Abstract
Background
Thrombotic and bleeding complications are major concerns during orthopedic surgery. Given the frequency of orthopedic surgical procedures and the limited data in the literature, we sought to investigate the incidence and risk factors for thrombotic (myocardial necrosis and infarction) and bleeding events in patients undergoing orthopedic surgery.
Methods and Results
We performed a retrospective cohort analysis of 3,082 consecutive subjects ≥ 21 years of age undergoing hip, knee, or spine surgery between November 1, 2008, and December 31, 2009. Patient characteristics were ascertained using ICD-9 diagnosis coding and retrospective review of medical records, and laboratory/blood bank databases. In-hospital outcomes included myocardial necrosis (elevated troponin), major bleeding, coded myocardial infarction (MI), and coded hemorrhage as defined by ICD-9 coding. Of the 3,082 subjects, mean age was 60.8 ± 13.3 years and 59% were female. Myocardial necrosis, coded MI, major bleeding, and coded hemorrhage occurred in 179 (5.8%), 20 (0.7%), 165 (5.4%), and 26 (0.8%) subjects, respectively. Increasing age (P<0.001), CAD (P<0.001), cancer (P=0.004), and chronic kidney disease (P=0.01) were independent predictors of myocardial necrosis, while procedure type (P<0.001), cancer (P<0.001), female sex (P<0.001), CAD (P<0.001), and COPD (P=0.01) were independent predictors of major bleeding.
Conclusion
There is a delicate balance between thrombotic and bleeding events in the perioperative period following orthopedic surgery. Perioperative risk of both thrombosis and bleeding deserve careful attention in preoperative evaluation and future prospective studies aimed at attenuating this risk are warranted.
Keywords: arterial thrombosis, major bleeding, perioperative management
The competing risks of thrombotic and hemorrhagic complications are a major concern following orthopedic surgery. Perioperative thrombotic complications are a major cause of morbidity and mortality, occurring in 1–53% of cases depending on type of surgery, comorbidities present, and outcome ascertainment1–9. On the opposite end of the haemostatic equilibrium, major perioperative bleeding increases the risk of reoperation, overall length of stay, and hospital costs10. Despite the significant consequences of perioperative bleeding, there is a lack of information about baseline risk factors and incidence of major bleeding following orthopedic surgery.
With the ageing population, up to 25% of patients undergoing orthopedic surgery have underlying coronary artery disease (CAD)6, 11, 12. Subjects with established CAD are at increased risk for both perioperative thrombotic and bleeding complications4, 13, and the potential benefit of antiplatelet therapy to decrease thrombotic events must be weighed against increased risk of bleeding during the perioperative period.
To properly assess the potential benefit/risk trade-off during the perioperative period, one must know the incidence of both thrombotic and bleeding events following surgery. Most studies investigating the incidence of perioperative myocardial infarction (MI) in non-cardiac surgery were done in vascular surgery, a high-risk population that is likely to differ from the orthopedic population14–17. Furthermore, there are few data on the perioperative risk of bleeding in orthopedic surgery. Given the frequency of orthopedic surgical procedures and the limited data in the literature, we undertook this study to investigate the incidence of thrombotic (myocardial necrosis and/or MI) and major bleeding events in a cohort of patients with and without established coronary CAD who underwent orthopedic surgery in a tertiary medical center.
Methods
Study Design
We performed a retrospective cohort analysis of 3,294 consecutive subjects at a tertiary care academic medical center between November 1, 2008 and December 31, 2009. Patients older than 21 years of age who underwent hip, knee, or spine surgery were eligible. 212 subjects younger than 21 years of age were excluded leaving 3,082 subjects in this cohort. This study was approved by the Institutional Review Board with an informed consent waiver.
Data Sources
Data for this study were obtained from the hospital administrative database, hospital laboratory database, hospital blood bank database, and from retrospective medical record review. Data quality was assessed by reviewing a random sample of 10% of all medical records, blood bank database cases, and laboratory database cases.
Patient Characteristics
Patient characteristics and comorbidities were ascertained using the hospital administrative dataset. Information was collected on the following baseline demographics: age, sex, race/ethnicity, admission type (elective, urgent, or emergent surgery), and discharge disposition (alive, transferred to hospice, or expired). International Classification of Disease (ICD)-9 procedure codes were used to ascertain spinal fusion (81.0x), refusion of spine (81.3x), joint replacement of lower extremity (81.5x), other procedures on spine (81.6x). ICD-9 diagnosis codes were used to ascertain comorbidities on admission: history of coronary artery disease (412, 414.x), hypertension (401.x, 402, 403.x, 404, 405.x), cancer (140–208.9), diabetes mellitus (250.x), chronic obstructive pulmonary disease (COPD) (491.x, 496), chronic kidney disease (CKD) (585.x), peripheral vascular disease (PVD) (440.x, 443.x).
Approximately 11% of all subjects undergoing orthopedic surgery had CAD as determined by an ICD-9 diagnosis code indicating previous myocardial infarction (412.x), previous cardiac revascularization procedure (coronary artery bypass graft surgery or percutaneous coronary intervention, 36.x), or coronary artery disease (412, 414.x). Retrospective medical record review was carried out for all cases with a diagnosis of coronary artery disease (CAD) to obtain detailed information on demographics, clinical risk factors, and preoperative medications. History of heart failure (HF) and the preoperative use of aspirin (within 3 days of surgery) were additionally collected in the CAD cohort.
Outcomes
Myocardial necrosis was defined by an elevation in troponin level above the upper reference limit of the laboratory. Major bleeding was defined according to a modified definition of the International Society on Thrombosis and Haemostasis (ISTH) perioperative definition as transfusion of two or more units of packed red blood cells within 1 calendar day of surgery. We performed a medical record review of all blood bank data and obtained number and date of transfusions18. Troponin level was collected from laboratory data. Additional endpoints collected included discharge diagnosis codes for myocardial infarction (410.x), postoperative hemorrhage (998.11), and stroke (430, 431, 432.9, 433.x, 434.x, 435.x, 436, 674.x, 997.02), all not present on admission, all-cause mortality, and length of stay.
Statistical Analysis
Baseline variables with a continuous outcome were summarized as means ± standard deviation (SD) and categorical variables as frequencies and percentages overall and stratified by presence of CAD. Continuous variables were compared using Student’s t test and categorical variables were compared by χ2 Chi-square test/Fisher exact test. Baseline characteristics associated with thrombotic and bleeding events were estimated with univariate logistic regression models and reported as odd ratios (ORs) with corresponding to 95% confidence intervals (CIs). Those with a value of P ≤ 0.2 were introduced in a multivariate logistic regression model to identify independent predictors for thrombotic and major bleeding events.
All p values were obtained from two-sided tests. Statistical significance was defined as P < 0.05.
Funding Sources
The study was an investigator initiated study. It was supported in part by grant 1UL1RR029893 from the National Center for Research Resources, National Institutes of Health. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Overall Population
A total of 3,082 subjects who underwent orthopedic surgery of the spine (38%), knee (33%) and hip (30%) were included. Baseline characteristics are described in Table 1. The mean age of the cohort was 60.8 ± 13.3 years, 59% were female, and 65% were Caucasian. A history of coronary artery disease (CAD) was present in 327 (11%) subjects. Forty-nine percent of subjects had a history of hypertension, 15% diabetes, 3% kidney disease, 2% cancer, and 1% peripheral vascular disease.
Table 1.
Baseline characteristics in the overall population
| Overall Population (n=3082) | |
|---|---|
| Mean Age (yr) | 60.8 (± 13.3) |
| Female | 1822 (59.1) |
| Race | |
| White | 2005 (65.1) |
| Black | 439 (14.2) |
| Hispanic | 416 (13.5) |
| Other | 222 (7.2) |
| Admission Type | |
| Elective | 2902 (94.1) |
| Emergency or Urgent | 181 (5.9) |
| Procedure | |
| Spine | 1161 (37.7) |
| Knee | 1001 (32.5) |
| Hip | 920 (29.9) |
| Hypertension | 1513 (49.1) |
| CAD | 327 (10.6) |
| Cancer | 69 (2.2) |
| Diabetes Mellitus | 447 (14.5) |
| COPD | 69 (2.2) |
| CKD | 82 (2.7) |
| PVD | 23 (0.8) |
CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; PVD, peripheral vascular disease
Adverse perioperative events are recorded in Table 2. Myocardial necrosis and coded myocardial infarction (MI) occurred in 179 (5.8%), and 20 (0.7%) subjects, respectively. Any transfusion, ISTH defined perioperative major bleeding, and coded hemorrhage occurred in 781 (25.3%), 165 (5.4%), and 26 (0.8%) subjects, respectively. Stroke and all-cause mortality or transfer to hospice occurred in 4 (0.1%) and 5 (0.2%) subjects, respectively. The mean length of stay was 5.3 ± 5.6 days.
Table 2.
Adverse perioperative events in the overall population
| Overall Population (n=3082) | |
|---|---|
| Myocardial Necrosis | 179 (5.8) |
| Coded MI | 20 (0.7) |
| Major Bleeding* | 165 (5.4) |
| Coded Bleeding | 26 (0.8) |
| Any Transfusion w/in 1 day of surgery | 215 (7.0) |
| Any Transfusion | 781 (25.3) |
| Coded Stroke | 4 (0.1) |
| All-cause mortality | 5 (0.2)† |
Defined by ISTH guidelines18
1 subject passed away and 4 were discharged to hospice
MI, myocardial infarction
Perioperative Myocardial Necrosis
Myocardial necrosis occurred in 179 subjects. Subjects with myocardial necrosis were older (72.3 ± 11.3 years vs 60 ± 13.1 years, P<0.001) than those without myocardial necrosis. There were no significant differences in sex and type of surgery between groups. As expected, subjects with myocardial necrosis had more cardiovascular risk factors (Table 3). To determine the independent factors related to the incidence of myocardial necrosis, we performed multivariate logistic regression analysis (Table 3). Increasing age, history of CAD, history of cancer, and kidney disease were independent predictors of myocardial necrosis. Increasing age, history of CAD, and history of cancer had the highest Wald’s chi square values and therefore were most strongly correlated with myocardial necrosis. Procedure type and clinical urgency were not independently associated with the risk of myocardial necrosis. Subjects with perioperative myocardial necrosis had a 2-fold increased length of stay compared to subjects without myocardial necrosis (10.8 days ± 10.3 vs 5.0 days ± 4.9), P<0.001). Although occurring less frequently, we performed a multivariable analysis for the endpoint of coded MI. Similar to the findings for the myocardial necrosis endpoint, age and cancer were most strongly correlated with coded MI (data not shown).
Table 3.
Univariate and multivariate regression analysis in subjects with and without myocardial necrosis in overall population
| Myocardial Necrosis (n=179) | No Myocardial Necrosis (n=2903) | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | |||
| Mean Age (yr) | 72.3 (± 11.3) | 60.0 (± 13.1) | 1.09 [1.07–1.10] | <0.001 | 1.07 [1.05–1.09] | <0.001 |
| Female | 97 (54.2) | 1725 (59.4) | 0.81 [0.60–1.10] | 0.17 | 0.89 [0.63–1.25] | 0.49 |
| Race | 0.02 | 0.94 | ||||
| White | 133 (74.3) | 1872 (64.5) | 1.06 [0.62–1.95] | 1.07 [0.60–2.06] | ||
| Black | 14 (7.8) | 425 (14.6) | 0.49 [0.23–1.05] | 0.90 [0.40–2.05] | ||
| Hispanic | 18 (10.1) | 398 (13.7) | 0.67 [0.33–1.40] | 0.96 [0.45–2.10] | ||
| Other (reference level) | 14 (7.82) | 208 (7.17) | 1 | 1 | ||
| Admission Type | <0.001 | 0.12 | ||||
| Elective (reference level) | 146 (81.6) | 2755 (94.9) | 1 | 1 | ||
| Emergency or Urgent Surgery | 33 (18.4) | 148 (5.1) | 4.21 [2.75–6.29] | 1.51 [0.89–2.50] | ||
| Procedure | 0.09 | 0.38 | ||||
| Spine (reference level) | 64 (35.8) | 1097 (37.8) | 1 | 1 | ||
| Knee | 49 (27.4) | 952 (32.8) | 0.88 [0.60–1.29] | 0.74 [0.49–1.13] | ||
| Hip | 66 (36.9) | 854 (29.4) | 1.32 [0.93–1.89] | 0.89 [0.60–1.32] | ||
| CAD | 63 (35.2) | 264 (9.1) | 5.43 [3.88–7.54] | <0.001 | 2.58 [1.75–3.77] | <0.001 |
| Hypertension | 102 (57) | 1411 (48.6) | 1.40 [1.03–1.90] | 0.03 | 1.17 [0.82–1.70] | 0.39 |
| Cancer | 15 (8.4) | 54 (1.9) | 4.83 [2.58–8.52] | <0.001 | 2.86 [1.36–5.70] | 0.004 |
| Diabetes Mellitus | 39 (21.8) | 408 (14.1) | 1.70 [1.16–2.44] | 0.005 | 1.20 [0.78–1.79] | 0.39 |
| COPD | 11 (6.2) | 58 (2.0) | 3.21 [1.57–6.00] | 0.001 | 1.55 [0.70–3.16] | 0.25 |
| CKD | 21 (11.7) | 61 (2.1) | 6.19 [3.60–10.27] | <0.001 | 2.56 [1.30–4.86] | 0.01 |
| PVD | 7 (3.9) | 16 (0.6) | 7.34 [2.79–17.45] | <0.001 | 2.66 [0.91–7.14] | 0.06 |
CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; PVD, peripheral vascular disease
Perioperative Major Bleeding
Perioperative major bleeding defined by the ISTH perioperative major bleeding definition occurred in 165 subjects18. Characteristics of subjects with and without major bleeding are described in Table 4. To determine the independent factors related to the incidence of major bleeding, we performed multivariate logistic regression analysis (Table 4). Procedure type, cancer, female sex, CAD, and COPD were independent predictors of major bleeding. Procedure type, cancer, female sex, and CAD had the highest Wald’s chi square values, thus were most strongly correlated with major bleeding. Subjects with a major bleeding event had a significantly longer length of stay versus those without a major bleeding event (9.0 days ± 7.1 vs 5.1 days ± 5.4, P<0.001). After multivariable analysis for the endpoint of coded hemorrhage, cancer and CAD were the strongest correlates (data not shown).
Table 4.
Univariate and multivariate regression analysis in subjects with and without major bleeding in overall population
| Bleeding (n=165) | No Bleeding (n=2917) | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | |||
| Mean Age (yr) | 61.5 (± 15.5) | 60.7 (± 13.2) | 1.00 [0.99–1.02] | 0.49 | ||
| Female | 114 (69.1) | 1708 (58.6) | 1.58 [1.13–2.24] | 0.01 | 2.34 [1.63–3.41] | <0.001 |
| Race | 0.02 | 0.15 | ||||
| White | 122 (73.9) | 1883 (64.6) | 0.89 [0.53–1.62] | 1.10 [0.63–2.07] | ||
| Black | 14 (8.5) | 425 (14.6) | 0.45 [0.21–0.96] | 0.68 [0.31–1.49] | ||
| Hispanic | 14 (8.5) | 402 (13.8) | 0.48 [0.23–1.02] | 0.65 [0.29–1.43] | ||
| Other (reference level) | 15 (9.1) | 207 (7.1) | 1 | 1 | ||
| Admission Type | <0.001 | 0.48 | ||||
| Elective (reference level) | 140 (84.9) | 2761 (94.7) | 1 | 1 | ||
| Emergency or Urgent Surgery | 25 (15.2) | 156 (5.3) | 3.16 [1.97–4.90] | 1.23 [0.68–2.11] | ||
| Procedure | <0.001 | <0.001 | ||||
| Spine (reference level) | 110 (66.7) | 1051 (36.0) | 1 | 1 | ||
| Knee | 19 (11.5) | 982 (33.7) | 0.18 [0.11–0.30] | 0.19 [0.11–0.31] | ||
| Hip | 36 (21.8) | 884 (30.3) | 0.39 [0.26–0.57] | 0.35 [0.23–0.52] | ||
| CAD | 33 (20.0) | 294 (10.1) | 2.23 [1.47–3.29] | <0.001 | 2.49 [1. 58–3.85] | <0.001 |
| Hypertension | 80 (48.5) | 1433 (49.1) | 0.97 [0.71–1.33] | 0.87 | ||
| Cancer | 21 (12.7) | 48 (1.7) | 8.72 [4.99–14.76] | <0.001 | 6.85 [3.57–12.84] | <0.001 |
| Diabetes Mellitus | 28 (17.0) | 419 (14.4) | 1.22 [0.79–1.82] | 0.36 | ||
| COPD | 10 (6.1) | 59 (2.0) | 3.13 [1.48–5.96] | 0.001 | 2.69 [1.21–5.42] | 0.01 |
| CKD | 9 (5.5) | 73 (2.5) | 2.25 [1.03–4.35] | 0.03 | 1.53 [0.65–3.24] | 0.30 |
| PVD | 5 (3.0) | 18 (0.6) | 5.03 [1.65–12.80] | 0.002 | 2.63 [0.81–7.25] | 0.08 |
CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; PVD, peripheral vascular disease
CAD Population
In the CAD population, the perioperative incidence of both thrombotic and bleeding events was higher than in subjects without CAD (Appendix Table 1). Of the 63 subjects who developed myocardial necrosis, 36 (57.1%) had a troponin elevation greater than or equal to two times the upper limit of normal, and 7 (13.2%) subjects were given a discharge diagnosis of acute MI not present on admission.
Preoperative aspirin use was infrequent among patients with CAD (7.6%), and not significantly different in subjects with and without myocardial necrosis or major bleeding.
Discussion
There have been few studies on thrombotic and bleeding complications following orthopedic surgery. This lack of information complicates the preoperative evaluation and makes it difficult to assess the thrombotic and bleeding risks of surgery. Furthermore, differences in both the study population and the definitions used for thrombotic and bleeding complications limits the comparison between studies. Our study showed an overall incidence of myocardial necrosis and major bleeding of 5.8% and 5.4%, respectively. Previous studies have shown an incidence of perioperative myocardial necrosis ranging from 1% to 53%3–9, depending on the study population. For example, Chong et al found an incidence of perioperative myocardial necrosis of 53% in subjects with a mean age of 79 years undergoing emergency orthopedic surgery4. Dawson et al and Fisher et al found perioperative incidences of 39% and 29%, respectively, in the elderly population7, 9. Few studies have simultaneously investigated the incidence of both thrombotic and bleeding complications in orthopedic surgery, and in those studies, the majority of data were ascertained from coding alone without any biomarker data13, 19–22.
Our study used troponin elevation as a definition of myocardial necrosis. Coded myocardial infarction was defined by ICD-9 diagnosis coding. We found an incidence of myocardial necrosis of 5.8%, while coded myocardial infarction was noted in 0.7%. A recent study showed that nearly two-thirds of subjects having a perioperative MI will not experience ischemic symptoms17. The high incidence of myocardial necrosis not acknowledged in diagnosis coding may be secondary to a small cardiac insult, blunting of symptoms from perioperative pain medication and anesthesia, and post-surgical complications23. Understanding that patients in the perioperative period may not demonstrate clinical signs or symptoms of myocardial infarction emphasizes the point that diagnosing myocardial ischemia based on ICD-9 coding will lead to underascertainment of myocardial ischemia.
Like myocardial necrosis, perioperative bleeding is a major complication of surgery that is often difficult to define. The International Society on Thrombosis and Haemostasis (ISTH) proposed guidelines for the definition of major bleeding in surgical patients18. We found an incidence of ISTH major bleeding of 5.4%; however, coded hemorrhage was only found in 0.8%. This underestimation of major bleeding reflects that coded hemorrhage does not identify all patients with major bleeding. The ISTH standardized definition of perioperative bleeding is a useful option for future studies in the in the perioperative period.
Our study demonstrates several important findings. Increasing age, CAD, cancer, and CKD were independent predictors of myocardial necrosis, while procedure type, cancer, female sex, CAD, and COPD were independent predictors of major bleeding. Given the delicate balance between thrombotic and bleeding events during the perioperative period, it is important to understand the baseline risk for each of these complications. It is worthwhile to note that CAD and cancer were independent predictors of both myocardial necrosis and major bleeding (Figure 1). While CAD is a well-known risk factor for arterial thrombosis and bleeding complications, cancer may be an under recognized risk factor for perioperative complications that likely deserves attention in preoperative evaluation.
Figure 1.
Figure 1a. The incidence of in-hospital myocardial necrosis and major bleeding in subjects undergoing orthopedic surgery when stratified by coronary artery disease
Subjects with CAD were significantly more likely to experience myocardial necrosis (19.3% vs 4.2%, P<0.001) and major bleeding (10.1% vs 4.8%, P<0.001) than subjects without CAD.
CAD, n=327
No CAD, n=2755
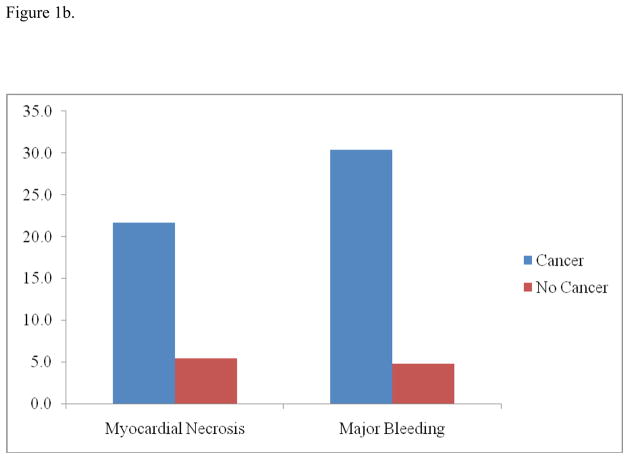
Figure 1b. The incidence of in-hospital myocardial necrosis and major bleeding in subjects undergoing orthopedic surgery when stratified by cancer
Subjects with cancer were significantly more likely to experience myocardial necrosis (21.7% vs 5.4%, P<0.001) and major bleeding (30.4% vs 4.8%, P<0.001) than subjects without cancer.
Cancer, n=69
No Cancer, n=3013
The question then arises of whether these perioperative complications have an impact on short- and long-term prognosis. We found that subjects with either an elevation in troponin or bleeding complication had approximately a 2-fold increased length of stay compared to subjects without these complications. Given the morbidity and expense that occurs with remaining in the hospital, a better understanding of the risk of perioperative complications may allow for a reduction in the length of stay. Although our study shows the overall incidence and risk factors for perioperative complications, it does not determine whether these complications impact prognosis. Previous studies have shown that perioperative troponin elevation, regardless of etiology, is associated with short- and long-term mortality4, 6, 15–17, 24, 25. While troponin elevation may have important prognostic implications, future studies are needed to assess the potential clinical benefit and/or cost-effectiveness of adopting routine troponin measurements in orthopedic surgery.
Similarly, major bleeding in the non-operative setting has been associated with increases in cardiovascular events and long-term mortality26, 27; however, data in the perioperative setting is lacking. The negative prognostic impact of perioperative troponin elevation and major bleeding emphasizes the importance of understanding the risk factors for perioperative complications.
There were several limitations to our study. First, this was an observational study and therefore many confounders may exist. Second, the data are limited to variables captured by the hospital laboratory, blood bank and ICD-9 coding, therefore subject to misclassification bias and underascertainment of medical comorbidities and endpoints. Third, troponin value was not obtained in all subjects, and there was no uniform time assessment of measuring troponin. Our data likely leads to an underestimation of myocardial necrosis, since we use all subjects in the dataset as the denominator. If only higher risk patients had a troponin checked, the denominator would be smaller and thus the incidence of myocardial necrosis would be larger. Fourth, while we tried to use the ISTH bleeding definition for surgical patients, we used 1 calendar day instead of 48 hours, because it is often difficult to determine the precise time of surgery and therefore we attempted to be more conservative to avoid overestimating the incidence of major bleeding. We extracted electronic blood bank data for all 3,082 patients to determine the number and date of transfusions. However, it was not feasible to perform a complete medical record review for all subjects to determine symptoms/signs of bleeding. Moreover, many times this information is not available in the medical chart.
In conclusion, our study provides an overall incidence of perioperative thrombotic and bleeding complications, using standardized definitions, of the general population undergoing orthopedic surgery. Increasing age, CAD, cancer, and CKD were independent predictors of myocardial necrosis, while procedure type, cancer, female sex, CAD, and COPD were independent predictors of major bleeding. Given the delicate balance between thrombotic and bleeding events during the perioperative period, as well as the long-term prognostic implications, it is important to understand the association between baseline risk factors and thrombotic and bleeding complications. We found a similar incidence of myocardial necrosis and major bleeding, thus highlighting the importance of a patient-centered approach to perioperative evaluation. Perioperative risk for thrombosis and bleeding deserves careful evaluation in preoperative evaluation and future prospective studies aimed at attenuating this risk are warranted.
Acknowledgments
Dr Berger was partially funded by an American Heart Association Fellow to Faculty Award (0775074N) and a Doris Duke Clinical Scientist Award (2010055).
Funding Sources: The study was an investigator initiated study. It was supported in part by grant 1UL1RR029893 from the National Center for Research Resources, National Institutes of Health.
Appendix
Table 1.
Adverse perioperative events in subjects with and without coronary artery disease
| CAD (n=327) | No CAD (n=2755) | P-value | |
|---|---|---|---|
| Myocardial Necrosis | 63 (19.3) | 116 (4.2) | <0.001 |
| Coded MI | 7 (2.1) | 13 (0.5) | 0.003 |
| Major Bleeding* | 33 (10.1) | 132 (4.8) | <0.001 |
| Coded Bleeding | 7 (2.1) | 19 (0.7) | 0.02 |
| Any Transfusion w/in 1 day of surgery | 39 (11.9) | 176 (6.4) | <0.001 |
| Any Transfusion | 147 (45.0) | 634 (23.0) | <0.001 |
| Coded Stroke | 2 (0.6) | 2 (0.1) | 0.06 |
| All-cause mortality† | 3 (0.9) | 2 (0.1) | 0.01 |
Defined by ISTH guidelines18
1 subject passed away and 4 were discharged to hospice
CAD, coronary artery disease; MI, myocardial infarction
Footnotes
Disclosures: There are no conflicts of interest for any of the submitting authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153–84. doi: 10.1097/00000542-199001000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med. 1996;335(23):1713–20. doi: 10.1056/NEJM199612053352301. [DOI] [PubMed] [Google Scholar]
- 3.Ausset S, Auroy Y, Verret C, Benhamou D, Vest P, Cirodde A, et al. Quality of postoperative care after major orthopedic surgery is correlated with both long-term cardiovascular outcome and troponin Ic elevation. Anesthesiology. 113(3):529–40. doi: 10.1097/ALN.0b013e3181eaacc4. [DOI] [PubMed] [Google Scholar]
- 4.Chong CP, Lam QT, Ryan JE, Sinnappu RN, Lim WK. Incidence of post-operative troponin I rises and 1-year mortality after emergency orthopaedic surgery in older patients. Age Ageing. 2009;38(2):168–74. doi: 10.1093/ageing/afn231. [DOI] [PubMed] [Google Scholar]
- 5.Urban MK, Jules-Elysee K, Loughlin C, Kelsey W, Flynn E. The one year incidence of postoperative myocardial infarction in an orthopedic population. HSS J. 2008;4(1):76–80. doi: 10.1007/s11420-007-9070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ausset S, Auroy Y, Lambert E, Vest P, Plotton C, Rigal S, et al. Cardiac troponin I release after hip surgery correlates with poor long-term cardiac outcome. Eur J Anaesthesiol. 2008;25(2):158–64. doi: 10.1017/S0265021507001202. [DOI] [PubMed] [Google Scholar]
- 7.Dawson-Bowling S, Chettiar K, Cottam H, Worth R, Forder J, Fitzgerald-O’Connor I, et al. Troponin T as a predictive marker of morbidity in patients with fractured neck of femur. Injury. 2008;39(7):775–80. doi: 10.1016/j.injury.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Jules-Elysee K, Urban MK, Urquhart B, Milman S. Troponin I as a diagnostic marker of a perioperative myocardial infarction in the orthopedic population. J Clin Anesth. 2001;13(8):556–60. doi: 10.1016/s0952-8180(01)00337-3. [DOI] [PubMed] [Google Scholar]
- 9.Fisher AA, Southcott EN, Goh SL, Srikusalanukul W, Hickman PE, Davis MW, et al. Elevated serum cardiac troponin I in older patients with hip fracture: incidence and prognostic significance. Arch Orthop Trauma Surg. 2008;128(10):1073–9. doi: 10.1007/s00402-007-0554-x. [DOI] [PubMed] [Google Scholar]
- 10.Vera-Llonch M, Hagiwara M, Oster G. Clinical and economic consequences of bleeding following major orthopedic surgery. Thromb Res. 2006;117(5):569–77. doi: 10.1016/j.thromres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Salerno SM, Carlson DW, Soh EK, Lettieri CJ. Impact of perioperative cardiac assessment guidelines on management of orthopedic surgery patients. Am J Med. 2007;120(2):185, e1–6. doi: 10.1016/j.amjmed.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Ackland GL, Harris S, Ziabari Y, Grocott M, Mythen M. Revised cardiac risk index and postoperative morbidity after elective orthopaedic surgery: a prospective cohort study. Br J Anaesth. 105(6):744–52. doi: 10.1093/bja/aeq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanes S, Fraeman K, Meyers A, Wood Ives J, Huang HY. Incidence rates for thromboembolic, bleeding and hepatic outcomes in patients undergoing hip or knee replacement surgery. J Thromb Haemost. 9(2):325–32. doi: 10.1111/j.1538-7836.2010.04155.x. [DOI] [PubMed] [Google Scholar]
- 14.Adesanya AO, de Lemos JA, Greilich NB, Whitten CW. Management of perioperative myocardial infarction in noncardiac surgical patients. Chest. 2006;130(2):584–96. doi: 10.1016/S0012-3692(15)51881-3. [DOI] [PubMed] [Google Scholar]
- 15.Landesberg G, Shatz V, Akopnik I, Wolf YG, Mayer M, Berlatzky Y, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol. 2003;42(9):1547–54. doi: 10.1016/j.jacc.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Kim LJ, Martinez EA, Faraday N, Dorman T, Fleisher LA, Perler BA, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation. 2002;106(18):2366–71. doi: 10.1161/01.cir.0000036016.52396.bb. [DOI] [PubMed] [Google Scholar]
- 17.Devereaux PJ, Xavier D, Pogue J, Guyatt G, Sigamani A, Garutti I, et al. Characteristics and Short-Term Prognosis of Perioperative Myocardial Infarction in Patients Undergoing Noncardiac Surgery: A Cohort Study. Ann Intern Med. 154(8):523–528. doi: 10.7326/0003-4819-154-8-201104190-00003. [DOI] [PubMed] [Google Scholar]
- 18.Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8(1):202–4. doi: 10.1111/j.1538-7836.2009.03678.x. [DOI] [PubMed] [Google Scholar]
- 19.Hagen TP, Vaughan-Sarrazin MS, Cram P. Relation between hospital orthopaedic specialisation and outcomes in patients aged 65 and older: retrospective analysis of US Medicare data. BMJ. 340:c165. doi: 10.1136/bmj.c165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cram P, Cai X, Lu X, Vaughan-Sarrazin MS, Miller BJ. Total knee arthroplasty outcomes in top-ranked and non-top-ranked orthopedic hospitals: an analysis of Medicare administrative data. Mayo Clin Proc. 87(4):341–8. doi: 10.1016/j.mayocp.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 308(12):1227–36. doi: 10.1001/2012.jama.11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibrahim SA, Stone RA, Han X, Cohen P, Fine MJ, Henderson WG, et al. Racial/ethnic differences in surgical outcomes in veterans following knee or hip arthroplasty. Arthritis Rheum. 2005;52(10):3143–51. doi: 10.1002/art.21304. [DOI] [PubMed] [Google Scholar]
- 23.Devereaux PJ, Goldman L, Yusuf S, Gilbert K, Leslie K, Guyatt GH. Surveillance and prevention of major perioperative ischemic cardiac events in patients undergoing noncardiac surgery: a review. CMAJ. 2005;173(7):779–88. doi: 10.1503/cmaj.050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy M, Heels-Ansdell D, Hiralal R, Bhandari M, Guyatt G, Yusuf S, et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta-analysis. Anesthesiology. 114(4):796–806. doi: 10.1097/ALN.0b013e31820ad503. [DOI] [PubMed] [Google Scholar]
- 25.Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, Villar JC, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 307(21):2295–304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 26.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–17. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 27.Berger JS, Bhatt DL, Steg PG, Steinhubl SR, Montalescot G, Shao M, et al. Bleeding, mortality, and antiplatelet therapy: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Am Heart J. 162(1):98–105. e1. doi: 10.1016/j.ahj.2011.04.015. [DOI] [PubMed] [Google Scholar]