Abstract
To determine whether visceral fat (VF), independent of other confounders, is causally linked to intestinal tumorigenesis, we surgically removed VF in Apc1638/N+ mice. At 15 wks of age, male and female Apc1638/N+ mice were randomized to one of three groups: sham operated (AL), VF removal (VF-), or sham operated and caloric restricted (CR), and were studied for effects on tumorigenesis and survival. As compared to AL, VF removal and CR reduced macroadenomas to a similar extent (P<0.05), but only CR significantly improved survival (P<0.05). Given that a significant group × gender interaction was observed, we next examined males and females separately. In females, macroadenomas were markedly attenuated by VF removal (1.33±0.23 mean±SE; P<0.05), but not by CR (2.35±0.25; P=0.71), as compared to AL (2.50±0.34). In males, however, CR (1.71±0.26; P<0.01), but not VF removal (2.94±0.42; P=0.29), reduced macroadenomas, as compared to AL males (3.47±0.30). In females, both VF- (P=0.05) and CR (P<0.01) improved survival, but not in male mice (P=0.15). The benefits observed with CR were consistent with favorable metabolic adaptations, but protection conferred in VF- females was in spite of lower adiponectin levels (P<0.05), and failure to reduce body mass, total adiposity, glucose, insulin, leptin and CXCL-1 levels. In conclusion, these data provide the first causal evidence linking VF to intestinal cancer risk, and suggest that factors, other than known metabolic mediators, may impact tumor development. Furthermore, these data emphasize that strategies designed to deplete VF stores in humans should be considered in the prevention of intestinal cancer.
Keywords: obesity, visceral fat, colon cancer, insulin resistance, adiponectin
Introduction
Obesity rates are at epidemic levels in the U.S. and other developed countries (1, 2). Obese individuals are at increased risk for developing metabolic syndrome, type 2 diabetes, cardiovascular disease (3) and cancer at many sites, including colon, kidney, thyroid, endometrium, liver and oesophagus (4-10). Excess adiposity poses an even greater risk for cancer mortality, an observation that is nearly universal among the most common forms of cancer (9). For example, obesity dramatically increases the risk of death from colon cancer by 46% in women and 84% in men, breast cancer by two-fold, liver and kidney cancer by more than four-fold, and uterine cancer by more than six-fold (9).
Since the obesity-cancer link first emerged, studies across disciplines have worked toward establishing the biologic underpinnings explaining this association. Epidemiologic studies (4, 5, 11) strongly suggest a relationship via endocrine and other metabolic effects (12, 13), while in vitro (14) and in vivo studies (15, 16) have provided evidence with respect to possible mechanisms. The most commonly studied pathways linking obesity to cancer include the ability of excess adipose tissue to promote insulin resistance and contribute to a chronic, low-grade, pro-inflammatory state through the secretion of adipokines (17). However, establishing the independent contribution of these mediators to site-specific cancer risk has been difficult as their importance seems to vary by cancer type and stage, and is further complicated by interactions with other hormones (14, 18-20). A second unresolved issue is the lack of in vivo evidence causally linking adipose tissue per se to cancer. The predominant method utilized in obesity-cancer studies employs high-fat feeding in rodents to cause excess gains in weight and adiposity, but this strategy introduces other confounders, including changes in dietary components, nutrient flux and energy balance. Furthermore, it is known that nutrients can interact with adipose tissue to provoke the expression and secretion of pro-inflammatory cytokines (21), thus interventions that limit this influx of nutrients, such as caloric restriction or bariatric surgery, for example, may work in part by attenuating this interaction.
Since the relationship between obesity and disease first emerged, a more careful examination of body fat distribution has revealed that the risk posed by obesity in humans to disease and mortality is primarily harbored by the extent of visceral fat (VF) accretion (22). Utilizing a surgical model of VF removal in rats, our group first demonstrated that this relationship in regards to insulin resistance, type 2 diabetes and lifespan is causal (23-25). Given that abdominal obesity has been shown to more strongly predict cancer risk and mortality, including colon cancer (26, 27), then general obesity, we addressed whether VF is a direct modulator of intestinal tumor development. We specifically utilized our surgical approach of depleting VF in a mouse model of intestinal cancer (Apc1638/N+), in order to distinguish the contribution of visceral adiposity to tumorigenesis, independent of other important confounders. Here we show that VF removal independently protects against the development of macroadenomas in female Apc1638/N+ mice.
Methods
Animals
Male Apc1638/N+ mice on a C57BL/6 background were bred with C57BL6/J female mice (Jackson Laboratory, Bar Harbor, ME). Genotyping of Apc1638/N+ offspring was performed as described (28). At weaning (3 wks old), mice were group housed in same-sex cages and fed a purified 45% high-fat diet (cat#D12451, Research Diets Inc., New Brunswick, NJ) to induce weight gain. Animals were housed at standard temperature (~22°C) and humidity-controlled conditions under a standard light/dark photoperiod (14L:10D). All experiments were approved by the Institutional Animal Care and Use Committee at the Albert Einstein College of Medicine.
Experimental Design
At 15 wks of age, which we found was an age that fat can be ablated without significant re-growth, ~two-thirds of mice were randomly assigned to sham abdominal surgery, and ~one-third to VF removal (VF-). Following a 1 wk recovery, sham-operated animals were further randomized to either AL or CR groups, resulting in three experimental groups (AL; n=45 total, males=21, females=24), VF removal and ad libitum fed (VF-; n=40 total, males=21, females=19), or sham operated and 40% caloric restricted (CR; n=38 total, males=18, females=20). Mice were then monitored daily for up to 12 wks (28 wks of age) for tumor development. Animals were removed early from the study and sacrificed if evidence of decreased food intake and weight loss (>20%), coupled with signs of sickness and lethargy were observed. In all cases, mice removed early from the experiment due to these criteria were found to harbor intestinal tumors.
Sham and fat removal surgery
For each surgery, mice were anesthetized with isoflurane (2% induction and maintenance) and a small mid-line incision was made in the lower abdomen under aseptic conditions, as previously described (25). For VF- mice, all visible gonadal (epididymal in males, periovarian in females) and perinephric adipose tissue was carefully removed by blunt dissection, weighed and recorded. For the sham operation, a similar incision was made and soft tissues were disturbed in a similar manner as VF-, but no fat was removed. Analgesic was given (Buprenorphine, 0.1mg/kg SQ) peri-operatively and as needed for up to 72 hrs post-operatively. Recovery was >90% for both the sham and fat removal procedures.
Food intake, body weight and body composition
Following surgery, mice were singly housed for the duration of the experiment. Body weight and food intake was monitored weekly. Animals assigned to the CR group were provided a weighed food pellet daily between 1200h-1700h. Since AL male mice consumed slightly more food than AL female mice, the amount of food provided to male and female CR mice, respectively, was calculated as ~60% of the intake consumed by same-sex AL controls. Body composition was assessed by quantitative magnetic resonance (qMR) for determination of fat and lean mass (Echo Medical Systems, Houston, TX) at baseline (-1 wk), 4, 8 and 12 wks in study. At sacrifice, VF pads (perinephric, mesenteric, epididymal/periovarian) were removed, weighed and recorded.
Necropsy and tissue processing
For determination of tumors, mice were sacrificed and the gastrointestinal tract was separated from the mesenteric fat depot, and divided into four segments: duodenum, ileum, jejunum and colon. Each segment was opened longitudinally, rinsed in phosphate-buffered saline, and laid out flat for examination of tumor multiplicity with the aid of a dissecting magnifying lens. Macroadenomas (~>0.5mm diameter) were counted in the proximal and distal region of each segment of intestinal tissue and recorded. Tissues were then rolled and fixed overnight in 10% neutral-buffered formalin at 4°C, processed through a series of alcohols and xylenes, and embedded in paraffin. Sections (5 μm) were stained with hematoxylin and eosin and assessed by a pathologist for histological changes following consensus recommendations for assessing intestinal tumors in rodents (29).
Four-week cohort for insulin tolerance tests and serum collection
In order to minimize the confounding effects of cancer morbidity on metabolic outcomes, a separate cohort of mice were included and used for insulin tolerance tests (ITTs) at 3 wks in study and sacrificed for serum collection at 4 wks in study (i.e. 20 wks of age). ITTs were performed in random-fed mice, early in their light cycle (~0700h-0800h), as described (30). Briefly, following a baseline glucose measurement (One Touch Ultra, LifeScan, Inc., Milpitas, CA), mice were intraperitoneally injected with 0.75U/kg insulin and blood glucose was measured at 15, 30, 45 and 60 min later. For serum collection, food was removed at ~0600h and mice were killed 5-6 hrs later by decapitation without anesthesia. Blood was allowed to clot at room temperature, and serum was separated by centrifugation and stored at -80°C until analysis.
Serum measures
At sacrifice, glucose was measured in whole blood with a handheld glucose analyzer (One Touch Ultra). Serum insulin was measured in duplicate using a high-sensitivity mouse ELISA (Alpco Inc, Salem, NH). 17β-Estradiol levels in serum were measured in duplicate by EIA in female mice (Cayman Inc, Ann Arbor, MI). Serum leptin and adiponectin were measured in duplicate by ELISA (Alpco Inc) as described (16). Other cytokines and chemokines, including IL-1β, IL-10, IL-12-p70, IL-6, IFN-γ and CXCL-1 were measured in duplicate using an electrochemiluminescence assay with sensitivities of 0.75, 11.0, 35.0, 4.5, 0.38, and 3.3 pg/mL, respectively (Meso Scale Discovery, Gaithersburg, MD).
Statistics
Tumor multiplicity was analyzed by two-way ANOVA (group × gender) and post hoc comparisons were conducted when appropriate. A total of five statistical outliers (>2SD from the group mean) were identified and excluded from the macroadenoma dataset (one each from Male AL, VF-, CR and Female AL and VF- groups, respectively). Histological outcomes were analyzed by the Kruskal-Wallis procedure and post hoc comparisons conducted with the Mann-Whitney U test when appropriate. Survival to 12 wks in study was performed using the Kaplan-Meier method and significant differences in survival distribution among groups was tested by a log-rank test. Longitudinal measures were assessed by repeated-measures ANOVA and cross sectional measures were assessed by one-way ANOVA. When significance was detected for the main effect, planned contrasts were carried out when appropriate. All analyses were performed using either SPSS (SPSS Inc, Chicago, IL) or JMP software version 9 (SAS Institute Inc., Cary, NC). A P≤0.05 was considered statistically significant.
Results
Caloric restriction or visceral fat removal resulted in a similar and significant reduction in macroadenomas
At sacrifice, tumor multiplicity was determined in mice by counting macroadenomas in the small and large intestine. Macroadenomas were primarily confined to the duodenum and jejunum, less frequently in the ileum, and only rarely observed in the colon. No differences in macroadenoma number by intestinal region (i.e. proximal or distal duodenum, ileum, jejunum, and colon) were detected among groups (data not shown), but a significant Group effect was observed for total macroadenomas (P<0.01). Comparisons among groups revealed a similar and significant reduction in tumor multiplicity in VF- and CR mice, as compared to AL mice (Fig. 1A; P<0.05).
Figure 1.
Tumor multiplicity and survival to 12 wks in AL, VF- and CR mice. A, At necropsy, the number of macroadenomas in the intestinal tract were counted with the assistance of a dissecting magnifying lens. VF- and CR interventions led to a significant reduction in macroadenomas, as compared to AL mice (P<0.05). B, Survivorship to 12 wks was determined using the Kaplan-Meier method. Events were defined as the early removal of an animal based upon pre-defined criteria (see Methods). As compared to AL mice (62.2% remaining), CR significantly improved survival (89.5% remaining, P<0.05) while a tendency toward improved survival was observed with VF removal (80.0% remaining; P=0.10). C, In females, VF removal, but not CR, led to a significant reduction in macroadenomas, as compared to AL mice (P<0.05). D, When assessing survivorship to 12 wks in study for female mice, the percentage of CR mice (100% remaining; P<0.01) and VF- mice (94.7% remaining; P=0.05) surviving to 12 wks, was significantly greater than AL females (79.2% remaining). E, In males, CR significantly reduced macroadenomas, as compared to AL (P<0.01) and VF- male mice (P<0.05). F, In males, 42.9% of AL mice survived to 12 wks in study, as compared to 66.7% of VF-mice and 77.8% of CR mice, but the overall model effect did not reach significance (P=0.15). Bars are means±SE. Different letters denote a significant difference between groups (P<0.05).
Caloric restriction significantly improved survival in Apc1638/N+ mice
Using criteria described in Methods, we next examined the effect of interventions on survival to 12 wks in study (28 wks of age). Analysis of survivorship revealed that CR significantly improved survival (89.5% remaining; P<0.05), but no significant difference was observed between VF- and AL animals (80.0% v 62.2% remaining, respectively; Fig. 1B; P=0.10). In addition, a significant main effect for Gender (P<0.05) and a Group × Gender interaction (P<0.01) were detected for tumor multiplicity, leading to a secondary analysis within female and male groups, respectively.
Visceral fat removal, but not caloric restriction, reduced the number of macroadenomas in Apc1638/N+ female mice
In females, macroadenoma numbers were significantly reduced by VF removal (P<0.05), but not by CR (P=0.71), as compared to AL females (Fig. 1C). When intestinal tissue sections were evaluated histologically by a pathologist, no differences were observed in the severity of crypt hyperplasia or the frequency of dysplasia among female groups, but a marked increase in microadenomas was observed in VF- female mice, as compared to AL females (Table 1; P<0.05), while CR female mice had an intermediary level, relative to AL and VF- females. Taken together, these findings suggest that in VF- females, but not in CR females, the progression of microadenomas to macroadenomas was significantly attenuated.
Table 1.
Histopathology of the gastrointestinal tract in AL, VF- and CR female mice
| AL | VF- | CR | |
|---|---|---|---|
| Hyperplasia, crypt epithelial† | 0.20±0.13 | 0.18±0.13 | 0.07±0.07 |
| Dysplasia‡ | 0.20±0.13 | 0.27±0.15 | 0.21±0.11 |
| Microadenomas‡ | 0.30±0.15a | 1.27±0.30b | 0.77±0.21ab |
| Carcinomas‡ | 0.30±0.15 | 0.36±0.15 | 0.46±0.17 |
Data are means±SE. Different letters denote a significant difference between groups, P<0.05.
Value based on the pathologic severity using a 1-4 scale, with 4 being most severe.
Value indicates the number of identified foci per section.
Caloric restriction and visceral fat removal significantly improved survival in Apc1638/N+ female mice
When a survival analysis truncated at 12 wks, was performed in female mice, the percentage of both CR (100% remaining; P<0.01) and VF- female mice (94.7% remaining; P=0.05) surviving to 12 wks, was significantly greater than AL females (79.2% remaining; Fig. 1D).
Caloric restriction, but not visceral fat removal, reduced the number of macroadenomas in Apc1638/N+ male mice
In contrast to females, CR male mice (P<0.01), but not VF- male mice (P=0.29), had markedly fewer total macroadenomas than AL males (Fig. 1E). In addition, CR males had significantly fewer macroadenomas than VF- mice (P<0.05). Histopathology revealed that the severity of crypt hyperplasia was lowest in VF- male mice (Table 2; P<0.05), while CR males had the lowest incidence of microadenomas, but the greatest occurrence of crypt dysplasia (Table 2; P<0.05). Collectively, these findings suggest that the progression of dysplastic cells toward adenoma formation was abrogated only in CR male mice, leading to development of fewer micro- and macroadenomas in this group. When assessing survivorship in males, the percentage of AL male mice still remaining at 12 wks was 42.9%, while the percentage of VF- and CR male mice surviving to 12 wks were 66.7% and 77.8%, respectively, but no significant model effect was observed (Fig. 1F; P=0.15).
Table 2.
Histopathology of the gastrointestinal tract in AL, VF- and CR male mice
| AL | VF- | CR | |
|---|---|---|---|
| Hyperplasia, crypt epithelial† | 0.66±0.19b | 0.11±0.11a | 0.63±0.30b |
| Dysplasia‡ | 0.33±0.14a | 0.67±0.37a | 1.13±0.52b |
| Microadenomas‡ | 2.08±0.31b | 2.44±0.50b | 0.75±0.25a |
| Carcinomas‡ | 0.25±0.18 | 0.78±0.32 | 0.46±0.17 |
Data are means±SE. Different letters denote a significant difference between groups, P<0.05.
Denotes the severity score, based upon a 1-4 scale (4 being most severe).
Denotes the number of foci per section.
Caloric restriction, but not visceral fat removal, significantly altered phenotypic characteristics in Apc1638/N+ female mice
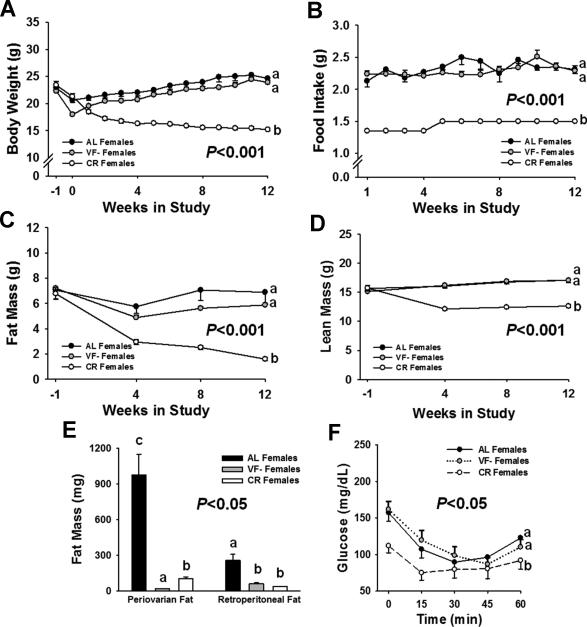
On average, 730.4±61.7mg periovarian fat and 331.5±61.7mg perinephric fat (1061.9mg total) was surgically removed from VF- female mice. As expected, no significant differences were detected for body weight (Fig. 2A), food intake (Fig. 2B), lean mass (Fig. 2C) or total adiposity (Fig. 2D) over the course of the study, between AL and VF- females. In contrast, CR females consumed nearly 40% fewer calories, weighed significantly less, and had reduced amounts of lean and fat mass, as compared to AL and VF- females (Fig. 2A-D; P<0.001). At 12 wks in study, individual fat pads were weighed, which confirmed both the successful surgical ablation of VF pads in VF- females, and the significant depletion in these same depots by CR, as compared to AL female mice (Fig. 2E; P<0.05).
Figure 2.
Phenotypic characteristics of AL, VF- and CR female mice. A, Body weight, and B, Food intake were monitored on a weekly basis throughout the course of the study. No significant differences were observed between AL and VF- females, but CR mice weighed less and consumed ~40% fewer calories. Body composition was assessed by qMR at baseline (-1 wk) and at 4-5 wk intervals throughout the study for determination of C, Fat mass and D, Lean mass. CR females had significantly less fat and lean mass, as compared to AL and VF- mice. E, Periovarian and retroperitoneal fat pads were weighed and at 12 wks in study. These fat pads did not return in VF- mice while CR also significantly reduced fat pad weights, as compared to AL females. F, Insulin sensitivity was measured by ITT in female mice. No differences were found between AL and VF- mice, while glucose levels remained significantly lowest in CR mice. Values are means±SE. Different letters denote a significant difference between groups (P<0.05).
Visceral fat removal resulted in hyperinsulinemia, hyperleptinemia, and lower adiponectin levels in Apc1638/N+ female mice
Serum measures in females at 4 wks in study are presented in Table 3 and Fig. 2F. Glucose and leptin levels were significantly reduced in CR females, as compared to AL and VF- females (Table 3; P<0.01). In contrast, VF- females were hyperinsulinemic, hyperleptinemic, and had significantly lower levels of adiponectin, as compared to AL and CR female mice (Table 3; P<0.05). Insulin sensitivity, as determined by ITTs, was greatest in CR female mice, but no distinguishable difference was observed between AL and VF- females (Fig. 2F). In addition, CXCL-1, which is thought to play a role in angiogenesis and metastasis, was markedly reduced in CR females (Table 3; P<0.01), but no significant differences were observed among groups for other serum cytokines and chemokines, including IFN-γ, IL-6, IL-10, IL-12-p70, and IL-1β. Furthermore, no differences were observed in estradiol levels among female groups (Table 3).
Table 3.
Serum measures in AL, VF- and CR female mice at 4 wks in study
| AL | VF- | CR | |
|---|---|---|---|
| Glucose (mg/dL) | 166.8±6.9b | 167.9±6.0b | 128.3±6.5a |
| Insulin (ng/mL) | 0.61±0.06a | 0.86±0.10b | 0.64±0.06a |
| Leptin | 1.75±0.32b | 3.76±0.69 c | 0.53±0.18a |
| Adiponectin (ug/mL) | 56.2±5.4b | 42.2±1.7a | 56.8±5.3b |
| Estradiol (pg/mL) | 984.5±34.2 | 1014.1±18.8 | 1018.3±35.1 |
| IFN-γ (pg/mL) | 1.25±0.47 | 2.73±0.72 | 1.67±0.61 |
| IL-10 (pg/mL) | 36.2±4.8 | 56.7±13.6 | 26.4±9.3 |
| IL-12-p70 (pg/mL) | 51.5±17.8 | 130.0±47.4 | 73.1±34.8 |
| IL-1β (pg/mL) | 1.55±0.58 | 0.78±0.21 | 1.68±0.51 |
| IL-6 (pg/mL) | 12.7±3.8 | 26.1±8.8 | 26.4±7.0 |
| CXCL-1 (pg/mL) | 92.8±6.5b | 100.6±6.1b | 59.6±3.9a |
Data are means±SE. Different letters denote a significant difference between groups, P<0.05.
Caloric restriction, but not visceral fat removal, significantly altered phenotypic characteristics in Apc1638/N+ male mice
Similar to females, VF- males had an average of 1237.9±120.0mg of epididymal fat and 377.0±38.1mg of perinephric fat surgically removed. Despite removing ~1.6g of fat, no significant differences were observed over the course of the study for body weight (Fig. 3A), food intake (Fig. 3B), lean mass (Fig. 3C) or total adiposity (Fig. 3D) between AL and VF- male mice. As in females, males caloric restricted by ~40% weighed significantly less (Fig. 3A), and had reduced amounts of lean and fat mass (Fig. 3C-D), as compared to AL and VF- males (P<0.001). We confirmed at 12 wks that epididymal and perinephric fat pads did not return in VF- males, and these fat pads were markedly lower in both VF- and CR males, as compared to AL male mice (Fig. 3E; P<0.05).
Figure 3.
Phenotypic characteristics of AL, VF- and CR male mice. A, Body weight, and B, Food intake were monitored weekly throughout the course of the experiment. No significant differences were observed between AL and VF- males, but CR mice weighed less as a result of consuming ~40% fewer calories. Body composition was assessed by qMR at baseline (-1 wk) and at 4-5 wk intervals throughout the study for determination of C, Fat mass and D, Lean mass. CR males had significantly less fat and lean mass, as compared to AL and VF- mice. E, Epididymal and retroperitoneal fat pads were weighed and at 12 wks in study and found to not return in VF- mice, and were also significantly reduced in CR mice, as compared to AL males. F, Insulin sensitivity was measured by ITTs at 3 wks in study. While CR mice were the most insulin sensitive, VF-also tended to improve insulin action in males (P=0.09). Values are means±SE. Different letters denote a significant difference between groups (P<0.05).
Caloric restriction, but not visceral fat removal, led to beneficial metabolic adaptations in Apc1638/N+ male mice
Serum measures of hormones, cytokines, chemokines and metabolites in male mice from the 4-wk cohort are presented in Table 4. CR male mice had lower glucose, insulin and leptin levels, (P<0.05), while adiponectin levels tended to be greater in this group, as compared to AL (P=0.27) and VF- males (P=0.03; Table 4). In contrast to the observed trends for VF- female mice (see Table 3 for female data), VF removal in male mice did not significantly alter insulin (P=0.55) or leptin levels (P=0.45), as compared to AL male controls, while total adiponectin levels were numerically lowest in this group (P=0.16), similar to what was found in VF- females. ITTs in male mice revealed a tendency for improved insulin sensitivity in VF- males, as compared to AL mice (Fig. 3F; P=0.09), but glucose levels during the ITT were only significantly improved in CR male mice (Fig. 3F; P<0.001). Similar to female CR mice, CXCL-1 levels were markedly reduced in CR males (Table 4; P<0.01), but no significant differences were observed among groups for IFN-γ, IL-1β, IL-6, IL-10 and IL-12-p70 levels (Table 4).
Table 4.
Serum measures in AL, VF- and CR male mice at 4 wks in study
| AL | VF- | CR | |
|---|---|---|---|
| Glucose (mg/dL) | 183.7±14.2b | 178.9±12.5b | 122.4±3.7a |
| Insulin (ng/mL) | 1.38±0.29b | 1.17±0.21ab | 0.89±0.08a |
| Leptin | 5.94±2.33b | 3.81±1.22 b | 0.36±0.17a |
| Adiponectin (ug/mL) | 24.8±3.1ab | 19.4±1.6a | 34.9±5.3b |
| IFN-γ (pg/mL) | 2.87±1.80 | 1.09±0.40 | 1.29±0.80 |
| IL-10 (pg/mL) | 69.8±33.1 | 34.5±5.0 | 27.3±3.7 |
| IL-12-p70 (pg/mL) | 132.4±96.9 | 27.2±15.2 | 26.2±8.8 |
| IL-1β (pg/mL) | 0.94±0.32 | 0.90±0.49 | 0.49±0.33 |
| IL-6 (pg/mL) | 32.0±20.0 | 34.7±18.6 | 24.2±13.6 |
| CXCL-1 (pg/mL) | 112.8±16.4b | 129.5±8.4b | 69.1±13.5a |
Data are means±SE. Different letters denote a significant difference between groups, P<0.05.
Discussion
This study establishes the importance of visceral adiposity in obesity-associated intestinal tumorigenesis in the Apc1638/N+ mouse model. Numerous epidemiologic and pre-clinical studies, including a prior effort in this model (31), have linked the obese state to increased colon cancer risk and/or mortality. However, obesity is a complex phenotype, characterized not only by excess weight gain and adiposity, but also by dietary factors and a sedentary lifestyle (32). Collectively, this has made isolating the individual contribution of adipose tissue to cancer risk challenging for the field. Here, using a surgical approach to deplete VF, we have circumvented these issues, providing causal evidence linking visceral adiposity per se to intestinal tumorigenesis, independent of other important confounders related to energy balance. Similarly, Lu et al found that partial removal of VF protected female SKH-1 mice against UVB-induced skin carcinogenesis (33). Importantly, these data provide yet another line of evidence directly linking VF to the etiology of aging (25) and age-related diseases (23, 24, 33, 34).
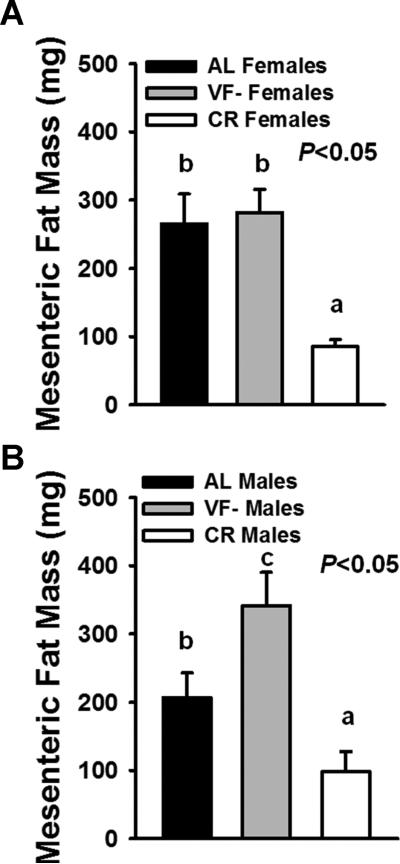
Remarkably, we found that VF removal was not only effective at attenuating macroadenoma development, but this reduction was comparable to that observed in CR mice. This was accompanied by improved survival in CR mice, with a similar, albeit non-significant trend observed for increased survival in VF- mice, as compared to AL mice. However, we also observed a clear effect of sex differences in the efficacy of these interventions on tumor initiation, promotion and survival. Perhaps most striking was the marked protection conferred by VF removal for development of macroadenomas in females, but not in males. While it is possible that the inability of VF removal to protect against macroadenomas in males was simply due to inherent sex differences, we also observed a marked shift in fat distribution among VF- males that could also explain this difference. Indeed, when we evaluated mesenteric fat mass in females (Fig. 4A) and males (Fig. 4B), we observed a distinct increase in mesenteric fat in VF- males, but not in VF- females. Given the hazardous nature of the mesenteric fat depot, coupled with anatomic location of this depot lying in close proximity to the intestine, it is plausible that mesenteric fat accretion abrogated the benefits of removing the epididymal and perinephric fat depots via endocrine and/or paracrine mechanisms.
Figure 4.
Mesenteric fat mass in male and female AL, VF- and CR mice. Mesenteric fat is believed to be the most closely related VF depot to humans, both anatomically and due to its portal access. However, unlike other VF depots, this fat pad cannot be surgically removed due to the extensive vascularization and neural innervation to the tissue. We recovered and weighed this fat depot at sacrifice. A, In females, CR significantly decreased mesenteric fat mass while no differences were observed between AL and VF- mice. B, In males however, while CR similarly decreased mesenteric fat, VF removal led to a marked re-distribution of fat to this depot, as compared to AL males. Bars are means±SE. Different letters denote a significant difference between groups (P<0.05).
Interestingly, whereas VF removal conferred protection against macroadenoma development in female mice, it was unexpectedly accompanied by a greater incidence of microadenomas. We believe the most likely reason for this finding is that VF removal led to a systemic change in factor(s) that blocked the progression of tumor development at the microadenoma-macroadenoma transition. In contrast, CR was associated with increased dysplasia in males, but was protective against the development of both micro- and macroadenomas. However, CR had no apparent effect on tumorigenesis in females. This suggests that energy availability may play a unique role in tumor initiation and early promotion in male mice, but CR was also beneficial for females, perhaps at later stages, as demonstrated by their improved survival (see Fig. 1D). Future studies are needed to explore the mechanisms whereby abdominal obesity and nutrient availability act independently during stages of initiation, promotion and progression, and how these interactions are modulated by gender.
We also noted that while both AL males (24.3% fat) and females (26.3% fat) were obese, due to consuming a 45% high-fat diet, AL females developed fewer tumors (P<0.05) and had improved survival (P<0.05), as compared to AL males. Indeed, consistent evidence from human studies shows a stronger risk posed by obesity and visceral obesity to colon cancer incidence and mortality in men, as compared to women (9, 27). The reason for this sex difference is not clear, but may be related to reproductive hormone status, as indicated by protection conferred from oral contraceptive use in women (35), and evidence that ovariectomized female mice injected with colon cancer cells have increased fat mass, insulin resistance and tumor growth (30). Females in our study also had approximately 50% lower insulin levels than males, and nearly two-fold greater adiponectin levels, which may also have also contributed to these differences.
Another important observation from this study was the observed hyperinsulinemia, hyperleptinemia, and reduced adiponectin levels with VF removal in females, which appears to be at odds with the reduction in macroadenomas. Indeed, the modest, but significant increase in fasting insulin was unexpected given that our group has previously shown improved hepatic insulin action with VF removal in chow-fed male rats (24), while Shi et. al (34) showed that removing just a single VF depot (periovarian) was sufficient to improve glucose tolerance in high-fat fed female mice. It should be pointed out however, that any potential difference in insulin action here between AL and VF-female mice was likely limited to basal conditions as we did not detect any difference in response to an insulin challenge (ITTs).
We also observed that VF removal in males and females resulted in an ~21% reduction in adiponectin, which was not unexpected, given that a significant amount of fat tissue was removed. While there is in vitro and in vivo evidence to support a protective role for increased adiponectin levels in colon cancer (16), rodent studies linking low adiponectin levels with colon cancer risk are mostly derived from models of constitutive adiponectin deficiency (adiponectin knock-out mice) (36). Thus, a much more modest 21% reduction in adiponectin may not be sufficient to predispose to tumorigenesis, at least in females, in which levels are two-fold higher than in male mice. However, an adverse effect in males cannot be ruled out and along with the compensatory increase in mesenteric fat, may explain why VF removal was ineffective in male mice.
Leptin has also been widely implicated in linking obesity to colon as well as other cancers (32), and we observed that VF- females had greater leptin levels, which may be indicative of leptin resistance in these mice. However, this may be a complex effect since evidence for leptin as a stimulator of proliferation is clear only for colon cancer cells in vitro (32, 37). We also measured several other inflammatory mediators in serum and observed a reduction in the chemokine, CXCL-1, which has been linked to angiogenesis and metastasis, in both male and female CR mice, but no other consistent patterns emerged. It is important to note that Apc1638/N+ mice presented with splenomegaly, which is commonly observed in these mice (38), and corresponded with elevated cytokine levels in some animals, making it difficult to distinguish the contribution of adipose tissue to the pro-inflammatory milieu.
The evidence implicating obesity and its related sequelae to cancer risk spans a wide array of models and systems. Yet, efforts to elucidate mechanism(s) linking the obese state to site-specific cancers such as breast, colon, prostate and skin, particularly in vivo, have revealed a more complex biology then perhaps was initially anticipated. For example, studies utilizing the fatless A-ZIP/F-1 mouse model demonstrated that adipokines are not absolutely required for tumor development. Indeed, despite the absence of adipose tissue and adipose-derived peptides such as leptin, these mice present with several other features of the obese phenotype, including hyperglycemia, hyperinsulinemia, and elevated levels of pro-inflammatory cytokines. When subjected to a two-stage skin carcinogenesis procedure, these mice develop more skin papillomas (39, 40), but this effect is not seen in the severely obese, ob/ob mouse model (40).
A-ZIP/F-1 mice also have accelerated development of mammary tumors when crossed with C3(1)/T-Ag transgenic mice (39), while a similar effect was observed when MKR mice, which are lean but diabetic, were crossed with MMTV-PyVmT mice (41). In contrast, MMTV-TGFα/db/db, which are obese and insulin resistant, but leptin-receptor deficient, fail to develop mammary tumors (20), but have increased incidence of intestinal neoplasms (31, 42). Thus, our finding that protection conferred from intestinal tumors in VF- females occurs despite a failure to produce favorable changes in factors suggested to play an important role in obesity-associated tumor growth is not without precedent. Collectively, these data demonstrate the complexity of the obesity-cancer interface and emphasize the need for continued efforts to delineate how specific perturbations to endocrine and other factors (43) by obesity, contribute to site-specific cancer risk.
In summary, these data provide causal evidence linking VF to intestinal cancer risk. The protection conferred by VF removal was preferentially seen in female mice, despite a lack of favorable changes in leptin, insulin, adiponectin, and several pro-inflammatory cytokines and chemokines, suggesting that other unknown mechanisms may underlie the obesity-colon cancer link. However, since the genetic model used here develops tumors predominantly in the small intestine, rather than the colon, further work on the underlying mechanisms will need to focus on a model in which the colon is the principal site of tumor development. Given that VF accrual and subcutaneous fat depletion represent a common hallmark of aging (44), it is tempting to conclude that the nearly logarithmic increase in cancer incidence and mortality with age is driven in part by these unfavorable changes in fat re-distribution. Therefore, strategies designed to deplete VF stores in abdominally-obese individuals, including pharmacologic and/or behavioral strategies, such as diet and exercise, may be an important cancer prevention strategy as well as an adjuvant therapy for improving outcomes following a cancer diagnosis.
Acknowledgements
This work has been supported by grants from the Prevent Cancer Foundation and National Institute on Aging (AG037574) to D.M.H., and the Einstein Nathan Shock Center Healthy Aging Physiology Core (P30AG038072) and the Diabetes Research and Training Center (DK20541) at the Albert Einstein College of Medicine. C.S.M. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants 58785, 79929 and 81913 as well as Award Number 1I01CX000422-01A1 from the Clinical Science Research and Development Service of the VA Office of Research and Development. The authors are indebted to Dr. Rani Sellers for assistance with the histopathologic evaluation of tissue sections and Youngmei Zhao and Hongquian Liang for technical support.
Abbreviations
- AL
ad libitum
- VF
visceral fat
- CR
caloric restriction
- SQ
subcutaneous
- ITTs
insulin tolerance tests
- qMR
quantitative magnetic resonance
- ANOVA
analysis of variance
- ELISA
enzyme-linked immunosorbent assay
- IL-1β
interleukin-1 beta
- IL-10
interleukin-10
- IL-12-p70
interleukin-12
- IL-6
interleukin-6
- IFN-γ
interferon-gamma
- CXCL-1
chemokine (C-X-C motif) ligand 1
Footnotes
Author Contribution
DMH planned and conceived the study, obtained funding, managed, performed and supervised the experiments, acquired, analyzed and interpreted data, and drafted the manuscript. LHA assisted with the experimental design and methodologies, interpreted data, and critically reviewed the manuscript. XZ and JJL assisted with conducting the experiments and reviewed the manuscript. GA assisted with statistical analysis and critically reviewed the manuscript. JPC assisted with performing assays and reviewed the manuscript. CSM assisted with coordinating assay measurements, data interpretation, and critically reviewed the manuscript.
Competing Interest
All authors state that there is no conflict of interest related to this research paper.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. Jama. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. Jama. 2012;307:483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys Acta. 2009;1790:1117–23. doi: 10.1016/j.bbagen.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsson SC, Permert J, Hakansson N, Naslund I, Bergkvist L, Wolk A. Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish population-based cohorts. Br J Cancer. 2005;93:1310–5. doi: 10.1038/sj.bjc.6602868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapp K, Schroeder J, Klenk J, Stoehr S, Ulmer H, Concin H, et al. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93:1062–7. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. 2006;55:285–91. doi: 10.1136/gut.2005.073163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11:1531–43. [PubMed] [Google Scholar]
- 8.Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proc Nutr Soc. 2008;67:128–45. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- 9.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 10.Carroll KK. Obesity as a risk factor for certain types of cancer. Lipids. 1998;33:1055–9. doi: 10.1007/s11745-998-0305-8. [DOI] [PubMed] [Google Scholar]
- 11.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 12.Hursting SD, Hursting MJ. Growth signals, inflammation, and vascular perturbations: mechanistic links between obesity, metabolic syndrome, and cancer. Arterioscler Thromb Vasc Biol. 2012;32:1766–70. doi: 10.1161/ATVBAHA.111.241927. [DOI] [PubMed] [Google Scholar]
- 13.Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–94. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rondini EA, Harvey AE, Steibel JP, Hursting SD, Fenton JI. Energy balance modulates colon tumor growth: Interactive roles of insulin and estrogen. Mol Carcinog. 2011;50:370–82. doi: 10.1002/mc.20720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barzilai N, Gupta G. Revisiting the role of fat mass in the life extension induced by caloric restriction. J Gerontol A Biol Sci Med Sci. 1999;54:B89–96. doi: 10.1093/gerona/54.3.b89. discussion B7-8. [DOI] [PubMed] [Google Scholar]
- 16.Moon HS, Liu X, Nagel JM, Chamberland JP, Diakopoulos KN, Brinkoetter MT, et al. Salutary effects of adiponectin on colon cancer: in vivo and in vitro studies in mice. Gut. 2012 doi: 10.1136/gutjnl-2012-302092. [DOI] [PubMed] [Google Scholar]
- 17.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28:4058–65. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunez NP, Perkins SN, Smith NC, Berrigan D, Berendes DM, Varticovski L, et al. Obesity accelerates mouse mammary tumor growth in the absence of ovarian hormones. Nutr Cancer. 2008;60:534–41. doi: 10.1080/01635580801966195. [DOI] [PubMed] [Google Scholar]
- 19.Fenton JI, Birmingham JM, Hursting SD, Hord NG. Adiponectin blocks multiple signaling cascades associated with leptin-induced cell proliferation in Apc Min/+ colon epithelial cells. Int J Cancer. 2008;122:2437–45. doi: 10.1002/ijc.23436. [DOI] [PubMed] [Google Scholar]
- 20.Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp Biol Med (Maywood) 2004;229:182–93. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- 21.Einstein FH, Atzmon G, Yang XM, Ma XH, Rincon M, Rudin E, et al. Differential responses of visceral and subcutaneous fat depots to nutrients. Diabetes. 2005;54:672–8. doi: 10.2337/diabetes.54.3.672. [DOI] [PubMed] [Google Scholar]
- 22.Matsuzawa Y, Shimomura I, Nakamura T, Keno Y, Tokunaga K. Pathophysiology and pathogenesis of visceral fat obesity. Ann N Y Acad Sci. 1995;748:399–406. doi: 10.1111/j.1749-6632.1994.tb17336.x. [DOI] [PubMed] [Google Scholar]
- 23.Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51:2951–8. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- 24.Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J, et al. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes. 1999;48:94–8. doi: 10.2337/diabetes.48.1.94. [DOI] [PubMed] [Google Scholar]
- 25.Muzumdar R, Allison DB, Huffman DM, Ma X, Atzmon G, Einstein FH, et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7:438–40. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjonneland A, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006;98:920–31. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 27.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–65. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 28.Fodde R, Edelmann W, Yang K, van Leeuwen C, Carlson C, Renault B, et al. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci U S A. 1994;91:8969–73. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–77. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 30.Yakar S, Nunez NP, Pennisi P, Brodt P, Sun H, Fallavollita L, et al. Increased tumor growth in mice with diet-induced obesity: impact of ovarian hormones. Endocrinology. 2006;147:5826–34. doi: 10.1210/en.2006-0311. [DOI] [PubMed] [Google Scholar]
- 31.Gravaghi C, Bo J, Laperle KM, Quimby F, Kucherlapati R, Edelmann W, et al. Obesity enhances gastrointestinal tumorigenesis in Apc-mutant mice. Int J Obes (Lond) 2008;32:1716–9. doi: 10.1038/ijo.2008.149. [DOI] [PubMed] [Google Scholar]
- 32.Drew JE. Molecular mechanisms linking adipokines to obesity-related colon cancer: focus on leptin. Proc Nutr Soc. 2012;71:175–80. doi: 10.1017/S0029665111003259. [DOI] [PubMed] [Google Scholar]
- 33.Lu YP, Lou YR, Bernard JJ, Peng QY, Li T, Lin Y, et al. Surgical removal of the parametrial fat pads stimulates apoptosis and inhibits UVB-induced carcinogenesis in mice fed a high-fat diet. Proc Natl Acad Sci U S A. 2012;109:9065–70. doi: 10.1073/pnas.1205810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi H, Strader AD, Woods SC, Seeley RJ. The effect of fat removal on glucose tolerance is depot specific in male and female mice. Am J Physiol Endocrinol Metab. 2007;293:E1012–20. doi: 10.1152/ajpendo.00649.2006. [DOI] [PubMed] [Google Scholar]
- 35.Bosetti C, Bravi F, Negri E, La Vecchia C. Oral contraceptives and colorectal cancer risk: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:489–98. doi: 10.1093/humupd/dmp017. [DOI] [PubMed] [Google Scholar]
- 36.Fujisawa T, Endo H, Tomimoto A, Sugiyama M, Takahashi H, Saito S, et al. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut. 2008;57:1531–8. doi: 10.1136/gut.2008.159293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aparicio T, Kotelevets L, Tsocas A, Laigneau JP, Sobhani I, Chastre E, et al. Leptin stimulates the proliferation of human colon cancer cells in vitro but does not promote the growth of colon cancer xenografts in nude mice or intestinal tumorigenesis in Apc(Min/+) mice. Gut. 2005;54:1136–45. doi: 10.1136/gut.2004.060533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kucherlapati M, Nguyen A, Kuraguchi M, Yang K, Fan K, Bronson R, et al. Tumor progression in Apc(1638N) mice with Exo1 and Fen1 deficiencies. Oncogene. 2007;26:6297–306. doi: 10.1038/sj.onc.1210453. [DOI] [PubMed] [Google Scholar]
- 39.Nunez NP, Oh WJ, Rozenberg J, Perella C, Anver M, Barrett JC, et al. Accelerated tumor formation in a fatless mouse with type 2 diabetes and inflammation. Cancer Res. 2006;66:5469–76. doi: 10.1158/0008-5472.CAN-05-4102. [DOI] [PubMed] [Google Scholar]
- 40.Ablamunits V, Cohen Y, Brazee IB, Gaetz HP, Vinson C, Klebanov S. Susceptibility to induced and spontaneous carcinogenesis is increased in fatless A-ZIP/F-1 but not in obese ob/ob mice. Cancer Res. 2006;66:8897–902. doi: 10.1158/0008-5472.CAN-05-4679. [DOI] [PubMed] [Google Scholar]
- 41.Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010;70:741–51. doi: 10.1158/0008-5472.CAN-09-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hata K, Kubota M, Shimizu M, Moriwaki H, Kuno T, Tanaka T, et al. C57BL/KsJ-db/db-Apc Mice Exhibit an Increased Incidence of Intestinal Neoplasms. Int J Mol Sci. 2011;12:8133–45. doi: 10.3390/ijms12118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klopp AH, Zhang Y, Solley T, Amaya-Manzanares F, Marini F, Andreeff M, et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res. 2012;18:771–82. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys Acta. 2009;1790:1117–23. doi: 10.1016/j.bbagen.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]