Abstract
The dentate gyrus (DG), in addition to its role in learning and memory, is increasingly implicated in the pathophysiology of anxiety disorders. Here, we show that, dependent on their position along the dorso-ventral axis of the hippocampus, DG granule cells (GCs) control specific features of anxiety and contextual learning. Using optogenetic techniques to either elevate or decrease GC activity, we demonstrate that GCs in the dorsal DG control exploratory drive and encoding, not retrieval, of contextual fear memories. In contrast, elevating the activity of GCs in the ventral DG has no effect on contextual learning but powerfully suppresses innate anxiety. These results suggest that strategies aimed at modulating the excitability of the ventral DG may be beneficial for the treatment of anxiety disorders.
Introduction
Lesion studies in both humans and animals, have demonstrated an essential role for the hippocampus in episodic memory formation (Burgess et al., 2002). Although this mnemonic function remains undisputed, recent studies have suggested that the hippocampus might also contribute to emotional behavior as neuroimaging studies have implicated hippocampal dysfunction in mood and anxiety disorders (Campbell et al., 2004; Dannlowski et al., 2012; Gilbertson et al., 2002; Irle et al., 2010; Kitayama et al., 2005).
Consistent with its proposed roles in both cognitive and emotional domains, the hippocampus shows marked variation along its dorso-ventral axis in terms of both afferent and efferent connectivity (Bannerman et al., 2004; Fanselow and Dong, 2010; Gray and McNaughton, 2000). Most strikingly, the dorsal hippocampus projects extensively to associational cortical regions whereas the ventral hippocampus projects to regions implicated in autonomic, neuroendocrine and motivational responses to emotionally charged stimuli, such as prefrontal cortex (PFC), amygdala and hypothalamus (Fanselow and Dong, 2010; Gray and McNaughton, 2000; Moser and Moser, 1998; Swanson and Cowan, 1977). Selective lesion studies have shown that removal of the dorsal hippocampus disrupted spatial memory, while lesion of the ventral pole spared spatial learning but had an anxiolytic effect (Bannerman et al., 2002; Bannerman et al., 1999; Kjelstrup et al., 2002; Moser et al., 1995; Richmond et al., 1999). However, it is not clear how the anatomical heterogeneity of the hippocampus mediates its differential contributions to memory processing and to anxiety-like behavior, or more generally, if changes in hippocampal activity can impact anxiety levels. In addition, it remains unclear whether the three subregions of the hippocampus (dentate gyrus (DG), CA3 and CA1) perform the same operations along the dorso-ventral axis.
In this study we sought to examine the effects of acutely increasing or decreasing activity in the dentate gyrus (DG), on cognitive and emotional behavior. We focused on the DG as multiple lines of evidence implicate it in affective processing. For example, DG granule cells (GCs) are especially susceptible to damage by elevated stress hormone levels (McEwen, 1999) whereas adult neurogenesis, a unique feature of the DG, is increased by factors such as exercise or antidepressant treatment, that elevate mood and is decreased by stress (Dranovsky et al., 2011; Malberg et al., 2000; van Praag et al., 1999). Furthermore, ablation of neurogenesis blocks certain behavioral effects of antidepressants and some responses to stress (David et al., 2009; Santarelli et al., 2003; Snyder et al., 2011; Surget et al., 2011). To manipulate the DG with high temporal resolution and cell-type precision we employed optogenetic techniques. First, we genetically targeted inhibitory and excitatory opsins to the DG’s principal cell type, the granule cell (GC). And secondly we optically targeted either the dorsal or ventral DG, so as to evaluate the effect of acutely reducing or elevating activity in GCs in awake behaving animals in tests of contextual learning and anxiety-like behavior.
Results
Optogenetic control of DG GCs
To target opsin expression selectively to GCs we employed a mouse line that specifically expresses Cre recombinase in this cell type; the proopiomelanocortin (POMC)– bacterial artificial chromosome (BAC) Cre recombinase line (McHugh et al., 2007). This line was crossed to conditional mouse lines that contained either the yellow light-activated chloride pump halorhodopsin (eNpHR3.0 (Zhang et al., 2007), ROSA26-CAG-stopflox- eNpHR3.0-YFP (Madisen et al., 2012)) or the blue light-activated cation channel channelrhodopsin (ChR2)(Boyden et al., 2005) (ROSA26-CAG-stopflox-ChR2(H134R)-tdTomato (Madisen et al., 2012), Figure 1A, E). POMC-eNpHR3.0 and POMC-ChR2 mice were compared to single transgenic littermate control animals throughout.
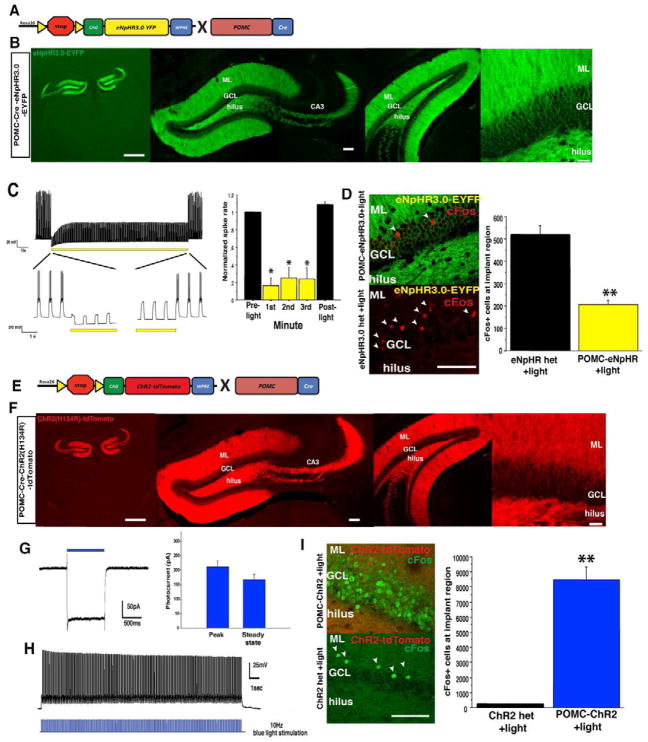
Figure 1. Optogenetic control of dentate gyrus granule cells.
(A) Genetic design for eNpHR3.0-YFP expression in DG GCs. (B) Expression of eNpHR3.0 in DG GCs, scale bar left, 1mm, middle 100um, right 30um. (C) Extended 3-minute illumination blocks evoked spiking in GCs, quantified on the right (n = 6, Mann-Whitney tests, planned comparisons, p < 0.05 for each minute). (D) Yellow light illumination of the dorsal DG in vivo reduces the number of cells positive for the immediate early gene cFos in the GCL region below the implanted fiber optic (scale bar, 100um), quantified on right (n=4/geno, t6= 6.7, p<0.001) (E) Genetic design for ChR2-tdTomato expression in DG GCs. (F) Expression of ChR2-tdTomato in DG GCs, scale bar left, 1mm, middle 100um, right 30um. (G) Voltage-clamp trace showing cationic current evoked by 1s pulse of 473nm light, average peak and steady state current amplitudes are quantified on the right. (H) Example current clamp record of spiking GC following a 10Hz train of 20 msec pulses. (I) Blue light illumination of the dorsal DG (10Hz, 20ms pulses) led to induction of cFos in a cohort of DG GCs (scale bar, 100um), quantified on the right (n=3–4/geno, t5=8.2, p<0.001). **p<0.01.
POMC-eNpHR3.0 mice showed robust and selective membrane expression of eNpHR3.0-eYFP in GCs along the entire dorso-ventral axis of the DG with pronounced expression in their dendrites and mossy fiber axons that project to CA3 (Figure 1B). Whole-cell recordings from GCs in brain slices from POMC-eNpHR3.0 mice confirmed that a brief pulse of yellow (594 nm) light elicited a robust membrane hyperpolarization that effectively suppressed action potential generation (Figure 1C, and Figure S1). To confirm the functional impact of eNpHR3.0 activation in vivo, the DG was illuminated with yellow light while animals explored a novel environment (for behavior during stimulation, see Figure S2), a task that in eNpHR3.0 single transgenic control animals induced a sparse but robust expression of the activity-induced immediate early gene cFos in the DG (Figure 1D). In POMC-eNpHR3.0 mice ~60% fewer cells were cFos positive in the region below the implanted fiber optic, indicating effective local inhibition of GC recruitment (Figure 1D). This local suppression of activity was confirmed with both dorsal and ventral DG illumination in vivo (dorsal DG, Figure 2B, ventral DG, Figure 2F)
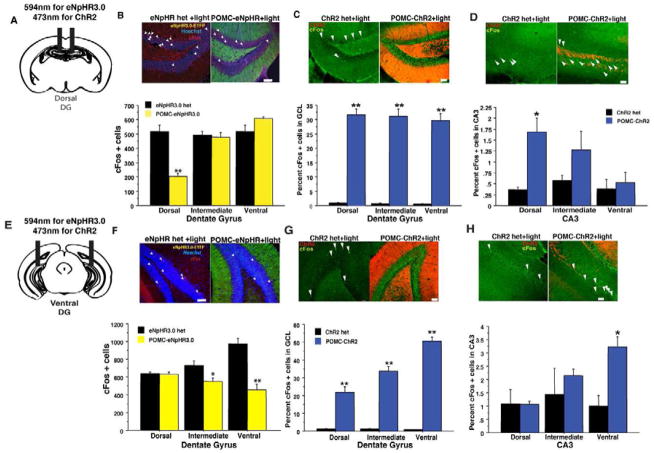
Figure 2. Illumination of the DG in POMC-eNpHR3.0 and POMC-ChR2 mice in vivo modulates cFos levels in the hippocampus in a region specific manner.
Yellow light Illumination of the dorsal (B) or ventral (F) DG of POMC-eNpHR3.0 mice during exploration of a novel environment (20min constant illumination) reduces total number of cFos immunoreactive cells in the GCL in the region below the implanted fiber optic. (dorsal implants, n=4/geno, repeated measures ANOVA, genotypeXregion interaction F(2,12)=45.1, p<0.01, ventral implants, n-4–5/geno, F(2,14)=23.8, p<0.01. (C, G) Blue light illumination of the (5min, 10 Hz illumination in a novel environment) of the dorsal (C), or ventral DG (G) in POMC-ChR2 mice can increase percent of cFos+ cells (% Hoechst 33342) throughout the GCL, but leads to modest induction in CA3 in a region-specific manner (D, H), in the region of the implanted fiber optic, most likely via direct stimulation of mossy fiber axons projecting towards CA3 (dorsal implants DG: n=3–4/geno, repeated measures ANOVA, genotype effect, F(1,5)= 148.4, p<0.0001, geno X region F(2,10)=0.5, p=0.6, CA3:, repeated measures ANOVA, geno X region F(2,10)=6.8, p=0.01. Ventral implants DG: repeated measures ANOVA, genotype effect, F(1,3)= 297.5, p<0.01, geno X region, F(2,6)=1321, p<0.001, CA3:, repeated measures ANOVA, geno X region F(2,6)=5.2, p<0.05. Scale bars represent 50um, *p<0.05, **p<0.01. All error bars are +/− SEM.
The POMC-ChR2 mice exhibited robust and selective expression of ChR2-tdTomato in DG GCs, in their dendrites and mossy fiber axons, and along the DG’s entire dorso-ventral axis (Figure 1E–F). Whole-cell recordings of GCs from POMC-ChR2 mice confirmed that blue (473nm) light elicited inward currents (Figure 1G). Currents were sufficiently large to bring a subpopulation of GCs to action potential threshold to elicit time-locked spiking to light pulses (Figure 1H), and a population in which ChR2 activation generated a robust depolarization from resting potential (Figure S1). Consistently, in the DG of control animals, exploration paired with blue light (for behavior during stimulation see Figure S2), induced the characteristic sparse expression of cFos whereas in POMC-ChR2 mice, 30–50% of GCs expressed this activity marker (Figure 1I, and 2C, G). Interestingly, blue light stimulation of the DG elicited cFos induction in regions rostral and caudal to the implanted fiber optic (Figures 2C, G), most likely due to local recurrent excitatory connections within the DG. However, increased cFos expression in CA3 was only observed locally at the level of the implant site, indicating region-specific elevation of DG output to CA3 (Figures 2D, H).
Selective role of GCs in the dorsal DG in contextual encoding but not retrieval
We began an examination of the specific contribution of DG GCs to behavior by testing if inhibiting GCs in a region specific manner modulated either the encoding or retrieval of contextual fear memories. First, POMC-eNpHR3.0 and control mice were implanted bilaterally with fiber optics targeted to the dorsal DG (Figure 3A). To test the requirement for dorsal DG activity during the encoding of a context, GC activity was locally suppressed by constant yellow light as mice explored a conditioning context (conditioned stimulus, CS) for three minutes prior to receiving a single foot shock (unconditioned stimulus, US) (Figure 3B). POMC-eNpHR3.0 and control mice responded similarly to the footshock, but POMC-eNpHR3.0 mice exhibited more exploration of the conditioning chamber prior to the shock (Figure S3A). When mice were exposed to the context without light stimulation 24 hrs later, POMC-eNpHR3.0 mice exhibited a robust impairment in freezing to the context (Figure 3B). This decrease in freezing was also present when mice were tested with light on during both encoding and retrieval phases (Figure S3B). Encoding deficits were selective for contextual learning, as inhibition of the dorsal DG did not impair the encoding of a tone-shock association (Figure S3C).
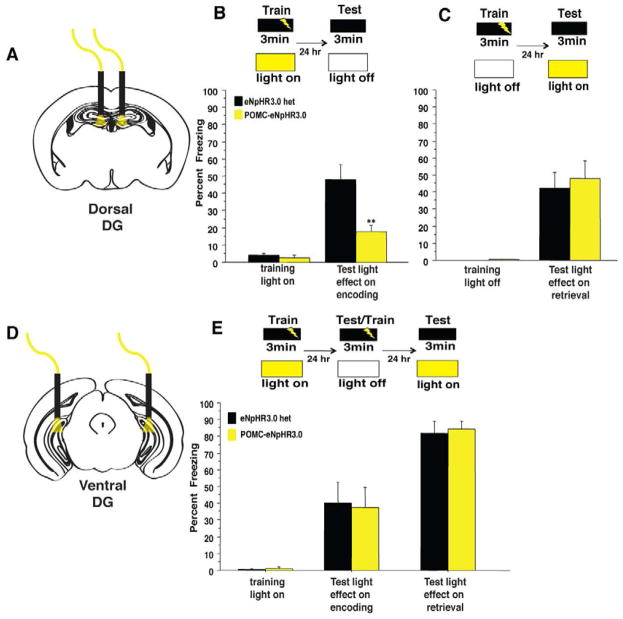
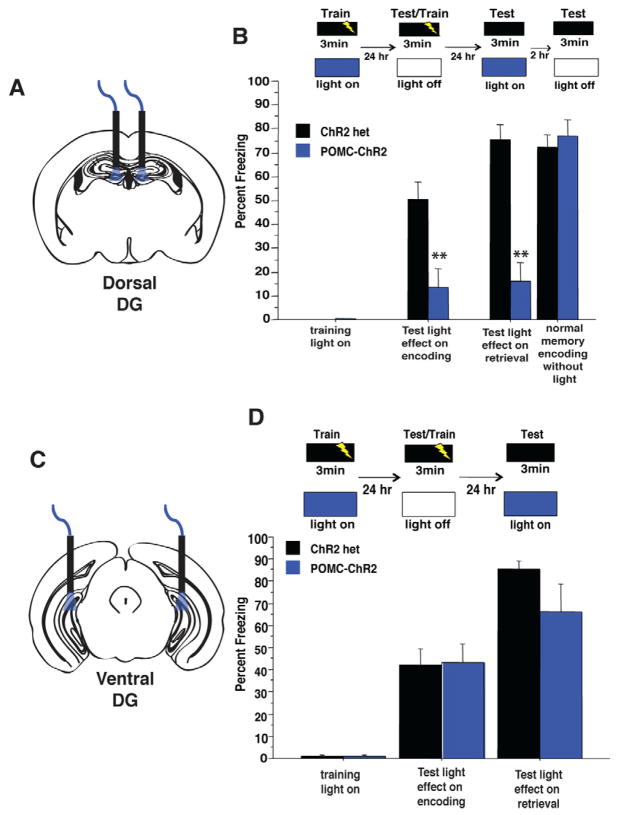
Figure 3. GCs in the dorsal but not ventral DG selectively control the encoding of contextual fear memories.
(A–B) Yellow light illumination of the dorsal DG during training blocked the encoding of context fear as POMC-eNpHR3.0 mice exhibited reduced freezing when tested 24hr later in the absence of light (n=6–7/geno, repeated measures ANOVA, genotype effect F(1,11)=13.2, p=0.004, genotypeXtraining interaction F(1,11)=9.4, p=0.01 (t11=3.4, p=0.006) (C) Optogenetic inhibition of the dorsal DG selectively impairs the encoding of contextual fear memories, as yellow light illumination of the dorsal DG during a recall session did not impair the retrieval of contextual fear memories. (n=7–8/geno, repeated measures ANOVA, geno effect F(1,13)= 0.19, p=0.7, training effect F(1,13)= 42.7, p=<0.0001, genotype X training interaction F(1,13)= 0.19, p=0.7 (t13=−.4, p=0.7) (D–E) POMC-eNpHR3.0 mice received yellow light stimulation of the ventral DG during encoding, were tested and retrained in the absence of light, then tested for light effects on retrieval. (E) Optogenetic inhibition of the ventral DG had no impact in acquisition or retrieval of contextual fear memories. (n=6/geno light on during encoding, repeated measures ANOVA, geno effect F(1,10)= 0.01, p=0.9, training effect F(1,10)= 19.5, p<0.01, genotype X training interaction F(1,10)= 0.03, p=0.9, light on during retrieval, geno effect F(1,10)= 0.1, p=0.8, genotype X training interaction F(1,10)= 0.05, p=0.8, (t10=−.3, p=0.8). **p<0.01. All error bars are +/− SEM.
To test if the dorsal DG is also involved in the retrieval of contextual memories, POMC-eNpHR3.0 mice were trained in contextual fear conditioning in the absence of yellow light, then tested twenty-four hours later under optical inhibition of the dorsal DG. Interestingly, POMC-eNpHR3.0 mice froze at similar levels to single transgenic controls, indicating that inhibition of the dorsal DG does not impair the retrieval of already learned contextual fear memories (Figure 3C). Together these data support a specific role for the dorsal DG in the rapid encoding, but not the retrieval, of contextual fear memories.
As fear conditioning elicits a robust emotional response, we hypothesized that manipulations of the ventral DG, which has been implicated in emotional control, might similarly affect fear learning. To test this, GC activity in the ventral or intermediate DG was locally suppressed in vivo by constant yellow light (Figure 3 and S3E) as mice explored a conditioning chamber prior to a foot shock (Figure 3E). Local suppression on the ventral DG did not impact exploration of the conditioning chamber or response to the footshock (Figure S3D). Twenty-four hours later the mice were returned to the same context without light stimulation to test for freezing, and to retrain in the absence of light stimulation. Surprisingly, POMC-eNpHR3.0 and controls froze similarly to the training context regardless of whether they received light during the training or retrieval sessions (Figure 3E). Thus, silencing of GCs in either the ventral or intermediate DG impaired neither the encoding nor the retrieval of contextual fear memories.
GCs in the dorsal DG modulate cognitive flexibility
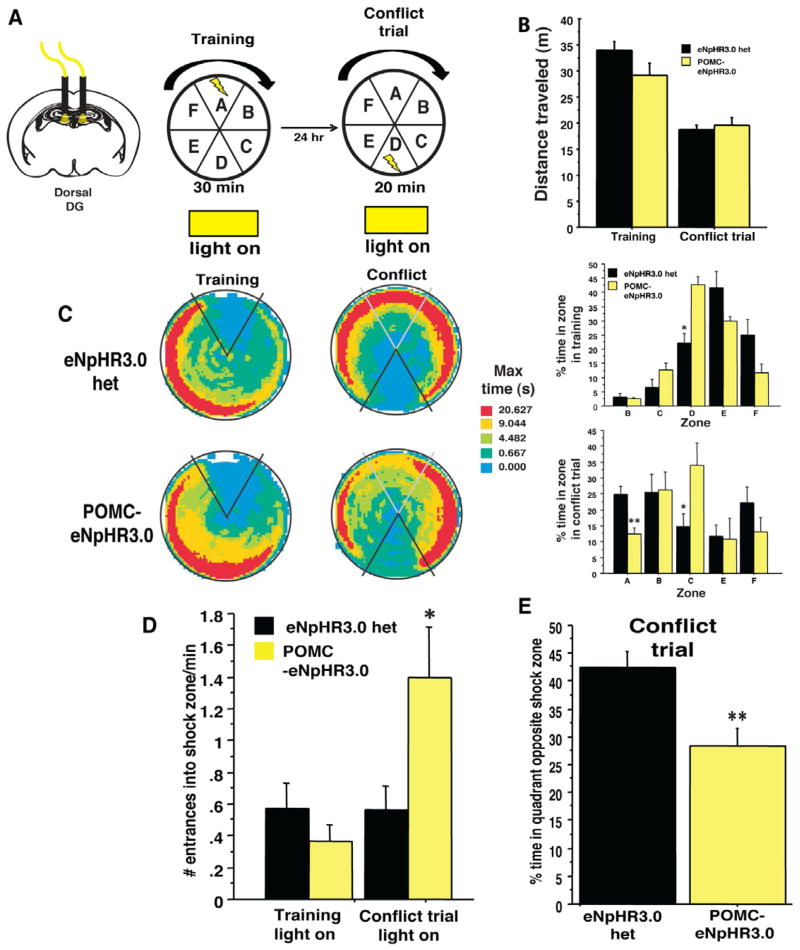
To test the contribution of GCs in the dorsal DG to spatial learning, POMC-eNpHR3.0 with dorsally implanted fiber optics were trained in active place avoidance, a spatial task that has been shown to be sensitive to DG manipulations (Burghardt et al., 2012). In this task, mice were placed on a rotating arena and trained to avoid a stationary shock zone while GC activity in the dorsal DG was locally suppressed with constant yellow light (Figure 4A and S4). Optogenetic inhibition of the dorsal DG did not impact exploration, acquisition or retrieval of the spatial memory (Figure 4B and S4). In addition, analysis of the percent time in each of the zones did not reveal any difference in the first two training sessions (Figure S4D–F), though a small effect was seen in the percent time in one of the zones opposite the shock zone in the third training trial before the switch (Figure 4C). This difference did not impact avoidance of the shock zone during this training session (Fig. 4C–D). However, when the location of the shock zone was switched to the opposite side of the room, a variant of the task that required animals to rapidly encode a new contingency and resolve a conflicting memory, POMC-eNpHR3.0 mice showed marked deficits. During suppression of dorsal GC activity, mice entered the new shock zone significantly more (Figure 4D) and spent less time in the safe, opposite zone than non-suppressed controls (Figure 4 C, E). These data suggest that GCs in the dorsal DG modulate cognitive flexibility in the resolution of conflicting memories.
Figure 4. Optogenetic inhibition of the dorsal DG impairs discrimination in an active place avoidance task.
(A) Experimental design. Shown are the last training trial and the conflict trial. (B) Optogenetic inhibition of the dorsal DG did not impact exploration of the apparatus in either training or conflict trial phases (n=5–6/geno, repeated measures ANOVA, geno effect F(1,9)= 1.1, p=0.3, training effect F(1,9)= 1.3, p=0.3, genotype X training interaction F(1,9)= 2.7, p=0.1 (C) Time-in-location heat maps for POMC-eNpHR3.0 and control mice during the first 20 min of the last training trial and the 20min conflict trial. (D) Percent time in each of the zones during the training trial and the conflict trial. In training, a slightly different strategy was used, but both groups effectively avoided the shock zone (genotype X training interaction F(5,45)= 5.4, p<0.01, t-test on zone D, t9=−3.4, p<0.05). In the conflict trial, POMC-eNpHR3.0 mice spent significantly less time in the zone opposite the shock zone and more time adjacent to the shock zone (genotype X training interaction F(5,45)= 2.5, p<0.05, t-test on zone D, t9=4.1, p<0.05, zone C, t9=−2.3, p<0.05) (D–E) As a consequence, POMC-eNpHR3.0 mice exhibited an increased number of entrances into the shock zone after switching its location (unpaired t test, t9=−2.3, p<0.05, and the percent time in the quadrant opposite the new shock zone (t9=3.4, p<0.01). **p<0.01, *p<0.05. All error bars are +/− SEM.
Elevating activity in the dorsal, but not ventral, DG impairs contextual fear learning
We next tested the impact of stimulating GCs rather than inhibiting them. GCs are characterized by a sparse activation pattern (Leutgeb et al., 2007); a behavioral experience (such as exposure to a novel environment or contextual fear conditioning) induces expression of activity dependent immediate early genes in only ~5% of the GC population (Chawla et al., 2005). By stimulating DG GCs in POMC-ChR2 mice we increased dramatically overall DG activity (Figure 1I), and examined the impact of this manipulation on contextual learning and innate emotional behavior.
To assess the consequence of elevating activity within the DG GC population on the encoding of contextual fear, mice explored a conditioning chamber for three minutes while receiving 10 Hz blue light illumination of the dorsal DG (Figure 5A). POMC-ChR2 and control mice responded similarly to the foot shock, yet POMC-ChR2 mice exhibited significantly more exploration of the conditioning chamber (Figure S5A). Twenty-four hours later, mice were placed back in the conditioning chamber in the absence of light stimulation to test if stimulation had affected encoding. Optogenetic stimulation of the dorsal DG robustly impaired the encoding of contextual fear, as POMC-ChR2 mice showed a marked decrease in freezing to the conditioning context as compared to controls (Figure 5B). This decrease in freezing was also seen when blue light was given during both encoding and retrieval phases (Fig S5B)
Figure 5. Optogenetic stimulation of GCs in the dorsal, but not ventral, DG impairs the encoding and retrieval of contextual fear memories.
(A–B) Blue light illumination of the dorsal DG during training blocked the context-shock association as POMC-ChR2 mice froze less to the training context when tested in the absence of light 24hr after training n=7–9/geno, repeated measures ANOVA, genotype effect F(1,14)=11.7, p=0.004, training effect, F(1,14)= 35.06, p<0.0001, training X genotype interaction F(1,14)=11.8, p=0.004, t-test on test day t14=3.4, p=0.004. Mice were retrained in the absence of light in this session by providing a single footshock at the end of the session. 24hr later, mice were tested for light effects on retrieval, where blue light impaired retrieval in POMC-ChR2 mice, but not single transgenic controls,(repeated measures ANOVA, genotype effect F(1,14)=35.9, p<0.0001, training effect, F(1,14)= 83.6, p<0.0001, training X genotype interaction F(1,14)=36.108, p<0.0001, t-test on test day t14=9.2, p<0.0001). Mice were tested 2hr later to confirm normal encoding of the memory during the retraining session, and that light during retrieval did not permanently erase the memory. POMC-ChR2 mice froze similar to control mice in this session, confirming an intact memory for the conditioning context, (Repeated measures ANOVA, genotype effect F(1,14)=0.3, p=0.58, training effect, F(1,14)= 31.1, p<0.0001, training X genotype interaction F(1,14)=0.29 p=0.6, t-test on test day t14=−0.6, p=0.59). (C–D) Optogenetic stimulation of the ventral DG did not impact the encoding or retrieval of contextual fear memories. Mice received blue light stimulation of the ventral DG during encoding, were tested and retrained in the absence of light, then tested for light effects on retrieval. POMC-ChR2 mice froze similar to single transgenic controls in all phases of the experiment (n=9/geno, light on during encoding, repeated measures ANOVA, genotype effect F(1,16)=0.01, p=0.9, training effect, F(1,6)= 60.7, p<0.0001, training X genotype interaction F(1,16)=0.002, p=0.97, t-test on test day t16=.06, p=0.95, light on during retrieval, repeated measures ANOVA, genotype effect F(1,16)=2.1, p=0.17, training effect, F(1,6)= 130.4, p<0.0001, training X genotype interaction F(1,16)=2.2, p=0.16, t-test on test day t16=−1.4, p=0.16. **p<0.01, all error bars are +/− SEM.
Next, we probed the effect of optogenetic stimulation of GCs in the dorsal DG on retrieval of conditioned fear. Unlike eNpHR3.0-mediated inhibition, optogenetic activation of these cells robustly impaired the retrieval of contextual fear memories, as POMC-ChR2 mice exhibited decreased freezing to the conditioning context as compared to controls (Figure 5B). In addition, optogenetic stimulation of the GCs during retrieval could block the expression of an already initiated freezing response (Figure S5C). Yet, elevation of activity in this proportion of GCs did not permanently erase the memory as when mice were retested the same day, in the absence of light, the memory for the context was intact, as both genotypes froze similarly to the conditioning context (Figure 5B). Similar results were obtained when stimulating the intermediate portion of DG (Figure S5F). To rule out the possibility that optical stimulation of the dorsal DG impairs any ability to learn or express a freezing response, we tested the effects of light stimulation of GCs on encoding or retrieving a tone-shock association. The encoding and retrieval of cued fear memories were intact, demonstrating that the elevation of activity in DG GCs selectively impairs the encoding and retrieval of a hippocampus-dependent contextual fear memory (Figure S5D).
We next asked whether elevating DG activity in the ventral DG would produce similar impairments in contextual fear encoding and retrieval. POMC-ChR2 mice were implanted with fiber optics targeted to the ventral DG (Figure 5C) and trained in contextual fear conditioning in an identical fashion as mice implanted dorsally. Optogenetic activation of the ventral DG did not impact exploration or response to the footshock (Figure S5E). Surprisingly, ChR2-mediated stimulation of GCs in the ventral DG had no effect on either the encoding or retrieval of fear memories (Figure 5D), further implicating the specific contribution of the dorsal DG to contextual encoding.
Acute control of anxiety through elevation of activity in DG GCs
Finally we tested the impact of modulating activity in the DG on innate anxiety-related behavior. Mice were tested in two well-validated tests of conflict anxiety, the elevated plus maze (EPM) and the open field test (OFT) (Belzung and Griebel, 2001). Due to an innate aversion to bright, open spaces, mice in these tests avoid the open arms of the EPM and the center of the OFT, such that entrances into or time in these areas is inversely related to anxiety levels. We first tested whether inhibition of either the dorsal or ventral DG influenced anxiety-like behavior. POMC-eNpHR3.0 mice were implanted with fiber optics targeted to either the dorsal or ventral DG, and tested for consequences of light-induced suppression of activity on anxiety state. In neither the EPM or OFT did suppression of activity in any portion of the DG impact anxiety-related behaviors or locomotor levels regardless of whether light was provided after an initial light off epoch (Figure S6), or if light was given at the beginning of the test (data not shown). This suggests that brief suppression of DG activity does not impact baseline anxiety state.
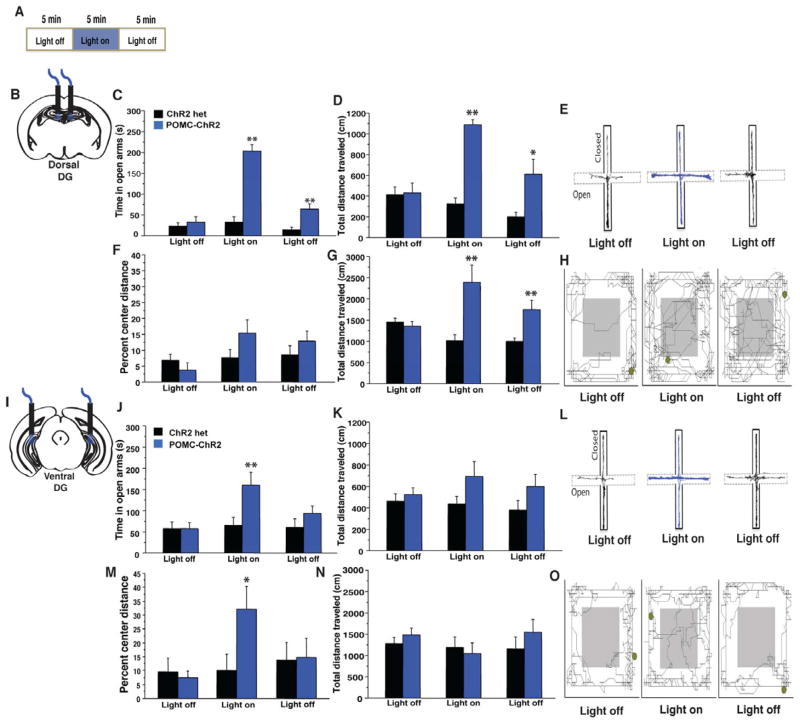
Next, we examined the effect of localized ChR2-mediated stimulation of GCs on baseline anxiety state. First, POMC-ChR2 mice were implanted bilaterally with fiber optics targeting the dorsal DG and tested for acute effects of light stimulation on anxiety-related behaviors (Figure 6A). In both tests, activation of the dorsal DG elicited a dramatic increase in exploration of novel environments: stimulation increased time in the open arms of the EPM (Figure 6C), and total exploration of the OFT and EPM (Figure 6D, G), but did not significantly alter the percent distance travelled in the center of the open field (Figure 6F). Interestingly, this increase in exploration extended beyond the light stimulation epoch, which may be due to either induction of plasticity of mossy fiber-CA3 synapses as a consequence of the stimulation, or due to the decreased aversion to the testing apparatus due to increased exploration during the light-on period. This robust elevation of exploratory drive was specific for novel environments, as exploration induced by novel objects or another mouse was unaffected by light stimulation (Figure S6D). The blue light-induced increase in exploration in a novel environment was absent in mice treated with the dopamine D1 receptor antagonist, SCH23390 demonstrating that it required intact dopaminergic function (Figure S6E). When mice were tested for light effects in home cage exploration, the effect of light stimulation was attenuated as compared to testing in a novel environment, yet habituation to the testing conditions was impaired in POMC-ChR2 mice as compared to control mice (Figure S6F).
Figure 6. GCs in the ventral DG control conflict anxiety, while those in the dorsal DG drive exploratory behavior.
A) Experimental design. POMC-ChR2 mice and single transgenic littermate controls were implanted in the dorsal (B–G) or ventral (I–O) DG then tested for 15min in the EPM and OFT with 5min light off, 5min light on, 5min light off epochs. (C–D) Optical stimulation of the dorsal DG in POMC-ChR2 mice but not single transgenic controls increased total time in the open arms of the EPM (C, repeated measures ANOVA, genotype effect F(1,15)=54.1, p<0.0001, light effect, F(2,30)= 42.2, p<0.0001, light X genotype interaction F(2,30)=30.2, p<0.0001, t test light on t15= −8.4, p<0.0001) as well as total distance traveled in the maze (genotype effect F(1,15)=18, p<0.001, light effect, F(2,30)= 16.3, p<0.0001, light X genotype interaction F(2,30)=20.3, p<0.0001, t test light on t15= −9.9, p<0.0001) Track trace represented in (E) (F–G) Optical stimulation of the dorsal DG in POMC-ChR2 mice but not single transgenic controls increased total exploration in the OFT but not percent distance traveled in the center of the arena (n=8–9/geno, total distance traveled, repeated measures ANOVA, genotype effect F(1,15)=8.3, p=0.01, light effect, F(2,30)= 3.2, p=0.05, light X genotype interaction F(2,30)=14.3, p<0.0001, t test light on t15= 3.3, p=0.004, percent center distance F(1,15)=1.1, p=0.31, light effect, F(2,30)= 3.5, p=0.04, light X genotype interaction F(2,30)=2.4, p=0.1, t test light on t15= 1.5, p=0.14). Track trace in (H). J–K) Ventral DG stimulation was acutely anxiolytic in the EPM as light stimulation increased time POMC-ChR2 mice spent in open arms of the maze (n=8/geno, repeated measures ANOVA, genotype effect F(1,14)=3.2, p=0.1, light effect, F(2,28)= 4.6, p=0.02, light X genotype interaction F(2,28)=4, p=0.03, t-test on light epoch t14=−2.4, p=0.03), but did not impact total distance traveled in the maze, genotype effect F(1,14)=3.4, p=0.1, light effect, F(2,28)= 0.7, p=0.5, light X genotype interaction F(2,28)=1, p=0.4). Track trace in L). M–N) OFT. Ventral DG stimulation did not impact total locomotor activity, but increased the percent distance traveled in the center of the arena (n=8–9/geno, repeated measures ANOVA, total distance traveled, genotype effect F(1,15)=0.3, p=0.6, light effect, F(2,30)= 1.9, p=0.2, light X genotype interaction F(2,30)=1.6, p=0.2, percent center distance, genotype effect F(1,15)=0.9, p=0.2, light effect, F(2,30)= 6.4, p=0.005, light X genotype interaction F(2,30)=6.9, p=0.003, t test light on t15=2.2, p=0.04, track trace in O). *p<0.05, **p<0.01. All error bars are +/− SEM.
We next asked whether stimulation of GCs in the ventral DG would have a similar effect. POMC-ChR2 mice implanted with fiber optics targeted to the ventral portion of the DG were tested for acute effects of light stimulation. Light stimulation of GCs in the ventral DG induced a robust, acute, and reversible anxiolytic response in the EPM and OFT, with mice spending significantly more time in the open arms of the maze during the light-on epoch (Figure 6J, Supplemental video 1), and travelling more in the center of the OFT (Figure 6M). In contrast to dorsal stimulation, light activation of the ventral DG did not influence overall exploratory activity, as total distance traveled in the OFT and the EPM did not differ between genotypes in the light-on epoch (Figure 6K, N). Stimulation of the intermediate portion of the DG produced a hybrid effect, by modestly increasing exploration, and decreasing anxiety (Figure S6G). These results suggest that the DG modulates exploration via its dorsal pole and anxiety-like behavior via its ventral pole.
Discussion
By using optogenetic techniques that allow neural activity in DG GCs to be acutely, reversibly and bidirectionally manipulated, we have shown that the dorsal and ventral poles of the hippocampus are functionally distinct and demonstrate that hippocampal activity not only has a mnemonic function but can also strongly influence anxiety-related behaviors. Specifically, dorsal GCs were shown to contribute to spatial and contextual learning, where they were required for rapid encoding of contextual information, but not for memory retrieval. Surprisingly, elevating dorsal DG activity also induced a dramatic increase in exploratory behavior in novel environments. The ventral DG was not required for contextual fear learning, but was found to exert a major influence on innate anxiety-like behavior (Table 1).
Table 1.
Summary of effects of DG manipulations on learning and anxiety.
| Contextual learning | Innate responses | |||
|---|---|---|---|---|
| encoding | retrieval | exploration | anxiety | |
| Inhibition of GCs in dorsal DG | ⬇ | ⚊ | ⚊ | ⚊ |
| Inhibition of GCs in ventral DG | ⚊ | ⚊ | ⚊ | ⚊ |
| Activation of GCs in dorsal DG | ⬇ | ⬇ | ⬆ | ↓ |
| Activation of GCs in ventral DG | ⚊ | ⚊ | ⚊ | ⬇ |
As our experimental design allowed epoch-selective manipulations of GC activity, we could directly demonstrate that DG GCs are required for the encoding of contextual information, but not its retrieval. Computational models of the hippocampus have proposed a selective role for the DG in encoding novel information (Rolls, 1996; Treves and Rolls, 1992). However, experimental evidence is mixed, with some studies reporting a role of the DG in the encoding of contextual information (Lassalle et al., 2000; Lee and Kesner, 2004b), others in both contextual encoding and retrieval (Lee and Kesner, 2004a), and more recently, no effect has been seen in contextual fear learning after long-term genetic inactivation of GCs (Nakashiba et al., 2012). Through optogenetic modulation of the DG with high temporal and regional precision during either the encoding or retrieval phase we determined that the DG GCs were selectively required for rapid contextual encoding. In contrast to encoding, the retrieval of contextual memories does not require the DG to be on-line suggesting that retrieval of contextual memories is mediated either by direct cortical activation of the collateral network of pyramidal neurons in CA3 (Gilbert and Brushfield, 2009; Nakazawa et al., 2002), or through the temporoammonic pathway from EC to CA1 (Brun et al., 2002). In the ChR2 activation experiment, the impairment in retrieval we observe is likely to be caused by the indiscriminate activation of GCs, which in turn leads to altered activity in CA3 (Figure 2) or further downstream in the hippocampal circuit. Altering dorsal DG activity did not impact tone-shock association, consistent with a requirement of the hippocampus in encoding of contextual information but not discrete cues (Phillips and LeDoux, 1992). Our data supports a requirement for dorsal DG in rapid contextual encoding, rather than for the ability to form the context-shock association, which likely occurs in downstream amygdala nuclei (McNish et al., 1997)
Here we show that either suppression or elevation of activity in the dorsal DG impairs contextual encoding. DG GCs are characterized by a very sparse pattern of activation, allowing for incoming contextual representations to be encoded in non-overlapping populations of GCs. Either elevation or suppression of activity within the DG disrupt this coding structure in the GCL likely contributing to the deficits in proper encoding of the context. In addition, we found that both manipulations impacted exploration of the conditioning chamber prior to the footshock, which may contribute to the impaired contextual encoding in both manipulations.
Our studies revealed that epoch selective regulation of activity in the ventral DG did not influence contextual encoding or retrieval. Whether contextual information is represented in the ventral DG remains unknown, but place fields in ventral hippocampus have been reported to be significantly larger and less numerous than those in dorsal CA1 (Jung et al., 1994; Kjelstrup et al., 2008), indicating that spatial information is differentially processed along the dorsal-ventral axis. Lesion and pharmacological interventions in the ventral hippocampus have produced conflicting results regarding the role of the ventral hippocampus in contextual encoding (Czerniawski et al., 2012; Hunsaker and Kesner, 2008; Maren and Holt, 2004; Richmond et al., 1999; Wang et al., 2012). Our studies differ from these, as here we have employed real-time, brief modulation of activity within the ventral DG, only during the 3 minutes of encoding or retrieval, rather than the long-term manipulations such as pharmacological interventions or lesions. In addition, our manipulations are specific to DG GCs, which leaves open the possibility that other areas of ventral hippocampus, most notably vCA1, may contribute to contextual fear conditioning or unconditioned anxiety.
In the active place avoidance task, we found that suppression of activity in dorsal GCs impaired the ability to discriminate between conflicting memories. Inactivation studies indicate that the hippocampus modulates the encoding of spatial memories in the active place avoidance task (Cimadevilla et al., 2000; Cimadevilla et al., 2001), yet this is likely via hippocampal subregions outside the dorsal DG, as our results suggest that the GCs in the dorsal DG do not contribute significantly to this process. Yet, the impaired performance in the conflict variant of this task indicated that the DG does significantly influence cognitive flexibility, the ability to resolve conflicting memories and select the appropriate response to changing contingencies. This dissociation between encoding of spatial memories and resolving conflicting memories has recently been shown in mice lacking adult neurogenesis in the DG, and in mice lacking NMDA receptors in the DG (Bannerman et al., 2012; Burghardt et al., 2012). These results indicate that the DG plays a significant role in both rapid contextual encoding and discrimination between conflicting spatial memories, highlighting the specific nature of the dorsal DG in cognitive information processing. This role of the DG in rapid and flexible encoding of novel information may impact not only cognitive but also emotional processes. For example, impaired contextual processing in fearful situations may underlie the overgeneralization observed in a number of anxiety disorders such as panic disorder and post-traumatic stress disorder (PTSD) (Gilbertson et al., 2007; Kheirbek et al., 2012; Lissek et al., 2010; Sahay et al., 2011).
Surprisingly, activation of the dorsal DG resulted in a dramatic increase in exploratory behavior and activation of the ventral DG caused an equally robust decrease in anxiety-related behavior (Table 1). Given that CA3 was only recruited at the same dorso-ventral level as the GC stimulation (Figure 2) these differential behavioral effects may be due to driving hippocampal output in a region-specific manner, yet future studies are required to determine the regions mediating these behavioral effects. Potential candidates for ventral DG stimulation may be the amygdala (Bienvenu et al., 2012; Kishi et al., 2006; Tye et al., 2011) and PFC (Adhikari et al., 2010), while dorsal DG may modulate structures involved in exploration such as the lateral septum and ventral tegmental area (Luo et al., 2011; Swanson, 2000). These studies may also provide a framework to explain how changes in neurogenesis within the DG can impact both cognition and mood (Sahay and Hen, 2007). Specifically, we postulate a differential contribution of neurogenesis along the dorso-ventral DG axis to learning and anxiety-related behaviors (Kheirbek and Hen, 2011).
Recent studies employing deep brain stimulation to ameliorate symptoms of treatment resistant depression highlight the effectiveness of circuit based approaches for the treatment of psychiatric illness (Mayberg et al., 2005). Our results provide the first evidence that increasing activity in the ventral DG can reduce innate anxiety without affecting learning. The clear dissociation between the contributions of the dorsal and ventral poles of the DG to cognitive function and anxiety offers a rationale for pursuing strategies that target the ventral DG to treat anxiety with minimal cognitive side effects. In addition, given the immediate behavioral impact of our manipulations, these strategies are likely to have a faster onset of therapeutic efficacy than current treatments such as serotonin reuptake inhibitors.
Materials and methods
Mice and Stereotactic Surgery
ROSA26-CAG-stopflox-ChR2(H134R)- tdTomato (Ai27), ROSA26-CAG-stopflox- eNpHR3.0-YFP (Ai39) and POMC-Cre were generated as previously described (Madisen et al., 2012; McHugh et al., 2007). Male mice were surgically implanted with fiber optic cannulas at 8–10 weeks of age using published protocols (Sparta et al., 2012). Behavioral experiments commenced >3 wks after surgery to allow for recovery. Mice were implanted bilaterally with chronically dwelling optical fibers targeted to the dentate gyrus (dorsal implants: +/−1mm ML +/−1.5mm AP, −1.7mm DV, ventral implants: +/−2.5mm ML +/−3.7mm AP, −2mm DV, intermediate implants: +/−2 mm ML +/−2.9mm AP −1.9mm DV). All experiments were approved by the IACUC at the New York State Psychiatric Institute.
Construction of Optical Fibers
We employed use of published techniques for the construction of chronically dwelling optical fibers and patch cables for behavioral procedures (Sparta et al., 2012). For all experiments a 200um core, 0.37 numerical aperture (NA) multimode fiber (ThorLabs) was used for optical stimulation via a patch cable connected to either a 100mw 593.5 or 473nm laser diode (OEM laser systems).
Slice electrophysiology
Brains were taken from 12–14 week old mice following halothane anesthesia and decapitation. Brains were chilled in ice-cold dissection solution (in mM: sucrose 195, NaCl 10, KCl 2.5, NaH2PO4 1, NaHCO3 25, glucose 10, MgCl2 6, CaCl2 0.5) then 350 μm horizontal slices were cut on a Leica VT1000 vibratome. Slices were recovered in an intermediate solution (in mM: sucrose 70, NaCl 80, KCl 2.5, NaH2PO4 1, NaHCO3 25, Glucose 10, MgCl2 4, CaCl2 2) in a submerged chamber at 37 °C for 45 min and then at room temperature until use in ACSF (in mM: NaCl 124, KCl 2.5, NaH2PO4 1, NaHCO3 25, Glucose 20, MgCl2 1, CaCl2 2). Whole-cell patch-clamp recordings were made in ACSF at 31–32 °C using borosillicate glass pipettes (initial resistance 5–6 MΩ) filled with an internal solution that contained (in mM): KMeSO4 130, KCl 10, HEPES 10, NaCl 9, EGTA 0.1, MgATP 4, Na2GTP 0.3, phosphocreatine 10. Junction potentials were not corrected for. Voltage-clamp recordings were made at a holding potential of −65 mV and current clamp recordings at the cell’s resting potential. Light was delivered via a cleaved 200 um core, 0.37NA fiber optic (~10–15mW at tip of optic) held at approximately 30° to the slice surface with its tip ~150 μm from the recorded cell. DG GCs were recorded from at random. For recordings from POMC-ChR2 mice, functional channel expression was confirmed with 1 sec blue light pulses and then responses to 10 Hz stimulation for 2 sec using pulse durations of 5, 10 and 20 msec were recorded. In a subset of cells, it was confirmed that ChR2 responded to 10 Hz stimulation over a 3 minute period (data not shown). In POMC-eNpHR3.0 mice, functional pump expression was checked using a 1 sec yellow light pulse pulse and then three stimulation paradigms were used to assay the suppression of action potential generation: 1) light was delivered for 800 msec and incremental depolarizing current injections of 500 were given during illumination 2) 1.5 sec incremental depolarizing current injections were delivered and yellow light was given for the middle 0.5 sec 3) 250 msec 150 pA current injections were given at 1 Hz for 20 sec without light and then throughout a 3 min yellow light pulse and for 20 sec after light. All protocols were repeated 3–6 times (twice for 3 min illuminations) for each cell and the average taken to represent that cell’s response.
Behavioral experiments
For anxiety tests, mice were quickly attached to the fiber optic patch cables (bilaterally) via a zirconia sleeve, then placed in testing apparatus. The patch cables were interfaced to an FC/PC rotary joint (Doric lenses), which was attached on the other end to either a 593.5 or 473 nm laser diode that was controlled by a Master-8 stimulator (AMPI). Hardware configuration was identical for all tests. Sessions lasted for 15 min consisting of three 5 min epochs: light off, light on, and light off. Open field (Kinder Scientific) was tested with high lux illumination (600lux) and was collected and analyzed with MotorMonitor software, with total distance traveled and percent of that distance traveled in the center of the arena documented. In the EPM, sessions were videotaped, and analyzed for time spent in closed arms, open arms and center of the maze using TopScan software. For home cage exploration, mice were singly housed for 1 week before being brought into a novel testing room, and tested for light effects on distance traveled in their home cage using TopScan software. For social interaction and novel object investigation, an experimenter blind to treatment condition analyzed total approaches and time investigating the novel mouse or object during light on and off epochs.
Fear conditioning took place in Coulbourn Instruments fear conditioning boxes that contained one clear plexiglass wall, three aluminum walls and a stainless steel grid as a floor. Mice were brought in to the testing room in a novel cage, attached to the fiber optic patch cables then placed in fear conditioning boxes. The training session began with the onset of the houselight and fan, and anise scent was placed under the grid floor. In this one-trial contextual fear conditioning protocol, mice received light stimulations as described in the text, and 180 s after placement of the mouse in the training context and onset of houselight and fan, mice received single 2-s foot shock of 0.75 mA. All freezing was measured before the single footshock. The mouse was taken out 15 s after termination of the foot shock and returned to its home cage. The grid and the waste tray were cleaned with Sanicloths between runs. Mice were recorded by digital video cameras mounted above the conditioning chamber, and were scored for freezing by an investigator blind to the genotype of the animal. For cued fear conditioning, mice were trained in the same context as in contextual fear conditioning, except that a 20s, 80db, 2 kHz pure tone was provided as the discrete cue CS, and a 2s footshock that co-terminated with the tone was provided. 24hr later, mice were tested for cued fear in a novel context, in which the conditioning chamber was altered, the stainless steel grid floor was covered with a plastic panel and novel cage bedding, the chamber walls were covered and made circular using plastic inserts, the house fan and lights were turned off, and a mild lemon scent was placed below the floor. The chamber door was left ajar during testing. Mice were brought into the testing room in white transport buckets. Mice were given the tone, and an investigator blind to genotype scored freezing before the first tone presentation and during tone presentations as a measure of cued fear.
Active place avoidance
We tested active place avoidance using methods previously described (Burghardt et al., 2012). Briefly, mice were attached to fiber optic cables and placed on a circular (40 cm diameter) platform that rotated clockwise at a speed of 1 rpm. The rotating platform was exposed to the room environment with multiple visual cues, including a black curtain, a white piece of cardboard, a black and white stripped piece of paper, and a cream colored cloth. A shock zone was defined within a 60° region of the stationary room. Entrance into the shock zone resulted in a brief constant current footshock (500ms, 60Hz, 0.2mA) that was scrambled across pairs of parallel rods located on the platform floor. If the mouse remained in the shock zone, it received additional shocks of the same intensity and duration every 1.5 seconds. The position of the mouse was tracked by PC-based software that analyzed images from an overhead camera and delivered shocks appropriately (Tracker, Bio-Signal Group Corp., Brooklyn, NY). Mice were trained for 3 sessions (30 min session, sessions 1 and 3, light on, session 2 light off). In the conflict trial, the shock zone was flipped to the opposite quadrant and mice were tested for 20 minutes with the light on. Active place avoidance was measured as the number of times a mouse entered the shock zone, which was computed by Track Analysis software (Bio-Signal Group Corp., Brooklyn, NY), and time in place heat maps were generated as previously described (Burghardt et al., 2012).
Immunohistochemistry
For all cFos induction experiments, mice were separated and singly housed for at least 24hr before tested for light effects on cFos induction. Mice were placed in a novel arena, and then stimulated with light. For ChR2 experiments mice received 5 min of stimulation (10 Hz, 20ms pulses, 8mw light power) then returned to their home cage. For eNpHR3.0 experiments, mice received 20min of constant yellow light stimulation (15mw), then returned to their home cage. 90min after the onset of stimulation, mice were perfused, and sections were labeled for cFos. An investigator blind to treatment condition counted cFos+ cells in the GCL across the dorso-ventral axis of the dentate gyrus.
Statistical Methods
All data are represented as mean +/− SEM. Data was analyzed in Microsoft Excel, Clampfit 10, StatView, and SigmaPlot 12. All statistical tests used are noted in the text, which included t-tests and ANOVAs with post-hoc t-test where applicable. All main effects and interactions are noted in the text. A p<0.05 was considered significant.
Supplementary Material
Highlights.
Inhibition of activity in dorsal dentate gyrus blocks the rapid encoding of contexts
Modulating activity in ventral dentate gyrus does not impact contextual learning
Activation of dorsal dentate gyrus elicits robust exploration of novel environments
Activation of ventral dentate gyrus powerfully suppresses innate anxiety
Acknowledgments
We thank J. Ledoux and A. Losonczy for comments on previous versions of this manuscript, G. Stuber, J. Britt, and A. Bonci for assistance in optogenetic manipulation, S. Tonegawa for POMC-Cre mice, K. Deisseroth for ChR2 and eNpHR constructs, and C. Dupre, A. Goldberg and E. Balough for technical assistance. This work was supported by NIMH 1K01MH099371-01, a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award and a Sackler Institute Award (M.A.K.); and NARSAD, the New York Stem Cell Initiative (NYSTEM C026430), NIH R37 MH068542, and Hope for Depression Research Foundation grants (R.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Bus T, Taylor A, Sanderson DJ, Schwarz I, Jensen V, Hvalby O, Rawlins JN, Seeburg PH, Sprengel R. Dissecting spatial knowledge from spatial choice by hippocampal NMDA receptor deletion. Nature neuroscience. 2012;15:1153–1159. doi: 10.1038/nn.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behavioral neuroscience. 2002;116:884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neuroscience and biobehavioral reviews. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behavioral neuroscience. 1999;113:1170–1188. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behavioural brain research. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Bienvenu TC, Busti D, Magill PJ, Ferraguti F, Capogna M. Cell-type-specific recruitment of amygdala interneurons to hippocampal theta rhythm and noxious stimuli in vivo. Neuron. 2012;74:1059–1074. doi: 10.1016/j.neuron.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature neuroscience. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012 doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. The American journal of psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- Cimadevilla JM, Fenton AA, Bures J. Functional inactivation of dorsal hippocampus impairs active place avoidance in rats. Neuroscience letters. 2000;285:53–56. doi: 10.1016/s0304-3940(00)01019-3. [DOI] [PubMed] [Google Scholar]
- Cimadevilla JM, Wesierska M, Fenton AA, Bures J. Inactivating one hippocampus impairs avoidance of a stable room-defined place during dissociation of arena cues from room cues by rotation of the arena. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3531–3536. doi: 10.1073/pnas.051628398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Ree F, Chia C, Otto T. Dorsal versus ventral hippocampal contributions to trace and contextual conditioning: Differential effects of regionally selective nmda receptor antagonism on acquisition and expression. Hippocampus. 2012;22:1528–1539. doi: 10.1002/hipo.20992. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, et al. Neurogenesis-dependent and - independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranovsky A, Picchini AM, Moadel T, Sisti AC, Yamada A, Kimura S, Leonardo ED, Hen R. Experience dictates stem cell fate in the adult hippocampus. Neuron. 2011;70:908–923. doi: 10.1016/j.neuron.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Brushfield AM. The role of the CA3 hippocampal subregion in spatial memory: a process oriented behavioral assessment. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33:774–781. doi: 10.1016/j.pnpbp.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature neuroscience. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Williston SK, Paulus LA, Lasko NB, Gurvits TV, Shenton ME, Pitman RK, Orr SP. Configural cue performance in identical twins discordant for posttraumatic stress disorder: theoretical implications for the role of hippocampal function. Biological psychiatry. 2007;62:513–520. doi: 10.1016/j.biopsych.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. 2. Oxford; New York: Oxford University Press; 2000. [Google Scholar]
- Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiology of learning and memory. 2008;89:61–69. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irle E, Ruhleder M, Lange C, Seidler-Brandler U, Salzer S, Dechent P, Weniger G, Leibing E, Leichsenring F. Reduced amygdalar and hippocampal size in adults with generalized social phobia. J Psychiatry Neurosci. 2010;35:126–131. doi: 10.1503/jpn.090041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Hen R. Dorsal vs ventral hippocampal neurogenesis: implications for cognition and mood. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:373–374. doi: 10.1038/npp.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nature neuroscience. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Yokota S, Yasui Y. Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. The Journal of comparative neurology. 2006;496:349–368. doi: 10.1002/cne.20919. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassalle JM, Bataille T, Halley H. Reversible inactivation of the hippocampal mossy fiber synapses in mice impairs spatial learning, but neither consolidation nor memory retrieval, in the Morris navigation task. Neurobiology of learning and memory. 2000;73:243–257. doi: 10.1006/nlme.1999.3931. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus. 2004a;14:301–310. doi: 10.1002/hipo.10177. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus. 2004b;14:66–76. doi: 10.1002/hipo.10167. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin S, Heller RE, Lukenbaugh D, Geraci M, Pine DS, Grillon C. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. The American journal of psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ, 3rd, Gu X, Zanella S, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nature neuroscience. 2012 doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behavioral neuroscience. 2004;118:97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- McNish KA, Gewirtz JC, Davis M. Evidence of contextual fear after lesions of the hippocampus: a disruption of freezing but not fear-potentiated startle. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:9353–9360. doi: 10.1523/JNEUROSCI.17-23-09353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Barrera VR, Chittajallu R, Iwamoto KS, McBain CJ, et al. Young Dentate Granule Cells Mediate Pattern Separation, whereas Old Granule Cells Facilitate Pattern Completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J, Bannerman DM. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behavioral neuroscience. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A theory of hippocampal function in memory. Hippocampus. 1996;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nature neuroscience. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Stamatakis AM, Phillips JL, Hovelso N, van Zessen R, Stuber GD. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nature protocols. 2012;7:12–23. doi: 10.1038/nprot.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, et al. Antidepressants recruit new neurons to improve stress response regulation. Molecular psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain research. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. The Journal of comparative neurology. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Wang SH, Finnie PS, Hardt O, Nader K. Dorsal hippocampus is necessary for novel learning but sufficient for subsequent similar learning. Hippocampus. 2012 doi: 10.1002/hipo.22036. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.