 CFU/mouse) followed by intra-alveolar delivery of recombinant mGM-CSF (20 μg/mouse) or vehicle at 3 hours post-infection. At 6 hours post-infection, mice were subjected to bronchoalveolar lavage, and flow-sorted alveolar macrophages and neutrophils were subjected to real-time RT-PCR (A, B) or western blot analysis of iNOS mRNA or protein expression (C), respectively. (A) Fold change in iNOS mRNA levels in S. pneumoniae-infected mice treated with GM-CSF relative to S. pneumoniae-infected, vehicle treated mice. (B) Fold change in iNOS mRNA levels of S. pneumoniae-infected vehicle-versus GM-CSF-treated mice relative to uninfected mice. (C) iNOS protein expression in flow-sorted alveolar macrophages of mice treated with GM-CSF (black bars) or vehicle (white bars) at 3 h after infection with S. pneumoniae for 24 h. iNOS protein is expressed as fold change relative to GAPDH. Data in A-C are shown as mean±SEM of n=3 mice per treatment group. (D) Pneumococcal killing by alveolar macrophages infected with serotype 19 S. pneumoniae (MOI 50) for 30 minutes, followed by incubation with or without GM-CSF for the indicated time points. Values are shown as mean ± SEM of triplicate determinations, and the experiment was repeated twice.
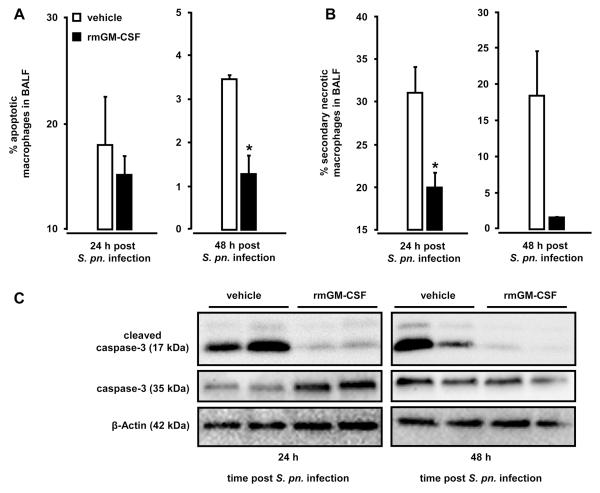
Treatment of mice with GM-CSF protects alveolar macrophages from S. pneumoniae-induced apoptosis
GM-CSF has been shown to be an important survival factor for leukocytes. Therefore, we next questioned whether therapeutic treatment of mice with GM-CSF would render alveolar macrophages more resistant to S. pneumoniae-induced apoptosis, similar to our observations made in Ad-GM-CSF treated mice. As shown in Fig. 8, we found that S. pneumoniae-infected mice therapeutically treated with GM-CSF at 3 hours post-infection demonstrated significantly reduced numbers of apoptotic and secondary necrotic macrophages in their bronchoalveolar space both at 24 hours and 48 hours post-infection (Fig. 8A, B). This reduced apoptotic rate and secondary necrosis induction in macrophages was due to substantially suppressed caspase 3 cleavage induction in alveolar macrophages of GM-CSF treated but not vehicle treated mice (Fig. 8C).
Figure 8. Apoptosis and secondary necrosis in alveolar macrophages of S. pneumoniae-infected mice after therapeutic treatment with GM-CSF.
Mice were infected with serotype 19 S. pneumoniae (5×106 CFU/mouse) followed by intratracheal treatment with either vehicle (white bars) or recombinant murine GM-CSF (20 μg/mouse, black bars) at 3 hours post-infection. At 24 hours and 48 hours post pneumococcal challenge, mice were subjected to bronchoalveolar lavage and the percentage of apoptotic (A) and secondary necrotic (B) alveolar macrophages was determined by flow cytometry. (C) Immunoblot analysis of caspase 3 and cleaved caspase 3 expression in BAL cells of S. pneumoniae-infected mice treated with vehicle or GM-CSF at 3 h post-infection, as indicated. β-actin was used to control for similar protein loading of the gel (C). The given western blot is representative of 3 independent experiments. Values are shown as mean ± SEM for n=3 mice per time point and treatment group and experiments were repeated twice with similar results. * indicates significant difference of p<0.05 relative to vehicle treated mice.
Discussion
In this study we evaluated the potential of GM-CSF to improve protective immunity against the major lung pathogen S. pneumoniae in vivo, which would be of particular benefit to immunocompromized patients. For the first time, we show that GM-CSF in the lung protects mice from lethal pneumococcal pneumonia, using adenoviral mediated gene transfer of GM-CSF either 14 or even 28 days before infection. More specifically, intra-alveolar GM-CSF caused a sustained increase of PU.1 protein levels and STAT5 signaling in lung sentinel cells, particularly alveolar macrophages, for a period of 28 days, and enhanced macrophage-dependent iNOS induction in response to infection. It is well established that increased iNOS expression contributes to antimicrobial activity of alveolar macrophages against pneumococci and is needed for effective lung antibacterial activity during pneumococcal pneumonia (41-43). In line with this, we found that macrophage activation mediated by intra-alveolar GM-CSF was associated with a reduction of bacterial loads in the lungs of mice challenged with S. pneumoniae. With increased GM-CSF bioavailability in the lungs, the leukocytic recruitment profiles shifted from a neutrophil-dominated towards a macrophage-rich phenotype during infection. This was accompanied by a substantially decreased release of proinflammatory cytokines and neutrophil chemoattractants, along with reduced apoptosis and secondary necrosis of macrophages. Reduced apoptosis rates were also observed in mice treated with recombinant GM-CSF. Together, we show here that prophylactic administration of GM-CSF in a mouse model of pneumococcal pneumonia results in a shift from a neutrophil-dominated towards a macrophage-rich inflammatory response with long lasting effects on bacterial clearance and survival. These data support a strong immunostimulatory effect of intra-alveolar GM-CSF augmenting lung innate immunity against bacterial pneumonia in mice.
GM-CSF was initially described in 1977 as a factor contained in conditioned medium from mouse lung mediating growth and survival of professional phagocytes derived from hematopoietic progenitors (44). Since then, multiple studies have characterized GM-CSF as a pleiotropic growth factor and cytokine with a wide range of biological activities under normal and disease conditions (9). Studies in GM-CSF knockout and transgenic mice revealed the critical importance of GM-CSF in surfactant homeostasis and its importance in antibacterial defense against a wide range of bacterial, viral and fungal pathogens (14-24, 45). Like G-CSF, GM-CSF is frequently used as adjuvant therapy in patients with hematological malignancies to augment bone marrow stem cell engraftment and expansion subsequent to whole body irradiation (46). GM-CSF is further employed as a valuable adjuvant therapy to compensate for autoantibody-induced endogenous GM-CSF neutralization in patients with pulmonary alveolar proteinosis (47-49). More recently, delivery of recombinant human GM-CSF by inhalation was found to be effective for the treatment of human pulmonary alveolar proteinosis, and at the same time was also well tolerated by healthy control individuals (50). Only very limited data are available that characterize the usefulness of prophylactic GM-CSF as a novel approach to augment antibacterial host defense mechanisms particularly in immunocompromized patients (45). Individuals with increased risk of acquiring lung bacterial infections include HIV patients, or patients with hematological malignancies undergoing whole body irradiation regimen, or lung transplant recipients. To date, preventive strategies are limited to vaccination approaches against major lung pathogens such as S. pneumoniae, with clinical protection rates of no more than 60-70 %. This suboptimal situation underscores the need for novel adjuvant therapies. Therefore, we evaluated whether or not prophylactic GM-CSF has the potential to enhance protective immunity against pneumococcal pneumonia in a mouse model. We found that 90 to 100 % of the mice survived otherwise lethal pneumococcal pneumonia when pretreated with Ad-GM-CSF 14 and 28 days prior to infection with S. pneumoniae. Prophylactic administration of recombinant GM-CSF was only effective in improving lung innate immunity against pneumococcal pneumonia when given by inhalation, whereas systemic delivery had no protective effects in our studies.
The protective efficacy of inhaled GM-CSF against pneumococci was limited to those serotypes causing localized focal pneumonia (serotype 19) but did not prevent the development of invasive pneumococcal disease caused by sepsis-inducing serotype 2 S. pneumoniae (data not shown). This finding may be due to different time kinetics by which intratracheally delivered GM-CSF triggers antibacterial activities in professional phagocytes relative to the short time that serotype 2 S. pneumoniae needs to cause invasive pneumococcal disease. Consistent with our experimental data, a clinical trial in preterm neonates also showed that systemic administration of GM-CSF is not able to reduce sepsis rates or improve survival (51). In contrast, intravenous infusion of GM-CSF in patients with severe sepsis and respiratory failure significantly improved oxygenation and had a positive impact on the development of ARDS, which was associated with improved function of blood and alveolar neutrophils (52). This suggests that GM-CSF is most effective when administered directly to the site of infection, which may explain why systemic delivery of GM-CSF was not protective in mice with pneumococcal pneumonia. Further studies are needed to evaluate the efficacy of intravenous GM-CSF on sepsis rates in mice infected with sepsis-inducing, highly virulent serotype 2 S. pneumoniae.
We observed that pulmonary overexpression of GM-CSF shifted the leukocyte recruitment profiles from a neutrophil-dominated towards a macrophage-dominated phenotype during infection with S. pneumoniae. This shift was characterized by reduced release of proinflammatory cytokines and chemokines along with decreased numbers of apoptotic and secondary necrotic macrophages. In addition, prophylactic overexpression of GM-CSF prior to pneumococcal infection substantially reduced interstitial lung inflammation and neutrophilic alveolitis subsequent to pneumococcal challenge. Both secondary necrotic macrophages and secondary necrotic neutrophils contribute to increased systemic inflammatory responses to bacterial challenge, which in turn promotes lung tissue damage and increased death in mice and humans (5, 27, 53). Thus, we believe that the substantial GM-CSF-mediated inhibition of apoptosis and secondary necrosis in alveolar macrophages contributes to the overall dampened proinflammatory immune responses resulting in overall increased survival of pneumococcal pneumonia in our model.
In the current study, we observed a macrophage-dominated lung innate immune response to pneumococcal pneumonia in mice prophylactically exposed to Ad-GM-CSF, resulting in increased survival. In this regard, we and others (5, 54) recently reported that in a condition where exudate macrophages accumulate in the alveolar space, e.g. in CCL2 transgenic mice, lung bacterial loads are substantially reduced, along with dampened pulmonary inflammation and improved survival (5). In addition, Schabbauer and colleagues recently demonstrated in myeloid-cell PTEN-deficient mice that reduced numbers of lung-infiltrating neutrophils were offset by enhanced phagocytic properties of alveolar macrophages, resulting in improved bacterial clearance and augmented resolution of inflammation in response to S. pneumoniae (54). Whether or not the shift from a neutrophil- towards a macrophage-dominated immune response as observed in GM-CSF-pretreated or PTEN KO mice represents a principal mechanism by which lung pathology caused by excessive inflammatory response following bacterial infections might be attenuated to improve survival warrants further investigation.
In the current study, adenoviral vectors were just employed as an efficient gene delivery system with specificity for bronchial and alveolar epithelial cells, leading to strong transgene expression specifically within the bronchoalveolar compartment of mice. Clinically, prophylactic inhalation of GM-CSF might be an effective adjuvant in the management of pulmonary infections with multidrug-resistant bacterial pathogens, where GM-CSF therapy could be used in combination with newer antibiotics such as daptomycin, which per se has limited antibacterial efficacy in the bronchoalveolar space of mice and humans (55). Repetitive inhaled GM-CSF applications could be considered for stimulation of innate immune responses in patients at risk of acquiring bacterial infections (50). We found prophylactic intra-alveolar deposition of GM-CSF to exert a long-lasting protection against pneumococcal pneumonia, while therapeutic administration of recombinant GM-CSF was less effective when initiated at later time points post-infection. Therefore, we believe that particularly prophylactic application of GM-CSF might be useful for prevention of pneumococcal pneumonia in immunocompromized patients.
In summary, we show for the first time that prophylactic delivery of GM-CSF triggers long-lasting immunostimulatory effects in the murine lung and protects mice from lethal pneumococcal pneumonia. These data may direct the development of novel antibiotic-independent immunostimulatory therapies to protect immunocom-promized patients against bacterial pneumonias.
Supplementary Material
Footnotes
This study has been supported by the German Research Foundation, grant SFB 587, to UAM and TW.
References
- 1.Akins RL, Haase KK. Gram-positive resistance: pathogens, implications, and treatment options: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2005;25:1001–1010. doi: 10.1592/phco.2005.25.7.1001. [DOI] [PubMed] [Google Scholar]
- 2.Ortqvist A, Hedlund J, Kalin M. Streptococcus pneumoniae: epidemiology, risk factors, and clinical features. Semin Respir Crit Care Med. 2005;26:563–574. doi: 10.1055/s-2005-925523. [DOI] [PubMed] [Google Scholar]
- 3.Reinert RR. The antimicrobial resistance profile of Streptococcus pneumoniae. Clin Microbiol Infect. 2009;15(Suppl 3):7–11. doi: 10.1111/j.1469-0691.2009.02724.x. [DOI] [PubMed] [Google Scholar]
- 4.Denzel A, Maus UA, Rodriguez Gomez M, Moll C, Niedermeier M, Winter C, Maus R, Hollingshead S, Briles DE, Kunz-Schughart LA, Talke Y, Mack M. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 5.Winter C, Taut K, Srivastava M, Langer F, Mack M, Briles DE, Paton JC, Maus R, Welte T, Gunn MD, Maus UA. Lung-specific overexpression of CC chemokine ligand (CCL) 2 enhances the host defense to Streptococcus pneumoniae infection in mice: role of the CCL2-CCR2 axis. J Immunol. 2007;178:5828–5838. doi: 10.4049/jimmunol.178.9.5828. [DOI] [PubMed] [Google Scholar]
- 6.Clement CG, Evans SE, Evans CM, Hawke D, Kobayashi R, Reynolds PR, Moghaddam SJ, Scott BL, Melicoff E, Adachi R, Dickey BF, Tuvim MJ. Stimulation of lung innate immunity protects against lethal pneumococcal pneumonia in mice. Am J Respir Crit Care Med. 2008;177:1322–1330. doi: 10.1164/rccm.200607-1038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metcalf D. Hematopoietic cytokines. Blood. 2008;111:485–491. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey B, Trapnell BC. The molecular basis of pulmonary alveolar proteinosis. Clin Immunol. 2010;135:223–235. doi: 10.1016/j.clim.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trapnell BC, Whitsett JA. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 10.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15:557–567. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Yamada Y, Nakata K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Moczygemba M, Doan ML, Elidemir O, Fan LL, Cheung SW, Lei JT, Moore JP, Tavana G, Lewis LR, Zhu Y, Muzny DM, Gibbs RA, Huston DP. Pulmonary alveolar proteinosis caused by deletion of the GM-CSFRalpha gene in the X chromosome pseudoautosomal region 1. J Exp Med. 2008;205:2711–2716. doi: 10.1084/jem.20080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki T, Sakagami T, Rubin BK, Nogee LM, Wood RE, Zimmerman SL, Smolarek T, Dishop MK, Wert SE, Whitsett JA, Grabowski G, Carey BC, Stevens C, van der Loo JC, Trapnell BC. Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. J Exp Med. 2008;205:2703–2710. doi: 10.1084/jem.20080990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 15.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballinger MN, Paine R, 3rd, Serezani CH, Aronoff DM, Choi ES, Standiford TJ, Toews GB, Moore BB. Role of granulocyte macrophage colony-stimulating factor during gram-negative lung infection with Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 2006;34:766–774. doi: 10.1165/rcmb.2005-0246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berclaz PY, Zsengeller Z, Shibata Y, Otake K, Strasbaugh S, Whitsett JA, Trapnell BC. Endocytic internalization of adenovirus, nonspecific phagocytosis, and cytoskeletal organization are coordinately regulated in alveolar macrophages by GM-CSF and PU.1. J Immunol. 2002;169:6332–6342. doi: 10.4049/jimmunol.169.11.6332. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Juarrero M, Hattle JM, Izzo A, Junqueira-Kipnis AP, Shim TS, Trapnell BC, Cooper AM, Orme IM. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J Leukoc Biol. 2005;77:914–922. doi: 10.1189/jlb.1204723. [DOI] [PubMed] [Google Scholar]
- 19.LeVine AM, Reed JA, Kurak KE, Cianciolo E, Whitsett JA. GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infection. J Clin Invest. 1999;103:563–569. doi: 10.1172/JCI5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paine R, 3rd, Preston AM, Wilcoxen S, Jin H, Siu BB, Morris SB, Reed JA, Ross G, Whitsett JA, Beck JM. Granulocyte-macrophage colony-stimulating factor in the innate immune response to Pneumocystis carinii pneumonia in mice. J Immunol. 2000;164:2602–2609. doi: 10.4049/jimmunol.164.5.2602. [DOI] [PubMed] [Google Scholar]
- 21.Riopel J, Tam M, Mohan K, Marino MW, Stevenson MM. Granulocyte-macrophage colony-stimulating factor-deficient mice have impaired resistance to blood-stage malaria. Infect Immun. 2001;69:129–136. doi: 10.1128/IAI.69.1.129-136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seymour JF, Lieschke GJ, Grail D, Quilici C, Hodgson G, Dunn AR. Mice lacking both granulocyte colony-stimulating factor (CSF) and granulocyte-macrophage CSF have impaired reproductive capacity, perturbed neonatal granulopoiesis, lung disease, amyloidosis, and reduced long-term survival. Blood. 1997;90:3037–3049. [PubMed] [Google Scholar]
- 23.Wu H, Suzuki T, Carey B, Trapnell BC, McCormack FX. Keratinocyte growth factor augments pulmonary innate immunity through epithelium-driven, GM-CSF-dependent paracrine activation of alveolar macrophages. J Biol Chem. 2011;286:14932–14940. doi: 10.1074/jbc.M110.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhan Y, Lieschke GJ, Grail D, Dunn AR, Cheers C. Essential roles for granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF in the sustained hematopoietic response of Listeria monocytogenes-infected mice. Blood. 1998;91:863–869. [PubMed] [Google Scholar]
- 25.Briles DE, Hollingshead SK, Paton JC, Ades EW, Novak L, van Ginkel FW, Benjamin WH., Jr. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J Infect Dis. 2003;188:339–348. doi: 10.1086/376571. [DOI] [PubMed] [Google Scholar]
- 26.Henken S, Bohling J, Ogunniyi AD, Paton JC, Salisbury VC, Welte T, Maus UA. Evaluation of biophotonic imaging to estimate bacterial burden in mice infected with highly virulent compared to less virulent Streptococcus pneumoniae serotypes. Antimicrob Agents Chemother. 2010;54:3155–3160. doi: 10.1128/AAC.00310-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maus UA, Backi M, Winter C, Srivastava M, Schwarz MK, Ruckle T, Paton JC, Briles D, Mack M, Welte T, Maus R, Bohle RM, Seeger W, Rommel C, Hirsch E, Lohmeyer J, Preissner KT. Importance of phosphoinositide 3-kinase gamma in the host defense against pneumococcal infection. Am J Respir Crit Care Med. 2007;175:958–966. doi: 10.1164/rccm.200610-1533OC. [DOI] [PubMed] [Google Scholar]
- 28.Taut K, Winter C, Briles DE, Paton JC, Christman JW, Maus R, Baumann R, Welte T, Maus UA. Macrophage Turnover Kinetics in the Lungs of Mice Infected with Streptococcus pneumoniae. Am J Respir Cell Mol Biol. 2008;38:105–113. doi: 10.1165/rcmb.2007-0132OC. [DOI] [PubMed] [Google Scholar]
- 29.Herbold W, Maus R, Hahn I, Ding N, Srivastava M, Christman JW, Mack M, Reutershan J, Briles DE, Paton JC, Winter C, Welte T, Maus UA. Importance of CXC chemokine receptor 2 in alveolar neutrophil and exudate macrophage recruitment in response to pneumococcal lung infection. Infect Immun. 2010;78:2620–2630. doi: 10.1128/IAI.01169-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maus UA, Srivastava M, Paton JC, Mack M, Everhart MB, Blackwell TS, Christman JW, Schlondorff D, Seeger W, Lohmeyer J. Pneumolysin-induced lung injury is independent of leukocyte trafficking into the alveolar space. J Immunol. 2004;173:1307–1312. doi: 10.4049/jimmunol.173.2.1307. [DOI] [PubMed] [Google Scholar]
- 31.Maus UA, Koay MA, Delbeck T, Mack M, Ermert M, Ermert L, Blackwell TS, Christman JW, Schlondorff D, Seeger W, Lohmeyer J. Role of resident alveolar macrophages in leukocyte traffic into the alveolar air space of intact mice. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1245–1252. doi: 10.1152/ajplung.00453.2001. [DOI] [PubMed] [Google Scholar]
- 32.Maus UA, Waelsch K, Kuziel WA, Delbeck T, Mack M, Blackwell TS, Christman JW, Schlondorff D, Seeger W, Lohmeyer J. Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: role of the CCL2-CCR2 axis. J Immunol. 2003;170:3273–3278. doi: 10.4049/jimmunol.170.6.3273. [DOI] [PubMed] [Google Scholar]
- 33.Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW, Seeger W, Welte T, Lohmeyer J. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol. 2006;35:227–235. doi: 10.1165/rcmb.2005-0241OC. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava M, Meinders A, Steinwede K, Maus R, Lucke N, Buhling F, Ehlers S, Welte T, Maus UA. Mediator responses of alveolar macrophages and kinetics of mononuclear phagocyte subset recruitment during acute primary and secondary mycobacterial infections in the lungs of mice. Cell Microbiol. 2007;9:738–752. doi: 10.1111/j.1462-5822.2006.00824.x. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava M, Jung S, Wilhelm J, Fink L, Buhling F, Welte T, Bohle RM, Seeger W, Lohmeyer J, Maus UA. The inflammatory versus constitutive trafficking of mononuclear phagocytes into the alveolar space of mice is associated with drastic changes in their gene expression profiles. J Immunol. 2005;175:1884–1893. doi: 10.4049/jimmunol.175.3.1884. [DOI] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Srivastava M, Steinwede K, Kiviranta R, Morko J, Hoymann HG, Langer F, Buhling F, Welte T, Maus UA. Overexpression of cathepsin K in mice decreases collagen deposition and lung resistance in response to bleomycin-induced pulmonary fibrosis. Respir Res. 2008;9:54–66. doi: 10.1186/1465-9921-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahn I, Klaus A, Maus R, Christman JW, Welte T, Maus UA. Dendritic cell depletion and repopulation in the lung after irradiation and bone marrow transplantation in mice. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2010-0279OC. (doi: 10.1165/rcmb.2010-0279OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paton JC. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 1996;4:103–106. doi: 10.1016/0966-842X(96)81526-5. [DOI] [PubMed] [Google Scholar]
- 40.Rubins JB, Charboneau D, Fasching C, Berry AM, Paton JC, Alexander JE, Andrew PW, Mitchell TJ, Janoff EN. Distinct roles for pneumolysin’s cytotoxic and complement activities in the pathogenesis of pneumococcal pneumonia. Am J Respir Crit Care Med. 1996;153:1339–1346. doi: 10.1164/ajrccm.153.4.8616564. [DOI] [PubMed] [Google Scholar]
- 41.Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 43.Kerr AR, Wei XQ, Andrew PW, Mitchell TJ. Nitric oxide exerts distinct effects in local and systemic infections with Streptococcus pneumoniae. Microb Pathog. 2004;36:303–310. doi: 10.1016/j.micpath.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Burgess AW, Camakaris J, Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977;252:1998–2003. [PubMed] [Google Scholar]
- 45.Hebert JC, O’Reilly M. Granulocyte-macrophage colony-stimulating factor (GM-CSF) enhances pulmonary defenses against pneumococcal infections after splenectomy. J Trauma. 1996;41:663–666. doi: 10.1097/00005373-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Metcalf D. The colony-stimulating factors and cancer. Nat Rev Cancer. 2010;10:425–434. doi: 10.1038/nrc2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kavuru MS, Sullivan EJ, Piccin R, Thomassen MJ, Stoller JK. Exogenous granulocyte-macrophage colony-stimulating factor administration for pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2000;161:1143–1148. doi: 10.1164/ajrccm.161.4.9906044. [DOI] [PubMed] [Google Scholar]
- 48.Seymour JF, Presneill JJ, Schoch OD, Downie GH, Moore PE, Doyle IR, Vincent JM, Nakata K, Kitamura T, Langton D, Pain MC, Dunn AR. Therapeutic efficacy of granulocyte-macrophage colony-stimulating factor in patients with idiopathic acquired alveolar proteinosis. Am J Respir Crit Care Med. 2001;163:524–531. doi: 10.1164/ajrccm.163.2.2003146. [DOI] [PubMed] [Google Scholar]
- 49.Venkateshiah SB, Yan TD, Bonfield TL, Thomassen MJ, Meziane M, Czich C, Kavuru MS. An open-label trial of granulocyte macrophage colony stimulating factor therapy for moderate symptomatic pulmonary alveolar proteinosis. Chest. 2006;130:227–237. doi: 10.1378/chest.130.1.227. [DOI] [PubMed] [Google Scholar]
- 50.Tazawa R, Trapnell BC, Inoue Y, Arai T, Takada T, Nasuhara Y, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ishii H, Yokoba M, Tanaka N, Yamaguchi E, Eda R, Tsuchihashi Y, Morimoto K, Akira M, Terada M, Otsuka J, Ebina M, Kaneko C, Nukiwa T, Krischer JP, Akazawa K, Nakata K. Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2010;181:1345–1354. doi: 10.1164/rccm.200906-0978OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carr R, Brocklehurst P, Dore CJ, Modi N. Granulocyte-macrophage colony stimulating factor administered as prophylaxis for reduction of sepsis in extremely preterm, small for gestational age neonates (the PROGRAMS trial): a single-blind, multicentre, randomised controlled trial. Lancet. 2009;373:226–233. doi: 10.1016/S0140-6736(09)60071-4. [DOI] [PubMed] [Google Scholar]
- 52.Presneill JJ, Harris T, Stewart AG, Cade JF, Wilson JW. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Respir Crit Care Med. 2002;166:138–143. doi: 10.1164/rccm.2009005. [DOI] [PubMed] [Google Scholar]
- 53.Winter C, Herbold W, Maus R, Langer F, Briles DE, Paton JC, Welte T, Maus UA. Important role for CC chemokine ligand 2-dependent lung mononuclear phagocyte recruitment to inhibit sepsis in mice infected with Streptococcus pneumoniae. J Immunol. 2009;182:4931–4937. doi: 10.4049/jimmunol.0804096. [DOI] [PubMed] [Google Scholar]
- 54.Schabbauer G, Matt U, Gunzl P, Warszawska J, Furtner T, Hainzl E, Elbau I, Mesteri I, Doninger B, Binder BR, Knapp S. Myeloid PTEN promotes inflammation but impairs bactericidal activities during murine pneumococcal pneumonia. J Immunol. 2010;185:468–476. doi: 10.4049/jimmunol.0902221. [DOI] [PubMed] [Google Scholar]
- 55.Henken S, Bohling J, Martens-Lobenhoffer J, Paton JC, Ogunniyi AD, Briles DE, Salisbury VC, Wedekind D, Bode-Boger SM, Welsh T, Bange FC, Welte T, Maus UA. Efficacy profiles of daptomycin for treatment of invasive and noninvasive pulmonary infections with Streptococcus pneumoniae. Antimicrob Agents Chemother. 2010;54:707–717. doi: 10.1128/AAC.00943-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.