Abstract
The local and systemic alterations induced by Bothrops atrox snake venom (BaV) injection in mice were studied. BaV induced superoxide production by migrated neutrophils, mast cell degranulation and phagocytosis by macrophages. Moreover, BaV caused hemorrhage in dorsum of mice after 2hr post- injection. Three hours post-injection in gastrocnemius muscle, we also observed myonecrosis, which was assessed by the determination of serum and tissue CK besides the release of urea, but not creatinine and uric acid, indicating kidney alterations. BaV also induced the release of LDH and transaminases (ALT and AST) indicating tissue and liver abnormalities. In conclusion, the data indicate that BaV induces events of local and systemic importance.
Keywords: Snake venom, Bothrops atrox, leukocytes, hemorrhage, myonecrosis, renal failure, liver abnormalities
INTRODUCTION
Envenomation by snakes is a public health problem that it deserves attention from health authorities. In Latin America, snakes of the genus Bothrops (Viperidae) are responsible for the majority of snakebites (Gutiérrez et al, 2010). This environmental and occupational disease affects mainly agricultural workers especially in Brazil (WHO, 2007; Kasturiratne et al, 2008). Recently, snakebite envenoming was incorporated in World Health Organization list of neglected diseases (www.who.int/neglected_diseases/diseases/en) because it fulfills the criteria of a ‘neglected tropical disease’ as it affects mainly poor people living in rural areas in tropical countries (Gutiérrez et al, 2010).
There is a large variation in molecular composition and biological activities in Bothrops spp. Venoms, as well as anti-bothropic venoms do not neutralize the toxic activities of several bothropic venoms (Queiroz et al, 2008). Variations may occur even among the venoms from the same species living in different geographical regions. Since human therapy of bothropic bite in Brazil has been done with the administration of anti-bothropic venom produced with a pool of venoms (B. alternatus, B. jararaca, B. jararacussu, B. moojeni and B. neuwiedii species), this therapeutic approach may not have effect on all alterations induced by a different Bothrops species, such as B. atrox.
B. atrox venoms cause local effects, such as swelling, hemorrhage and necrosis besides systemic effects, including alterations in blood coagulation and bleeding distant from the bite site (Borges et al, 1999). In Brazilian Amazon region, especially in Rondonia, B.atrox is responsible for the majority (80-90%) of snakebites treated at Tropical Medicine Center of Rondonia (Porto Velho-RO).
Taken together, these observations demand additional studies in order to investigate further biological effects of the B. atrox venom, providing not only new data about the venom action, but also helping to seek new approaches for the treatment. Thus our study was aimed at assessing local and systemic changes induced by intraperitoneal and intramuscular injection of B. atrox venom in the gastrocnemius muscle focusing on cellular influx, leukocyte activation, hemorrhage, prothrombin time (PT) and activated partial thrombosplatin time (APTT), creatine kinase (CK), lactate dehydrogenase (LDH), aspartate aminotransferase (AST/GOT), alanine aminotransferase (ALT/GPT), uric acid, creatinine and urea levels.
MATERIAL AND METHODS
Chemicals
All kits were purchased from Labtest Diagnostica SA (Brazil). PT and APTT were from Wiener Lab (Argentina). Trypan blue, RPMI-1640, gentamicin, L-glutamine, zymosan, halothane, safranin, compound 48/80, toluidine blue and nitroblue tetrazolium (NBT) from Sigma (USA); and fetal bovine serum obtained from Cultilab (Brazil). All salts used (low endotoxin or endotoxin-free grades) were from Merck (Germany).
Animals and venom
Male Swiss mice (18-20gm) and Wistar rats (200-250gm) were used, housed in temperature-controlled rooms with water and food ad libitum. The experimental procedures were approved by the Experimental Animals Committee of IPEPATRO (protocol 2008/3) in accordance with the statements from Universities Federation for Animal Welfare. Venom was obtained from adult specimens of B. atrox collected in Rondonia-Brazil, lyophilized and diluted in sterile isotonic saline and filtered in 0.22µm membranes before use.
Harvesting of macrophages
Thioglycollate–elicited macrophages (TG-macrophages) were harvested 4 days after intraperitoneal (i.p.) injection of 1ml of 3% (w/v) thioglycollate according to Setubal et al (2011).
Cytotoxic assay
Cell viability was measured by Trypan blue exclusion according to Setubal et al (2011).
Phagocytic activity of elicited peritoneal macrophages
Phagocytic activity was determined according to Setubal et al (2011).
Induction of inflammatory reaction and leukocyte harvesting
BaV (0.2mg/kg) or sterile saline were injected by i.p. route. After 6hr, the animals were euthanized under halothane atmosphere and the inflammatory exudate was withdrawn after washing the cavities with 2ml of phosphate-buffered saline (PBS) containing heparin (10U/ml) according to Zuliani et al (2005).
Superoxide anion production
Leukocytes were collected as described in Zuliani et al (2005). 2x105/200μl leukocytes were incubated for 1hr with 0.1% (v/v) NBT at 37°C, 5% (v/v) CO2. After leukocytes were centrifuged (400xg for 5min) in cytospin the slides were fixed with 100% (v/v) methanol for 5min, and stained with 1% (w/v) safranin for 5min. At least 100 leukocytes were counted by microscopic observation in each determination and those containing crystals of formazan were positive for superoxide production. Results were expressed as percentage of cells positive for superoxide anion production.
Histological assessment of mesenteric mast cell degranulation
In this assay male Wistar rats (200-250gm) were used. Mesenteric mast cell degranulation was determined according to Zuliani et al (2011).
Determination of hemorrhage
The hemorrhagic activity was performed according to the method described by Nikai et al (1984). Mice received an intradermal injection in dorsum of PBS (50µl) or BaV (6.5µg/50µl). The venom dose used corresponded to 10 times the minimum hemorrhagic dose, which is an amount of venom that carries out a hemorrhagic area of 10mm in diameter (Colombini et al, 2001). Two hours after the injection, animals were euthanized by cervical dislocation, their skin removed and the hemorrhagic area measured in mm on the inner surface. The skin was weighed and placed in Drabkin solution (8ml/gm tissue, for 24hr) and the absorbance measured at 540nm. The concentration of hemoglobin was estimated from a specific standard curve and represented as mg/ml.
Determination of systemic enzymatic alterations
Mice received intramuscular (i.m.) injection in the right thigh of BaV (50µg/50µl) and left thigh received sterile PBS (control). After 3hr or 12hr mice were euthanized by ether inhalation and their thoracic cavity was opened. Blood samples were obtained by cutting the aorta. After clotting, serum was separated by centrifugation and the following assays were performed: CK; LDH; AST; ALT; creatinine; urea; uric acid. To procedure the PT and APTT assays, citrated plasma was used.
Statistical analysis
The obtained data were compared by ANOVA, followed by Tukey test with significance probability levels in less than 0.05.
RESULTS
Effect of BaV on macrophage viability and phagocytosis
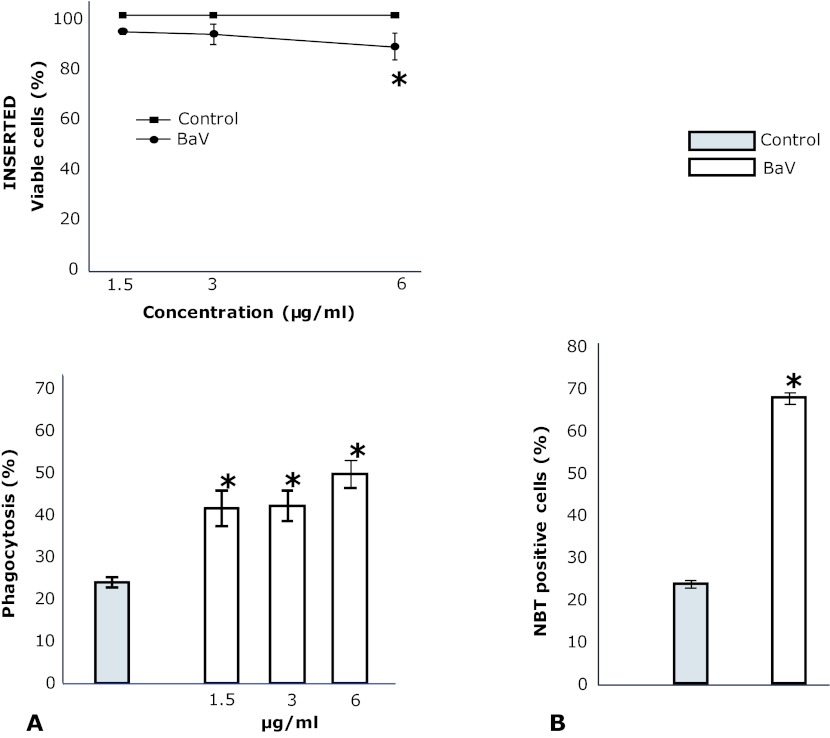
As shown in Figure 1 (inserted), BaV (1.5 and 3µg/ml), did not affect the viability of TG-macrophages. On the other hand, at 6µg/ml, BaV reduced the viability of macrophages to a significant extent. TG-macrophages incubated with RPMI showed an average of phagocytosis of SOZ particles of 25% (Figure 1A). Incubation of macrophages with BaV (1.5 and 3µg/ml) caused an increase of this rate to 60%.
Figure 1.
Inserted. Effect of BaV on cell viability. Thioglycollate-elicited macrophages were collected 96hr after i.p. injection of thioglycollate. Cells (2x105) were incubated with different concentrations of BaV or RPMI (control) for 60min at 37°C under 5% (v/v) CO2, after which cytotoxicity was assessed by Trypan blue exclusion. Values represent the mean ±SEM from four animals. *p<0.05 compared to controls (ANOVA). A. Phagocytosis of opsonized zymosan particles by thioglycollate-elicited macrophages. Cells were harvested 96hr after i.p. injection of thioglycollate and were incubated with BaV or RPMI (control) for 60min before addition of opsonized zymosan particles. Values represent the mean ±SEM from four animals. *p<0.05 compared to controls (ANOVA). B. Effect of BaV on superoxide anion production by migrated leukocytes. The cells were collected from mice peritoneal cavity 6hr after BaV (0.2mg/kg) or sterile saline alone (0.15M NaCl) (control) injection. 2x105cells/ml were incubated for 40min with NBT. Cells were then stained with safranin and centrifugated (400xg/5min) in cytospin. NBT positive cells were evaluated by cell count crystals that formed inside of the cell by phase contrast microscope. Values represent the mean ±SEM of 4 animals. *p<0.01 compared to control (ANOVA).
Effect of BaV on leukocyte influx and activation
Since PMN influx was observed 6hr after BaV i.p. injection, we evaluated the superoxide production at this time point. As shown in Figure 1B, control peritoneal leukocytes showed a percentage of superoxide production of 23.7 ±0.88%. After BaV injection, 67.7 ±1.45% peritoneal leukocytes harvested stained for superoxide production which was different from control.
BaV (0.2mg/kg) was able to induce an inflammatory infiltrate with neutrophils representing the predominant cells, whereas the population of mononuclear leukocytes (MN) did not show a significant increment (Figure 2A). BaV induced also degranulation of mast cells when compared to data from tissues incubated only with Tyrode (negative control) (Table 1).
Figure 2.

A. Leukocyte accumulation into the mouse peritoneal cavity after injection of BaV. BaV (0.2mg/kg) or sterile saline alone (0.15M NaCl) (control) were injected into the mouse peritoneal cavity in a final volume of 1ml. Total leukocyte, polymorphonuclear (PMN) and mononuclear (MN) cell counts were determined in peritoneal washes harvested 6hr after these injections as described in Materials and Methods. Values represent the mean ±SEM of 5-7 animals. *p<0.05 when compared to control (ANOVA). B. Hemorrhagic effect of BaV. Swiss male mice were injected via i.d. with 50µl of BaV (6.5µg/animal) or pyrogen-free PBS (control animals). After 2hr, the animals were euthanized by cervical dislocation and the skin removed for measuring the hemorrhagic area and tissue hemoglobin concentration. Values represent the mean ±SEM 5-7 animals.*p<0.01 compared to control (ANOVA).
Table 1.
Mast cell degranulation induced by Bothrops atrox venom.
P<0.05 compared to Tyrode group.
(n=5 for all groups).
Effect of BaV on hemorrhage
As demonstrated in Figure 2B, BaV induced hemorrhage in the skin of mice compared to control animals. The PT was extremely prolonged in envenomed animals at different time points, being considered incoagulable after 2min of measurement, while the control group showed a PT of 13.5sec. As well APTT showed a pattern homologous to PT being considered incoagulable after 120sec of measurement, significantly increased compared with the control group (APTT: 38sec) (data not shown).
Systemic effects of BaV injection
Table 2 summarizes the systemic effects of 3hr and 12hr after BaV injection in mice. The i.m. injection of PBS caused a leakage of CK into the bloodstream of 427.4U/l, while BaV caused an extravasation of 2125U/l. In agreement with CK results, the LDH levels show raised reaching 1857.7U/l while the control group showed a serum LDH of 12.5U/l. The levels of residual CK after i.m. injection of PBS were 5786.5U/l and after BaV was 1908U/l, significantly different from control. The i.m. injection of PBS caused a leakage of CK-MB into the bloodstream of 334U/l, while BaV caused an extravasation of 497.5U/l. The level of AST after i.m. injection of PBS was 58.3U/ml. After 12hr of BaV i.m. injection an extravasation of 92.9U/ml of AST, statistically different from control, was observed. The serum ALT after i.m. injection of PBS was 42.2U/ml. Twelve hours after BaV i.m. injection an extravasation of 59.5U/ml of ALT, significantly different from control, was observed. The urea concentration after PBS i.m. injection was 53mg/l. BaV caused an increase of urea to 53.7% (81.5mg/l) significantly different from control. The PBS i.m. injection showed a serum creatinine concentration of 0.29mg/dl. After BaV the serum creatinine concentration was 0.15mg/dl. The PBS i.m. injection showed a serum uric acid concentration of 4.8mg/dl, which was not modified by BaV administration.
Table 2.
Systemic effects induced by Bothrops atrox venom.
| Control (PBS) | BaV (50μg) | |
|---|---|---|
| Plasma CK (U/l) | 427.4 ±94.2 | 2125.0 ±118.9* |
| Residual Muscle CK (U/gm tissue) | 5786.5 ±449.5 | 1908.0 ±246.9* |
| CK-MB (U/l) | 334.0 ±25.5 | 497.5 ±62.4 |
| LDH (U/l) | 1251.0 ±6.9 | 1851.7 ±117.9* |
| Urea (mg/l) | 53.04 ±2.5 | 81.5 ±3.6* |
| Creatinine (mg/dl) | 0.29 ±0.05 | 0.15 ±0.01 |
| AST (U/l) | 85.6 ±7.1 | 68 ±10.3 |
| ALT (U/l) | 51.4 ±2.5 | 32.5 ±4.3 |
| AST (U/l) - 12hr | 58.3 ±3.9 | 92.9 ±3.8* |
| ALT (U/l) - 12hr | 42.2 ±1.2 | 59.5 ±1.2* |
P<0.05 compared to control group.
(n=5 for all groups).
DISCUSSION
The tissue injury induced by snake venom has been studied by several authors. In the case of Bothrops venom, the tissue injury appear to involve the action of toxins like PLA2, metalloproteases and various other toxins that directly or indirectly, induces inflammation characterized by swelling, pain, leukocyte infiltration and hemorrhage (Gutiérrez and Lomonte, 1989, Gutiérrez et al, 2009, Teixeira et al, 2009). Here, we found that BaV induces some of the alterations that venoms from another species of Bothrops can trigger. A number of previous studies have described inflammatory infiltration after bothropic envenomation in mice (Barravieira et al, 1995a; Zamuner et al, 2001), and neutrophils predominated at 6hr to 24hr (Teixeira et al, 2009). Our results showed a marked neutrophil influx into peritoneal cavity 6hr after of BaV injection. These data corroborate those obtained by Escocard et al (2006) which showed that BaV induced local afflux of inflammatory cells, one neutrophil-rich peak after 6hr, in BALB/c mice.
Thus, we evaluated the BaV effects on the release of superoxide anions by peritoneal migrated cells. Our data showed a significant release of superoxide anion indicating that the BaV stimulates neutrophils to activate the respiratory burst that is in accordance with Zamuner et al (2001). From these data we conducted assays to verify the BaV effect in adhered TG-macrophages in phagocytosis via complement receptor using SOZ particles. Our results demonstrated that BaV stimulated macrophages phagocytosis like B. alternatus venom (Setubal et al, 2011). According to our results, Zamuner et al (2001) observed an increase of phagocytosis of SOZ particles by peritoneal leukocytes induced by B.asper and B.jararaca venons 12hr and 48hr after an i.p. injection. The fact that snake venom activates the process of phagocytosis suggests that leukocyte function is an essential event for the elimination of venom in a bitten individuals.
Mast cells are very important on inflammation development, and they are identified as participants of edema induced by PLA2 and crude venom from Bothrops genus snakes (Kanashiro et al 2002; Guimarães et al, 2004). Recently, Galvão Nascimento et al (2010) found that mast cells contribute to the edema developed by B. moojeni venom through the release of histamine and probably prostaglandin D2. We found hemorrhage after BaV injection and, although we discussed here that it could be derived from detachment of endothelial cells probably by venom metalloproteases, we cannot exclude that activation of mast cells could induces hemorrhage (Kwasniewski et al, 2008).
Amorin et al (1951) indicated that bleeding and swelling appears in the following minutes after B. jararaca venom inoculation. Our results demonstrated that 2hr after BaV injection induced a marked hemorrhagic halo on the backs of mice, confirmed by the quantification of hemoglobin into the skin. These data corroborate the data obtained by Da Silva et al (2007) which showed that B. jararaca and B. jararacussu venoms induce bleeding in the skin of mice.
The synergistic action of Bothrops venom components are responsible for changes in hemostasis and may induce local or systemic hemorrhagic (Kamiguti et al, 1995), characterized by changes in the vessel permeability, change in platelet aggregation and fibrinogen depletion (Hati et al, 1999). In vitro, this can be measured indirectly by determination of PT and APTT which showed markedly altered, indicating that both extrinsic and intrinsic pathway were interpreted as an impaired deficiency in serum levels of fibrinogen.
In order to assess local damage caused by BaV, we used a dose below the lethal dose given by intramuscular route, since the sting reaches, in most of the victims, the intramuscular or subcutaneous region. The results showed that BaV injection in the gastrocnemius muscle induced the release of CK into the bloodstream after 3hr. Most data concerning the release of CK after i.m. inoculation indicates that this action seems to be concentrated within 3hr to 4hr after injection of Bothrops venom (Gutiérrez, Ownby, 2003). In addition, data on residual CK present in muscle of animals confirm the data on serum CK. In an attempt to determine the extension of tissue damage induced by BaV other enzyme activity was measured. Plasma levels on LDH achieved high levels 3hr after BaV injection, which is in accordance to Gonçalves et al (2008) and Zeni et al (2007).
The mechanism by which BaV induces myotoxicity is not clear. However, previous work showed that the appearance of lesions delta, the onset of muscle degeneration, is not a feature observed only in changes caused by PLA2 snake venoms enzymes. The formation of small triangular areas adjacent to the plasma membrane is very similar to those observed in myonecrosis induced by detergents (Pestronk et al, 1982) and in Duchenne muscular dystrophy (Mokri, Engel, 1975). In these cases, the presence of these lesions is an indication that the initial site of action is the plasma membrane. Thus, the myotoxicity observed in our study, can be attributed to PLA2, since BaV has been shown to have two isoforms (Kanashiro et al 2002), and they constitute important factors for the observed effect.
Since Bothrops venoms cause renal toxicity (Linardi et al, 2011), we evaluate the effect of BaV on creatinine, urea and uric acid blood levels. Results showed that 3hr after i.m. injection of BaV on gastrocnemius muscle mice led to an accumulation of urea in the blood. Moreover, this effect was not observed for creatinine. These data suggest that an increase in blood levels of urea induced by BaV, leads a difficulty in excreting nitrogenous catabolites by kidneys as may be a result of renal dysfunction.
AST and ALT enzymes are markers for cellular damage and ALT enzyme is essentially present in hepatocytes. These enzymes are of importance in assessing and monitoring liver inflammation and necrosis which result in the release of both enzymes in circulation due to increased permeability of the cell membrane or breakdown of the cells (Talwer et al, 1989). Animals inoculated with BaV showed an increase in AST and ALT levels as demonstrated by Gonçalves et al (2008) for B. jarararaca in rats and Chaves et al (1992) for B. asper in mice. Moreover, liver damage was found in patients bitten by species of Crotalus genus (Barraviera et al, 1989; Barraviera et al, 1995b). Barraviera et al (1995b) showed that only in patients bitten by Crotalus hepatic lesion was present. Our results and that from Gonçalves et al (2008) and Chaves et al (1992) studies are in apparent disagree when compared with Barraviera et al (1995b). Despite the obvious subject difference, the victims studied by Barraviera et al (1995b) were from São Paulo state (in the Southeast region of the country) and were probably bitten by B. jararaca, B. alternatus or B. newiedii, while B. atrox is an important species found in the north region of Brazil and B. asper is found in Central America and in some regions of South America. The increase in AST levels is further evidence that Bothrops venom induces muscle damage together with the concomitant increase in plasma LDH, an indirect evidence of hemolysis, and CK levels.
It is possible that the observed effect is triggered by PLA2 action present in the venom and acts on the endogenous PLA2, present in the membrane of hepatocytes. Reactive oxygen species and toxins can interact with polyunsaturated fatty acids of membrane phospholipids of hepatocytes, increasing the degradation of these lipid membranes. The hepatocyte necrosis is common in acute lesions caused by toxins, their extent and location may be useful to assess the severity of the damage. Likewise, the proliferation of bile ducts and changes in hepatic circulation may help in differential diagnosis of various liver diseases. Changes in blood supply to the hepatocytes may also act as a propagator of liver damage. Some toxins, such as the metalloproteases present in the BaV, increased vascular permeability, since it acts on the basal membrane of the vessels, triggering prolongation of clotting time and changing the hepatic blood flow (Monteiro et al, 2001).
Overall, BaV induces significant systemic and local events. The acute phase reaction was characterized by superoxide production by migrated neutrophils, mast cell degranulation and phagocytosis by macrophages. The bleeding appears to be due to the presence of proteins, such as metalloproteases, that cleave the extracellular matrix components. In addition, BaV induces myonecrosis at site of its injection, and PLA2 seem to be the main protein responsible for this event. Still, BaV induces systemic alterations characterized by the release of urea, AST and ALT. This study extends the understanding of the pathogenesis of local and systemic actions induced by BaV an endemic snake from the north region of Brazil. Therefore, it opens new perspectives for therapeutic interventions and in seeking specific targets of the inflammatory reaction to treat local and systemic effects.
ACKNOWLEDGMENTS
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológco (CNPq) and Secretaria de Planejamento de Rondonia (SEPLAN-RO).
COMPETING INTERESTS
None declared.
REFERENCES
- Amorim MF, Bohm GM, Bonta IL. Envenenamento botrópico e crotálico. Contribuição para o estudo comparado das lesões. Mem Inst Butantan. 1951;23:63–108. [PubMed] [Google Scholar]
- Barraviera B, Bonjorno Jr JC, Arakaki D, Meira DA. A retrospective study of 40 victims of Crotalus snakebites. Analysis of the hepatic necrosis observed in one patient. Rev Soc Bras Med Trop. 1989;22:5–12. doi: 10.1590/s0037-86821989000100002. [DOI] [PubMed] [Google Scholar]
- Barraviera B, Coelho KY, Curi PR, Meira DA. Liver dysfunction in patients bitten by Crotalus durissus terrificus (Laurenti, 1768) snakes in Botucatu (State of São Paulo, Brazil) Rev Inst Med Trop Sao Paulo. 1995b;37:63–69. [PubMed] [Google Scholar]
- Barraviera B, Lomonte B, Tarkowski A, Hanson LA, Meira DA. Acute-phase reactions, including cytokines, in patients bitten by Bothrops spp. and Crotalus durissus terrificus in Brazil. J Venom Anim Toxins. 1995a;1:11–22. [Google Scholar]
- Borges CC, Sadahiro M, Santos MC. Aspectos epidemiológicos e clínicos dos acidentes ofídicos ocorridos nos municípios do Estado do Amazonas. Rev Soc Bras Med Trop. 1999;32:637–646. [PubMed] [Google Scholar]
- Chaves F, Gutiérrez JM, Brenes F. Pathological and biochemical changes induced in mice after intramuscular injection of venom from newborn specimens of the snake Bothrops asper (Terciopelo) Toxicon. 1992;30:1099–109. doi: 10.1016/0041-0101(92)90055-a. [DOI] [PubMed] [Google Scholar]
- Colombini M, Fernandes I, Cardoso DF, Moura-Da-Silva AM. Lachesis muta muta venom: immunological differences with Bothrops atrox venom and importance of specific antivenom therapy. Toxicon. 2001;39:711–719. doi: 10.1016/s0041-0101(00)00201-4. [DOI] [PubMed] [Google Scholar]
- Da Silva NM, Arruda EZ, Murakami YL, et al. Evaluation of three Brazilian antivenom ability to antagonize myonecrosis and hemorrhage induced by Bothrops snake venoms in a mouse model. Toxicon. 2007;50:196–205. doi: 10.1016/j.toxicon.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Escocard RDEC, Kanashiro MM, Petretski JH, et al. Neutrophils regulate the expression of cytokines, chemokines and nitric oxide synthase/nitric oxide in mice injected with Bothrops atrox venom. Immunobiology. 2006;211:37–46. doi: 10.1016/j.imbio.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Galvão Nascimento N, Sampaio MC, Amaral Olivo R, Teixeira CFP. Contribution of mast cells to the oedema induced by Bothrops moojeni snake venom and a pharmacological assessment of the inflammatory mediators involved. Toxicon. 2010;55:343–352. doi: 10.1016/j.toxicon.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Gonçalves DA, Silva EV, Graça FA, et al. In vivo effects of Bothrops jararaca venom on metabolic profile and on muscle protein metabolism in rats. Am J Trop Med Hyg. 2008;79:771–778. [PubMed] [Google Scholar]
- Guimarães AQ, Cruz-Höfling MA, Ferreira De Araújo PM, Bon C, Lôbo De Araújo A. Pharmacological and histopathological characterization of Bothrops lanceolatus (Fer de lance) venom-induced edema. Inflamm Res. 2004;53:284–291. doi: 10.1007/s00011-004-1258-0. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Escalante T, Rucavado A. Experimental pathophysiology of systemic alterations induced by Bothrops asper snake venom. Toxicon. 2009;54:976–987. doi: 10.1016/j.toxicon.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Lomonte B. Local tissue damage induced by Bothrops snake venoms: a review. Mem Inst Butantan. 1989;51:211–223. [Google Scholar]
- Gutiérrez JM, Ownby CL. Skeletal muscle degeneration induced by venom phospholipases A2: insights into the mechanisms of local and systemic myotoxicity. Toxicon. 2003;42:915–931. doi: 10.1016/j.toxicon.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Williams D, Wen FH, Warrell DA. Snakebite envenoming from a global perspective: towards an integrated approach. Toxicon. 2010;56:1223–1235. doi: 10.1016/j.toxicon.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Hati R, Mitra P, Sarker S, Bhattacharyya KK. Snake venom hemorragins. Crit Rev Toxicol. 1999;29:1–19. doi: 10.1080/10408449991349168. [DOI] [PubMed] [Google Scholar]
- Kamiguti AS, Sano-Martins IS. South American snake venom affecting haemostasis. J Toxicol Toxin Rev. 1995;14:359–374. [Google Scholar]
- Kanashiro MM, De Cássia M, Escocard R, et al. Biochemical and biological properties of phospholipases A2 from Bothrops atrox snake venom. Biochem Pharmacol. 2002;64:1179–1186. doi: 10.1016/s0006-2952(02)01288-1. [DOI] [PubMed] [Google Scholar]
- Kasturiratne A, Wickremasinghe AR, Silva N, et al. The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PloS Medicine. 2008;5:1591–1604. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwasniewski FH, Landgraf RG, Jancar S. Small bowel injury associated to allergy is triggered by platelet-activating factor, mast cells, neutrophils and protected by nitric oxide. Int Immunopharmacol. 2008;8:371–378. doi: 10.1016/j.intimp.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Linardi A, Rocha e Silva TA, Miyabara EH, et al. Histological and functional renal alterations caused by Bothrops alternatus snake venom: expression and activity of Na+/K+-ATPase. Biochim Biophys Acta. 20111810:895–906. doi: 10.1016/j.bbagen.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Mokri B, Engel AG. Duchenne dystrophy: electron microscopic findings pointing to a basic or early abnormality in the plasma membrane of the muscle fiber. Neurology. 1975;25:1111–1120. doi: 10.1212/wnl.25.12.1111. [DOI] [PubMed] [Google Scholar]
- Monteiro HAS, Silva IMSC, Martins AMC, Fonteles MC. Actions of Crotalus durissus terrificus venom and crotoxin on the isolated rat kidney. Braz J Med Biol Res. 2001;34:1347–1352. doi: 10.1590/s0100-879x2001001000017. [DOI] [PubMed] [Google Scholar]
- Nikai T, Mori N, Kishida M, Sugihara H, Tu AT. Isolation and biochemical characterization of hemorrhagic toxin from the venom of Crotalus atrox (western diamondback rattlesnake) Arch Biochem Biophys. 1984;231:309–319. doi: 10.1016/0003-9861(84)90393-x. [DOI] [PubMed] [Google Scholar]
- Pestronk A, Drachman DB, Adams RN. Treatment of ongoing experiment. Muscle Nerve. 1982;5:79–84. doi: 10.1002/mus.880050115. [DOI] [PubMed] [Google Scholar]
- Queiroz GP, Pessoa LA, Portaro FCV, Furtado MFD, Tambourgi DV. Interspecific variation in venom composition and toxicity of Brazilian snakes from Bothrops genus. Toxicon. 2008;52:842–851. doi: 10.1016/j.toxicon.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Setubal SS, Pontes AS, Furtado JL, Kayano AM, Stábeli RG, Zuliani JP. Effect of Bothrops alternatus snake venom on macrophage phagocytosis and superoxide production: participation of protein kinase C. J Venom Anim Toxins incl Trop Dis. 2011;17:430–441. [Google Scholar]
- Talwer GP, Scrivastava LM, Moudgil KD. 2nd ed. Prentice Hall of Indian; New Delhi: 1989. Textbook of Biochemistry and Human Biology. [Google Scholar]
- Teixeira C, Cury Y, Moreira V, Picolo G, Chaves F. Inflammation induced by Bothrops asper venom. Toxicon. 2009;54:988–997. doi: 10.1016/j.toxicon.2009.05.026. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Rabies and Envenomings. A Neglected Public Health Issue 2007, Geneva. http://www.who.int/neglected_diseases/diseases/snakebites/en/ Available. Accessed 08 May, 2012.
- Zamuner SR, Gutiérrez JM, Muscará MN, Teixeira AS, Teixeira CFP. Bothrops asper e Bothrops jaracaca snake venoms trigger microbicidal functions of peritoneal leukocytes in vivo. Toxicon. 2001;39:1505–1513. doi: 10.1016/s0041-0101(01)00123-4. [DOI] [PubMed] [Google Scholar]
- Zeni ALB, Becker A, Krug M, Albuquerque CAC. Histological and biochemical effects induced by sublethal doses of Bothrops jararacussu venom in mice. J Venom Anim Toxins incl Trop Dis. 2007;13:664–676. [Google Scholar]
- Zuliani JP, Fernandes CM, Zamuner SR, Gutiérrez JM, Teixeira CFP. Inflammatory events induced by Lys-49 and Asp-49 secretory phospholipases A2: Role of catalytic activity. Toxicon. 2005;5:335–346. doi: 10.1016/j.toxicon.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Zuliani JP, Freitas TA, Conceição IM, Kwasniewski FH. Tityus serrulatus venom increases vascular permeability in selected airway tissues in a mast cell-independent way. http://dx.doi.org/10.1016/j.etp.2011.08.010. Exp Toxicol Pathol. 2011 doi: 10.1016/j.etp.2011.08.010. in press. [DOI] [PubMed]