Abstract
The four JAKS comprise a family of intracellular, non-receptor tyrosine kinases that first gained attention as signaling mediators of the type I and type II cytokine receptors. Subsequently, the JAKs were found to be involved in signaling downstream of the insulin receptor, a number of receptor tyrosine kinases, and certain G-protein coupled receptors. Although a number of cytoplasmic targets for the JAKs have been identified, their predominant action was found to be the phosphorylation and activation of the STAT transcription factors. Through the STATs, the JAKs activate gene expression linked to cellular stress, proliferation, and differentiation. The JAKs are especially important in haematopoiesis, inflammation, and immunity, and aberrant JAK activity has been implicated in a number of disorders including rheumatoid arthritis, psoriasis, polycythemia vera, and myeloproliferative diseases. Although once thought to reside strictly in the cytoplasm, recent evidence shows that JAK1 and JAK2 are present in the nucleus of certain cells often under conditions associated with high rates of cell growth. Nuclear JAKs have now been shown to affect gene expression by activating other transcription factors besides the STATs and exerting epigenetic actions, for example, by phosphorylating histone H3. The latter action derepresses global gene expression and has been implicated in leukemogenesis. Nuclear JAKs may have a role as well in stem cell biology. Here we describe recent developments in understanding the noncanonical nuclear actions of JAK1 and JAK2.
Introduction
The JAK family of intracellular nonreceptor tyrosine kinases consists of 4 mammalian members, JAK1, JAK2, JAK3, and TYK2. While the other JAKs are ubiquitously expressed, JAK3 is primarily restricted to hematopoietic cells and is functionally associated only with cytokine receptors that use the common gamma chain (1). There is no normal functional role for JAK3 outside of the immune system. The JAKs are critical signaling components of the type I and type II cytokine receptor families, as well as insulin signaling, a number of receptor tyrosine kinases (e.g., epidermal growth factor receptor), and certain G-protein coupled receptors (2). As such the JAKs play key roles in a wide-variety of cellular responses important for inflammation, growth, regulation of metabolism, and contractility that involve both short-lived phosphorylation events, as well as gene transcription (1,3). The latter is most notably mediated by their recruitment and activation of the STAT (Signal Transducers and Activators of Transcription) protein family of transcription factors, which might be considered the canonical action of the JAKs.
Until recently, the involvement of the JAKs in gene expression was assumed to be restricted to their role as activators of the STATs, which was thought to occur in the cytoplasm. However, over the last several years, numerous studies have established the presence of the JAKs within the nucleus and have provided evidence that these kinases phosphorylate not only other transcription factors besides the STATs, but that the JAKs (at least JAK1 and JAK2) function as epigenetic regulators of gene expression. Emerging evidence indicates that the latter noncanonical role may be of particular significance under physiological and pathological conditions of heightened cellular growth.
JAK Facts
JAKs are comprised of 4 major functional domains (1). Closest to the amino terminal is the FERM (band 4.1, ezrin, radixin, and moesin domain) domain, followed by an SH2 domain, a pseudokinase domain, and a kinase domain at the carboxyl terminal. The pseudokinase domain has a role in regulating the catalytic activity of the kinase domain (1). The tandem arrangement of the kinase and pseudokinase domains was reminiscent of the opposing faces of the Roman god Janus, leading to the origin of JAKs as an abbreviation for Janus kinases (4), although at first JAK was an acronym for Just Another Kinase (4,5).
Loss-of-function mutations in JAK1 or JAK2 are presumably lethal in humans based on transgenic animal models, whereas such mutations in JAK3 or TYK2 result in primary immunodeficiencies in humans. Humans with rare TYK2 deficiencies exhibit an immunodeficiency syndrome that includes Hyper IgE Syndrome (HIES) and an increased susceptibility to infections (6). Loss-of-function mutations in JAK3 can result in a broad clinical spectrum of immune disorders (7), including Severe Combined Immunodeficiency (SCID). Activating point mutations in the JAKs (JAK2 in particular) or chromosomal translocations have been implicated in a number of inflammatory disorders and hematopoietic malignancies (8–13). Somatic hyperactivating mutations in JAK1 were identified in adult patients with acute lymphoblastic leukemia, and these mutations were associated with significantly decreased survival outcomes (14). Perhaps the most prominent activating mutation is the JAK2 V617F missense mutation associated with a spectrum of myeloproliferative disorders including essential thrombocythemia, polycythema vera, and primary myelofibrosis (15), which has been the major focus of recent JAK-targeted drug development efforts. Ruxolitinib (also known as INC424) is a JAK1/JAK2-selective inhibitor drug currently in late-stage clinical trials for the treatment of myelofibrosis (16); success of these trials has prompted the manufacturer to seek early a priority review from the Food and Drug Administration for market approval. Other JAK-selective inhibitors, such as the JAK3/JAK1-selective inhibitor tofacitinib (also known as CP-690,550), are undergoing clinical evaluation as therapies for autoimmune diseases such as rheumatoid arthritis (17,18) and psoriasis (19).
As far as substrate preference, the JAKs appear to be rather promiscuous compared to most kinases (20,21). Evidence suggests that JAK1 and JAK2 may have both the same and different substrates, which may be influenced as well by cell type. Besides the JAKs themselves and cytosolic regions of various receptors, purported non-STAT targets of JAK1 and/or JAK2 phosphorylation, as well as the two other Janus kinases, include: Pyk2 (22), cytosolic phospholipase A2(23), various non-receptor protein-tyrosine kinases (24–28), the methyltransferase JBP1 (29), insulin receptor substrate (IRS)-1 and IRS-2 (30,31), RAF1 (32,33), phosphatidylinositol 3-kinase p85, beta subunit (34), phospholipase Cγ (35), and SH2-containing inositol 5′-phosphatase (SHIP/SHIP1) (36). The specific substrates that are phosphorylated by the JAKs may be determined in no small part by scaffold proteins that associate with JAKs and assemble multiprotein complexes (1,20,37).
Early evidence that JAK is in the nucleus
The original report that JAK1 and JAK2 were present in the nucleus was a logical follow-up to the observation that the growth hormone (GH) receptor undergoes ligand-dependent nuclear translocation, since both JAK2 and JAK1 associate with, and phosphorylate upon ligand binding, the GH receptor (38). This study was performed using CHO cells stably transfected with the (rat) GH receptor. The presence of both JAKs in the nucleus was detected by Western blot analysis, immunogold electron microscopy, and immunocytochemistry. Surprisingly, both JAKs were found to be constitutively present in the nucleus. Although ligand treatment did not further increase amounts of JAK protein in the nucleus, levels of tyrosine phosphorylated (activated) JAKs were increased. About the same time, others reported the presence of JAK2 in pancreatic islet cell nuclei (39) and subsequent studies reported JAK2 in nuclei of cultured liver cells and rat liver in vivo (40), as well as COS-7 cells(41). JAK2 was also observed in the nuclei of mature mouse unfertilized oocytes and one-cell and two-cell embryos; interestingly, JAK2 appeared to co-localize with chromosomes (42).
However, others reported being unsuccessful in detecting the presence of JAKs in the nuclei of several different human cell lines (43), as well as in CHO cells expressing the (rabbit) GH receptor (44). Differences in culturing conditions might explain the disparate findings, but the nuclear presence of JAKs may be a more prominent fixture of cells that are especially affected by cytokine signaling. The latter possibility is consistent with recent studies that have detected JAKs in the nuclei of hematopoietic cells (45,46).
Hitching a ride: how JAKs may enter the nucleus
Being too large (120-140 kDa) to simply diffuse freely between the cytosol and nucleus, the JAKs would require involvement of the nuclear import machinery and consequently a nuclear localization sequence (NLS). In fact, the nuclear localization of JAK1, JAK2, and Tyk2 have been ascribed to an arginine-rich NLS-like motif within the region mediating interaction with cytokine receptors (38,47). But to the best of our knowledge, mutational analysis that defines the importance of the purported NLS to the nuclear localization of the JAKs has not been reported.
Ligand-dependent nuclear translocation of cell surface receptors, with or without bound ligand, offers another explanation for the presence of JAKs within the nucleus. A number of receptors that bind JAK1 and/or JAK2 on their cytoplasmic region have been shown to translocate to the perinuclear region of the cell or the nucleus. These include receptors for growth hormone (48,49), prolactin (50,51), insulin (52–54), and epidermal growth factor (55), as well as the angiotensin II type 1 receptor (AT1) (56,57). However, as mentioned, in the case of the GH receptor, ligand-induced nuclear translocation was not associated with an increase in the size of the nuclear pool of JAK1 and JAK2 (38). In addition, neither receptor phosphorylation nor JAK activity were needed to elicit the nuclear trafficking of the GH receptor (58), but surprisingly, evidence indicated that JAK kinase activity was needed for removal of GH from the nucleus.
Another possible association partner for the JAKs is SOCS1, which binds and inhibits JAK1 and JAK2 and which contains an NLS (59–61). Other possible associations could be envisioned as well to explain the localization of the JAKs to the nucleus. For instance, JAK2 was shown to interact with Hsp70/Hsc70 (62), which shuttles between nuclei and cytoplasm (63). In general, the triggers that cause nuclear JAK translocation are poorly understood and largely unidentified. However, there is an intriguing report showing that anoxia results in JAK2/STAT5 nuclear translocation in bovine retinal capillary endothelial cells suggesting that the mechanism is under regulatory control (64).
Nuclear Actions
In light of the persuasive evidence that JAKs do indeed reside in the nucleus, one must wonder what are the roles and functions of nuclear JAK. The presence of GH receptor within the nucleus has been implicated in cellular proliferation within the context of hepatocyte regeneration, tumorigenesis, and metastasis (65,66). In this model, the role of the nuclear JAKs (principally JAK2) would be ostensibly to initiate signaling off the receptor, including in particular the activation of STAT5 (66,67). In contrast, while still involving nuclear JAK2, GH-induced STAT3 signaling may be less dependent on the presence of the GH receptor within the nucleus (40). Such differences in the nuclear anchoring of JAK2 might play a role in the differential activation of STAT3 and STAT5 by GH as a function of dosage level and time (40). As far as we know, noncanonical targets of the nuclear JAKs, however, have not been identified for GH signaling.
In response to prolactin stimulation, nuclear JAK2 was found to phosphorylate the transcription factor nuclear factor 1-C2 (NF1-C2) in mammary epithelial cells, thereby preventing its proteasomal degradation (68). NF1-C2 plays an important role in milk gene expression and the temporal pattern of activated JAK2 in the nucleus and sustainability of NF1-C2 protein levels were consistent with a role for nuclear JAK2 in the initiation, as opposed to the maintenance, of milk gene expression. In these cells, another downstream consequence for increased NF1-C2 due to activated nuclear JAK2 may be the increased expression of tumor suppressor protein p53 (68). These investigators subsequently provided evidence suggesting that nuclear JAK2/NF1-C2 suppresses breast cancer progression and metastasis by opposing the effects of FoxF1 (69).
Members of the helicase-like transcription factor (HLTF) family would seem to be another target for nuclear JAK2 downstream of prolactin signaling in uterine epithelial cells and thereby forming the basis for prolactin-induced augmentation of progesterone-dependent transcriptional activity (70). The rabbit HLTF ortholog RUSH-1α was shown to be tyrosine phosphorylated by nuclear JAK2 thereby enhancing DNA binding, while JAK2 inhibition blocked enhancement of progesterone-dependent gene induction by prolactin (70,71). These findings support the existence and relevance of a JAK-RUSH/HLTF signaling pathway operating in parallel to the better known JAK-STAT pathway.
It is interesting to note that while nuclear JAK2 may play a role in preventing the epithelial-to-mesenchymal transition (EMT) associated with carcinoma metastasis (69), accumulating evidence in the last few years has revealed JAK1 and JAK2 as epigenetic regulators of gene expression that may result in leukemogenesis. JAK overactivation was found to globally disrupt heterochromatic gene silencing in a Drosophila melanogaster hematopoietic tumor model, while JAK loss of function enhanced heterochromatic gene silencing (72). This action of JAK was circumvented by mutations in genes important in heterochromatic gene silencing through histone H3 methylation. Subsequently, others showed that JAK2 was present in the nucleus of hematopoietic cells and phosphorylated histone H3 on Y41. Phosphorylation of H3Y41 prevented binding of heterochromatin protein 1α (HP1α), which functions in the repression of heterochromatic genes (45). Moreover, in the human erythroleukemia (HEL) cell line JAK2 activity directly correlated with expression of the lmo2 oncogene and H3Y41 phosphorylation at the lmo2 promoter, but inversely correlated with HP1α binding at the promoter.
Suggestive evidence was recently reported that the epigenetic actions of JAK2 that are mediated through H3Y41 phosphorylation may have physiological relevance, in this case in embryonic stem (ES) cell self-renewal (73). The interleukin 6 type cytokine leukemia inhibitory factor (LIF) is a potent activator of JAK-STAT signaling and is used to maintain mouse embryonic stem cells in an undifferentiated state. Mouse ES cells that were engineered to contain the JAK2 V617F mutant allele were able to self-renew in the absence of LIF. In these cells, levels of HP1α bound to chromatin were lower, but were increased by JAK2 inhibition and this was associated with reduced histone H3Y41 phosphorylation. Furthermore, JAK2 inhibition reduced levels of Nanog, a transcription factor important for maintaining pluripotency, and this was associated with reduced H3Y41 phosphorylation and increased HP1α levels at the Nanog promoter.
Oncogenic JAK2 mutant kinases may exert epigenetic actions on chromatin structure in myeloproliferative neoplasms by phosphorylating the arginine methyltransferase PRMT5, which was originally identified as JAK-binding protein 1 (74). Myeloproliferative neoplasms are stem cell disorders in which most patients express the JAK2V617F (or less commonly exon12 mutations) constitutively activated tyrosine kinase. These oncogenic JAK2 mutants show an enhanced association with PRMT5 compared to wild type JAK2 (74). In addition, unlike wild type JAK2, the oncogenic JAKs were found to phosphorylate PRMT5 in HEL cells, resulting in reduced PRMT5 methyltransferase activity and decreased global histone H2A/H4 R3 methylation. The mutant JAKs were shown to impair PRMT5 activity by negatively impacting on its association with methylosome protein 50 (MEP50), which plays a role in enhancing the enzymatic activity of PRMT5. Enhanced phosphorylation of PRMT5 was detected in CD34+ cells and granulocytes of JAK2 V617F-positive patients. Moreover, knockdown of PRMT5 in human CD34+ cells increased colony formation and erythroid differentiation, providing evidence that PRMT5 phosphorylation contributes to myeloproliferative phenotype associated with the oncogenic JAK2 mutants. Intriguingly, PRMT5, MEP50, and the mutant JAK2 kinases were detected in both the nucleus and cytoplasm, raising the possibility of different consequences of JAK2-mediated PRMT5 phosphorylation depending upon the cellular compartment (74).
Still not firmly established is whether wild type JAK2 (or JAK1 for that matter) is normally present in the nucleus of hematopoietic cells. A recent report established the presence of nuclear JAK2 in CD34+ bone marrow cells of patients with myeloproliferative neoplasia harboring the JAK2 V617F mutation, but not in those patients with wild type JAK2 (46). In all patients, JAK2 was predominately cytoplasmic in differentiated granulocytic, megakaryocytic and erythroid cells. Again a correlation was noted between nuclear JAK2 activity and lmo2 expression. One possible explanation for these findings is that activation (phosphorylation) of JAK2, which is a fixture of undifferentiated CD34+ progenitor cells, is required for its nuclear accumulation (46).
A growing appreciation of the broadening scope of functional roles of nuclear JAK offers new paradigms for phenomena incompletely explained by canonical JAK-STAT models (Fig. 1). The mitogenic role of JAK, especially JAK2, provides a good illustration. JAK2 is essential for the prolactin-stimulated proliferation of mammary epithelial cells, partially through a STAT-mediated activation of cyclin D1 gene transcription (75), partially by promoting the nuclear accumulation of cyclin D1 protein (76), and partially by direct phosphorylation of the cyclin-dependent kinase inhibitor p27Kip1, which simultaneously impairs its ability to inhibit cyclin-dependent kinases and initiates a cascade of events culminating in proteasomal degradation of p27Kip1 (77). While it has not yet been unambiguously established that JAK2 catalyzes some of these critical phosphorylations in the nucleus, it is intriguing to note that conditions such as anoxia/hypoxia which are conducive to cyclin D1 gene expression (78) have also been shown to trigger nuclear localization of JAK2 in other cell types (64). Thus, understanding the functional role(s) of nuclear JAK2 may lead not only to a better understanding of epigenetic control, but also of cell cycle control (Fig 1).
Figure 1. Broadening scope of a functional role for nuclear JAK.
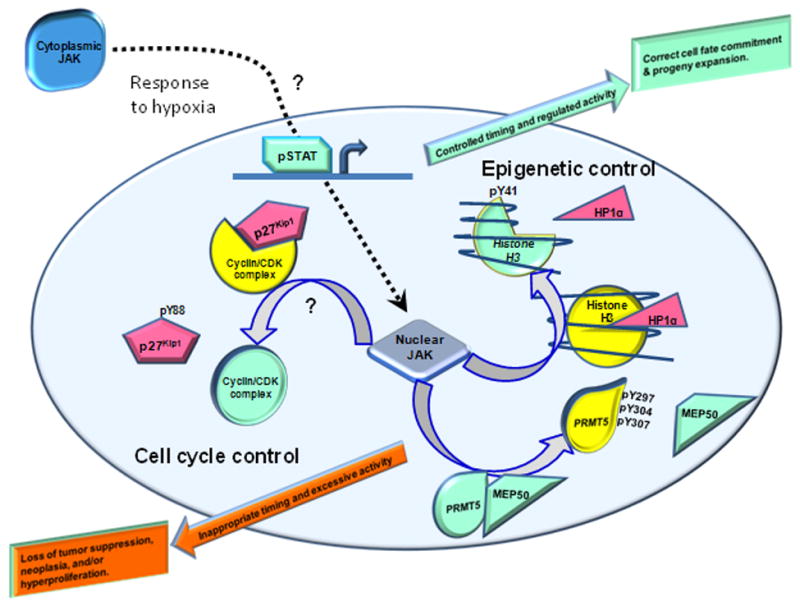
Certain triggers, such as hypoxia, can result in nuclear localization of JAK1 or JAK2 through poorly defined mechanisms. Canonical STAT activation by cytokine-stimulated JAK, a driving force in cell differentiation, is a well-known function of extra-nuclear JAK; conceivably (but not clearly established) nuclear translocation of JAK may occur through association with ligand-activated cytokine receptors or some chaperone protein. Due to promiscuous substrate recognition, JAK can phosphorylate nuclear substrates having pivotal roles in controlling cell cycle progression and epigenetics. When these events occur during critical windows in cellular development, and when the amplitude and duration of JAK activity are properly regulated, these roles are essential in determining cell fate and proliferation. Excessive, prolonged and ill-timed nuclear JAK activity may result in hyperproliferative disorders, neoplastic transformation and tumor progression. Evidence exists that nuclear JAK is responsible for phosphorylating substrates involved in epigenetic regulation, such as PRMT5 (thereby impairing binding of its methyltransferase-enhancing binding partner MEP50) or histone H3 (so as to displace transcriptional repressor heterochromatin protein 1α (HP1α)). In other instances, such as in the phosphorylation of the cyclin-dependent kinase inhibitor p27Kip1, the involvement of nuclear JAK is conceivable but remains to be determined. For simplicity, nuclear JAK-mediated phosphorylation of HLTF and NF1-C2 transcription factors are not shown (see Text).
When trying to fully understand the biological significance of nuclear JAK, it is advisable to simultaneously consider JAK's cytoplasmic actions, as well as to consider the cell's developmental stage when JAK-catalyzed events occur, the magnitude and duration of JAK-catalyzed phosphorylation events and, of course, the specific substrates phosphorylated by JAK. We postulate that when these events are orchestrated during the correct stage of development and the activity levels are tightly controlled, nuclear and cytoplasmic JAK activities are essential; when these events are ill-timed, excessive, and/or prolonged, the results can be disastrous, leading to cancers and related diseases (Fig. 1).
Conclusions and Future perspectives
Evidence indicates that the JAKs (JAK1 and JAK2 in particular) are present in the nucleus of certain cells under conditions associated with high rates of cell growth. Besides their canonical role as activators of the STAT transcription factors, the JAKs are now known to affect gene expression by activating other transcription factors and exerting epigenetic actions by phosphorylating histone H3. The consequence of the latter action is derepressed gene expression, which has been implicated in leukemogenesis. Unresolved, is what role the epigenetic actions of the JAKS have during human inflammatory maladies associated with increased JAK catalytic activity, including arthritis (3), atherosclerosis (79), and possibly coronary heart disease (80). It is tantalizing to suspect that the epigenetic actions of JAKs also come into play during normal physiological growth in particular in stem cell expansion and differentiation. Other nuclear targets for the JAKs within the nucleosome, enhanceosome, or transcriptional machinery may exist as well and await discovery.
Acknowledgments
This work was supported by a grant from NHLBI to GWB (5R01HL088101-05) and a grant from NIDDK to RJD (1R56DK082781-01).
Footnotes
Declaration of Interest: None of the authors have any conflict of interest to disclose.
References
- 1.Kurdi M, Booz GW. JAK redux: a second look at the regulation and role of JAKs in the heart. Am J Physiol Heart Circ Physiol. 2009;297:H1545–H1556. doi: 10.1152/ajpheart.00032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- 3.Quintás-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov. 2011;10:127–140. doi: 10.1038/nrd3264. [DOI] [PubMed] [Google Scholar]
- 4.Wilks AF, Harpur AG, Kurban RR, Ralph SJ, Zürcher G, Ziemiecki A. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol. 1991;11:2057–2065. doi: 10.1128/mcb.11.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilks AF. The JAK kinases: not just another kinase drug discovery target. Semin Cell Dev Biol. 2008;19:319–328. doi: 10.1016/j.semcdb.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S, Takada H, Hara T, Kawamura N, Ariga T, Kaneko H, Kondo N, Tsuge I, Yachie A, Sakiyama Y, Iwata T, Bessho F, Ohishi T, Joh K, Imai K, Kogawa K, Shinohara M, Fujieda M, Wakiguchi H, Pasic S, Abinun M, Ochs HD, Renner ED, Jansson A, Belohradsky BH, Metin A, Shimizu N, Mizutani S, Miyawaki T, Nonoyama S, Karasuyama H. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–755. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Frucht DM, Gadina M, Jagadeesh GJ, Aksentijevich I, Takada K, Bleesing JJ, Nelson J, Muul LM, Perham G, Morgan G, Gerritsen EJ, Schumacher RF, Mella P, Veys PA, Fleisher TA, Kaminski ER, Notarangelo LD, O'Shea JJ, Candotti F. Unexpected and variable phenotypes in a family with JAK3 deficiency. Genes Immun. 2001;2:422–432. doi: 10.1038/sj.gene.6363802. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson LR, Han DY, Fraser AG, Huebner C, Lam WJ, Morgan AR, Duan H, Karunasinghe N. Genetic factors in chronic inflammation: single nucleotide polymorphisms in the STAT-JAK pathway, susceptibility to DNA damage and Crohn's disease in a New Zealand population. Mutat Res. 2010;690:108–115. doi: 10.1016/j.mrfmmm.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffé M, Berthou C, Lessard M, Berger R, Ghysdael J, Bernard OA. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 10.Peeters P, Raynaud SD, Cools J, Wlodarska I, Grosgeorge J, Philip P, Monpoux F, Van Rompaey L, Baens M, Van den Berghe H, Marynen P. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood. 1997;90:2535–2540. [PubMed] [Google Scholar]
- 11.Reiter A, Walz C, Watmore A, Schoch C, Blau I, Schlegelberger B, Berger U, Telford N, Aruliah S, Yin JA, Vanstraelen D, Barker HF, Taylor PC, O'Driscoll A, Benedetti F, Rudolph C, Kolb HJ, Hochhaus A, Hehlmann R, Chase A, Cross NC. The t(8;9)(p22;p24) is a recurrent abnormality in chronic and acute leukemia that fuses PCM1 to JAK2. Cancer Res. 2005;65:2662–2667. doi: 10.1158/0008-5472.CAN-04-4263. [DOI] [PubMed] [Google Scholar]
- 12.Bousquet M, Quelen C, De Mas V, Duchayne E, Roquefeuil B, Delsol G, Laurent G, Dastugue N, Brousset P. The t(8;9)(p22;p24) translocation in atypical chronic myeloid leukaemia yields a new PCM1-JAK2 fusion gene. Oncogene. 2005;24:7248–7252. doi: 10.1038/sj.onc.1208850. [DOI] [PubMed] [Google Scholar]
- 13.Murati A, Gelsi-Boyer V, Adélaïde J, Perot C, Talmant P, Giraudier S, Lodé L, Letessier A, Delaval B, Brunel V, Imbert M, Garand R, Xerri L, Birnbaum D, Mozziconacci MJ, Chaffanet M. PCM1-JAK2 fusion in myeloproliferative disorders and acute erythroid leukemia with t(8;9) translocation. Leukemia. 2005;19:1692–1696. doi: 10.1038/sj.leu.2403879. [DOI] [PubMed] [Google Scholar]
- 14.Flex E, Petrangeli V, Stella L, Chiaretti S, Hornakova T, Knoops L, Ariola C, Fodale V, Clappier E, Paoloni F, Martinelli S, Fragale A, Sanchez M, Tavolaro S, Messina M, Cazzaniga G, Camera A, Pizzolo G, Tornesello A, Vignetti M, Battistini A, Cavé H, Gelb BD, Renauld JC, Biondi A, Constantinescu SN, Foà R, Tartaglia M. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. J Exp Med. 2008;205:751–758. doi: 10.1084/jem.20072182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staerk J, Kallin A, Royer Y, Diaconu CC, Dusa A, Demoulin JB, Vainchenker W, Constantinescu SN. JAK2, the JAK2 V617F mutant and cytokine receptors. Pathol Biol (Paris) 2007;55:88–91. doi: 10.1016/j.patbio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S, Vaddi K, Levy R, Tefferi A. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen S, Zwillich SH, Chow V, Labadie RR, Wilkinson B. Co-administration of the JAK inhibitor CP-690,550 and methotrexate is well tolerated in patients with rheumatoid arthritis without need for dose adjustment. Br J Clin Pharmacol. 2010;69:143–151. doi: 10.1111/j.1365-2125.2009.03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, Krishnaswami S, Burgos-Vargas R, Wilkinson B, Zerbini CA, Zwillich SH. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895–1905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 19.Boy MG, Wang C, Wilkinson BE, Chow VF, Clucas AT, Krueger JG, Gaweco AS, Zwillich SH, Changelian PS, Chan G. Double-blind, placebo-controlled, dose-escalation study to evaluate the pharmacologic effect of CP-690,550 in patients with psoriasis. J Invest Dermatol. 2009;129:2299–2302. doi: 10.1038/jid.2009.25. [DOI] [PubMed] [Google Scholar]
- 20.Sanz A, Ungureanu D, Pekkala T, Ruijtenbeek R, Touw IP, Hilhorst R, Silvennoinen O. Analysis of Jak2 catalytic function by peptide microarrays: the role of the JH2 domain and V617F mutation. PLoS One. 2011;6:e18522. doi: 10.1371/journal.pone.0018522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duhé RJ, Clark EA, Farrar WL. Characterization of the in vitro kinase activity of a partially purified soluble GST/JAK2 fusion protein. Mol Cell Biochem. 2002;236:23–35. doi: 10.1023/a:1016186907376. [DOI] [PubMed] [Google Scholar]
- 22.Benbernou N, Muegge K, Durum SK. Interleukin (IL)-7 induces rapid activation of Pyk2, which is bound to Janus kinase 1 and IL-7Rα. J Biol Chem. 2000;275:7060–7065. doi: 10.1074/jbc.275.10.7060. [DOI] [PubMed] [Google Scholar]
- 23.Flati V, Haque SJ, Williams BR. Interferon-α-induced phosphorylation and activation of cytosolic phospholipase A2 is required for the formation of interferon-stimulated gene factor three. EMBO J. 1996;15:1566–1571. [PMC free article] [PubMed] [Google Scholar]
- 24.Taler M, Shpungin S, Salem Y, Malovani H, Pasder O, Nir U. Fer is a downstream effector of insulin and mediates the activation of signal transducer and activator of transcription 3 in myogenic cells. Mol Endocrinol. 2003;17:1580–1592. doi: 10.1210/me.2002-0328. [DOI] [PubMed] [Google Scholar]
- 25.Zhou YJ, Magnuson KS, Cheng TP, Gadina M, Frucht DM, Galon J, Candotti F, Geahlen RL, Changelian PS, O'Shea JJ. Hierarchy of protein tyrosine kinases in interleukin-2 (IL-2) signaling: activation of syk depends on Jak3; however, neither Syk nor Lck is required for IL-2-mediated STAT activation. Mol Cell Biol. 2000;20:4371–4380. doi: 10.1128/mcb.20.12.4371-4380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oki S, Limnander A, Danial NN, Rothman PB. Functional involvement of Akt signaling downstream of Jak1 in v-Abl-induced activation of hematopoietic cells. Blood. 2002;100:966–973. doi: 10.1182/blood.v100.3.966. [DOI] [PubMed] [Google Scholar]
- 27.Jiang H, Foltenyi K, Kashiwada M, Donahue L, Vuong B, Hehn B, Rothman P. Fes mediates the IL-4 activation of insulin receptor substrate-2 and cellular proliferation. J Immunol. 2001;166:2627–2634. doi: 10.4049/jimmunol.166.4.2627. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi-Tezuka M, Hibi M, Fujitani Y, Fukada T, Yamaguchi T, Hirano T. Tec tyrosine kinase links the cytokine receptors to PI-3 kinase probably through JAK. Oncogene. 1997;14:2273–2282. doi: 10.1038/sj.onc.1201071. [DOI] [PubMed] [Google Scholar]
- 29.Pollack BP, Kotenko SV, He W, Izotova LS, Barnoski BL, Pestka S. The human homologue of the yeast proteins Skb1 and Hsl7p interacts with Jak kinases and contains protein methyltransferase activity. J Biol Chem. 1999;274:31531–1542. doi: 10.1074/jbc.274.44.31531. [DOI] [PubMed] [Google Scholar]
- 30.Johnston JA, Wang LM, Hanson EP, Sun XJ, White MF, Oakes SA, Pierce JH, O'Shea JJ. Interleukins 2, 4, 7, and 15 stimulate tyrosine phosphorylation of insulin receptor substrates 1 and 2 in T cells. Potential role of JAK kinases. J Biol Chem. 1995;270:28527–28530. doi: 10.1074/jbc.270.48.28527. [DOI] [PubMed] [Google Scholar]
- 31.Carvalheira JB, Calegari VC, Zecchin HG, Nadruz W, Jr, Guimarães RB, Ribeiro EB, Franchini KG, Velloso LA, Saad MJ. The cross-talk between angiotensin and insulin differentially affects phosphatidylinositol 3-kinase- and mitogen-activated protein kinase-mediated signaling in rat heart: implications for insulin resistance. Endocrinology. 2003;144:5604–5614. doi: 10.1210/en.2003-0788. [DOI] [PubMed] [Google Scholar]
- 32.Xia K, Mukhopadhyay NK, Inhorn RC, Barber DL, Rose PE, Lee RS, Narsimhan RP, D'Andrea AD, Griffin JD, Roberts TM. The cytokine-activated tyrosine kinase JAK2 activates Raf-1 in a p21ras-dependent manner. Proc Natl Acad Sci U S A. 1996;93:11681–11686. doi: 10.1073/pnas.93.21.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakatsume M, Stancato LF, David M, Silvennoinen O, Saharinen P, Pierce J, Larner AC, Finbloom DS. Interferonγ activation of Raf-1 is Jak1-dependent and p21ras -independent. J Biol Chem. 1998;273:3021–3026. doi: 10.1074/jbc.273.5.3021. [DOI] [PubMed] [Google Scholar]
- 34.Migone TS, Rodig S, Cacalano NA, Berg M, Schreiber RD, Leonard WJ. Functional cooperation of the interleukin-2 receptor beta chain and Jak1 in phosphatidylinositol 3-kinase recruitment and phosphorylation. Mol Cell Biol. 1998;18:6416–6422. doi: 10.1128/mcb.18.11.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang YJ, Holtzman MJ, Chen CC. Differential role of Janus family kinases (JAKs) in interferon-γ-induced lung epithelial ICAM-1 expression: involving protein interactions between JAKs, phospholipase Cγ, c-Src, and STAT1. Mol Pharmacol. 2004;65:589–598. doi: 10.1124/mol.65.3.589. [DOI] [PubMed] [Google Scholar]
- 36.Giallourakis C, Kashiwada M, Pan PY, Danial N, Jiang H, Cambier J, Coggeshall KM, Rothman P. Positive regulation of interleukin-4-mediated proliferation by the SH2-containing inositol-5′-phosphatase. J Biol Chem. 2000;275:29275–29282. doi: 10.1074/jbc.M002853200. [DOI] [PubMed] [Google Scholar]
- 37.Ubersax JA, Ferrell JE., Jr Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 38.Lobie PE, Ronsin B, Silvennoinen O, Haldosén LA, Norstedt G, Morel G. Constitutive nuclear localization of Janus kinases 1 and 2. Endocrinology. 1996;137:4037–4045. doi: 10.1210/endo.137.9.8756581. [DOI] [PubMed] [Google Scholar]
- 39.Sorenson RL, Stout LE. Prolactin receptors and JAK2 in islets of Langerhans: an immunohistochemical analysis. Endocrinology. 1995;136:4092–4098. doi: 10.1210/endo.136.9.7649117. [DOI] [PubMed] [Google Scholar]
- 40.Ram PA, Waxman DJ. Interaction of growth hormone-activated STATs with SH2-containing phosphotyrosine phosphatase SHP-1 and nuclear JAK2 tyrosine kinase. J Biol Chem. 1997;272:17694–17702. doi: 10.1074/jbc.272.28.17694. [DOI] [PubMed] [Google Scholar]
- 41.Lee S, Duhé RJ. Kinase activity and subcellular distribution of a chimeric green fluorescent protein-tagged Janus kinase 2. J Biomed Sci. 2006;13:773–786. doi: 10.1007/s11373-006-9111-9. [DOI] [PubMed] [Google Scholar]
- 42.Ito M, Nakasato M, Suzuki T, Sakai S, Nagata M, Aoki F. Localization of janus kinase 2 to the nuclei of mature oocytes and early cleavage stage mouse embryos. Biol Reprod. 2004;71:89–96. doi: 10.1095/biolreprod.103.023226. [DOI] [PubMed] [Google Scholar]
- 43.Behrmann I, Smyczek T, Heinrich PC, Schmitz-Van de Leur H, Komyod W, Giese B, Müller-Newen G, Haan S, Haan C. Janus kinase (Jak) subcellular localization revisited: the exclusive membrane localization of endogenous Janus kinase 1 by cytokine receptor interaction uncovers the Jak.receptor complex to be equivalent to a receptor tyrosine kinase. J Biol Chem. 2004;279:35486–35493. doi: 10.1074/jbc.M404202200. [DOI] [PubMed] [Google Scholar]
- 44.Moulin S, Bouzinba-Segard H, Kelly PA, Finidori J. Subcellular trafficking of growth hormone receptor and Jak2 under ligand exposure. Horm Metab Res. 2003;35:396–401. doi: 10.1055/s-2003-41619. [DOI] [PubMed] [Google Scholar]
- 45.Dawson MA, Bannister AJ, Göttgens B, Foster SD, Bartke T, Green AR, Kouzarides T. JAK2 phosphorylates histone H3Y41 and excludes HP1α from chromatin. Nature. 2009;461:819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rinaldi CR, Rinaldi P, Alagia A, Gemei M, Esposito N, Formiggini F, Martinelli V, Senyuk V, Nucifora G, Pane F. Preferential nuclear accumulation of JAK2V617F in CD34+ but not in granulocytic, megakaryocytic, or erythroid cells of patients with Philadelphia-negative myeloproliferative neoplasia. Blood. 2010;116:6023–6026. doi: 10.1182/blood-2010-08-302265. [DOI] [PubMed] [Google Scholar]
- 47.Ragimbeau J, Dondi E, Vasserot A, Romero P, Uzé G, Pellegrini S. The receptor interaction region of Tyk2 contains a motif required for its nuclear localization. J Biol Chem. 2001;276:30812–30818. doi: 10.1074/jbc.M103559200. [DOI] [PubMed] [Google Scholar]
- 48.Lobie PE, Wood TJ, Chen CM, Waters MJ, Norstedt G. Nuclear translocation and anchorage of the growth hormone receptor. J Biol Chem. 1994;269:31735–31746. [PubMed] [Google Scholar]
- 49.Hellgren G, Jansson JO, Carlsson LM, Carlsson B. The growth hormone receptor associates with Jak1, Jak2 and Tyk2 in human liver. Growth Horm IGF Res. 1999;9:212–218. doi: 10.1054/ghir.1999.0111. [DOI] [PubMed] [Google Scholar]
- 50.Clevenger CV, Russell DH, Appasamy PM, Prystowsky MB. Regulation of interleukin 2-driven T-lymphocyte proliferation by prolactin. Proc Natl Acad Sci U S A. 1990;87:6460–6464. doi: 10.1073/pnas.87.16.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu DW, Stark KC, Dunnington D, Dillon SB, Yi T, Jones C, Pelus LM. SH2-Containing protein tyrosine phosphatase-1 (SHP-1) association with Jak2 in UT-7/Epo cells. Blood Cells Mol Dis. 2000;26:15–24. doi: 10.1006/bcmd.2000.0273. [DOI] [PubMed] [Google Scholar]
- 52.Smith RM, Jarett L. Ultrastructural evidence for the accumulation of insulin in nuclei of intact 3T3-L1 adipocytes by an insulin-receptor mediated process. Proc Natl Acad Sci U S A. 1987;84:459–463. doi: 10.1073/pnas.84.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Podlecki DA, Smith RM, Kao M, Tsai P, Huecksteadt T, Brandenburg D, Lasher RS, Jarett L, Olefsky JM. Nuclear translocation of the insulin receptor. A possible mediator of insulin's long term effects. J Biol Chem. 1987;262:3362–3368. [PubMed] [Google Scholar]
- 54.Gual P, Baron V, Lequoy V, Van Obberghen E. Interaction of Janus kinases JAK-1 and JAK-2 with the insulin receptor and the insulin-like growth factor-1 receptor. Endocrinology. 1998;139:884–893. doi: 10.1210/endo.139.3.5829. [DOI] [PubMed] [Google Scholar]
- 55.Olayioye MA, Beuvink I, Horsch K, Daly JM, Hynes NE. ErbB receptor-induced activation of stat transcription factors is mediated by Src tyrosine kinases. J Biol Chem. 1999;274:17209–17218. doi: 10.1074/jbc.274.24.17209. [DOI] [PubMed] [Google Scholar]
- 56.Marrero MB, Schieffer B, Paxton WG, Heerdt L, Berk BC, Delafontaine P, Bernstein KE. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature. 1995;375:247–250. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- 57.Lu D, Yang H, Shaw G, Raizada MK. Angiotensin II-induced nuclear targeting of the angiotensin type 1 (AT1) receptor in brain neurons. Endocrinology. 1998;139:365–375. doi: 10.1210/endo.139.1.5679. [DOI] [PubMed] [Google Scholar]
- 58.Mertani HC, Raccurt M, Abbate A, Kindblom J, Törnell J, Billestrup N, Usson Y, Morel G, Lobie PE. Nuclear translocation and retention of growth hormone. Endocrinology. 2003;144:3182–3195. doi: 10.1210/en.2002-221121. [DOI] [PubMed] [Google Scholar]
- 59.Baetz A, Koelsche C, Strebovsky J, Heeg K, Dalpke AH. Identification of a nuclear localization signal in suppressor of cytokine signaling 1. FASEB J. 2008;22:4296–4305. doi: 10.1096/fj.08-116079. [DOI] [PubMed] [Google Scholar]
- 60.Koelsche C, Strebovsky J, Baetz A, Dalpke AH. Structural and functional analysis of a nuclear localization signal in SOCS1. Mol Immunol. 2009;46:2474–2480. doi: 10.1016/j.molimm.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 61.Sakamoto H, Kinjyo I, Yoshimura A. The janus kinase inhibitor, Jab/SOCS-1, is an interferon-γ inducible gene and determines the sensitivity to interferons. Leuk Lymphoma. 2000;38:49–58. doi: 10.3109/10428190009060318. [DOI] [PubMed] [Google Scholar]
- 62.Sarkar S, Pollack BP, Lin KT, Kotenko SV, Cook JR, Lewis A, Pestka S. hTid-1, a human DnaJ protein, modulates the interferon signaling pathway. J Biol Chem. 2001;276:49034–49042. doi: 10.1074/jbc.M103683200. [DOI] [PubMed] [Google Scholar]
- 63.Kodiha M, Chu A, Lazrak O, Stochaj U. Stress inhibits nucleocytoplasmic shuttling of heat shock protein hsc70. Am J Physiol Cell Physiol. 2005;289:C1034–C1041. doi: 10.1152/ajpcell.00590.2004. [DOI] [PubMed] [Google Scholar]
- 64.Dudley AC, Thomas D, Best J, Jenkins A. A VEGF/JAK2/STAT5 axis may partially mediate endothelial cell tolerance to hypoxia. Biochem J. 2005;390:427–436. doi: 10.1042/BJ20050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brooks AJ, Wooh JW, Tunny KA, Waters MJ. Growth hormone receptor; mechanism of action. Int J Biochem Cell Biol. 2008;40:1984–1999. doi: 10.1016/j.biocel.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 66.Swanson SM, Kopchick JJ. Nuclear localization of growth hormone receptor: another age of discovery for cytokine action? Sci STKE. 20072007:pe69. doi: 10.1126/stke.4152007pe69. [DOI] [PubMed] [Google Scholar]
- 67.Conway-Campbell BL, Wooh JW, Brooks AJ, Gordon D, Brown RJ, Lichanska AM, Chin HS, Barton CL, Boyle GM, Parsons PG, Jans DA, Waters MJ. Nuclear targeting of the growth hormone receptor results in dysregulation of cell proliferation and tumorigenesis. Proc Natl Acad Sci U S A. 2007;104:13331–13336. doi: 10.1073/pnas.0600181104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nilsson J, Bjursell G, Kannius-Janson M. Nuclear Jak2 and transcription factor NF1-C2: a novel mechanism of prolactin signaling in mammary epithelial cells. Mol Cell Biol. 2006;26:5663–5674. doi: 10.1128/MCB.02095-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nilsson J, Helou K, Kovács A, Bendahl PO, Bjursell G, Fernö M, Carlsson P, Kannius-Janson M. Nuclear Janus-activated kinase 2/nuclear factor 1-C2 suppresses tumorigenesis and epithelial-to-mesenchymal transition by repressing Forkhead box F1. Cancer Res. 2010;70:2020–2029. doi: 10.1158/0008-5472.CAN-09-1677. [DOI] [PubMed] [Google Scholar]
- 70.Helmer RA, Panchoo M, Dertien JS, Bhakta SM, Hewetson A, Chilton BS. Prolactin-induced Jak2 phosphorylation of RUSH: a key element in Jak/RUSH signaling. Mol Cell Endocrinol. 2010;325:143–149. doi: 10.1016/j.mce.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Helmer RA, Dertien JS, Chilton BS. Prolactin induces Jak2 phosphorylation of RUSHY195. Mol Cell Endocrinol. 2011;338:79–83. doi: 10.1016/j.mce.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 72.Shi S, Calhoun HC, Xia F, Li J, Le L, Li WX. JAK signaling globally counteracts heterochromatic gene silencing. Nat Genet. 2006;38:1071–1076. doi: 10.1038/ng1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Griffiths DS, Li J, Dawson MA, Trotter MW, Cheng YH, Smith AM, Mansfield W, Liu P, Kouzarides T, Nichols J, Bannister AJ, Green AR, Göttgens B. LIF-independent JAK signalling to chromatin in embryonic stem cells uncovered from an adult stem cell disease. Nat Cell Biol. 2011;13:13–21. doi: 10.1038/ncb2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu F, Zhao X, Perna F, Wang L, Koppikar P, Abdel-Wahab O, Harr MW, Levine RL, Xu H, Tefferi A, Deblasio A, Hatlen M, Menendez S, Nimer SD. JAK2V617F-mediated phosphorylation of PRMT5 downregulates its methyltransferase activity and promotes myeloproliferation. Cancer Cell. 2011;19:283–294. doi: 10.1016/j.ccr.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brockman JL, Schroeder MD, Schuler LA. PRL activates the cyclin D1 promoter via the Jak2/Stat pathway. Mol Endocrinol. 2002;16:774–784. doi: 10.1210/mend.16.4.0817. [DOI] [PubMed] [Google Scholar]
- 76.Sakamoto K, Creamer BA, Triplett AA, Wagner KU. The Janus kinase 2 is required for expression and nuclear accumulation of cyclin D1 in proliferating mammary epithelial cells. Mol Endocrinol. 2007;21:1877–1892. doi: 10.1210/me.2006-0316. [DOI] [PubMed] [Google Scholar]
- 77.Jäkel H, Weinl C, Hengst L. Phosphorylation of p27Kip1 by JAK2 directly links cytokine receptor signaling to cell cycle control. Oncogene. 2011;30:3502–3512. doi: 10.1038/onc.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joung YH, Lim EJ, Lee MY, Park JH, Ye SK, Park EU, Kim SY, Zhang Z, Lee KJ, Park DK, Park T, Moon WK, Yang YM. Hypoxia activates the cyclin D1 promoter via the Jak2/STAT5b pathway in breast cancer cells. Exp Mol Med. 2005;37:353–364. doi: 10.1038/emm.2005.45. [DOI] [PubMed] [Google Scholar]
- 79.Fenyo IM, Florea IC, Raicu M, Manea A. Tyrphostin AG490 reduces NAPDH oxidase activity and expression in the aorta of hypercholesterolemic apolipoprotein E-deficient mice. Vascul Pharmacol. 2011;54:100–106. doi: 10.1016/j.vph.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 80.Cucuianu A, Stoia M, Farcaş A, Dima D, Zdrenghea M, Paţiu M, Olinic D, Petrov L. Arterial stenosis and atherothrombotic events in polycythemia vera and essential thrombocythemia. Rom J Intern Med. 2006;44:397–406. [PubMed] [Google Scholar]


