Abstract
Proton therapy has been used in the treatment of prostate cancer for several decades, and interest surrounding its use continues to grow. Proton-based treatment techniques have evolved significantly over this time period, and several centers now routinely utilize technologies such as pencil beam scanning. However, whether the theoretical dosimetric advantages of the proton beam translate into clinically meaningful improvements for prostate cancer patients is unknown, and outcomes from single-arm experiences using whole courses of proton beam therapy in the treatment of early-stage prostate cancer have shown mixed results when compared to contemporary IMRT. A randomized trial comparing proton beam to IMRT in early-stage disease is open, and will be important in defining the role for proton therapy in this setting. We review the available evidence and present the current state of proton beam therapy for prostate cancer.
INTRODUCTION
External beam radiation continues to play a major role in the treatment of patients with prostate cancer. With the widespread implementation of PSA screening, an increasing proportion of patients are being diagnosed with early-stage disease and are candidates for definitive local therapy. For many of these patients, external beam radiation represents a highly effective treatment option with a well-defined toxicity profile. Continued improvements in radiation planning and delivery allow radiation oncologists to administer high doses of radiation to the prostate while minimizing toxicity from exposure of adjacent normal structures.
Proton beam radiation has been used in the treatment of prostate cancer for several decades, during which time planning and delivery techniques have also continued to evolve and improve. The advantageous dose distribution of protons makes their use attractive in the treatment of prostate cancer, and experience with proton therapy continues to grow.[1] However, randomized data comparing proton beam therapy to modern photon-based therapy in the treatment of early-stage prostate cancer is lacking, and non-randomized studies report mixed results. A randomized trial has recently opened and will provide insight regarding the relative value of proton beam therapy for these patients.
DOSIMETRIC CONSIDERATIONS
The unique properties of protons’ depth dose curve make them an appealing treatment modality for many cancers. For nearly all disease sites, the ability to deliver high doses to a target with relative sparing of nearby normal structures has theoretical advantages. Prostate cancer is no exception, but has several unique features that provide challenges in radiation targeting and delivery. Unlike most malignancies, prostate cancer is not visible on standard imaging, and thus the entire gland is currently targeted for treatment. The gland itself resides deep within the pelvis and is in intimate contact with several normal structures including the urethra, bladder, and rectum. Variations in rectal and bladder filling cause changes in position of the prostate that have important implications for treatment planning.
Despite these challenges, x-ray based treatments for prostate cancer have benefitted from several important technological advances over the last 30 years, and it is important to frame any comparison between proton and photon therapy in the context of contemporary x-ray based techniques. Today, both photon and proton-based treatments are based on 3D volumetric planning and often utilize image-guidance technology. Photon-based treatment has evolved from simple three- or four-field arrangements to the multiple, differentially-weighted beam design available with IMRT.
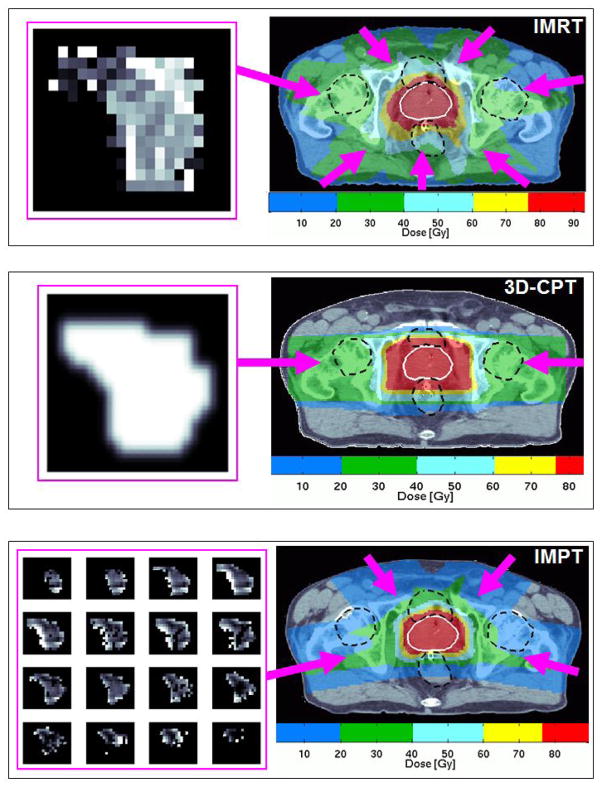
Currently, most proton treatments are delivered using a 3D-conformal technique, with plans created by combining beams of different energies to form a ‘spread out’ Bragg peak that covers the target at depth. The most common beam arrangement uses two opposed lateral beams, and custom apertures and compensators are fabricated to control the shape of the field and the depth of the dose. More conformal and complex ‘IMRT-like’ distributions can be delivered using proton pencil beam scanning (PBS, Figure 1). Prostate PBS treatments have recently commenced at select proton therapy centers, and are in the process of being implemented at others (including the authors’ institution).
Figure 1.
Comparison of IMRT, 3D-conformal proton therapy (3D-CPT), and intensity-modulated proton therapy (IMPT) plans. A representative axial CT image from each plan is shown for a prescription dose of 79.2 Gy(RBE). Rectum, bladder, and femoral heads are outlined with black hatched lines. Beam directions are represented by the pink arrows. For each plan, the fluence map for the indicated radiation portal is displayed to the left of the CT image. The IMPT plan delivers a sequence of fluences using proton beams of different energies.
Dosimetric analyses have been performed at several proton therapy centers to evaluate the relative advantages of contemporary IMRT and proton treatments. Trofimov et al compared proton treatment plans using two opposed lateral beams to IMRT plans using seven coplanar beams in ten patients with early-stage prostate cancer.[2] To account for intrafractional motion and set-up uncertainties, a 10-mm margin was added to the clinical target volume (CTV) to create the planning target volume (PTV). For a prescription dose of 79.2 Gy(RBE, adjusted for relative biological effectiveness) to the prostate, IMRT irradiated substantially greater volumes of normal tissue in the <30 Gy(RBE) range, including both the bladder and the rectum. However, patients treated with proton beam therapy had significantly larger normal tissue exposure in the 50–75 Gy(RBE) range. The volume of bladder receiving 50 and 60 GyE was significantly higher with the proton plans, but no difference in rectal volume was noted at these doses. In a study from the University of Florida, the CTV was expanded 5 mm in the axial and 8 mm in the craniocaudal dimensions to create the planning target volume (PTV). The irradiation directions were individually optimized to improve the PTV dose distribution and minimize the rectal and bladder dose, which resulted in the selection of lateral-oblique beams with small posterior angles. The low-dose (<35 Gy(RBE)) rectal and bladder exposure was significantly decreased with proton plans, and rectal volumes in the medium-high dose range of 35–80 Gy(RBE) were also significantly lower.[3] A separate analysis at the University of Texas employing similar 5–8 mm anisotropic margins for 10 patients treated with opposed lateral beams found no significant difference between IMRT and proton beam in the rectal and bladder volumes irradiated to 40 Gy(RBE) or higher.[4] Thus, the results of comparison of limited number of patients appear somewhat mixed, depending on such institution-specific factors as the choice of set-up and range margins, directions of irradiation, hardware design, and penumbra characteristics of the beam. The recently-opened randomized trial comparing IMRT to proton beam therapy standardizes treatment techniques across centers and is expected to provide important insight regarding the relative dosimetric advantages of the two treatment modalities.
In addition to dosimetric differences under ideal circumstances, practical issues such as daily anatomic variation and uncertainty in patient set-up have implications for proton treatment planning. The impact of inter- and intra-fractional movement on target dose for patients treated with opposed lateral proton fields has been studied by calculating the dosimetric consequences of movement in each direction both during and between fractions.[5, 6] The impact of target motion varies depending upon the direction of movement, but increasing movements were typically associated with a proportional decrease in target coverage. Differential effects of motion on IMRT versus proton plans were especially notable in the lateral direction where, for a shift of 6 mm, coverage decreased by 9% for the proton plan compared to only 1% for the IMRT plan.[6] The effects of target movement can be minimized by increasing the PTV margin, but this comes at the expense of increased normal tissue exposure. Even when image-guidance verification is used to minimize errors in set-up uncertainty, day-to-day variations of in vivo parameters such as tissue thickness and femur rotation angle, as well as uncertainty in estimating the proton stopping power of different tissues, necessitate the use of distal and proximal range margin and range compensation expansions.[7] With such expansions applied, the volume of prostate not covered by the fractional prescription dose (as determined by serial CT scans) averaged less than 3% per day, >98% of the prostate received the total prescription dose over the treatment course, and rectal and bladder constraints were met.[8]
One strategy to manage the range uncertainty problem for 3D-conformal proton treatment plans uses diode radiation detectors embedded on the anterior surface of a rectal balloon. The detectors record the radiation dose rate at the distal penumbra of an anterior-oriented proton field with millisecond resolution, thus providing in vivo range verification and allowing planners to minimize the required distal range uncertainty margin.[9] When compared to opposed lateral fields, a single anterior field plan designed using this approach decreased the anterior rectal wall V95% from 39% to 8% (p=0.002) and eliminated dose to the femoral heads. The volume of bladder wall irradiated was higher with a single anterior field, but could be decreased by using paired anterior oblique fields.[10]
Alternative field angles have also been described for 3D-conformal proton treatment of pelvic lymph nodes in high-risk patients. In these patients, the prostate PTV was covered by opposed lateral beams while right and left pelvic node PTVs were covered by lateral and posterior-oblique beams. Low-medium (10–40 Gy(RBE)) dose irradiation of the rectal wall, bladder wall, and small bowel was significantly lower with the proton plans than with IMRT, but the volumes receiving higher doses (50–80 Gy(RBE)) did not differ between plans. As expected, use of lateral proton beams resulted in significantly higher femoral head dose.[11]
The technology that offers the largest degree of flexibility in optimization of proton fluence across tissues is pencil beam scanning (PBS). PBS enables delivery of uniform doses that conform to the target both proximally and distally through the use of techniques such as uniform scanning and single-field-optimized uniform dose (SFUD). Furthermore, PBS can be used to deliver intensity-modulated proton therapy (IMPT), which is similar to IMRT in that complex non-uniform doses from individual fields sum to produce the desired target-conformal dose. Although not yet used for the treatment of prostate cancer, IMPT has potential to decrease the volume of rectum receiving high-dose radiation compared to a lateral field configuration.[2] However, due to the narrow depth-dose peak of the unmodulated beam and increased non-uniformity in the doses delivered from different directions, IMPT can be very sensitive to small variations in organ shape and position and may thus require extra measures to ensure set-up reproducibility.[12, 13]
DEVELOPMENT AND EFFICACY
The first description of protons in the treatment of prostate cancer was published in 1979 and reported the use of a perineal proton boost in 17 patients with localized prostate cancer treated at the Massachusetts General Hospital.[14] Treatment was well-tolerated, and this experience helped to set the stage for further investigation of proton beam therapy and dose-escalation.
The first randomized proton trial for prostate cancer compared a photon boost of 16.8 Gy to a proton boost of 25.2 Gy(RBE) following 50.4 Gy conventional four-field radiation for T3–4 tumors, and showed that dose escalation did not result in improvement in disease control or survival and was associated with worse toxicity.[15] The subsequent Proton Radiation Oncology Group (PROG) trial comparing 19.8 versus 28.8 Gy(RBE) proton boost following 50.4 Gy four-field photon radiation was one of two American randomized trials which demonstrated that dose escalation results in improved biochemical control rate (32.4% progression in the low-dose arm versus 16.7% progression in the high-dose arm at ten years), similar to photon dose escalation trials.[16] A subsequent case-matched analysis in which patients in the high-dose arm were compared with patients treated with brachytherapy at the same institution over the same time period did not, however, suggest that cancer control was superior with proton beam therapy.[17]
The largest retrospective single-institution experience using protons in the treatment of localized prostate cancer is from the Loma Linda University Medical Center.[18] Over 1200 patients were treated in the 1990s with a combination of photon and proton therapy to the prostate and pelvic lymph nodes or with proton therapy alone to the prostate (total dose 74–75 CGE). With a median follow-up of 62 months, the overall 5- and 8-year actuarial biochemical disease-free survival (bDFS) rates were 75% and 73%, respectively, and are comparable to similar cohorts of patients treated with conventional radiation over the same time period. Other single-institutions studies also show similar rates of biochemical control with whole courses of dose-escalated proton beam therapy, although follow-up is short.[19]
SAFETY AND TOXICITY
For all modalities of prostate radiation, toxicity primarily arises from exposure of normal tissues that lie in close proximity to the prostate. Rectal/bowel, bladder, and sexual dysfunction are the most commonly incurred toxicities following prostate radiation, and are described in detail in a recent comprehensive review.[20]
Toxicity data is available for many of the large reported series of prostate cancer patients treated with proton therapy. In the PROG 95-09 trial, RTOG grade ≥3 acute GU and GI toxicity were 3% and 1% for both high- and low-dose arms. Late grade ≥3 GU toxicity was 2% in each arm, while late grade ≥3 GI toxicity was 1% in the high-dose arm and absent in the low-dose arm. Post-hoc analysis revealed no significant differences in patient-reported bowel, bladder, or sexual function between low- and high-dose arms at a median follow-up of nine years.[21] Comparison to a similar cohort of patients treated with 3D conformal radiation (albeit to a slightly lower dose) also showed no meaningful differences in outcomes.
Similar analyses have been performed for patients receiving whole courses of proton beam therapy. In a Japanese study of patients receiving 74 Gy(RBE) for low or intermediate risk prostate cancer, acute grade 2 rectal and bladder toxicities occurred in 0.7% and 12% of patients, respectively.[22] For patients followed more than two years, the incidence of late grade 2 or greater rectal and bladder toxicity was 2% and 4%, respectively. The most commonly-reported rectal toxicity was bleeding followed by pain and urgency.
Long-term patient-reported quality of life (QOL) data from a series of 95 men with localized prostate cancer receiving a median dose of 78 Gy(RBE) showed significant changes in incontinence, bowel, and sexual dysfunction, but not obstructive/irritative voiding dysfunction.[23] When stratified by functional category, significant worsening occurred in those patients with normal function prior to treatment, emphasizing the importance of pre-treatment functional status in estimating risk. This study also highlights the importance of using patient-reported outcomes to characterize the long-term sequelae of treatment.
In general, toxicity and QOL outcomes from these proton therapy experiences are similar to outcomes for patients treated with photon-based techniques, although direct comparison is difficult due to differences in patient characteristics, dose, and treatment technique. The most relevant analyses are those that compare toxicity of proton therapy to the low toxicity reported in series using modern IMRT techniques. For instance, in a series of 145 men treated with IMRT to median dose of 75.6 Gy, rates of grade 3 GI and GU toxicity during treatment were 1% and 3%, respectively.[24] At a median of 5 years, 6% of patients had a chronic grade 3 GI toxicity while 1% had a chronic grade 3 GU toxicity.
A multi-institutional study of prospectively-collected data on patient QOL following treatment with radiotherapy (3DCRT, IMRT, and PBT) using validated instruments to assess patient-reported bowel and urinary toxicity found that patients report distinct patterns of treatment-related bowel and urinary QOL. In the acute setting, proton beam patients report minimal bowel morbidity whereas the same pattern was not observed in intensity-modulated and 3D conformal patients. By 24 months, all three cohorts reported small, clinically meaningful decrements in bowel QOL.[25] Another study used SEER data to identify procedures and diagnosis codes related to GI or GU complications following proton therapy or IMRT and found a significant reduction of GU complications at 6 months in patients treated with proton therapy, but no difference in GI toxicity at any time.[26]
Dose-escalation beyond 80 Gy has been attempted using both IMRT and proton techniques. For 561 patients treated with IMRT at Memorial Sloan-Kettering to a dose of 81 Gy, the 8-year actuarial likelihood of late grade ≥2 rectal and urinary toxicity was 1.6% and 15%, respectively.[27] A ten year update estimates risk of late grade 3 GU and GI toxicity to be 5% and 1%, respectively.[28] Although follow-up is shorter, the estimated rate of grade ≥3 urinary and rectal toxicity at 18 months was 7% and 1%, respectively, in a cohort of 84 patients treated to 82 Gy(RBE) using conformal proton radiation at MGH.[29] In an early analysis of patients treated with 78–82 Gy(RBE) on three Phase II trials at the University of Florida, 10% of patients experienced a Grade 2 or higher rectal toxicity in the first two years following treatment. In this study, the percentage of rectal wall receiving ≥70 Gy(RBE) was significantly associated with risk of GI toxicity on multivariate analysis.[19]
A recent SEER analysis of men with T1–2 prostate cancer showed that radiation was the most significant factor associated with an increased risk of GI toxicity.[30] Estimated GI toxicity rates per 1000 person-years were 9.3 for 3D-CRT, 8.9 for IMRT, and 20.1 for proton therapy, compared to 2.1 in patients not treated with radiation. However, only 337 patients in the analysis were treated with proton therapy compared to 11,770 with 3D-CRT and 4645 with IMRT. Due to the small number of proton centers nationwide and the geographical distribution of SEER representation, one institution likely represented the majority of the proton experience in this study. Notably, the rate of GI toxicity for both IMRT and proton therapy decreased markedly with later year of diagnosis, suggesting that experience and newer techniques may decrease toxicity rates for both modalities.
A separate SEER analysis compared usage, morbidity, and disease control for patients with non-metastatic prostate cancer diagnosed between 2000 and 2009 and treated with 3D-CRT, IMRT, or proton therapy.[31] (Notably, 79.2% of proton patients were residents of California, while the regional distribution of IMRT patients was more diverse.) There were no differences in rates of urinary diagnoses or procedures, erectile dysfunction, hip fractures, or additional cancer therapy between patients treated with IMRT versus proton beam. However, proton patients were more likely to have GI morbidity or undergo GI procedures.
These results suggest that rectal toxicity may actually be worse with proton beam therapy, either because of dosimetric compensations made to ensure adequate coverage at the end of the beam range, or because there is something unique about the biology of the proton beam. The relative biological effectiveness (RBE) of a proton beam is estimated to be 1.1, but may vary slightly depending upon fraction size, tissue type, and the distribution of proton Bragg peaks within the target. When treating to high doses, small differences in RBE estimates can have a large impact on toxicity.
A significant concern with therapeutic radiation is the risk of second malignancies, and population-based studies have shown an increased risk of several cancers, including bladder and rectal cancer, in patients who undergo external beam radiation for prostate cancer.[32] The risk of a radiation-induced neoplasm resulting from external beam treatment for prostate cancer has been estimated to be 1–2% beyond 10 years, and may be slightly higher for IMRT than for 3D-CRT due to increased exposure from low-dose and leakage radiation. Although it had been suggested that proton beam therapy administered via a passive scattering approach may have greater incidence of second malignancies than IMRT due to higher rates of neutron scatter, recent measurements confirmed the neutron production rates with passive scattering techniques to be substantially lower.[33, 34] A recent risk modeling comparison of proton and IMRT plans estimated the second malignancy risk from primary and secondary doses to be 26% to 39% lower with proton therapy.[35] The greatest risk for both modalities was within organs receiving direct radiation, but this risk was lower with protons due to significant sparing of portions of the rectum and bladder. Techniques such as PBS that improve dose conformality may further reduce the risk of secondary cancers, but the relationship between normal tissue dose and second cancer risk is not fully understood. If a decrease in second malignancy risk exists with proton therapy, the benefit is most likely to be clinically relevant in younger patients.
CURRENT & EVOLVING EVIDENCE
Improvements in targeting and dose delivery have led to improved disease control rates for patients receiving photon- or proton-based treatment. However, differences among patient characteristics, treatment techniques, and outcome measures have made it difficult to directly compare safety and efficacy endpoints across published studies. Both modern photon- and proton-based treatments have been shown to be effective with a well-defined toxicity profile, but it is not clear if one modality is superior to the other. Existing systematic reviews and the available data summarized above suggest similar tumor control with no clear benefit in terms of late effects and a suggestion that protons may be associated with higher incidence of late bowel toxicity.
A multicenter randomized trial comparing proton therapy versus IMRT in the treatment of low or low-intermediate risk prostate cancer is being conducted by partnering institutions, the Massachusetts General Hospital (study currently open) and University of Pennsylvania (study opening soon), with other proton centers also anticipated to participate in the future (http://clinicaltrials.gov/ct2/show/NCT01617161). Patients are randomized to receive 79.2 Gy(RBE) in 1.8 Gy(RBE) fractions via IMRT or proton beam. Patients who meet enrollment criteria but do not wish to be randomized or receive the assigned treatment will comprise a registry cohort. A prospective preference assessment of patients’ willingness to participate suggested that a substantial portion of eligible patients would be willing to participate, and accrual has recently begun.[36] The primary endpoint is post-treatment bowel function, with additional quality of life, clinical, biological, and physics endpoints. Cost-related outcomes will also be analyzed, and a full discussion of cost and usage issues related to proton therapy for prostate cancer is covered elsewhere in this issue.
FUTURE DIRECTIONS
Proton beam therapy is an expanding technology for the treatment of patients with localized prostate cancer. Whether the theoretical benefits of proton beam therapy apply to prostate irradiation, and if so, whether the benefits translate into clinically-meaningful improvement in outcomes for these patients remains a topic of intense and on-going investigation. Multi-center randomized trials will be vital in defining the role for proton therapy in these patients; however, efficacy and QOL data will take years to mature.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Efstathiou JA, Trofimov AV, Zietman AL. Life, liberty, and the pursuit of protons: an evidence-based review of the role of particle therapy in the treatment of prostate cancer. Cancer J. 2009;15(4):312–8. doi: 10.1097/PPO.0b013e3181b14ec0. [DOI] [PubMed] [Google Scholar]
- 2.Trofimov A, et al. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys. 2007;69(2):444–53. doi: 10.1016/j.ijrobp.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vargas C, et al. Dose-volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(3):744–51. doi: 10.1016/j.ijrobp.2007.07.2335. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, et al. Effect of anatomic motion on proton therapy dose distributions in prostate cancer treatment. Int J Radiat Oncol Biol Phys. 2007;67(2):620–9. doi: 10.1016/j.ijrobp.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon M, et al. Inter- and intrafractional movement-induced dose reduction of prostate target volume in proton beam treatment. Int J Radiat Oncol Biol Phys. 2008;71(4):1091–102. doi: 10.1016/j.ijrobp.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Yoon M, et al. Characteristics of movement-induced dose reduction in target volume: a comparison between photon and proton beam treatment. Med Dosim. 2009;34(3):191–201. doi: 10.1016/j.meddos.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Trofimov A, et al. Interfractional variations in the setup of pelvic bony anatomy and soft tissue, and their implications on the delivery of proton therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80(3):928–37. doi: 10.1016/j.ijrobp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, et al. Evaluation of the dosimetric impact of interfractional anatomical variations on prostate proton therapy using daily in-room CT images. Med Phys. 2011;38(8):4623–33. doi: 10.1118/1.3604152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu HM. A potential method for in vivo range verification in proton therapy treatment. Phys Med Biol. 2008;53:1413–1424. doi: 10.1088/0031-9155/53/5/016. [DOI] [PubMed] [Google Scholar]
- 10.Tang S, et al. Improvement of prostate treatment by anterior proton fields. Int J Radiat Oncol Biol Phys. 2012;83(1):408–18. doi: 10.1016/j.ijrobp.2011.06.1974. [DOI] [PubMed] [Google Scholar]
- 11.Chera BS, et al. Dosimetric study of pelvic proton radiotherapy for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75(4):994–1002. doi: 10.1016/j.ijrobp.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 12.Soukup M, et al. Study of robustness of IMPT and IMRT for prostate cancer against organ movement. Int J Radiat Oncol Biol Phys. 2009;75(3):941–9. doi: 10.1016/j.ijrobp.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 13.Trofimov A, et al. Visualization of a variety of possible dosimetric outcomes in radiation therapy using dose-volume histogram bands. Practical Radiation Oncology. 2012;2(3):164–171. doi: 10.1016/j.prro.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shipley WU, et al. Proton radiation as boost therapy for localized prostatic carcinoma. JAMA. 1979;241(18):1912–5. [PubMed] [Google Scholar]
- 15.Shipley WU, et al. Advanced prostate cancer: the results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys. 1995;32(1):3–12. doi: 10.1016/0360-3016(95)00063-5. [DOI] [PubMed] [Google Scholar]
- 16.Zietman AL, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95–09. J Clin Oncol. 2010;28(7):1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coen JJ, et al. Comparison of high-dose proton radiotherapy and brachytherapy in localized prostate cancer: a case-matched analysis. Int J Radiat Oncol Biol Phys. 2012;82(1):e25–31. doi: 10.1016/j.ijrobp.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 18.Slater JD, et al. Proton therapy for prostate cancer: the initial Loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004;59(2):348–52. doi: 10.1016/j.ijrobp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Mendenhall NP, et al. Early outcomes from three prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82(1):213–21. doi: 10.1016/j.ijrobp.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Budaus L, et al. Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of the literature. Eur Urol. 2012;61(1):112–27. doi: 10.1016/j.eururo.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 21.Talcott JA, et al. Patient-reported long-term outcomes after conventional and high-dose combined proton and photon radiation for early prostate cancer. JAMA. 2010;303(11):1046–53. doi: 10.1001/jama.2010.287. [DOI] [PubMed] [Google Scholar]
- 22.Nihei K, et al. Multi-institutional Phase II study of proton beam therapy for organ-confined prostate cancer focusing on the incidence of late rectal toxicities. Int J Radiat Oncol Biol Phys. 2011;81(2):390–6. doi: 10.1016/j.ijrobp.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Coen JJ, et al. Long-term quality of life outcome after proton beam monotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82(2):e201–9. doi: 10.1016/j.ijrobp.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 24.Vora SA, et al. Analysis of biochemical control and prognostic factors in patients treated with either low-dose three-dimensional conformal radiation therapy or high-dose intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68(4):1053–8. doi: 10.1016/j.ijrobp.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 25.Gray PJ, et al. Bowel and bladder toxicity patterns in patients with prostate cancer treated with proton beam versus intensity-modulated radiation therapy. J Clin Oncol. 2012;30:S5, abstr 22. [Google Scholar]
- 26.Yu JB, et al. Proton radiotherapy for prostate cancer in Medicare population: Patterns of care and comparison of early toxicity with IMRT. J Clin Oncol. 2012;30(suppl):abstr 4651. [Google Scholar]
- 27.Zelefsky MJ, et al. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176(4 Pt 1):1415–9. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Alicikus ZA, et al. Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2011;117(7):1429–37. doi: 10.1002/cncr.25467. [DOI] [PubMed] [Google Scholar]
- 29.Coen JJ, et al. Acute and late toxicity after dose escalation to 82 GyE using conformal proton radiation for localized prostate cancer: initial report of American College of Radiology Phase II study 03–12. Int J Radiat Oncol Biol Phys. 2011;81(4):1005–9. doi: 10.1016/j.ijrobp.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 30.Kim S, et al. Late gastrointestinal toxicities following radiation therapy for prostate cancer. Eur Urol. 2011;60(5):908–16. doi: 10.1016/j.eururo.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheets NC, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307(15):1611–20. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon K, et al. Cancer incidence after localized therapy for prostate cancer. Cancer. 2006;107(5):991–8. doi: 10.1002/cncr.22083. [DOI] [PubMed] [Google Scholar]
- 33.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65(1):1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Wroe A, Rosenfeld A, Schulte R. Out-of-field dose equivalents delivered by proton therapy of prostate cancer. Med Phys. 2007;34(9):3449–56. doi: 10.1118/1.2759839. [DOI] [PubMed] [Google Scholar]
- 35.Fontenot JD, Lee AK, Newhauser WD. Risk of secondary malignant neoplasms from proton therapy and intensity-modulated x-ray therapy for early-stage prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74(2):616–622. doi: 10.1016/j.ijrobp.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah A, et al. Prospective preference assessment of patients’ willingness to participate in a randomized controlled trial of intensity-modulated radiotherapy versus proton therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;83(1):e13–9. doi: 10.1016/j.ijrobp.2011.11.072. [DOI] [PubMed] [Google Scholar]