Abstract
The neurochemical changes underlying human emotions and social behavior are largely unknown. Here we report on the changes in the levels of two hypothalamic neuropeptides, hypocretin-1 (Hcrt-1) and melanin concentrating hormone (MCH), measured in the human amygdala. We show that Hcrt-1 levels are maximal during positive emotion, social interaction, and anger, behaviors that induce cataplexy in human narcoleptics. In contrast, MCH levels are minimal during social interaction, but are increased after eating. Both peptides are at minimal levels during periods of postoperative pain despite high levels of arousal. MCH levels increase at sleep onset, consistent with a role in sleep induction, whereas Hcrt-1 levels increase at wake onset, consistent with a role in wake induction. Levels of these two peptides in humans are not simply linked to arousal, but rather to specific emotions and state transitions. Other arousal systems may be similarly emotionally specialized.
Due to the link between the loss of hypocretin (Hcrt) neurons and human narcolepsy1-3, much work has focused on Hcrt’s putative role in maintaining waking. Its role in preventing cataplexy has remained unclear. Moreover, studies using Fos immunohistochemistry, Hcrt knockouts and Hcrt receptor blockers have suggested conflicting conclusions about the relation of Hcrt to food intake, anxiety, stress, positive vs. negative emotions and muscle tone increase vs. decrease4, 5. Some of these conflicts may stem from the limited temporal resolution of the Fos technique, which reflects activity occurring over the entire 2 h period prior to sacrifice. Others may be due to species differences and the brain reorganization that occurs in Hcrt mutant animals6.
Cataplexy, a sudden loss of muscle tone with maintained consciousness, is the unique symptom of narcolepsy. Triggers for cataplexy are strikingly species-specific. In human narcoleptics, cataplexy is most commonly triggered by laughter, next most commonly by angry interactions, less frequently by athletic exertion and rarely by eating, pain or anxiety7. In the narcoleptic sheep, cataplexy is most reliably triggered by the sound of dogs barking or by being knocked over, presumably anxiety-eliciting stimuli, but never by eating or by play behavior (our unpublished observations and 8). In narcoleptic dogs, cataplexy is triggered by eating favored foods or by “playful” running and pulling, never by aversive situations6. In narcoleptic cattle, aggressive behavior in the affected animal is the most frequent trigger9. In “narcoleptic” rats and mice, exploration and the eating of certain foods are the most reliable triggers of cataplexy10.
Although the activity of Hcrt cells may have a role in preventing cataplexy, these neurons are not solely active during behaviors associated with cataplexy, a symptom which typically happens less than once or twice a week in most human narcoleptics. In the normal rat, Hcrt neurons discharge at variable rates throughout the waking period11 and release of this peptide, measured in cats, varies considerably across the waking state12. Hcrt unit discharge rate, observed in rats is minimal in nonREM sleep. Although rates are low in REM sleep, occasional burst discharge of Hcrt cells is seen during this state11, 13.
The functional role of melanin-concentrating hormone (MCH) is also unclear. MCH is synthesized in non-Hcrt neurons which are anatomically intermixed with Hcrt neurons and these neurons are thought to interact 2, 14-16. Whereas most evidence suggests that Hcrt neurons are maximally active during waking, similar evidence suggests that MCH neurons are maximally active during sleep17, 18. The release of MCH across the sleep-wake cycle and its relation to waking behavior has not been measured in any species.
In the current paper, we first report on the levels of Hcrt-1 and MCH in the rat, showing that these levels are synchronously modulated in the amygdala and hypothalamus, increasing the likelihood that measurements of levels of these peptides in the human amygdala reflect release in widespread brain regions. We then report measurements of Hcrt-1 and MCH levels in the amygdala of human patients, each monitored continuously over several days during their complete range of spontaneous behaviors and with their assistance in quantifying their emotions. We find that maximal Hcrt levels occur during social interactions and subject-reported positive emotions or anger. Minimal Hcrt levels occur during pain and sleep. MCH levels are maximal at sleep onset and after eating. Minimal MCH levels occur during waking pain and during social interaction. This is the first study of neurotransmitter levels in relation to human emotions.
Results
Hcrt-1 and MCH levels in the rat brain
Hcrt neurons produce both Hcrt-1, which is stable and Hcrt-2, which is rapidly metabolized. Both peptides are derived from preprohypocretin19, 20. We first undertook studies of Hcrt-1 and MCH levels in 4 rats to determine whether there were strong regional differences between levels in the hypothalamus, the location of all Hcrt and most MCH cell bodies, and the amygdala, one of the many brain regions innervated by collaterals of Hcrt and MCH axons and the region sampled in all of our human subjects, as dictated by clinical considerations.
In the rat, average waking Hcrt-1 concentrations were 0.11±0.02 fmol/10 μl in the hypothalamus and 0.06±0.01 fmol/10 μl in the amygdala. Average waking MCH concentrations were 0.10±0.03 fmol/10 μl in the hypothalamus and 0.10±0.03 fmol/10 μl in the amygdala. Hcrt-1 levels in the hypothalamus were highly correlated with Hcrt-1 levels in the amygdala. MCH levels in the hypothalamus were also highly correlated with MCH levels in the amygdala (Table 1).
Table 1.
Rat number and correlation of Hcrt and MCH levels in the hypothalamus and amygdala.
| Correlation of Hcrt in Hypothalamus and Amygdala | Correlation of MCH in Hypothalamus and Amygdala | |||||
|---|---|---|---|---|---|---|
| Number of XY Pairs | R Value | P Value | Number of XY Pairs | R Value | P Value | |
| Rat 1 | 235 | 0.6575* | <0.0001 | 219 | 0.2372* | <0.0004 |
| Rat 2 | 244 | 0.7264* | <0.0001 | 243 | 0.6909* | <0.0001 |
| Rat 3 | 246 | 0.5924* | <0.0001 | 246 | 0.4904* | <0.0001 |
| Rat 4 | 256 | 0.4982* | <0.0001 | 256 | 0.2757* | <0.0001 |
Statistical analysis using Pearson product-moment correlation coefficient.
In all rats, Hcrt levels in the hypothalamus were significantly correlated with levels in the amygdala. MCH levels in the hypothalamus were also significantly correlated with MCH levels in the amygdala. Potential sources of variability in peptide level assessments include differences in placement of electrode within target region, protein deposition on the dialysis membrane and noise in RIA measurement of peptide levels.
Microdialysis setup in human patients
All eight patients were psychologically normal and had seizures of suspected unilateral temporal lobe origin. Patients were implanted with bilateral probes to localize the side which contained the primary seizure focus, for subsequent surgical removal. Using angiography and magnetic resonance imaging, electrode trajectory was adjusted to avoid major blood vessels. Electrodes generally passed though the temporalis muscle and dialysis probes were in the amygdala (Fig. 1). Some patients reported episodic pain localized to the muscle, whereas others did not have significant pain. Microdialysis experiments were performed between 2 and 5 days after electrode implantation. Only one patient had a seizure during the period in which we collected dialysate. No data from the 5 h period after seizure onset was used in the current study. Primary seizure foci were found to be in ipsilateral or contralateral cortical regions (See Table 2).
Figure 1.
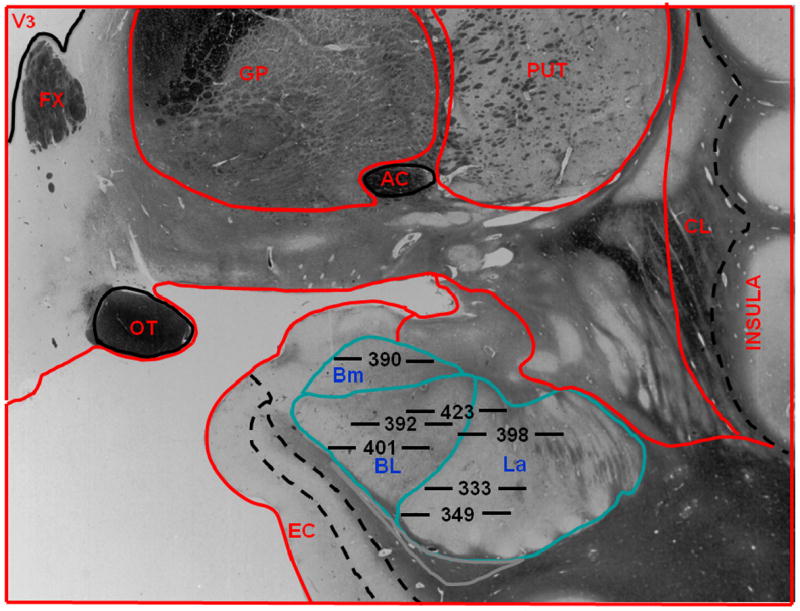
Location of microdialysis probes in human subjects. Placements of membranes at the tip of each probe are labeled by subject number. Horizontal lines on either side of each subject number are to scale, and their total length indicates the 5.0 mm area sampled by each membrane. The position of each membrane in the amygdala is drawn to scale. The anatomic outline graphic was adapted from the human brain atlas by Mai et al.58. Bm refers to basomedial nucleus of the amygdala; BL, basolateral nucleus of the amygdala, La, lateral nucleus of the amygdala; V3, third ventricle, FX, fornix; GP, globus pallidus; PUT, putamen; CL, claustrum; EC, entorhinal cortex; OT, optic tract; AC, anterior commissure.
Table 2.
Patient information and parameters of implanted electrodes.
| Patient | Patient ID | Probe location | Sex | Age | Number of samples (W,S) | Seizure Locus | Resection |
|---|---|---|---|---|---|---|---|
| 1 | d333 | LA | M | 46 | 31,27 | LTL | LTL |
| 2 | d349 | LA | F | 37 | 45,13 | RTL | RTL |
| 3 | d390 | LA | M | 20 | 98,6 | Bilateral | Not a surgical candidate |
| 4 | d392 | RA, LA | M | 23 | 104,28 | LTL | LTL |
| 5 | d398 | RA, LA | F | 41 | 106,47 | Not localizable, but excluded mesial temporal areas | Needs further monitoring |
| 6 | d401 | LA | M | 29 | 184,16 | Right hemisphere | Needs further monitoring |
| 7 | d423 | LA | F | 53 | 60,18 | LTL | LTL |
| 8 | d378 | LA | F | 39 | 37,14 | LTL | LTL |
In all cases, pre-surgical MRIs did not reveal abnormalities on either side sufficient to localize the pathology. None of the amygdalae from which we sampled exhibited seizure activity during the period of microdialysis sampling. In all of the patients, the amygdala was found to not be an independent seizure focus. Mesial temporal areas were later excised in 5 of the 8 patients studied.
Patient number, identification number (ID), probe location, sex, age, number of samples in waking and sleep (W, S), location of seizure focus and brain area resected following removal of electrodes (if resection was performed) are listed in the table. LA=left amygdala, RA=right amygdala, LTL=left temporal lobe, RTL=right temporal lobe. Patient 8 (Fig. 4) had a Hospal membrane and was not used in our statistical analyses. The other 7 patients, used in our statistical analyses, had Cuprophan membranes. All patients were withdrawn from seizure medication. Resection status reflects conditions 1 year after implantation.
Continuously recorded videos were scored for TV watching, social interactions (talking to physicians, nursing staff or family), eating, pain reports, clinical manipulations and sleep-wake transitions. Notes of activities were made throughout the study, every 15 min in synchrony with our 15 min microdialysis sample collection interval, by an experimenter in the patients’ room. The subjects rated their moods and attitudes on our questionnaire, administered every hour during waking (see Supplementary Methods).
Hcrt-1 and MCH levels in the human brain
Average waking (Awake, Fig. 2) Hcrt-1 concentration in samples from the amygdala of humans was 0.16±0.01 fmol/15 μl. Average waking MCH concentration was 0.09±0.01 fmol/15 μl. We compared levels in our 15 min microdialysis samples from the left and right amygdala of two patients to determine if changes in the levels of these peptides were bilaterally correlated. Hcrt-1 levels in the right and left amygdala were significantly correlated in the 24 h period monitored in both patients, as were MCH levels (Patient 4; Hcrt-1, r=0.81, p=0.0001, df=77; MCH, r=0.40, p=0.0003, df=77; Patient 5: Hcrt-1, r=0.60, p=0.0001, df=76; MCH, r=0.22, p=0.048, df=76, Pearson product-moment correlation coefficient). All the data presented below were derived from the left amygdala.
Figure 2.
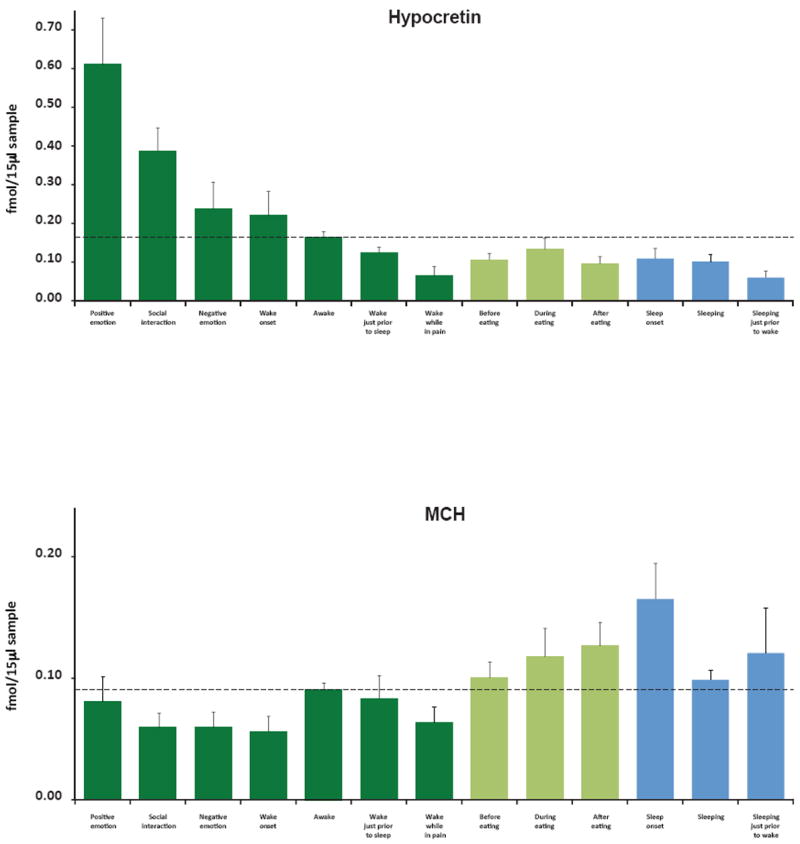
Hcrt and MCH levels across waking and sleep activities. (A) Maximal Hcrt levels in waking are seen during positive emotions, social interactions and awakening, minimal levels are seen prior to sleep and while reporting pain. Changes during and after eating are smaller than those during monitored non-eating related activities. Waking values in shades of green, sleep in blue. Awake indicates samples in which subjects were awake but were not exhibiting social interaction or reporting emotion. (B) Maximal MCH levels are seen at sleep onset and after eating. Minimal levels are seen during wake onset, social interaction and pain. Error bars represent ±SEM.
Hcrt-1 and MCH levels correlate with emotional state
After log transformation, one-way ANOVA of peptide levels measured during the 13 behaviors evaluated showed significant differences for both Hcrt-1 (F=7.4, p<0.0001) and MCH (F=3.4, p<0.001). The number of observations for the ANOVAs and Fisher’s Least Significant Difference post-hoc comparisons are provided in Table 3. We found that high levels of Hcrt-1 in humans consistently occurred during social interactions (p<0.002), comparing the mean Hcrt-1 levels during social interaction with awake levels (awake=waking without social interaction or emotion reports; Fig.2A, Fig.3D, Fig.4). Waking periods with positive emotion had significantly higher levels than waking periods with negative emotion (p<0.02; Fig 2A).
Table 3.
Measurements of Hcrt and MCH levels across behaviors.
| Positive emotion | Social interaction | Negative emotion | Wake onset | Awake | Awake just prior to sleep | Awake while In pain | Before eating | During eating | After eating | Sleep onset | Sleep | Sleep just prior to wake | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hcrt | mean | 0.611 | 0.388 | 0.237 | 0.220 | 0.164 | 0.124 | 0.064 | 0.106 | 0.133 | 0.096 | 0.109 | 0.101 | 0.060 |
| SEM | 0.120 | 0.059 | 0.069 | 0.062 | 0.014 | 0.014 | 0.024 | 0.017 | 0.028 | 0.018 | 0.026 | 0.017 | 0.017 | |
| MCH | mean | 0.081 | 0.059 | 0.059 | 0.056 | 0.091 | 0.083 | 0.063 | 0.100 | 0.118 | 0.127 | 0.165 | 0.099 | 0.121 |
| SEM | 0.021 | 0.012 | 0.013 | 0.012 | 0.006 | 0.019 | 0.013 | 0.013 | 0.023 | 0.020 | 0.029 | 0.008 | 0.037 | |
| n | 21 | 90 | 21 | 19 | 383 | 16 | 16 | 77 | 51 | 75 | 17 | 149 | 14 |
Data plotted in Fig 2.
Figure 3.
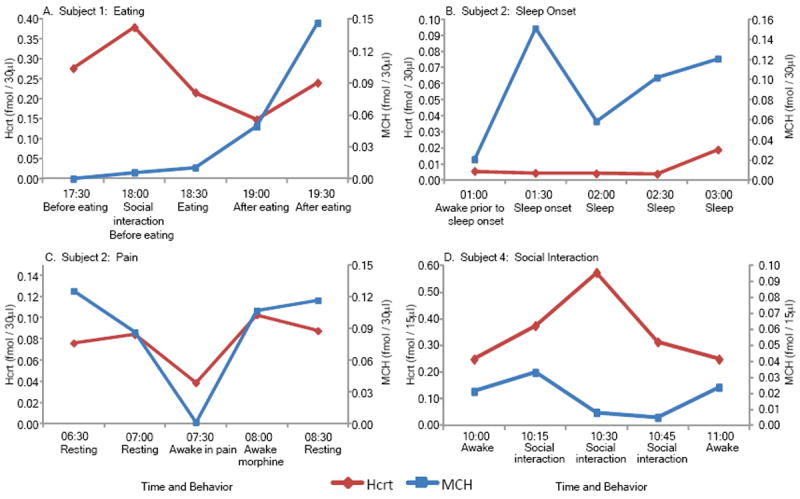
Examples of single case raw data on Hcrt and MCH release from individual patients. Release pattern over time shows that (A) MCH levels increase after eating, (B) MCH levels increase at sleep onset, (C) both Hcrt and MCH levels are low during pain and (D) Hcrt levels increase with social interaction. With the exception of the example shown in C, all data in this study were collected during morphine-free periods. See figure 2 for average levels in each condition.
Figure 4.
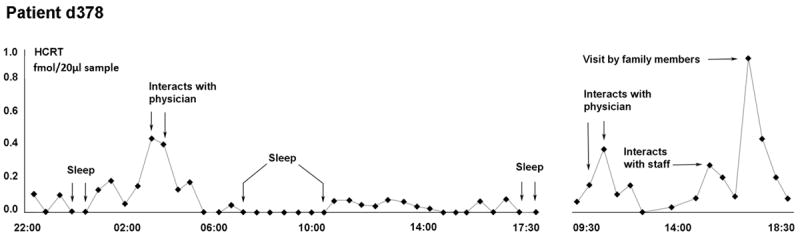
Time course of Hcrt release over a 20 h period in patient d378. A Hospal dialysis membrane was used in this subject who was therefore not used in the statistical comparisons with the other 7 subjects. However the results with these two membrane types were indistinguishable. Hcrt release is minimal during sleep and maximal during periods of social interaction.
Average Hcrt-1 levels in the 15 min period of wake onset (Fig 2A) were significantly higher than levels during sleep (p<0.01) and during the 15 min period prior to sleep (p<0.05). Surprisingly, average Hcrt-1 values during periods of waking pain were as low as sleep values (Fig. 2A, Fig 3C, Table 3).
MCH levels were significantly increased after eating compared to awake levels (p<0.01; Fig 2B, Fig3A). We found an interesting dissociation between Hcrt-1 and MCH levels at sleep onset. Hcrt-1 levels did not significantly change at sleep onset because Hcrt-1 levels were reduced prior to sleep onset and remained below awake levels throughout sleep (p<0.01, Fig. 2A). Conversely, MCH level was maximal at sleep onset (Fig. 2B, Fig 3B) and this value significantly exceeded wake onset levels (p<0.01).
We found a significant negative relation between MCH levels and the level of questionnaire-indicated pain (r=-0.32, df=45, p=0.03). There was also a significant positive correlation between Hcrt-1 levels and the level of questionnaire-indicated anger (r=0.49, df=45, p=0.043), consistent with anger being a common trigger of human cataplexy7.
Discussion
In the current study, we found that Hcrt-1 level is not a simple function of arousal. Hcrt-1 levels were higher when subjects showed laughter or excitement than when subjects reported feeling frustrated or sad. Furthermore, we found that Hcrt levels were low during waking pain. Allowing for species differences, these human data bear considerable resemblance to data on Hcrt cell activity in animals. Our prior studies of Fos expression, an indication of elevated rates of neuronal discharge, in Hcrt neurons of wild type mice found that these neurons are maximally active during performance of rewarded behaviors5 and that Hcrt knockout mice were strikingly deficient in staying awake to perform rewarded behaviors. Two other studies reported a similar involvement of Hcrt neurons in positive reinforcement21, 22. Our studies of Fos expression in wild type mice also showed that Hcrt neurons were not activated beyond baseline levels during foot shock, or foot shock avoidance behavior, despite high levels of EEG arousal5. We have also reported that Hcrt unit activity in rats is suppressed in novel situations eliciting withdrawal, despite maximal levels of EEG activation. In contrast, Hcrt unit activity is high during grooming and exploration11. In studies in normal dogs, we found that treadmill running does not elevate Hcrt level, whereas play behavior of similar cardiovascular intensity greatly increases Hcrt level23, 24. Our prior studies of Fos expression in animals in combination with our current results in humans suggest the Hcrt system promotes arousal associated with positive emotion and anger, rather than arousal in general.
The amygdala is known to be a vital structure in sleep regulation25-30. We have found sleep active and cataplexy active neurons in the amygdala of narcoleptic dogs25 and evidence that pathology in the amygdala is linked to the symptoms of canine narcolepsy29. However, the pattern of Hcrt-1 release that we see in the amygdala is unlikely to be restricted to the amygdala. The branching pattern of individual Hcrt neurons31 suggests that a similar pattern of release is likely to be observed in all target regions. In prior investigations of arousal related systems with similar widely branching axons, we and others have found, for example, that serotonin, norepinephrine and dopamine release in the spinal cord are highly correlated with release in the brainstem32. In a prior study we have found that Hcrt release follows the same profile in the hypothalamus and the brainstem locus coeruleus12, and in the current study we see the same pattern in the amygdala and hypothalamus of rats.
Humans who have attempted suicide have reduced levels of Hcrt-1 in their cerebrospinal fluid33. Furthermore, a study of the performance of depressed humans in a structured task found that although they work as hard as non-depressed individuals to avoid loss of money, they do not work as hard as controls to acquire additional money34. This parallels our animal studies showing activation of Hcrt neurons during positively motivated tasks, but not during negatively motivated tasks5. The current results, in concert with these prior animal and human studies suggest that depression and reported difficulties with social interaction in narcolepsy and Parkinson’s 35 may be related to the loss of Hcrt cells in both of these conditions2, 14, 36. However, we cannot eliminate the indirect role of the disability that accompanies these disorders in generating depression in humans. Our data suggest that Hcrt antagonism may be a risk factor for depression and that Hcrt supplementation may have antidepressant effects.
We found that Hcrt levels do not decrease significantly at sleep onset. This suggests that a reduction in Hcrt-1 level may be linked to the quiet waking that precedes sleep, rather than being directly linked to sleep onset. Furthermore, our findings of low levels of Hcrt during pain in humans and low levels of Hcrt unit activity during anxiety in rats and mice5, 11 suggest that Hcrt antagonists, now being developed as hypnotic agents37, may not be effective in sleep induction in insomnia resulting from these aversive conditions, since the current data suggest that activity of the Hcrt system is already minimal at these times. On the other hand, our finding that MCH levels are maximal at sleep onset suggests that stimulation of the MCH system may alleviate insomnia.
Although we did not find that Hcrt-1 levels significantly decreased at sleep onset, Hcrt-1 levels showed a substantial increase at awakening. This is consistent with our prior work in rats showing that Hcrt neurons burst prior to awakening11 and with the finding that optogenetic activation of Hcrt cells can induce waking in mice38. Increased activity at wake onset, however, is not confined to Hcrt neurons, but is also seen in medial reticular39 noradrenergic, serotonergic40, histaminergic and other neurons39.
The concept of an ascending activating system and the subsequent realization that there are several components to this system led to suggestions that these systems worked in concert and redundantly, i.e. that cholinergic, adrenergic, histaminergic and hypocretinergic systems were active simultaneously to sustain waking 41. The current results suggest the existence of a substantial level of specialization for the Hcrt system and perhaps for other traditionally designated “arousal systems.” The minimal level of Hcrt-1 during pain indicates that other, as yet unidentified, systems must be responsible for pain-induced arousal in humans. Narcoleptics have been reported to be hyperalgesic42.
A large amount of data has suggested, but not proved, a reciprocal relationship between the activity of MCH cells and Hcrt cells15, 43-45. Our data are not inconsistent with the general idea of a reciprocal relationship. We see high levels of Hcrt-1 in relation to social activity and emotion, particularly positive emotion. We see minimal levels of MCH during emotion and during social interaction. However, our data show that the levels of these peptides are not always inversely related. The levels of both are minimal in pain. The major increase in MCH at sleep onset is not matched by a major decrease in Hcrt-1. Conversely the major increase in Hcrt-1 levels at wake onset is not matched by a major decrease in MCH levels. The activity of these peptides also differed after food consumption with a significant increase in MCH levels but no significant change in Hcrt-1 levels. A considerable amount of work has suggested that Hcrt release is linked to food intake20, but some recent work has raised doubts about the specificity of this relation4, 23, 46. In the current study we have directly compared the effects of eating with the effects of mood on Hcrt-1 level in humans and find that the latter has a far greater effect on Hcrt-1 levels.
Fos studies have produced conflicting conclusions regarding the determinants of Hcrt cell activation. When stress lasting for 5-30 minutes was followed by release back into the home cage, Fos was expressed in Hcrt neurons47. However, when stress was maintained for the entire 2h incubation period for Fos expression, little or no Fos expression was observed in Hcrt neurons48. Furlong et al.49 concluded that Fos expression in Hcrt neurons was maximal during exploration, consistent with our prior data on Hcrt unit activity in the rat11. These data suggest that the increased Fos expression in Hcrt neurons reported in a few papers with restraint, shock or “panic”50 may be due to increased waking and grooming subsequent to the aversive stimulus, compared to controls, rather than the aversive situation itself. Furthermore, studies of cerebrospinal fluid retrieved from the lumbar cistern50 are difficult to interpret since lumbar CSF levels represent an average of peptide release over the prior 6 hours.
Laughter, social interaction, and anger are the three most frequent triggers of human cataplexy, the most debilitating symptom of narcolepsy in many patients7. Animal studies cannot shed much light on why this is so, since important aspects of these behaviors are qualitatively unique to humans. Our current findings show that a common element in these three behaviors in non-narcoleptic humans is a surge of hypocretin levels, a peptide that is pathologically deficient in those who have cataplexy, i.e. narcoleptics1, 2.
Relatively little work has been done on the behavioral correlates of MCH cell activity. Studies with sleep deprivation and sleep rebound44 suggest that MCH activity may promote sleep in accordance with the current findings. However, these cells have not yet been studied during the performance of operant tasks in waking that might allow comparison of their discharge with Hcrt cells.
In summary we found that Hcrt-1 level, as assayed in the amygdala, was not linked to arousal per se, but was maximal during positive emotions, anger, social interaction and awakening in humans. Conditions that trigger cataplexy and high Hcrt-1 levels may be those that elicit approach. In contrast, we found that conditions associated with minimal Hcrt levels are those eliciting withdrawal, such as pain, or in the case of our prior animal work, anxiety5, 11. In contrast, MCH levels were maximal during sleep onset and minimal during social interactions. Together these results suggest a previously unappreciated emotional specificity in the activation of arousal and sleep induction systems in humans. Abnormalities in the pattern of activation of these systems may contribute to a number of psychiatric disorders.
Methods
Rat studies
All animal studies followed NIH guidelines and were approved by the Institutional Animal Care and Use Committee of the VA Greater Los Angeles Healthcare System. Rats were anaesthetized with a ketamine/xylazine injection (100 mg/kg and 15 mg/kg, i.p.). Anesthesia was maintained with 0.5-1.0% isoflurane. Using a stereotaxic frame under aseptic conditions, 2 guide cannulae were implanted in each rat. The cannulae tips were directly (0.5 mm) above the right hypothalamus (perifornical area; A -3.2, L -1.6, H -7.5) and left amygdala (central nucleus; A -2.5, L 4.1, H -7). Stainless steel screws were implanted bilaterally over the frontal and parietal areas for EEG recording, and EMG was recorded with stainless steel wires inserted into the dorsal neck muscles.
Microdialysis probes with 2 mm membranes (Eicom probes C-I-14-02, Hospal membrane, 40 kDa cut-off) were inserted through the guide cannulae (AG-14, Eicom) into the target areas. 1.0 mm Teflon tubing (JT-10-100, Eicom) was connected to the inflow and outflow portions of the probe. aCSF (Harvard Apparatus, MA, USA) with 0.025% rat serum albumin (Sigma) was continuously perfused at 2 μl/min using a syringe pump (EPS-64, Eicom). Samples were collected every 5 minutes using a fraction collector with an electronic cooler (EFC-82 and EFR-82, Eicom). Samples were stored at -80°C until analyzed with radioimmunoassay (RIA). Each sample was analyzed for both peptides by sequential multiple antigen solid-phase radioimmunoassay (RIA)51 providing IC50/limit of detection of 0.6/0.025 fmol and 1.5/0.025 fmol for Hcrt-1 and MCH, respectively. To determine probe recovery, a probe similar to the ones used in this study (2 mm membrane, C-I-14-02, Eicom) was placed in solutions of 0.1 nM, 1 nM and 10 nM unlabeled hypocretin-1 (Hcrt-1) and melanin concentrating hormone (MCH) (Phoenix Pharmaceuticals) in aCSF and 0.025% RSA and perfused at a rate of 2 μl/min. The in vitro recovery of Hcrt-1 and MCH across a 2.0mm long membrane was 1.5 +/- 0.2% for Hcrt-1 (n=4) and 3.5 +/- 0.8% for MCH (n=4). Measured concentrations can be assumed to reflect changes in release as well as any changes in the metabolic breakdown of the peptides.
Human studies
Eight patients (Table 2), diagnosed with pharmacologically resistant epilepsy were studied. These patients required implantation of depth electrodes to identify the brain area generating their seizures because pre-surgical diagnostic evaluation with scalp EEG, MRI and PET scans had localized no anatomically or functionally abnormal site for curative surgical resection. All patients gave their informed consent for participation in this study, and the protocol was approved by the UCLA Medical School Institutional Review Board. Because the area in the brain generating seizure activity is usually quite limited, in our experience, identification and subsequent removal of the seizure focus renders the great majority of patients free from seizures for the remainder of their lives 52. After the surgical implant the subjects recovered in the Neurological Intensive Care Unit for approximately 24 hours, and they were then transferred to the Medical-Surgical Specialty Unit where the studies were done.
The depth EEG electrode was 1.25 mm in diameter and consisted of MRI-compatible, flexible, polyurethane probes with six or seven 1.5-mm-wide platinum contacts with intercontact separations of 1.5 to 4 mm (Fig. 5a). It was stereotaxically inserted under general anesthesia through temporal lobe cortex into the amygdala (Fig. 5b, Fig 1). The lumen of the EEG electrode allowed insertion of the microdialysis probe. The microdialysis probe consisted of two fused silica tubes used for inflow (105 and 40 μm outer diameter (OD) and inner diameter (ID) respectively; length 39 cm) and outflow (150 and 75 μm OD, ID; length 39 cm) (Fig. 5a). The tubes were inserted into a 20 mm long Cuprophan (12 kD cutoff, 220 μm OD, 200 μm ID) membrane (made by Membrana GmbH, Wuppertal, Germany), or into a Hospal membrane in the case of patient d378. Dialysis membranes extended 5 mm beyond the EEG probe (Ad-Tech Medical Instrument Corp BF-7 Macro Depth Electrode) and connected to a fraction collector via fused silica tubing (Polymicro Technologies, 375 and 150 μm OD and ID; length 150 cm). A mini-pump (CMA 102) was used to perfuse sterile phosphate-buffered aCSF through the probes at a flow rate of 1 μl/min. Samples were collected continuously at 15 min intervals (30 min for patient d378) for a period of 2-4 days and stored at -80° C. The in vitro recovery of [125I] Hcrt-1 with a Cuprophan membrane constructed in the same manner as those used in this study was approximately 4.5%. The recovery of Hcrt-1 and MCH as measured by RIA with the Cuprophan probe was 2.4 +/- 0.7% for Hcrt-1 (n=10) and 1.4 +/- 0.3% for MCH (n=10). The actual probes used in the patients of this study could not be tested for peptide recovery as residual peptides could trigger an immune response when implanted into the brain. These probes were tested for glutamate and aspartate or monoamines and average recovery was found to be 29% for aspartate, 31% for glutamate and 23%, 27%, and 45% for norepinephrine, dopamine, and serotonin, respectively. Our early work establishing the feasibility of measuring peptides with microdialysis51 demonstrated that the thin-wall thickness version of the Cuprophan membrane was the optimal membrane for the current studies. A 12kDA cut-off is above the molecular weight of both peptides studied here (3,561 and 2,387 for Hcrt-1 and MCH, respectively).
Figure 5.
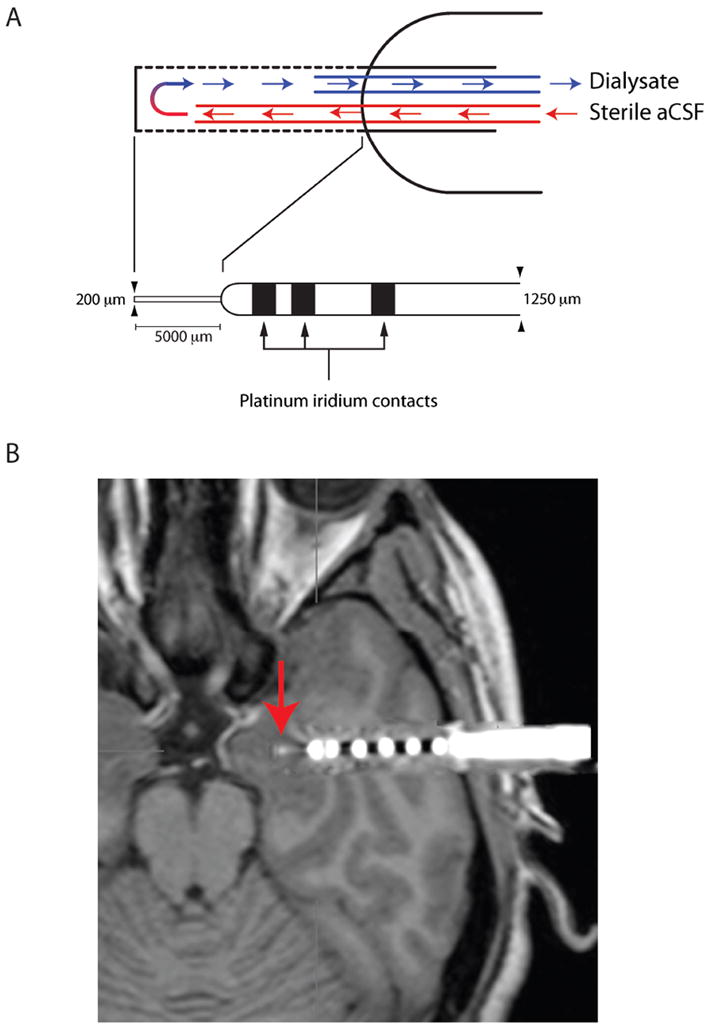
Design and placement of electrodes. (A) Electrode contained external contacts for localizing seizure focus and internal space for a 200μm diameter microdialysis membrane. The membrane protruded 5 mm from the outer cannula. aCSF flowed in through a 105μm outer diameter silica tube and flowed out of the Cuprophan membrane through a 150μm outer diameter silica tube. Dialysates were collected at 15 min intervals and immediately frozen to -80 degrees. Samples were subsequently analyzed for both Hcrt-1 and MCH using our multiple antigen solid state RIA51. (B) Image of implanted probe in the amygdala (metal contacts produce MRI artifact). CT image of electrode was superimposed on MRI after computer registration (alignment in 3 dimensions) of the two scans.
The statistical analyses presented above included only the seven patients implanted with the Cuprophan membranes (six in the case where one patient did not have data on an examined parameter). The results with the Hospal membrane (Fig. 4) were similar to those found with the Cuprophan membrane. A total of 843 15-min samples were collected and analyzed for both peptides (Table 2).
The microdialysis procedure was also being used in these patients to sample neurotransmitter levels prior to and during seizures to better understand the seizure initiation process and its variation across patients52-56. We found that Hcrt and MCH levels assayed with our Cuprophan membrane were stable for as long as 4 days. We have been able to measure glutamate and glutamine with these electrodes for up to 6 days 57.
Daytime light levels averaged 1000 lux and nighttime light levels were reduced to 50 lux. For the analysis of sleep onset, the pure wake sample preceding the transition and the following sample containing a transition to sleep were analyzed. For the analysis of wake onset, the pure sleep sample preceding the transition and the following sample containing a transition to wake were analyzed. State transitions were determined behaviorally. For analysis of the eating data, 60 minutes prior to and after eating were averaged to calculate peptide levels before and after eating. “During eating” included all samples which contained periods of eating. Eating periods ranged from 15-60 minutes. “Awake” samples were those not assigned to social interaction, emotion or sleep categories.
During the period of observation the subjects were free to walk about the room and hallways, watch television, play video games, make phone calls and receive visitors. However, most of our observations, including mood assays, eating and sleeping observations were made with the patients in bed or seated next to their bed. Our questionnaire was given to the subjects during waking at hourly intervals synchronized with the corresponding 15 min aliquot.
Behavior was scored as social interaction if the patient talked with another person or persons for at least one-third of the 15 min sample duration. Samples were scored for emotion if the patient stated a positive or negative emotion or openly and objectively showed some emotional expression such as laughing or crying at least once during the sample period. Positive emotion included laughing or excitement (example: watching a sports game and seeing the favored team score a home run) and negative emotion included sadness or frustration. In four patients, mood was also assessed with the questionnaire (Supplementary Methods), presented at hourly intervals. All behavioral judgments were recorded prior to analysis of the samples.
The samples used in the present study were all derived from amygdala electrodes that physiological analysis determined not to be an independent seizure focus. All data were collected during periods free of seizures.
Statistical analysis
Data with multiple comparisons were analyzed with one-way ANOVA and Fisher’s Least Significant Difference post-hoc comparisons after normalization and log transformation (GB-Stat, Silver spring, MD). Table 3 presents the number of samples for each category along with means and standard errors. Correlation studies of anger and pain were calculated across trials. Statistical significance of all findings was determined by p<0.05.
Supplementary Material
Acknowledgments
These studies were supported by NIH Grants MH064109, NS14610, NS33310, NS02808 and the Medical Research Service of the Department of Veterans Affairs. We thank Joyel Alamajano, Grace Barber, Josephine Ruidera, and Sandeep Sood for assistance with sample collection and Larry Ackerson, Tony Fields, Jo Hsieh, Yuval Nir, Ronald McGregor, Lalini Ramanathan and M.F. Wu for technical assistance.
Footnotes
Author contributions
AMB, IF, CLW and JMS designed the experiment; IF performed the neurosurgery; AMB, RJS, EJB, KÆK, JLL and JMS ran the experiment; HAL, AMB and NTM performed the RIA analyses; AMB, CLW and JMS wrote the paper.
Additional information
Supplementary information accompanies this paper at http://www.nature.com/naturecommunications
Competing financial interests: The authors declare no competing financial interests
Reference List
- 1.Peyron C, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 2.Thannickal TC, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu M, et al. Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice. J Neurosci. 2002;31:6028–6040. doi: 10.1523/JNEUROSCI.6069-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel JM. Hypocretin (orexin): role in normal behavior and neuropathology. Annual Rev of Psychol. 2004;55:125–148. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGregor R, Wu M-F, Barber G, Ramanathan L, Siegel JM. Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement vs. operant avoidance and light level. Journal of Neuroscience. 2011;31:15455–15467. doi: 10.1523/JNEUROSCI.4017-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu MF, Nienhuis R, Maidment N, Lam HA, Siegel JM. Role of the hypocretin (orexin) receptor 2 (Hcrt-r2) in the regulation of hypocretin level and cataplexy. J Neurosci. 2011;31:6305–6310. doi: 10.1523/JNEUROSCI.0365-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anic-Labat S, et al. Validation of a cataplexy questionnaire in 983 sleep-disorders patients. Sleep. 1999;22:77–87. [PubMed] [Google Scholar]
- 8.White EC, de Lahunta A. Narcolepsy in a ram lamb. Vet Rec. 2001;149:156–157. doi: 10.1136/vr.149.5.156. [DOI] [PubMed] [Google Scholar]
- 9.Strain GM, Olcott BM, Archer RM, McClintock BK. Narcolepsy in a Brahman bull. J Am Vet Med Assoc. 1984;185:538–541. [PubMed] [Google Scholar]
- 10.Willie JT, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 11.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiyashchenko LI, et al. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) and melanin concentrating hormone loss and the symptoms of Parkinson’s disease. Brain. 2008;131:e87. doi: 10.1093/brain/awm221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thannickal TC, Nienhuis R, Siegel JM. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep. 2009;32:993–998. doi: 10.1093/sleep/32.8.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagos P, Torterolo P, Jantos H, Chase MH, Monti JM. Effects on sleep of melanin-concentrating hormone (MCH) microinjections into the dorsal raphe nucleus. Brain Research. 2009;1265:103–110. doi: 10.1016/j.brainres.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi PH. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur J Neurosci. 2003;18:1627–1639. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- 18.Lagos P, Monti JM, Jantos H, Torterolo P. Microinjection of the melanin-concentrating hormone into the lateral basal forebrain increases REM sleep and reduces wakefulness in the rat. Life Sciences. 2012;90:895–899. doi: 10.1016/j.lfs.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 19.De Lecea L, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakurai T, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 21.Borgland SL, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. Journal of Neuroscience. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharf R, et al. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. 2010:753–760. doi: 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu MF, John J, Maidment N, Lam HA, Siegel JM. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1079–R1086. doi: 10.1152/ajpregu.00207.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu MF, Nienhuis R, Maidment N, Lam HA, Siegel JM. Cerebrospinal fluid hypocretin (orexin) levels are elevated by play but are not raised by exercise and its associated heart rate, blood pressure, respiration or body temperature changes. Arch ital Biol. 2011;149:492–498. doi: 10.4449/aib.v149i4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulyani S, Wu M-F, Nienhuis R, John J, Siegel JM. Cataplexy-related neurons in the amygdala of the narcoleptic dog. Neuroscience. 2002;112:355–365. doi: 10.1016/s0306-4522(02)00089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khatami R, Birkmann S, Bassetti CL. Amygdala dysfunction in narcolepsy-cataplexy. J Sleep Res. 2007;16:226–229. doi: 10.1111/j.1365-2869.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, et al. Antagonizing corticotropin-releasing factor in the central nucleus of the amygdala attenuates fear-induced reductions in sleep but not freezing. Sleep. 2011;34:1539–1549. doi: 10.5665/sleep.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nofzinger EA, et al. Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain. 2002;125:1105–1115. doi: 10.1093/brain/awf103. [DOI] [PubMed] [Google Scholar]
- 29.Siegel JM, et al. Neuronal degeneration in canine narcolepsy. J Neurosci. 1999;19:248–257. doi: 10.1523/JNEUROSCI.19-01-00248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der HE, et al. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr Biol. 2011;21:2029–2032. doi: 10.1016/j.cub.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Espana RA, Reis KM, Valentino RJ, Berridge CW. Organization of hypocretin/orexin efferents to locus coeruleus and basal forebrain arousal-related structures. J Comp Neurol. 2005;481:160–178. doi: 10.1002/cne.20369. [DOI] [PubMed] [Google Scholar]
- 32.Lai YY, Kodama T, Siegel JM. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis study. J Neurosci. 2001;21:7384–7391. doi: 10.1523/JNEUROSCI.21-18-07384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brundin L, Bjorkqvist M, Traskman-Bendz L, Petersen A. Increased orexin levels in the cerebrospinal fluid the first year after a suicide attempt. J Affect Disord. 2009;113:179–182. doi: 10.1016/j.jad.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cognition and Emotion. 2000;14:721–724. [Google Scholar]
- 35.Bayard S, et al. Decision making in narcolepsy with cataplexy. Sleep. 2011;34:99–104. doi: 10.1093/sleep/34.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thannickal TC, Siegel JM. Hypocretin pathology in human narcolepsy. In: Bassetti CL, Billiard M, Mignot E, editors. Narcolepsy and Hypersomnia. Informa Healthcare; New York: 2007. pp. 301–305. [Google Scholar]
- 37.Winrow CJ, et al. Pharmacological characterization of MK-6096 - a dual orexin receptor antagonist for insomnia. Neuropharmacology. 2012;62:978–987. doi: 10.1016/j.neuropharm.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de LL. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegel JM. The neurobiology of sleep. Seminars in neurology. 2009;29:277–296. doi: 10.1055/s-0029-1237118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu MF, et al. Activity of dorsal raphe cells across the sleep-waking cycle and during cataplexy in narcoleptic dogs. J Physiol. 2004;554:202–215. doi: 10.1113/jphysiol.2003.052134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegel JM. Behavioral functions of the reticular formation. Brain Res Rev. 1979;1:69–105. doi: 10.1016/0165-0173(79)90017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dauvilliers Y, et al. High pain frequency in narcolepsy with cataplexy. Sleep Medicine. 2002;12:572–577. doi: 10.1016/j.sleep.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Hassani OK, Lee MG, et al. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Modirrousta M, Mainville L, Jones BE. Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21:2807–2816. doi: 10.1111/j.1460-9568.2005.04104.x. [DOI] [PubMed] [Google Scholar]
- 45.Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Funato H, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metabolism. 2009;9:64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winsky-Sommerer R, Boutrel B, et al. Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol Neurobiol. 2005;32:285–294. doi: 10.1385/MN:32:3:285. [DOI] [PubMed] [Google Scholar]
- 48.Kiss A. Immobilization induced fos expression in the medial and lateral hypothalamic areas: a limited response of hypocretin neurons. Ideggyogy Sz. 2007;60:192–195. [PubMed] [Google Scholar]
- 49.Furlong TM, Vianna DM, Liu L, Carrive P. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci. 2009;30:1603–1614. doi: 10.1111/j.1460-9568.2009.06952.x. [DOI] [PubMed] [Google Scholar]
- 50.Johnson PL, et al. A key role for orexin in panic anxiety. Nature Medicine. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maidment NT, Evans CJ. Measurement of extracellular neuropeptides in the brain: Microdialysis linked to solid phase radioimmunoassays with subfemptomole limits of detection. In: Robinson TE, Justice JB, editors. Microdialysis in the Neurosciences. Elsevier; 1991. pp. 275–303. [Google Scholar]
- 52.Engel J. Epilepsy surgery. Neurology. 1994;7:140–147. doi: 10.1097/00019052-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Cornford EM, et al. Regional analyses of CNS microdialysate glucose and lactate in seizure patients. Epilepsia. 2002;43:1360–1371. doi: 10.1046/j.1528-1157.2002.01602.x. [DOI] [PubMed] [Google Scholar]
- 54.During MJ, Fried I, Leone P, Katz A, Spencer DD. Direct Measurement of Extracellular Lactate in the Human Hippocampus During Spontaneous Seizures. Journal of Neurochemistry. 1994;62:2356–2361. doi: 10.1046/j.1471-4159.1994.62062356.x. [DOI] [PubMed] [Google Scholar]
- 55.Wilson CL, et al. Comparison of seizure related amino acid release in human epileptic hippocampus versus a chronic, kainate rat model of hippocampal epilepsy. Epilepsy Res. 1996;26:245–254. doi: 10.1016/s0920-1211(96)00057-5. [DOI] [PubMed] [Google Scholar]
- 56.Scheyer RD, et al. Phenytoin concentrations in the human brain: an in vivo microdialysis study. Epilepsy Res. 1994;18:227–232. doi: 10.1016/0920-1211(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 57.Fried I, et al. Cerebral microdialysis combined with single-neuron and electroencephalographic recording in neurosurgical patients. Technical note. J Neurosurg. 1999;91:697–705. doi: 10.3171/jns.1999.91.4.0697. [DOI] [PubMed] [Google Scholar]
- 58.Mai JK, Assheuer J, Paxinos G. Atlas of the human brain. Elsevier Academic Press; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


