Abstract
Background
Kawasaki Disease (KD) can result in fatal coronary artery aneurysms especially in untreated patients. Our recent studies of KD vascular pathology revealed subacute/chronic vasculitis that began early in the illness with proliferation of smooth muscle cell derived-myofibroblasts in a complex extracellular matrix (ECM). We hypothesized that there is dysregulation of specific ECM and adhesion molecules in KD coronary arteries.
Methods
Gene expression profiling for ECM and adhesion molecules was performed on 6 acute KD and 8 control coronary arteries using a targeted real-time PCR array approach.
Results
Integrins alpha4 and alphaM (ITGA4, ITGAM), collagen 1A1 (COL1A1), and matrix metalloproteinase 7 (MMP-7) were significantly upregulated in KD coronary arteries compared with controls. Immunohistochemistry with anti- ITGAM antibodies revealed expression on inflammatory cells within the coronary artery wall in KD patients but not controls.
Conclusion
Integrins ITGA4 and ITGAM are upregulated in KD vasculopathy, likely promoting inflammatory recruitment that stimulates smooth muscle cell transition to myofibroblasts and their proliferation. MMP-7 likely enhances myofibroblast proliferation and luminal lesion expansion, and overexpression of COL1A1 may lead to coronary artery stenosis. Identification of the molecular pathogenesis of KD vasculopathy may lead to the development of circulating biomarkers and to directed therapeutic interventions.
INTRODUCTION
Gene expression profiling is a powerful tool in the study of disease pathogenesis, the development of biomarkers and prognostic indicators, and the identification of new therapeutic targets. Examples of its clinical applications in cardiovascular disease include peripheral blood gene expression profiling to identify individuals at low-risk of acute rejection following cardiac transplantation, reducing the need for biopsies (1,2). Expression profiling of myocardial biopsies in patients with cardiomyopathy have led to identification of disease-specific expression profiles (3,4).
The molecular pathogenesis of coronary artery (CA) abnormalities in Kawasaki Disease (KD), the leading cause of acquired heart disease in children in developed nations, is unknown, and gene expression profiling of acute KD CA has never been performed. We recently analyzed vascular pathology in 41 KD cases and identified three linked pathologic processes in KD vasculopathy (5). Necrotizing arteritis, the first process, is an acute self-limited neutrophilic process beginning and ending within the first two weeks of fever onset that progressively destroys the vessel wall from the endothelium into the adventitia, causing saccular aneurysms which can thrombose or rupture. This process is complete within two weeks of illness onset. Two other processes are also ongoing in the first two weeks, can continue indefinitely, and are likely particularly important in those KD patients who do not respond to intravenous immunoglobulin therapy and continue to have progressive coronary artery dilatation. These are subacute/chronic (SA/C) vasculitis, which consists of mostly small lymphocytes, but also plasma cells and eosinophils with some macrophages; and luminal myofibroblastic proliferation (LMP), a progressive stenosing intraluminal proliferative lesion whose pathognomonic cell is a smooth muscle cell-derived myofibroblast and its matrix products. These pathologic findings strongly suggest an important role for extracellular matrix (ECM) molecules in the pathophysiology of CA lesions in KD. Therefore, we hypothesized that specific ECM and cell adhesion molecules are dysregulated in acute KD CA when compared with childhood control coronary arteries. We used gene expression profiling to examine these genes in KD and control CA tissues, and immunohistochemistry to examine protein expression.
RESULTS
Clinical and pathologic findings in KD and control patients
The clinical and pathologic data on the KD and control patient tissues used in this study are provided in Tables 1 and 2. All KD patients had severe CA disease and died in the acute phase of illness (within 5 weeks of fever onset); pathologic examination showed SA/C and LMP (5). Control CA tissues were obtained from children who died of non-KD illnesses and had normal CA histology.
Table 1.
KD coronary artery tissues: clinical and pathologic information
| Case | Age (mos) | Time since onset (yr of death) | Gender | Ethnicity | KD therapy | Pathologic Features | Cause of death |
|---|---|---|---|---|---|---|---|
| KD1 | 11 | 2.5 wks (1997) | M | Caucasian | None | mild SA/C inflammation and marked LMP | Ruptured coronary artery aneurysm |
| KD2 | 10 | 4 wks (1984) | F | Black | None | mild to moderate SA/C, SA/C-LMP, thrombosis | Myocardial infarction |
| KD3 | 4 | 3 wks (2000) | M | Caucasian | IVIG | marked SA/C, mild LMP, severe periarteritis | Myocardial infarction |
| KD4 | 3.5 | 3–4 wks (2006) | M | Caucasian | IVIG, steroid | LMP-SA/C causing total occlusion | Thrombosed mesenteric aneurysm with small bowel infarction |
| KD5 | 4 | 5 wks (2005) | M | Unknown | IVIG, steroid | severe SA/C, mild LMP, and thrombosis | Myocardial infarction |
| KD6 | 4.5 | 4 wks (2008) | M | Hispanic | IVIG, steroid, infliximab | Medial SA/C and SA/C-LMP | Ruptured common iliac artery aneurysm |
Table 2.
Control coronary artery tissues: clinical information
| Case | Age | Gender | Diagnosis |
|---|---|---|---|
| C1 | 19 mo | M | Enterobacter sepsis, pulmonary hemorrhage, neurologic devastation from herpes simplex virus encephalitis |
| C2 | 11 mo | M | Hypoplastic left heart, respiratory syncytial virus infection |
| C3 | 7 yr and 5 wk (pooled) | M and F (pooled) | Pulmonary hypertension, demyelinating disease |
| C4 | 5 mo | M | Pneumococcal meningitis, disseminated intravascular coagulation |
| C5 | 10 mo | M | Prematurity, neurologic devastation secondary to Serratia meningitis, chronic lung disease |
| C6 | 12 day | F | Meconium aspiration, pulmonary hemorrhage |
| C7 | 9 yr | M | Developmental delay, seizures, fever |
| C8 | 4 yr | F | Small bowel obstruction, pneumonia |
Evaluating extracellular matrix and adhesion molecule gene expression
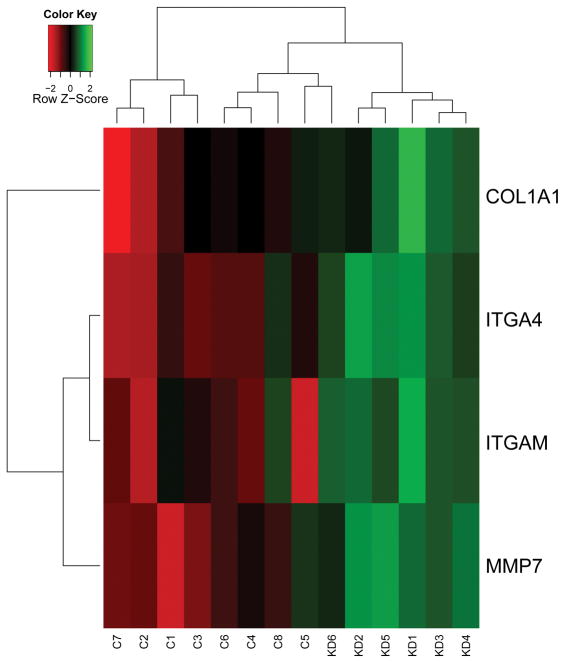
RNA isolated from six KD and eight control CA specimens were of adequate quality for PCR array analyses. The gene expression was normalized to the housekeeping gene HPRT1 and relative gene expression was evaluated in KD compared to control CA. Of 84 genes involved in cell-cell and cell-matrix interactions on the array, four were found to be statistically significantly upregulated in the KD specimens: ITGA4 (integrin, alpha 4), ITGAM (integrin, alpha M), COL1A1 (collagen, type I, alpha 1), and MMP7 (matrix metalloproteinase 7) (Table 3). Thus, array profiling of KD tissue revealed dysregulation of genes involved in extracellular matrix gene expression (COL1A1 and MMP7) and adhesion molecules (integrins alpha M and alpha 4). These genes clustered with KD diagnosis (Figure 1); this result was irrespective of KD therapy (Table 1).
Table 3.
Genes upregulated in KD CA
| Gene | Fold-change (95% CI) | p-value | q-value |
|---|---|---|---|
| ITGA4 | 11.89 (5.01,28.21) | <0.0001 | 0.003 |
| MMP7 | 172.65 (24.99, 1192.63) | 0.0001 | 0.003 |
| ITGAM | 6.18 (2.76, 13.83) | 0.0005 | 0.010 |
| COL1A1 | 8.94 (13.83, 33.24) | 0.0035 | 0.039 |
Figure 1.
Heat map of genes whose expression was significantly different in coronary arteries of KD patients and controls. There was clustering of KD samples, irrespective of therapy.
Expression of integrin alpha M protein in KD CA tissue
To determine if protein expression of integrin alpha M was also upregulated, we performed immunohistochemistry on 6 KD and 5 control CA tissues. We found that expression of ITGAM was observed in all 6 KD but none of the 5 control CA tissues (Figure 2). Integrin alpha M was expressed on inflammatory cells within KD tissues, predominantly macrophages (Figure 2). It was also expressed on spindle-shaped cells in LMP lesions (Figure 2C).
Figure 2.
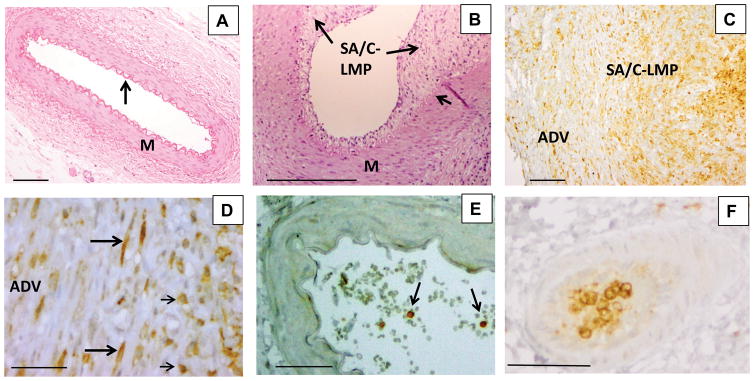
Histology and immunohistochemistry (IHC) of coronary arteries of KD and childhood control patients. Childhood control coronary artery (A) is free of inflammation and luminal proliferation and has a thin intima covering an undulating elastic lamina (arrow) and a uniform media. Hematoxylin and eosin stain (H&E), 10X objective, scale bar=200 μm. Portion of KD coronary artery (B) with luminal subacute/chronic vasculitis-luminal myofibroblastic proliferation (SA/C-LMP) (long arrows) of varying thickness. The underlying internal elastic lamina (short arrow) is mostly intact, while the media (M) is free of inflammation and somewhat tangentially sectioned. A small portion of visible adventitia in the lower right has subacute/chronic (SA/C) inflammation. H&E 10X objective, scale bar =200μm. IHC for integrin alpha M (ITGAM) (C) reveals positive cells (brown) in the adventitia (ADV), SA/C-LMP, and lumen of a damaged KD coronary artery.10X objective, scale bar =200μm. At higher magnification (D), the positive cells can be seen to be both spindle-shaped (long arrows) and mononuclear inflammatory cells (short arrows). 40X objective, scale bar =50μm. Childhood control coronary artery (E), no ITGAM expression in the arterial wall. A few positive staining circulating mononuclear cells are present in the lumen (arrows). IHC for ITGAM, 20X objective, scale bar =50μm. A section of a small artery located in KD coronary artery periadventitial tissue (F) is almost filled with ITGAM-positive mononuclear cells. IHC for ITGAM, 20X objective, scale bar =50μm.
DISCUSSION
Our recent pathologic study of vascular tissues from 41 KD cases demonstrated that smooth muscle cell-derived myofibroblasts actively proliferate in an uncontrolled fashion in the KD arterial wall and secrete or shed an active ECM. The proliferative LMP process can progress to life-threatening CA occlusion and myocardial ischemia over months to years (5). Although therapies are available to reduce thrombosis in KD vascular tissues, no therapy is available to reduce LMP. As the vast majority of KD fatalities occur after the second week of illness, when SA/C and LMP are the active processes in the vascular wall, the pathophysiology of the processes must be better understood in order to develop new preventative and therapeutic regimens. Here, we used expression profiling and report that four genes, ITGA4, MMP7, ITGAM, and COL1A1 were significantly upregulated in KD CA specimens. Only one prior study of gene expression profiling of KD CA tissues has been performed; this study included late-stage KD tissues only and did not focus on extracellular matrix and adhesion molecules (6).
The integrins are a family of receptors for extracellular matrix and cell surface ligands involved in cell migration and attachment to the ECM, composed of alpha and beta subunits. Bound integrins can both transmit and receive intracellular signals, regulating endothelial cell migration and survival as well as angiogenesis, linking components of the ECM, and modulating cellular proliferation, adhesion, and motility (7–9). We identified upregulation of ITGAM in KD patients, which is expressed on dendritic cells, macrophages, monocytes, and neutrophils when complexed with beta 2 integrin (10). ITGAM has multiple ligands and is involved in the regulation of neutrophil and monocyte adhesion and migration to damaged endothelium and endothelial- associated ECM. We also noted expression of ITGAM on spindle-shaped cells within KD LMP lesions, some of which may be myofibroblasts; we previously showed by transmission electron microscopy that the cellular component of LMP was a smooth muscle cell-derived myofibroblast (5). In this regard, two animal studies are of particular interest. ITGAM knockout mice subjected to endothelial denudation and arterial stretching were noted to have reduced intimal thickening and cell proliferation, as well as reduced leukocyte accumulation when compared to control mice (11). Another murine model of vascular injury demonstrated decreased inflammatory cell infiltrate and reduced long term intimal thickening in the presence of anti-alphaM antibodies (12). Interestingly, ITGAM was reported to be upregulated in the peripheral blood of KD patients who were refractory to initial therapy (13).
ITGA4 (alpha 4 integrin) was also noted to be upregulated in KD CA tissues. Expression of integrin α4 on peripheral blood leukocytes facilitates rolling and adhesion to activated endothelium and is thus implicated in inflammatory recruitment (14,15). ITGA4 is also expressed on endothelial cells, and binds to fibronectin and VCAM-1, promoting VEGF-A and B induced tumor lymphangiogenesis (16). ITGA4 has been noted to be upregulated in abdominal aortic aneurysms (17) and in the peripheral blood mononuclear cells of cardiac transplant patients with acute rejection (18). In KD coronary artery lesions ITGAM and ITGA4 are likely to promote SA/C vasculitis, leading to smooth muscle cell transition to myofibroblasts and their proliferation. Integrins have recently served as a therapeutic target in inflammatory diseases such as multiple sclerosis and Crohn’s disease; Natalizumab (Tysabri), an anti-α4 integrin monoclonal antibody, is FDA approved for the treatment of both conditions.
COL1A1 (Type I collagen) is an interstitial matrix molecule found in most connective tissues and involved in wound healing and remodeling. TGF-β1, -β2 and -β3, PDGF, IL-1α, -1β and -4, and mast cell tryptase increase production of Type I collagen (19). IL- 1-related genes are upregulated in KD peripheral blood during the acute phase of illness (20), and a murine model demonstrates a critical role for IL- 1 β in the development of vasculitis (21). Upregulation of type I collagen has been observed in injured vascular media (22); excessive collagen production likely contributes to CA stenosis in more severe KD cases.
MMP7 (matrilysin) is a matrix metalloproteinase which is involved in the breakdown of ECM proteins, including proteoglycans, elastin, laminin, fibronectin, gelatin, and entactin. Monocytes and macrophages have been noted to produce MMP7 in response to severe inflammation (23), and MMP7 is known to play a role in inflammatory recruitment of neutrophils via a chemotactic gradient (24). MMP7 has protective functions, including a role in innate immunity in gut mucosal tissues and wound healing (25). However, MMP-7 has also been implicated in pathologic processes such as pulmonary fibrosis, the development and progression of certain cancers, and promotion of thrombosis and plaque rupture in coronary atherosclerosis (26). Notably, increased plasma levels of MMP7 have been demonstrated in patients with coronary artery disease, metastatic colon and rectal cancer, lung cancer, and pancreatic cancer, suggesting its possible utility as a biomarker (27–31). Promising research is currently ongoing using matrix metalloproteinase inhibitors as therapeutic interventions for a variety of cancers (31). Future studies will focus on MMP7 as a possible prognostic and diagnostic biomarker in KD sera.
Our study does have several limitations. Because KD fatalities are not reportable and deaths are scattered, the number of tissue specimens available for study are limited. Two of our patients were untreated, but four others received several therapies which could have affected gene expression. However, in this small sample of patients, differences in therapy did not appear to significantly alter the expression of the 4 upregulated genes (Figure 1; Table 1). This may be because current therapies do not target expression of adhesion molecules and ECM proteins, or because these patients were so severely affected that therapeutic intervention was not efficacious. While control children in this study had pathologically normal coronary arteries, it is possible that their non-KD illnesses affected gene expression in the coronary arteries. This study was designed to examine the ECM and cell adhesion molecules using a commercially available array, and therefore was restricted to only those 84 genes on the array.
In conclusion, gene expression analysis of acute KD CA vasculopathy reveals upregulation of ITGA4, ITGAM, COL1A1, and MMP7; immunohistochemistry revealed strong expression of ITGAM in inflammatory cells of KD coronary arteries. Determining molecular events in the KD arterial wall is key to rational drug design of urgently needed new therapies for KD patients who do not respond to infusion of intravenous immunoglobulin, and secreted molecules that are upregulated in KD arterial tissues should be studied as possible circulating disease biomarkers.
METHODS
Tissues
KD and control CA tissues were de-identified autopsy samples; therefore, IRB approval and informed consent were not required because the study did not meet the criteria of human subjects research as defined by the U.S. Department of Health and Human Services.
RNA Extraction from FFPE tissues
RNA was extracted from formalin- fixed paraffin embedded (FFPE) tissue sections (7–10 microns) of CA from nine KD patients and eleven pediatric controls using the Qiagen RNeasy FFPE kit (Qiagen/SA Biosciences, Valencia, CA) as per manufacturer instructions, except that proteinase K lysis was performed for 1 hour at 56 C. RNA quantity was measured using a Nanodrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE).
cDNA Synthesis and cDNA quality assessment
Single strand cDNA was synthesized from 300 ng of extracted RNA using the Qiagen/SA Biosciences RT2 preAMP cDNA Synthesis Kit according to the manufacturer’s instructions. The quality of each cDNA sample was assessed in triplicate by real-time PCR using SYBR Green chemistry and primers for the RNA housekeeping gene RPL13A and for human genomic DNA contamination (HGDC) (Qiagen/SA Biosciences). Samples were considered to be of good quality if the C(t) values for HGDC were at least 3 higher (8 fold-change) than for RPL13A, and the RPL13A C(t) was less than 35.
ECM and Adhesion Molecule Array
Those cDNA samples that passed quality control underwent preamplification with array specific primers using the Qiagen/SA Biosciences RT2 preAMP Pathway Primer Mix according to manufacturer instructions and were applied to the ECM and Adhesion molecule array plate (Qiagen/SA Biosciences PAHS-013). Using a CFX96 X real-time PCR Detection system (Biorad, Hercules, CA), the following cycling program was used: 95 degrees for 10 minutes, then 40 cycles of 95 degrees for 15 seconds, and 60 degrees for 1 minute, then 95 degrees for 10 seconds with a melting curve performed from 65–95 degrees, with increments of 0.5 degrees every 5 seconds.
Immunohistochemistry
Immunohistochemistry was performed on KD and control CA FFPE tissue sections as previously described (32,33) for integrin alpha M (ITGAM) (Prestige Antibodies, 1:300, Sigma, St. Louis, MO). Briefly, antigen retrieval was performed in 0.01M sodium citrate buffer at a pH of 6.0 using a pressure cooker. The Vectastain Elite ABC system (Vector, Burlingame, CA) was used with diaminobenzidine (DAB) as the chromagen to yield a brown color.
Statistical Analysis
We compared the expression levels of each gene between KD and controls by calculating delta C(t) values. Fold changes were calculated by the delta-delta C(t) method. Using delta C(t) values, comparisons were made for each gene using t-tests. Multiple comparisons were accounted for by calculating false discovery rate (FDR) using q-values (34–36). Further, If ≥50% of either KD or control samples had undetected/undetermined C(t) values for a specific gene, then that gene was excluded from the study. For hierarchical clustering analysis, we used Euclidean distance as a metric for dissimilarity and summarized the results as a heatmap.
Acknowledgments
Statement of financial support: This work was supported by the National Institutes of Health grants HL 63771 and HL109955 to AHR, the Max Goldenberg Foundation, the Kawasaki Disease Fund, and the Center for Kawasaki Disease at the Ann and Robert H. Lurie Children’s Hospital of Chicago.
We thank Robin Biggs, Deidre Anderson, and Leslie Martin for assistance in identifying and dissecting control coronary artery samples.
References
- 1.Pham MX, Teuteberg JJ, Kfoury AG, et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362:1890–900. doi: 10.1056/NEJMoa0912965. [DOI] [PubMed] [Google Scholar]
- 2.Deng MC, Eisen HJ, Mehra MR, et al. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am J Transplant. 2006;6:150–60. doi: 10.1111/j.1600-6143.2005.01175.x. [DOI] [PubMed] [Google Scholar]
- 3.Kittleson MM, Minhas KM, Irizarry RA, et al. Gene expression analysis of ischemic and nonischemic cardiomyopathy: shared and distinct genes in the development of heart failure. Physiol Genomics. 2005;21:299–307. doi: 10.1152/physiolgenomics.00255.2004. [DOI] [PubMed] [Google Scholar]
- 4.Tan FL, Moravec CS, Li J, et al. The gene expression fingerprint of human heart failure. Proc Natl Acad Sci U S A. 2002;99:11387–92. doi: 10.1073/pnas.162370099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orenstein JM, Shulman ST, Fox LM, et al. Three linked vasculopathic processes characterize kawasaki disease: a light and transmission electron microscopic study. PLoS One. 2012;7:e38998. doi: 10.1371/journal.pone.0038998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukazawa R, Ikegam E, Watanabe M, et al. Coronary artery aneurysm induced by Kawasaki disease in children show features typical senescence. Circ J. 2007;71:709–15. doi: 10.1253/circj.71.709. [DOI] [PubMed] [Google Scholar]
- 7.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–17. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantor JM, Ginsberg MH, Rose DM. Integrin-associated proteins as potential therapeutic targets. Immunol Rev. 2008;223:236–51. doi: 10.1111/j.1600-065X.2008.00640.x. [DOI] [PubMed] [Google Scholar]
- 10.Hom G, Graham RR, Modrek B, et al. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. N Engl J Med. 2008;358:900–9. doi: 10.1056/NEJMoa0707865. [DOI] [PubMed] [Google Scholar]
- 11.Simon DI, Dhen Z, Seifert P, Edelman ER, Ballantyne CM, Rogers C. Decreased neointimal formation in Mac-1(−/−) mice reveals a role for inflammation in vascular repair after angioplasty. The J Clin Invest. 2000;105:293–300. doi: 10.1172/JCI7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Sakuma M, Chen Z, et al. Leukocyte engagement of platelet glycoprotein Ibalpha via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation. 2005;112:2993–3000. doi: 10.1161/CIRCULATIONAHA.105.571315. [DOI] [PubMed] [Google Scholar]
- 13.Abe J, Ebata R, Jibiki T, Yasukawa K, Saito H, Terai M. Elevated granulocyte colony-stimulating factor levels predict treatment failure in patients with Kawasaki disease. J Allergy Clin Immunol. 2008;122:1008–13. e8. doi: 10.1016/j.jaci.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Kitayama J, Fuhlbrigge RC, Puri KD, Springer TA. P-selectin, L-selectin, and alpha 4 integrin have distinct roles in eosinophil tethering and arrest on vascular endothelial cells under physiological flow conditions. J Immunol. 1997;159:3929–39. [PubMed] [Google Scholar]
- 15.Johnston B, Issekutz TB, Kubes P. The alpha 4-integrin supports leukocyte rolling and adhesion in chronically inflamed postcapillary venules in vivo. J Exp Med. 1996;183:1995–2006. doi: 10.1084/jem.183.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garmy-Susini B, Avraamides CJ, Schmid MC, et al. Integrin alpha4beta1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res. 2010;70:3042–51. doi: 10.1158/0008-5472.CAN-09-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenk GM, Tromp G, Weinsheimer S, Gatalica Z, Berguer R, Kuivaniemi H. Whole genome expression profiling reveals a significant role for immune function in human abdominal aortic aneurysms. BMC Genomics. 2007;8:237. doi: 10.1186/1471-2164-8-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernstein D, Williams GE, Eisen H, et al. Gene expression profiling distinguishes a molecular signature for grade 1B mild acute cellular rejection in cardiac allograft recipients. J Heart Lung Transplant. 2007;26:1270–80. doi: 10.1016/j.healun.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popper SJ, Shimizu C, Shike H, et al. Gene-expression patterns reveal underlying biological processes in Kawasaki disease. Genome Biol. 2007;8:R261. doi: 10.1186/gb-2007-8-12-r261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee Y, et al. IL-1E is crucial for induction of coronary artery inflammation in a mouse model of Kawasaki disease. Circulation. 2012;125:1542–50. doi: 10.1161/CIRCULATIONAHA.111.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190:300–9. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 23.Busiek DF, Baragi V, Nehring LC, Parks WC, Welgus HG. Matrilysin expression by human mononuclear phagocytes and its regulation by cytokines and hormones. J Immunol. 1995;154:6484–91. [PubMed] [Google Scholar]
- 24.Swee M, Wilson CL, Wang Y, McGuire JK, Parks WC. Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. J Leukoc Biol. 2008;83:1404–12. doi: 10.1189/jlb.0108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–29. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 26.Wielockx B, Libert C, Wilson C. Matrilysin (matrix metalloproteinase-7): a new promising drug target in cancer and inflammation? Cytokine Growth Factor Rev. 2004;15:111–5. doi: 10.1016/j.cytogfr.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson L, Jonasson L, Nijm J, Hamsten A, Eriksson P. Increased plasma concentration of matrix metalloproteinase-7 in patients with coronary artery disease. Clin Chem. 2006;52:1522–7. doi: 10.1373/clinchem.2006.067439. [DOI] [PubMed] [Google Scholar]
- 28.Back M, Ketelhuth DF, Agewall S. Matrix metalloproteinases in atherothrombosis. Prog Cardiovasc Dis. 2010;52:410–28. doi: 10.1016/j.pcad.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Kurokawa S, Arimura Y, Yamamoto H, et al. Tumour matrilysin expression predicts metastatic potential of stage I (pT1) colon and rectal cancers. Gut. 2005;54:1751–8. doi: 10.1136/gut.2005.071035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhlmann KF, van Till JW, Boermeester MA, et al. Evaluation of matrix metalloproteinase 7 in plasma and pancreatic juice as a biomarker for pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:886–91. doi: 10.1158/1055-9965.EPI-06-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–97. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown TJ, Crawford SE, Cornwall ML, Garcia F, Shulman ST, Rowley AH. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis. 2001;184:940–3. doi: 10.1086/323155. [DOI] [PubMed] [Google Scholar]
- 33.Gavin PJ, Crawford SE, Shulman ST, Garcia FL, Rowley AH. Systemic arterial expression of matrix metalloproteinases 2 and 9 in acute Kawasaki disease. Arterioscler Thromb Vasc Biol. 2003;23:576–81. doi: 10.1161/01.ATV.0000065385.47152.FD. [DOI] [PubMed] [Google Scholar]
- 34.Storey JD. A direct approach to false discovery rates. J R Stat Soc Series B Stat Methodol. 2002;64:479–98. [Google Scholar]
- 35.Storey JD, Taylor JE, Siegmund D. Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: a unified approach. J R Stat Soc Series B Stat Methodol. 2004;66:187–205. [Google Scholar]
- 36.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–5. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]