Abstract
Background
Fluorescence imaging hardware (SPY) has recently been developed for intraoperative assessment of blood flow via detection of probes emitting in the near-infrared (NIR) spectrum. This study sought to determine if this imaging system was capable of detecting micrometastatic head and neck squamous cell carcinoma (HNSCC) in preclinical models.
Methods
A NIR fluorescent probe (IRDye800CW) was covalently linked to a monoclonal antibody targeting EGFR (panitumumab) or non-specific IgG. HNSCC flank (SCC-1) and orthotopic (FADU and OSC19) xenografts were imaged 48-96hrs following systemic injection of labeled panitumumab or IgG. The primary tumor and regional lymph nodes were dissected using fluorescence guidance with the SPY system and grossly assessed with a charge-coupled NIR system (Pearl). Histologic slides were also imaged with a NIR charged-coupled device (Odyssey) and fluorescence intensity was correlated with pathologic confirmation of disease.
Results
Orthotopic tongue tumors were clearly delineated from normal tissue with tumor-to-background ratios of 2.9(Pearl) and 2.3(SPY). Disease detection was significantly improved with panitumumab-IRDye compared to IgG-IRDye800 (P<0.05). Tissue biopsies (average size=3.7mm) positive for fluorescence were confirmed for pathologic disease by histology and immunohistochemistry (n=25/25). Biopsies of non-fluorescent tissue were proven to be negative for malignancy (n=28/28). The SPY was able to detect regional lymph node metastasis (<1.0mm) and microscopic areas of disease. Standard histological assessment in both frozen and paraffin-embedded histologic specimens was augmented using the Odyssey.
Conclusions
Panitumumab-IRDye800 may have clinical utility in detection and removal of microscopic HNSCC using existing intraoperative optical imaging hardware and may augment analysis of frozen and permanent pathology.
INTRODUCTION
Due to the infiltrative nature of head and neck cancer, positive margins occur in 40%- 50% of cases after resection. This is likely attributed to the crude measures of tumor margin identification including gross palpation and visual inspection[1]. While there are a plethora of methods to image cancer preoperatively, including CT and PET-CT, there is currently no approved real-time method available to image tumors intraoperatively. A real-time imaging modality has the potential to decrease the rate of positive margins and spare uninvolved tissue by guiding surgical resection. To meet this need, there have been significant efforts in identifying the proper optical imaging agents and hardware for real-time intraoperative tumor detection. Clinical trials are underway in Europe evaluating the potential of targeting monoclonal antibodies labeled with fluorescent probes in oncological surgery. These trials are testing the ability of labeled antibodies to delineate tumor margins in the OR, to detect residual microscopic disease during resection, and to identify lymph node metastases [2].
The value of this technique will only be as accurate as the intra-operative imaging hardware, yet there is currently no consensus on which hardware is best suited for this purpose. Developing novel camera systems to detect IRDye800 fluorescence in real-time has been done by a few groups but their application to humans is complicated by development costs, overlapping intellectual property rights, large scale manufacturing, and FDA approval [3, 4]. Intraoperative fluorescence imaging systems have been developed for detecting blood flow with indocyanine green, and are currently used for plastic surgery procedures (SPY System, Novadaq, Toronto, Canada) and for next generation robotic consoles (Firefly, Intuitive Surgical, DaVinci). Because of their overlapping emission and absorption spectra, we undertook the current study to determine if operative imaging systems designed for indocyanine green can be used for IRDye800 imaging.
Selective delivery of pantitumumab-IRDye800 to the tumor has the additional benefit of improving post-resection assessment. Once removed, the tumor can be assessed on the ‘back table’ with the SPY system or the histological frozen sections can be imaged to improve the ability to identify small islands of disease. Preclinical models using fluorescence imaging of histological sections using an optical scanner (e.g., Odyssey, manufactured by LiCor) have been promising [5]. Such imaging could be used by pathologists to augment intraoperative margin evaluation and to improve sensitivity and specificity.
We hypothesize that both macroscopic and microscopic optical imaging modalities can be employed to detect microscopic disease when using antibody targeted fluorescence. This is the first study to investigate if current FDA-approved intraoperative imaging hardware (SPY) can detect microscopic disease and micrometastases from head and neck squamous cell carcinoma using bioconjugated panitumumab-IRDye800 in a preclinical murine model. Additionally, we investigated the use of fluorescence imaging (Odyssey) for detecting disease on frozen histological sections. This technology would provide a more efficient and accurate modality for intraoperative and histological detection of cancer.
MATERIALS AND METHODS
Cell Lines and Tissue Culture
Multiple HNSCC cell lines were used: SCC-1 (Thomas Carey, University of Michigan, Ann Arbor, Michigan), OSC-19 (Jeffrey Myers, The University of Texas M.D. Anderson Cancer Center, Houston, Texas), and FADU (ATCC). These cell lines were grown and maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and supplemented with 1% penicillin, streptomycin, and amphotericin B. They were incubated at 37°C in 5% CO2.
Reagents
Panitumumab (Vectibix; Amgen, Thousand Oaks, California; 147 kDa), a recombinant and fully humanized monoclonal antibody which binds to the extracellular domain of human EGFR, was the antibody used. Protein A purified IgG antibody (Innovative. Ir-Hu-Gf, #30010BM) (146kDa) was used as a control antibody.
NIR Fluorescence Agents
IRDye800CW (IRDye800CW-N-hydroxysuccinimide ester, LI-COR Biosciences, Lincoln, Nebraska) was the fluorescent probe. It has a NIR absorption and emission peak of 778nm/794nm. When conjugated to either panitumumab or IgG the absorbance and emission maximums decrease slightly (774nm/789nm).[6] Panitumumab and IgG were labeled according to the manufacturer’s protocol. Briefly, both antibodies were incubated with the IRDye800CW for 2h and the unconjugated dye was removed by desalting columns (Pierce, Zeba, #89891).
Antigen Detection Assay
A 96-well black plate was coated with recombinant EGFR (rEGFR; 50ng/well/100uL; Fc chimera, CF, #344-ER-050) overnight at 4°C. The wells were blocked for 1h at room temperature with 1% bovine serum albumin (BSA). Wells were then washed with PBS 3 times and incubated for 1h with varying concentrations (0.5nm to 64nM) of labeled or unlabeled panitumumab. As a control, uncoated plates were incubated with same concentrations of labeled panitumumab. Following incubation the wells were washed with PBS and imaged using the Pearl (LI-COR Biosciences, Lincoln, Nebraska). Well intensities were quantified using the Pearl Impulse Software Version 2.0.
Animal Models
Nude(nu/nu) and SCID female mice (Charles River Laboratories, Hartford, Connecticut), aged 4-6 weeks, were obtained and housed in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines. All experiments were conducted and the mice euthanized according to IACUC guidelines.
For the flank model, nude mice (n=2) received subcutaneous injections of SCC1 cells (2×106) suspended in media (200μL). Tumor size was monitored weekly until they reached 25mm2. The mice were then systemically injected with fluorescently labeled panitumumab or IgG (50-100μg). For the human split-thickness skin graft (STSG) model, the skin from the back of SCID mice was excised and the STSG sutured in place.
For the orthotopic tumor model, nude mice (n=3) were injected with OSC19 or FADU (2.5 x105) cells suspended in media (25μL) in the anterior portion of their tongues. Tumor growth was followed for 2 weeks after which time the mice received systemic administration of panitumumab-IRDye800.
For the human tumor model, SCID mice (n=3) were implanted on each flank with human tumor samples following IRB approval. Tumor growth was followed for 6 weeks after which the mice received systemic administration of panitumumab-IRDye800.
Fluorescence Imaging and Measurement
Table 1 summarizes the imaging modalities used. Figure 1 illustrates the sequence of real-time fluorescent-guided resection and fluorescent-guided histological processing. The SPY imaging system captures fluorescent light using a charged couple device video camera at 30 frames/second and displays it on a computer monitor[7]. The SPY system was used to guide tumor and lymph node resections by real-time fluorescence guidance 48-96 hours following injection of the panitumumab-IRDye800. Following resections, the excised tissues were placed in tissue cassettes and imaged with both the Pearl and SPY.
TABLE 1.
Imaging Modalities
|
Imaging
Modality |
Description |
Clinical
Translatability |
Designed to
image |
|---|---|---|---|
| Pearl Impulse Small Animal Imaging System |
In vivo fluorescence
imaging system that scans the whole body. |
No; restricted to small animals |
IRDye800 |
| Odyssey CLx Infrared Imaging System |
Ex vivo fluorescence
imaging system for histological and whole organ imaging |
Yes | IRDye800 |
| SPY Imaging System |
Intraoperative
fluorescence imaging system that allows surgeons to detect fluorescence in real-time. The SPY is FDA approved for use during a variety of surgical procedures. |
Yes | ICG |
Figure 1.

Use of fluorescent guided surgery in real-time using the SPY system and during histological processing using the Odyssey Scanner.
In vivo fluorescence detected with the SPY machine was quantified using ImageJ (http://rsb.info.nih.gov/ij/). Fluorescence intensity was measured by selecting several regions of interest (ROI) within the tumor and calculating the mean value. Subsequently, a tumor-to-background ratio (TBR) was calculated based on the fluorescence intensity from a sample of normal tissue adjacent to the tumor border. The Pearl Impulse small animal imager was used to verify the TBR measured by the SPY.
Fluorescent microscopy of histologic sections was performed using the Odyssey scanner (LI-COR Biosciences, Lincoln, Nebraska). Co-localization of the fluorescent signal with tumor was performed by overlaying the Odyssey-acquired fluorescent image with the microscopic H&E image.
Immunohistochemistry
Immunohistochemical analysis with CD147 (Millipore, clone 1S9-2A, mouse monoclonal IgG2ak) and cytokeratin (clone: AE1+AE3, Ventana, Tuscan, Arizona) was performed to confirm tumor cells. 5μm sections were cut from the paraffin blocks and antigen retrieval was accomplished by heating in 1mM EDTA, pH 9.0, for 10 minutes at 90°C. Samples were cooled to room temperature and blocked with 5% BSA in TBST for 5 minutes at room temperature. The primary antibodies (CD147 or cytokeratin) were applied at the recommended concentrations and incubated overnight at 4°C. The secondary antibody (Pierce, goat anti-mouse, horseradish peroxidase) was applied for 1 h in a humidified chamber at room temperature. Slides were then treated with the DAB substrate until the color developed.
Statistical analysis
For each model (flank, orthotopic, and human tumor), the average fluorescence of the tumor was compared to the average fluorescence of the surrounding tissue using a paired student’s t-test. Error bars represent the standard deviation. Statistically significant was considered P<0.05.
RESULTS
Detection of panitumumab-IRDye800
The panitumumab-IRDye bioconjugate maintained antigen specificity to EGFR in conventional antigen detection assays (data not shown). The specificity of in vivo tumor targeting of panitumumab-IRDye800 was assessed by comparison to a fluorescently labeled non-specific antibody (IgG-IRDye800) in SCC-1 flank xenografts. Tumor fluorescence was evaluated using the SPY intraoperative imaging system (Figure 2A) designed for indocyanine green imaging and compared to the gold standard for IRDye800 imaging, a small animal optical imaging system (Pearl, Table 1).
Figure 2. Panitumumab-IRDye versus aspecific control (IgG-IRDye).
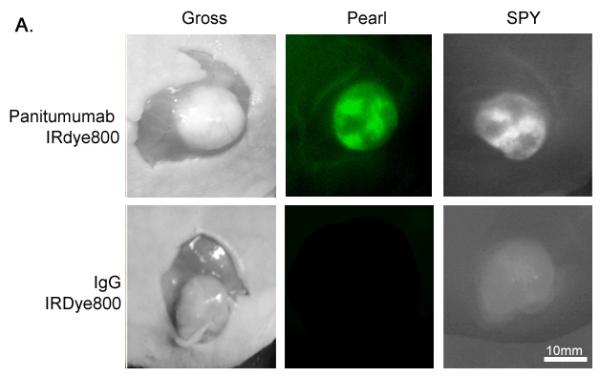
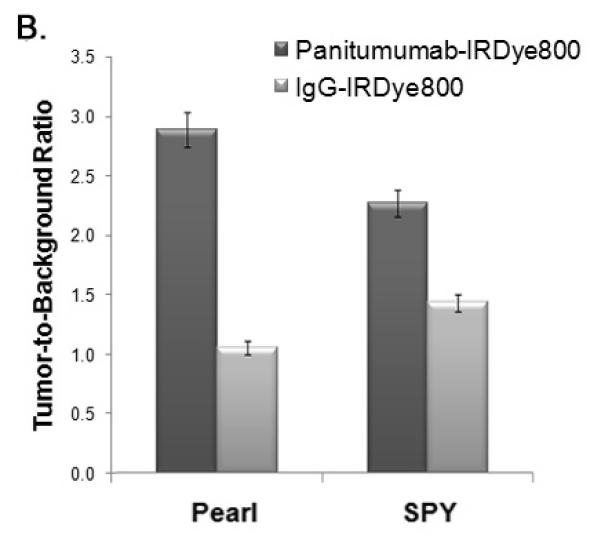
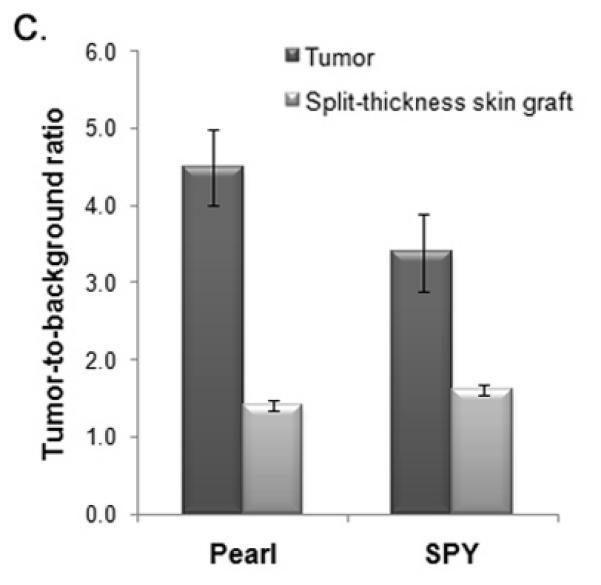
(A) Uptake of the near-infrared (NIR) fluorescence agent panitumumab-IRDye800 versus aspecific IgG-IRDye detected with the Pearl and SPY Systems in mice with flank SCC1 tumors. (B) The tumor-to-background is significantly higher versus the control for both the Pearl and SPY. (C) Panitumumab-IRDye demonstrates significantly greater fluorescence in a tumor versus the split-thickness skin graft for both the Pearl and SPY imager. Error bars indicate the SD.
Regardless of the imaging modality used, the mouse injected with panitumumab-IRDye800 had significantly greater fluorescence intensity compared to the control IgG-IRDye800 (Figure 2B). The tumor fluorescence after systemic injection with IgG-IRDye800 had a TBR of 1.1 (Pearl) and 1.4 (SPY). By comparison, systemic administration of panitumumab-IRDye800 resulted in a tumor-to-background ratio TBR of 2.9 (Pearl) and 2.3 (SPY), with the tumor demonstrating a statistically significant increase in fluorescence intensity versus the background (P<0.05).
Since rodent EGFR differs from human EGFR, we evaluated the uptake of panitumumab-IRDye800 in a tumor versus a human split-thickness skin-graft (STSG) to determine the expected background fluorescence in humans. Both the SPY and the Pearl detected significantly greater fluorescence in the tumor versus the STSG (Figure 2C) indicating that if used in humans, the panitumumab-IRDye800 is expected achieve a sufficient TBR to guide surgical resection.
Resection under NIR fluorescence-guided imaging
After systemic administration of panitumumab-IRDye800, orthotopic cell line xenografts or flank human tumor implants were resected by real-time guidance with the SPY System. Subclinical residual disease and lymph node metastases were also detected with the SPY. Residual areas of positive and negative fluorescence and lymph nodes were evaluated with conventional H&E histology and immunohistochemistry to identify the presence of tumor cells.
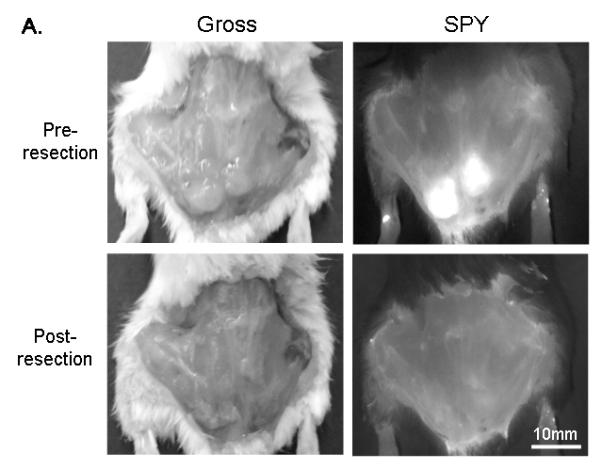
Orthotopic xenografts were imaged 48-96 h following systemic panitumumab-IRDye800 administration with both the Pearl and SPY systems (Figure 3A). Supplemental video 1 demonstrates in real-time how the fluorescent signal guides resection intraoperatively. The tumor-to-background ratio was 4.2 (Pearl) and 2.3 (SPY). Both systems demonstrated a significant difference between the tumor and background fluorescence intensities (P <0.01). Tongue tumors (n=3, average diameter: 4.25mm; range: 3.5-5mm) were resected under SPY guidance until no tumor was grossly visible and no fluorescence was detected. To verify this, several random biopsies of the non-fluorescent wound bed, which was presumed to be negative for cancer, were taken and sent to histology.
Figure 3. Orthotopic oral cancer and cervical metastasis in a mouse.
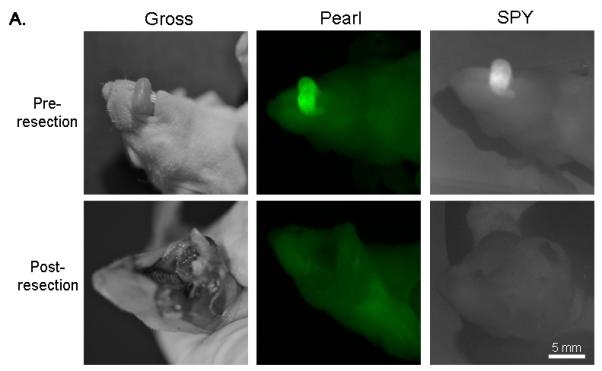
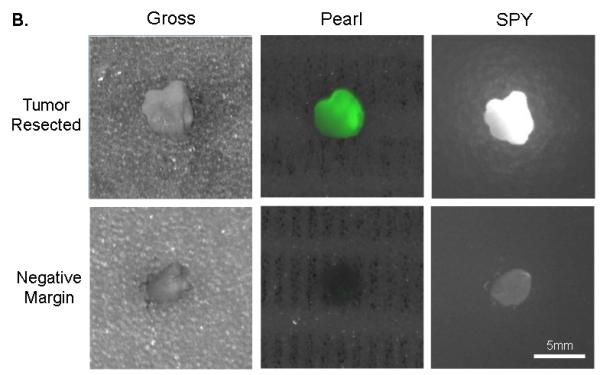
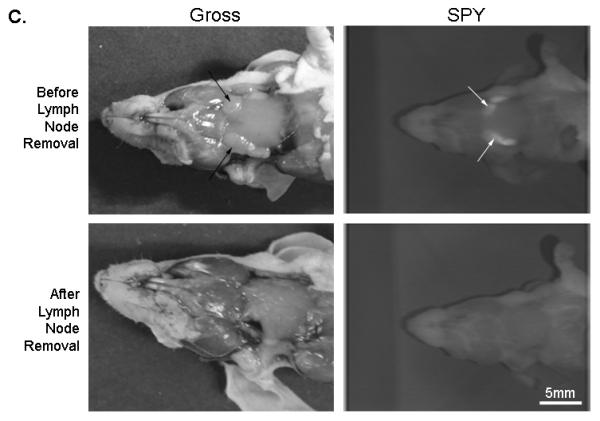
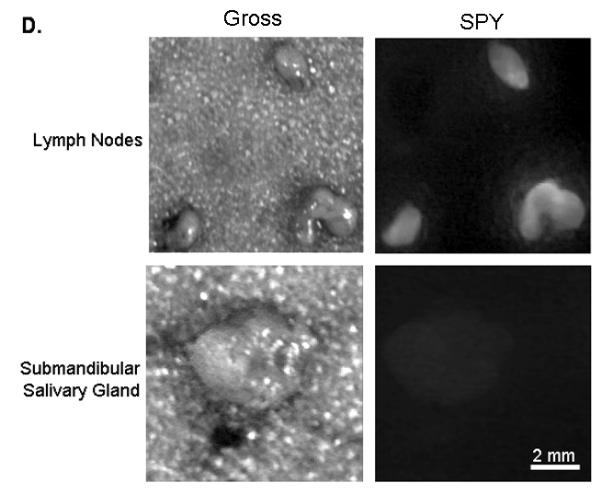
(A) Uptake of NIR panitumumab-IRDye800 before and after resection. The SPY imager was used to guide tumor resection in real-time. (B) Fluorescence imaging of tumor and negative margin in tissue cassettes. (C) Uptake of NIR panitumumab-IRDye800 before and after cervical lymph node resection. Tumor infiltration was not visible grossly but detectable using the SPY. SPY imager was used to guide resection in real-time of the fluorescent lymph nodes. (D) Gross and SPY fluorescent imaging of the resected lymph nodes and submandibular salivary gland (taken for control) in tissue cassettes.
Following resection, fluorescently positive and negative margins were placed in cassettes (mimicking tissue on the back table in the OR), measured, and assessed for fluorescence prior to histological sectioning (Figure 3B). Tumor fragments as small as 1mm were consistently detected by both imaging modalities. Tissue biopsies positive for fluorescence were found to correlate with pathologic disease on histologic analysis (H&E and immunohistochemistry) in 11/11 biopsies. Tissue margins absent for fluorescence were confirmed negative by histological analysis in 6/6 biopsies (histological representation not shown).
In some instances, following resection of the gross tumor, the SPY system detected fluorescence in tissue that did not appear to be cancerous on visual inspection or palpation. Histological analysis of these microscopic residual fragments demonstrated the presence of tumor cells (n=7/7). The smallest area of positive fluorescence that was detected with the SPY system was found to measure ~200μm in diameter.
Lymph nodes from the orthotopic model were also assessed using this technique as we have previously described [8]. Cervical lymph node metastases were not visualized grossly, however using the SPY and Pearl, a strong fluorescence signal could be detected (Figure 3C). The lymph nodes (n=6; average diameter: 1.9mm; range: 1-3mm) had a mean tumor-to-background ratio of 1.8 (Pearl) and 1.5 (SPY). In both modalities, the metastases had statistically significant higher fluorescence than the background (P<0.05). The lymph nodes were then placed in tissue cassettes for ex vivo fluorescence and histological processing (Figure 3D). Since small nests of tumor cells could not be consistently confirmed by H&E alone, malignancy was confirmed immunohistochemical analysis using CD147 and cytokeratin.
As a control, a non-fluorescent submandibular salivary gland was excised to confirm that areas with absent fluorescent imaging had no tumor (Figure 3D). As expected, histological analysis of the non-fluorescent tissue was negative for tumor.
Optical imaging to augment histologic analysis
To determine if panitumumab-IRDye800 could be used for imaging of histological sections after embedding in optimal cutting temperature (OCT) medium or paraffin, cut sections (H&E and unstained) from the tongue tumor were imaged for fluorescence using the Odyssey scanner. Optical scanning revealed that areas of fluorescence corresponded to areas of tumor infiltration in tissue (Figure 4A,B). Fluorescent microscopy of the negative margins confirmed the absence of tumor cells (data not shown).
Figure 4. Microscopic imaging of oral cancer.
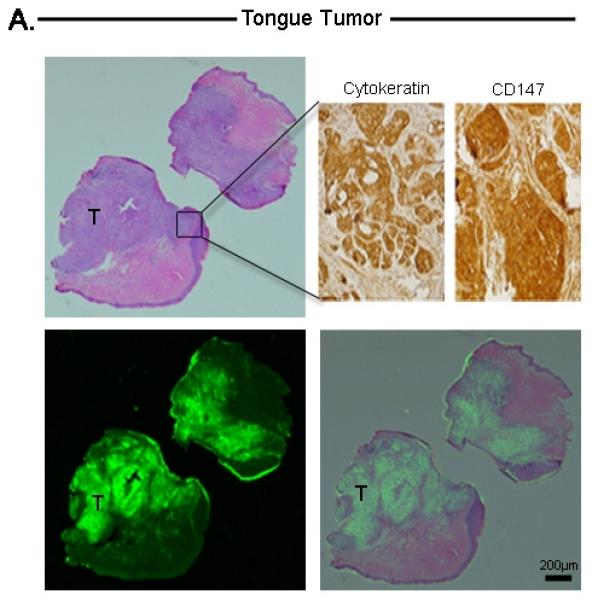
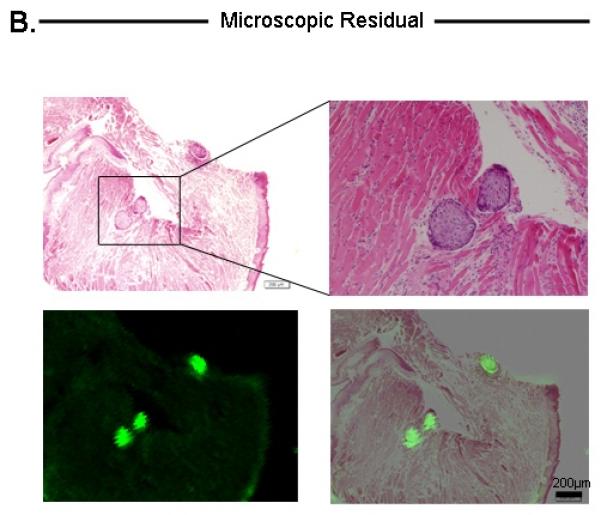
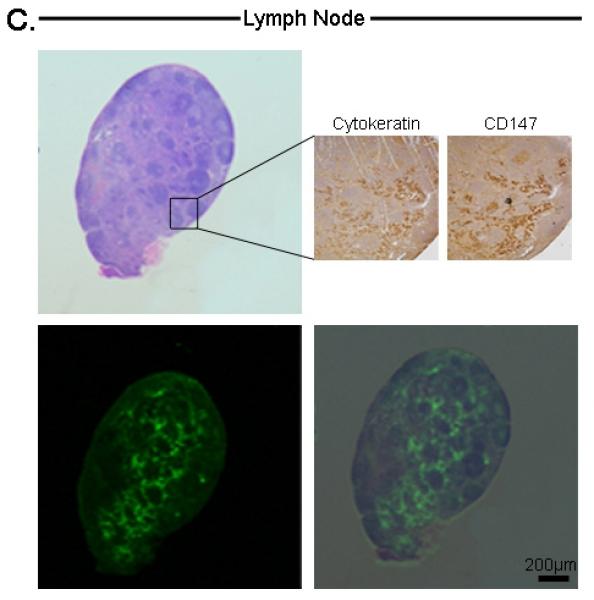
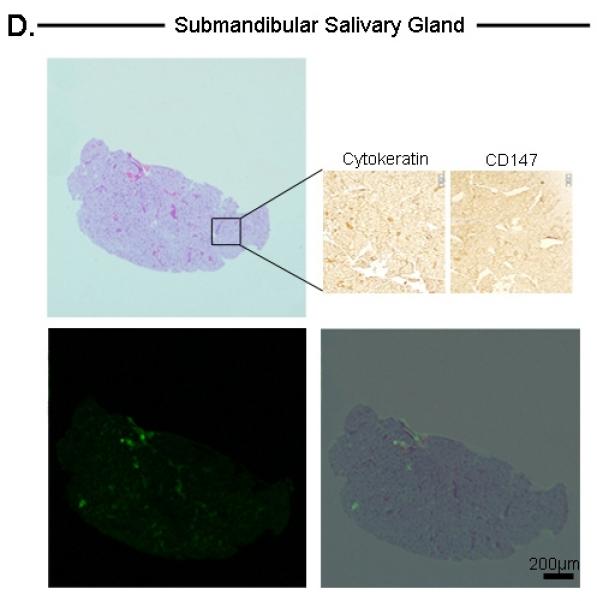
Histological analysis of tongue tumor (A), Microscopic residual disease (B), Lymph node metastases (C), and submandibular salivary gland (D). For each tissue, the top-left represents H&E, bottom-left represents Odyssey fluorescent microscopy, bottom-right represents an overlay of the H&E and Odyssey image demonstrating co-localization of the probe signal with tumor cells. For tongue and lymph node tissuse (A,C), immunohistochemical staining with cytokeratin and CD147 confirmed the presence of tumor cells (top-right images). For the submandibular salivary gland (D), immunohistochemical staining was negative for tumor. Microscopic residual disease (B) which was not grossly visualized, but detectable by the SPY system, was resected and sent for histological analysis. A detailed view (top-right of B) demonstrates conventional H&E of a small nest of tumor cells (original magnification 40x; magnified view (top-right) = 100x). Fluorescence detection using Odyssey scanner was sensitive enough to detect this small nest of tumor cells, emphasizing the importance and utility of such a technique in the pathology lab to ensure that cancer cells are not missed during tumor margin evaluation. T=Tumor
This technique also successfully detected microscopic disease in tissues with grossly unidentifiable residual disease. Tumor fragments measuring between 200μm to several millimeters were identified within the tongue, adherent to the mandible, or within the lymph nodes and confirmed by immunohistochemical analysis using CD147 and cytokeratin.
Similarly, the Odyssey detected a fluorescence signal within metastatic lymph nodes that co-localized with areas of tumor infiltration (Figure 4C). The non-fluorescent submandibular salivary gland lacked a fluorescence signal, consistent with the histology and immunohistochemical analysis (Figure 4D).
Human Tumor Model
In the human tumor implant model, we evaluated the efficacy of a fluorescence detection system in a heterogeneous cell population with mosaic patterns of EGFR expression and variations in cell types. The human tumor implants (n=6) had a mean TBR of 4.0 (Pearl) and 3.0 (SPY)(P <0.0001). As described above, the SPY was used to guide surgical resection and tumor pathology was confirmed by histology (Figure 5A). Fluorescence imaging of the tissue sections using the Odyssey scanner correlated with histological evidence of tumor (Figure 5B).
Figure 5. Human Tumor Model.
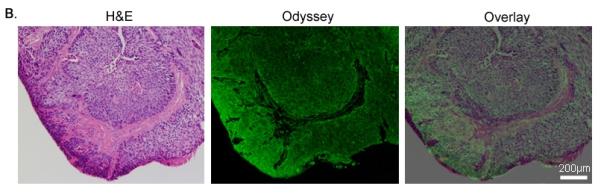
A lymph node metastasis from a patient with OCSCC was implanted into the flank of a SCID mouse. (A) Gross and SPY images following injection with the panitumumab IRDye800 before and after resection. (B) Histological analysis of the implanted tumor after resection with H&E staining, Odyssey imaging, and an overlay demonstrating co-localization of the probe signal with tumor cells.
DISCUSSION
Current methods for intra-operative assessment of primary tumor margins remain dependent on subjective assessment by the surgeon of subtle tissue changes and a final pathological confirmation several days later. Systemic administration of fluorescently labeled targeting molecules may permit real-time, cancer-specific imaging within the clinic and operating room. In fact, clinical trials using fluorescently labeled antibodies are underway in Europe in multiple cancer types[9, 10].
In our previous studies, we used the fluorophore Cy 5.5 (excitation, 678 nm; emission, 703 nm) conjugated to cetuximab, a therapeutic chimeric antibody directed against EGFR. Cetuximab-Cy 5.5 was used in vivo to detect primary tumors, residual disease, regional and distant metastasis, and to evaluate chemoradiotherapeutic treatment [8, 11-13]. Although we have assessed both cetuximab and panitumumab, we have compared the two in several in vitro and in vivo studies and have found that they exhibit similar imaging properties (unpublished data). Because panitumumab is fully humanized and has demonstrated an improved infusion reaction profile and a longer half-life, we have selected this FDA approved antibody for additional imaging studies. The choice of an optical probe in the present study was IRDye800 because it has emission and excitation properties that mimic indocyanine green, is non-toxic, has consistent protein labeling, and has superior fluorescence characteristics compared to Cy 5.5 for use in image-guided surgery [14, 15].
Panitumumab was chosen as the targeting molecule because of its potential clinical translation; it is fully humanized (unlike cetuximab), FDA approved for use in humans, and targets an epitope expressed on a wide variety of cancer types including the majority of head and neck cancers[16]. Other investigators have used recombinant human EGFR ligands, however these proteins may have activating properties at the receptor site, potentially promoting the malignant phenotype [5], whereas panitumumab is a therapeutic antibody.
Despite the significant effort to identify the optimal probe, successful imaging may depend as much on the probe choice as the appropriate hardware. A system that can accurately detect subclinical disease, is simple to use, and is readily translated to the operating room is ideal. Current clinical systems designed for the operating room are manufactured specifically to image indocyanine green, which cannot be linked to a tumor antibody[17]. Therefore, we sought to determine if these imaging systems could be used to image fluorescent dyes that can be linked to proteins, such as the IRDye800. To this end, we assessed a combination of macro and microscopic instrumentation that is currently available and can be readily implemented within the clinical setting.
Using a fluorescently labeled anti-EGFR targeting probe we assessed the potential of the SPY System (currently available in the OR) for detecting gross tumor in situ and the potential of an optical imaging scanner (Odyssey) for assessment of frozen and paraffin-embedded histological sections for detecting microscopic tumor ex vivo. In the current study we found the SPY System could detect subclinical or non-palpable disease measuring less than a millimeter in size, without appreciable false positives. Histological analysis using the Odyssey system consistently identified microscopic islands of tumor within normal tissues. The histological assessment can obviously be done by a trained pathologist, however, collections of only a few cells may be missed or uncertainty may be present particularly when using frozen sections, which are reversed on final pathology in 2-10% of cases [18, 19]. Optical scanning may provide crucial additional information that can be leveraged to make a more accurate intraoperative decision.
Strong tumor signal (high tumor-to-background ratio) is essential to limit false positives and perform successful image-guided cancer resections[20]. In the present study, we documented that the SPY System could detect in vivo fluorescence at a tumor-to-background ratio that varied between 1.5 and 3.0 depending on the organ being sampled. By comparison, the Pearl Impulse designed to image IRDye800 had a tumor-to-background ratio that was an average of 0.9 higher than the SPY (values varied from 1.8 to 4.0). However, the Pearl is restricted to small animals and, therefore, does not have the clinical translatability compared to the SPY system. While there are real-time NIR fluorescence camera systems, notably one by the group of Ntziachristos [3], these cameras are not currently available in the OR suite and access will be limited by regulatory agencies and manufacturing limitations [4]. The SPY imaging system, on the other hand, is already used in cardiothoracic surgery, plastic surgery, and various gastrointestinal procedures and therefore, is currently available for use in most tertiary care operating rooms. Furthermore, the SPY was still found to measure tumor fluorescence that was statistically significantly higher than normal tissue, likely because indocyanine green and IRDye800 have very similar spectral properties. In fact, the SPY detected fluorescence in a section of tissue <1mm which appeared normal when inspected grossly; on histological analysis, the island of tumor cells responsible for the fluorescence was pinpointed using the Odyssey and turned out to be ~200μm in diameter (Figure 4B).
A limitation to our study is the possibility of leaving behind residual cancer cells due to the antibody-probe not binding to the tumor cells or perhaps the fluorescent imaging devices were not sensitive enough to detect individual tumor cells. Future studies can avoid this with the use of Alu PCR which distinguishes residual human (cancer) cells from rodent cells with such a high sensitivity and specificity that it is able to detect the equivalent of one human tumor cell in 1 × 106 murine cells[21]. In addition, perhaps this technology will be most valuable after previous medical or surgical management where the tissue is fibrotic or contracted and tactile feedback is limited; however additional studies under these conditions may be necessary to clearly demonstrate this objectively.
In conclusion, this data suggests that a panitumumab-IRDye800 may have clinical utility in the detection and real-time surgical removal of HNSCC using existing intraoperative gross and microscopic optical imaging hardware.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Yolanda Hartman for running the western blot assays. This work was supported by grant from NIDCR (R21DE019232) and equipment donated by Novadaq.
Disclosure: work was supported by grant from NIDCR (R21DE019232) and equipment donated by Novadaq
REFERENCES
- 1.Spaulding DC, Spaulding BO. Epidermal growth factor receptor expression and measurement in solid tumors. Semin Oncol. 2002;29:45–54. doi: 10.1053/sonc.2002.35647. [DOI] [PubMed] [Google Scholar]
- 2.Terwisscha van Scheltinga AG, van Dam GM, Nagengast WB, et al. Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J Nucl Med. 2011;52:1778–1785. doi: 10.2967/jnumed.111.092833. [DOI] [PubMed] [Google Scholar]
- 3.Themelis G, Yoo JS, Soh KS, et al. Real-time intraoperative fluorescence imaging system using light-absorption correction. J Biomed Opt. 2009;14:064012. doi: 10.1117/1.3259362. [DOI] [PubMed] [Google Scholar]
- 4.Keereweer S, Kerrebijn JD, van Driel PB, et al. Optical image-guided surgery--where do we stand? Mol Imaging Biol. 2011;13:199–207. doi: 10.1007/s11307-010-0373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keereweer S, Kerrebijn JD, Mol IM, et al. Optical imaging of oral squamous cell carcinoma and cervical lymph node metastasis. Head Neck. 2011 doi: 10.1002/hed.21861. [DOI] [PubMed] [Google Scholar]
- 6.Biosciences L-C . 2007IRDye(R) 800CW Protein Labeling Kit - High MW. LI-COR Biosciences; Lincoln, Nebraska: 2007. pp. 1–9. [Google Scholar]
- 7.Reuthebuch O, Haussler A, Genoni M, et al. Novadaq SPY: intraoperative quality assessment in off-pump coronary artery bypass grafting. Chest. 2004;125:418–424. doi: 10.1378/chest.125.2.418. [DOI] [PubMed] [Google Scholar]
- 8.Gleysteen JP, Newman JR, Chhieng D, et al. Fluorescent labeled anti-EGFR antibody for identification of regional and distant metastasis in a preclinical xenograft model. Head Neck. 2008;30:782–789. doi: 10.1002/hed.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 10.Pleijhuis RG, Graafland M, de Vries J, et al. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: current modalities and future directions. Ann Surg Oncol. 2009;16:2717–2730. doi: 10.1245/s10434-009-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenthal EL, Kulbersh BD, Duncan RD, et al. In vivo detection of head and neck cancer orthotopic xenografts by immunofluorescence. Laryngoscope. 2006;116:1636–1641. doi: 10.1097/01.mlg.0000232513.19873.da. [DOI] [PubMed] [Google Scholar]
- 12.Gleysteen JP, Duncan RD, Magnuson JS, et al. Fluorescently labeled cetuximab to evaluate head and neck cancer response to treatment. Cancer Biol Ther. 2007;6:1181–1185. doi: 10.4161/cbt.6.8.4379. [DOI] [PubMed] [Google Scholar]
- 13.Kulbersh BD, Duncan RD, Magnuson JS, et al. Sensitivity and specificity of fluorescent immunoguided neoplasm detection in head and neck cancer xenografts. Arch Otolaryngol Head Neck Surg. 2007;133:511–515. doi: 10.1001/archotol.133.5.511. [DOI] [PubMed] [Google Scholar]
- 14.Marshall MV, Draney D, Sevick-Muraca EM, Olive DM. Single-dose intravenous toxicity study of IRDye 800CW in Sprague-Dawley rats. Mol Imaging Biol. 2010;12:583–594. doi: 10.1007/s11307-010-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams KE, Ke S, Kwon S, et al. Comparison of visible and near-infrared wavelength-excitable fluorescent dyes for molecular imaging of cancer. J Biomed Opt. 2007;12:024017. doi: 10.1117/1.2717137. [DOI] [PubMed] [Google Scholar]
- 16.Pomerantz RG, Grandis JR. The epidermal growth factor receptor signaling network in head and neck carcinogenesis and implications for targeted therapy. Semin Oncol. 2004;31:734–743. doi: 10.1053/j.seminoncol.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Withrow KP, Gleysteen JP, Safavy A, et al. Assessment of indocyanine green-labeled cetuximab to detect xenografted head and neck cancer cell lines. Otolaryngol Head Neck Surg. 2007;137:729–734. doi: 10.1016/j.otohns.2007.06.736. [DOI] [PubMed] [Google Scholar]
- 18.Remsen KA, Lucente FE, Biller HF. Reliability of frozen section diagnosis in head and neck neoplasms. Laryngoscope. 1984;94:519–524. doi: 10.1288/00005537-198404000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Gandour-Edwards RF, Donald PJ, Lie JT. Clinical utility of intraoperative frozen section diagnosis in head and neck surgery: a quality assurance perspective. Head Neck. 1993;15:373–376. doi: 10.1002/hed.2880150502. [DOI] [PubMed] [Google Scholar]
- 20.Keereweer S, Sterenborg HJ, Kerrebijn JD, et al. Image-guided surgery in head and neck cancer: current practice and future directions of optical imaging. Head Neck. 2012;34:120–126. doi: 10.1002/hed.21625. [DOI] [PubMed] [Google Scholar]
- 21.Zijlstra A, Mellor R, Panzarella G, et al. A quantitative analysis of rate-limiting steps in the metastatic cascade using human-specific real-time polymerase chain reaction. Cancer Res. 2002;62:7083–7092. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


