SUMMARY
Objective
To examine the relationship of knee osteoarthritis (OA) with cardiovascular and metabolic risk factors by obesity status and gender.
Methods
Data from 1,066 National Health and Nutrition Examination Survey III participants (≥60 years of age) was used to examine relationships of osteophytes-defined radiographic knee OA and cardiovascular and metabolic measures. Analyses were considered among obese [body mass index (BMI) ≥30 kg/m2] and non-obese (BMI < 30 kg/m2) men and women.
Results
The prevalence of osteophytes-defined radiographic knee OA was 34%. Leptin levels and homeostatic model assessment-insulin resistance (HOMA-IR), a proxy measure of insulin resistance, were significantly associated with knee OA; those with knee OA had 35% higher HOMA-IR values and 52% higher leptin levels compared to those without knee OA. The magnitude of the association between HOMA-IR and knee OA was strongest among men, regardless of obesity status; odds ratios (ORs) for HOMA-IR were 34% greater among non-obese men (OR = 1.18) vs obese women (OR = 0.88). Among obese women, a 5-µg/L higher leptin was associated with nearly 30% higher odds of having knee OA (OR = 1.28). Among men, ORs for the association of leptin and knee OA were in the opposite direction.
Conclusions
Cardiometabolic dysfunction is related to osteophytes-defined radiographic knee OA prevalence and persists within subgroups defined by obesity status and gender. A sex dimorphism in the direction and magnitude of cardiometabolic risk factors with respect to knee OA was described including HOMA-IR being associated with OA prevalence among men while leptin levels were most important among women.
Keywords: Osteoarthritis, Obesity, Leptin, Insulin resistance, Sex, Epidemiology
Introduction
Osteoarthritis (OA) is a highly prevalent disorder, affecting approximately 27 million Americans1. Estimates from a population-based study predict that one of every two elderly adults will have knee OA2. Arthritis generates substantial economic burden as a result of health care expenditures3, decrements in physical functioning4,5 and loss in productivity associated with physical disability6.
Obesity is a widely-acknowledged risk factor for knee OA7–9. There is a growing appreciation of the need to understand how obesity contributes to OA considering the increasing prevalence of obesity and overweight in the US and world-wide10,11, the underlying inflammatory component in both obesity and OA12, and the knowledge that few interventions have been successful without addressing either weight or the inflammatory response13.
Several mechanisms have been proposed by which obesity can influence OA onset and progression [reviewed by Sowers & Karvonen-Gutierrez14]. Some investigations have concluded that the primary role for obesity in OA etiology is as a sheer mechanical force leading to increased joint loading and subsequent articular cartilage damage15,16. However, this does not explain the associations observed between obesity and OA in non-weight bearing joints7,17–19 thereby motivating additional and alternative explanations of the OA-obesity relationship. The recognition that adipose tissue can contribute to changing metabolic environments has stimulated the consideration of hypotheses about the relationship of obesity and OA that extend beyond those of biomechanical loading. Emerging evidence about the active metabolic environment of chondrocytes, including glucose transport, cholesterol efflux and lipid metabolism [reviewed by Katz20] have led investigators to consider novel obesity-related biomarkers that may reflect underlying pathology between OA and the cardiovascular and metabolic diseases.
Findings from studies that have examined cardiovascular or metabolic risk factors and OA are mixed. Some21–23, but not all studies24,25 have found positive associations of OA with cardiovascular risk factors. Importantly, studies of OA and obesity that have included cardiometabolic measures have rarely incorporated measures of fat tissue metabolism such as leptin, an adipocytokine that is an important modulator of the inflammatory response26. Synthesis and secretion of leptin have been demonstrated in osteoblasts and chondrocytes27,28 and its receptors have been identified in articular cartilage29. Importantly, leptin levels are associated with markers of bone formation and leptin receptor is associated with greater cartilage loss30.
In an effort to further elucidate the role of cardiometabolic dysfunction with respect to OA, we report the relationship of cardiovascular and metabolic risk factors with radiographically-defined knee OA among a sample of men and women aged 60 and over, representative of the United States population. We hypothesized that individuals with greater insulin resistance, poorer lipid profiles, and greater leptin levels would have an increased odds of knee OA. We further hypothesized that the magnitude of these relationships would differ by gender.
Methods
Data source and sample
Data are from the National Health and Nutrition Examination Survey (NHANES) III, a survey of the civilian non-institutionalized US population conducted by the National Center for Health Statistics (NCHS) from 1988 to 1994. NHANES III utilized a stratified multistage probability sampling design, including a two-phase survey period. Adults aged 60+ years, African-Americans and Mexican Americans were oversampled to provide stable estimates of health characteristics for these subgroups.
These analyses address a subset of the total NHANES III sample as only adults aged 60+ years were recruited for the radiograph acquisition component and only Phase 2 (1991–1994) knee radiographs have been scored for osteophytes-defined OA severity. There were no medical, safety or other exclusions for the radiograph component; the overall completion rate for obtaining radiographs from eligible participants was 93%. Furthermore, only data from those with morning visits who were fasted and had blood assayed for leptin were considered for these analyses. Study subjects were randomly assigned to the morning group and samples were randomly selected for leptin assay. Thus, this report is based upon data from 1,066 adults aged 60 years or greater with knee OA data and fasted blood samples available for assay. Participants with data available to be included in this sample are more likely to be Caucasian but less likely to have a high school education as compared to the total NHANES sample. Further, they have a higher body mass index (BMI) and are older, as is expected given that age was part of the inclusion criterion for the radiograph protocol.
OA measures
Knee OA was defined using non-weight bearing anterio-posterior knee radiographs from a Centrix III x-ray unit with Kodak Lanex double screens and TML film (phototimed with 12:1 stationary grid). Radiographs were scored for osteophytes-defined OA severity using the Kellgren–Lawrence (KL) Atlas of Knee Radiographs of Arthritis31, where zero = normal, one = possible osteophyte, two = definite osteophyte, three = moderate multiple osteophytes, and four = large osteophytes, severe sclerosis. Those with a KL score ≥ 2 or those with knee joint replacements were considered to have osteophytes-defined radiographic OA.
Knee radiographs were read by one of two radiologists with additional scoring by a second reader if there was evidence of disease. Scores from the two readers were compared and discordant scores were subjected to consensus readings. The quality control program has been described32. For the KL scoring, Kappa statistics for inter-rater agreement were >0.71; for intra-rater agreement the Kappa scores were >0.84 and >0.82 for the primary and secondary reader, respectively.
Cardiometabolic measures
The primary independent variables were cardiometabolic risk factors, including a proxy indicator of insulin resistance homeostatic model assessment-insulin resistance (HOMA-IR), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), triglycerides, waist:hip ratio, blood pressure, and leptin. Each individual was classified as either non-obese (BMI < 30 kg/m2) or obese (BMI ≥ 30 kg/m2). Assay information is available as Online Supplemental Material (eMethods) and in the NHANES III reference documents33,34. HOMA-IR was calculated using the following formula: [Insulin uIU/mL × (Glucose mg/dL × 0.055)/22.5].
Blood pressure was measured three times by trained personnel and recorded to the nearest even number, according to a standardized protocol. The average of the three measurements was used in data analysis. Height (cm) was measured using a stadiometer. Weight (kg) was measured using a digital scale. BMI was calculated as [weight(kg)/height(m)2].Waist and hip circumference (cm) were measured using a nonstretching tape.
Other variables
Age was measured in years. Other demographic variables were analyzed as categorical variables, as follows: self-reported race/ethnic group (non-Hispanic White, non-Hispanic Black, Mexican American, and Other), gender, marital status (married vs not married), educational attainment (less than high school vs high school or more). Referent groups were non-Hispanic Whites, those not married and those with less than a high school education.
All data, including radiographs, specimen collection for assay of cardiometabolic measures, measurement of body size and self-reported demographic information were collected at the same visit. NHANES III was approved by the NCHS Institutional Review Board. Written informed consent was obtained from all participants.
Statistical analysis
NHANES III pseudo-stratum (SDPSTRA6), pseudo-cluster (SDPPSU6) and Phase 2 morning session subsample weight (WTPFSD2) variables were used in all analyses. WTPFSD2 was selected as the sample weight because only knee radiographs from Phase 2 were scored and because leptin was only assayed among participants from the morning session. Missing data was handled using case-wise deletion. Because of the small number of “Other” race/ethnicity, data from these participants were excluded from multivariate analyses. Potential demographic and cardiometabolic variables of interest were selected a priori given the availability of measures in the NHANES III datasets and their known relevance as risk factors for knee OA.
Univariate distributions of the continuous variables of age and the cardiometabolic measures were examined, overall and by osteophytes-defined radiographic knee OA status and reported as means and standard errors (SEs). Distributions were examined for normalcy. While leptin and HOMA-IR were not normally distributed, they were modeled on their original scale to ease with interpretation of results. Frequencies of the categorical variables were examined, overall and by osteophytes-defined radiographic knee OA group, to ensure sufficient sample sizes in individual cells for appropriate analyses. Categorical variables are reported as percentages and SEs.
The unadjusted relationships of the continuous cardiometabolic biomarkers and knee OA were characterized using logistic regression models (SAS PROC SURVEYLOGISTIC). Bivariate (unadjusted) associations of knee OA and categorical independent predictors were evaluated using Rao-Scott Chi-Square tests.
All variables were considered for inclusion in the multivariable analysis; variables were retained in the final models if the adjusted estimates changed by 10% or more as compared to the unadjusted analysis. Some variables were not retained in the final model due to collinearity concerns with other variables. Because HOMA-IR is calculated from glucose and insulin, these measures could not all be included in the final multivariable model. HOMA-IR, a proxy of insulin resistance, was selected for multivariable modeling. Similarly, diastolic blood pressure (DBP) was not included in the final model due to collinearity concerns with systolic blood pressure (SBP). Given that differences in DBP were relatively small between knee OA groups and not clinically significant, SBP was selected for inclusion in the final model. The two measures of body size, BMI and waist:hip ratio could not be included in the same model.
Covariate-adjusted models were stratified by obesity status and gender because of significant interactions between these variables and the independent cardiometabolic variables of interest. Regression diagnostics were examined for the final multivariable model and there was no evidence of collinearity among the included variables.
A sensitivity analysis was conducted to examine the consistency of the observations from the multivariable models after adjustment for waist:hip ratio instead of BMI within obesity and gender stratum. SAS Version 9.2 (SAS Institute, Cary, NC, USA) was used for all data analyses.
Results
This sample (N = 1,066) with knee radiographs included non-Hispanic Whites (83.2%), non-Hispanic Blacks (7.3%), Mexican Americans (2.3%) and Other race/ethnicity (7.2%). The design-adjusted mean age of the sample was 70.5 years (SE = 0.14) with 57% being female.
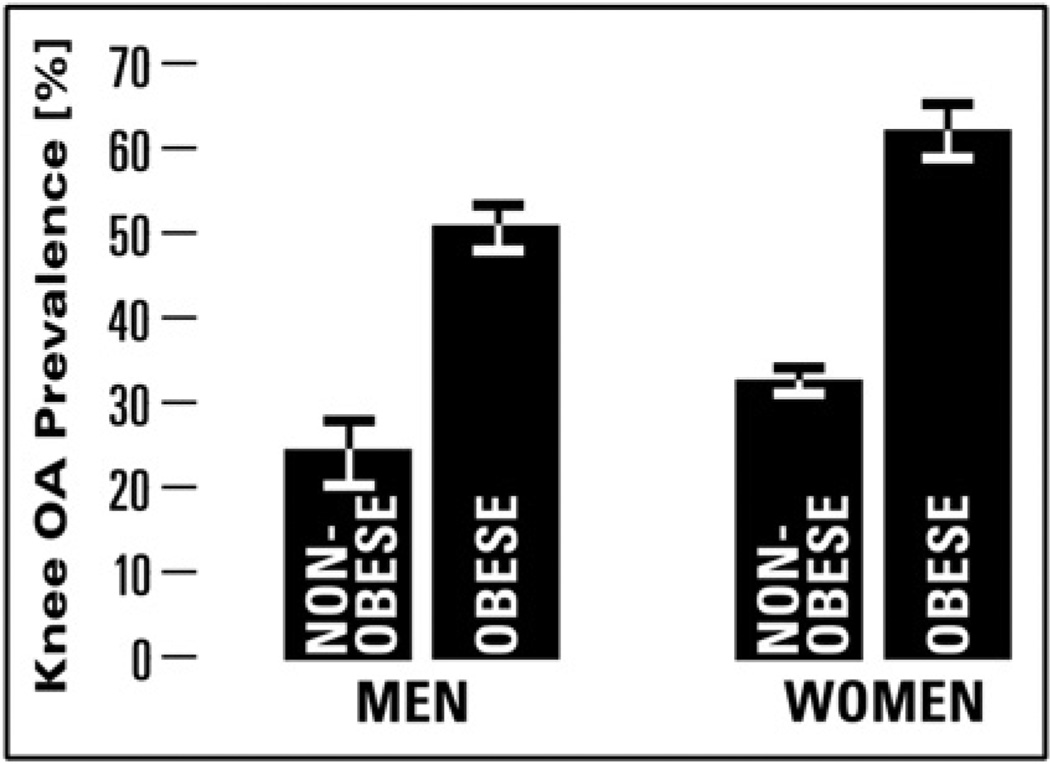
After considering the survey sampling design, 34.1% (SE = 0.60%) of individuals had osteophytes-defined radiographic knee OA as defined as a KL score ≥ 2; obese men and obese women had the greatest prevalence of osteophytes-defined radiographic knee OA (Fig. 1). Individuals with osteophytes-defined radiographic knee OA were, on average, 2 years older (P < 0.0001) and were more likely to be female, non-Hispanic Black, unmarried, less educated and a never smoker in comparison to those without knee OA (Table I).
Fig. 1.
Prevalence (95% CI) of osteophytes-defined radiographic knee OA by obesity status and gender among NHANES III sample.
Table I.
Demographic and cardiometabolic features of NHANES III participants aged 60+ years, by osteophytes-defined radiographic knee OA status*
| No osteophytes-defined radiographic knee OA N = 632 |
Osteophytes-defined radiographic knee OA N = 434 |
P-value knee OA vs no knee OA | |
|---|---|---|---|
| n (%, SE %) | n (%, SE %) | ||
| Sex | |||
| Male | 340 (69.6%, 1.3) | 172 (30.4%, 1.3) | <0.0001 |
| Female | 292 (58.4%, 1.1) | 262 (41.6%, 1.1) | |
| Race/ethnicity | |||
| Non-Hispanic White | 368 (63.5%, 1.0) | 242 (36.5%, 1.0) | <0.0001 |
| Non-Hispanic Black | 99 (52.0%, 2.2) | 94 (48.0%, 2.2) | |
| Mexican American | 133 (60.9%, 2.7) | 84 (39.1%, 2.7) | |
| Other | 32 (70.7%, 5.0) | 14 (29.3%, 5.0) | |
| Marital status | |||
| Married | 399 (65.3%, 0.6) | 235 (34.7%, 0.6) | <0.0001 |
| Not married | 232 (59.9%, 1.0) | 197 (40.1%, 1.0) | |
| Educational attainment | |||
| Less than high school | 346 (60.7%, 0.5) | 254 (39.3%, 0.5) | <0.0001 |
| High school or more | 284 (64.9%, 1.9) | 179 (35.1%, 1.9) | |
| Smoking status | |||
| Current | 119 (75.5%, 1.2) | 45 (24.5%, 1.2) | <0.0001 |
| Former | 243 (66.9%, 1.7) | 142 (33.1%, 1.7) | |
| Never | 270 (56.5%, 1.2) | 247 (43.5%, 1.2) | |
| BMI category | |||
| BMI< 25 kg/m2 | 253 (78.1%, 1.0) | 88 (21.9%, 1.0) | <0.0001 |
| BMI 25–29.9 kg/m2 | 268 (63.7%, 1.4) | 175 (36.3%, 1.4) | |
| BMI≥ 30 kg/m2 | 110 (41.4%, 1.2) | 170 (58.6%, 1.2) | |
| Mean (SE) | Mean (SE) | ||
| Age | 69.8 (0.14) | 71.7 (0.07) | <0.0001 |
| BMI, kg/m2 | 26.0 (0.03) | 29.4 (0.13) | <0.0001 |
| Cardiometabolic factors | |||
| Glucose (mg/dL) | 108.3 (0.39) | 116.2 (0.41) | <0.0001 |
| Insulin (uIU/mL) | 10.9 (0.08) | 13.9 (0.23) | <0.0001 |
| HOMA-IR | 3.1 (0.03) | 4.2 (0.05) | <0.0001 |
| HDL-cholesterol (mg/dL) | 52.6 (0.39) | 51.7 (0.35) | 0.02 |
| LDL-cholesterol (mg/dL) | 137.2 (0.48) | 143.4 (0.80) | <0.0001 |
| Triglycerides (mg/dL) | 155.1 (1.28) | 167.1 (1.43) | <0.0001 |
| Waist:hip ratio | 0.95 (0.001) | 0.96 (0.002) | 0.38 |
| SBP | 136.8 (0.37) | 141.6 (0.19) | <0.0001 |
| DBP | 73.6 (0.12) | 74.5 (0.08) | <0.0001 |
| Leptin (µg/L) | 11.8 (0.07) | 18.0 (0.28) | <0.0001 |
All data are design-adjusted (using weights, cluster and stratum variables) to account for the survey sample design.
Bold values indicate findings that are statistically significant.
Female participants were 1.1 years older, were less likely to be married but more likely to have completed high school as compared to male participants. Further, more women were never smokers whereas more men were former smokers (Table II). Women were more likely to be categorized as normal weight or obese as compared to men but there was no difference in the BMI between men and women. Women had lower glucose, triglyceride and DBP levels as compared to men but higher HDL-c, LDL-c, and SBP. Notably, design-adjusted average leptin levels were more than two times greater among women as compared to men (P < 0.0001).
Table II.
Demographic and cardiometabolic features of NHANES III participants aged 60+ years by gender*
| Male N = 517 |
Female N = 554 |
P-value male vs female |
|
|---|---|---|---|
| n (%, SE %) | n (%, SE %) | ||
| Race/ethnicity | |||
| Non-Hispanic White | 275 (83.2%, 1.0) | 335 (83.3%, 0.5) | 0.50 |
| Non-Hispanic Black | 91 (6.9%, 0.3) | 102 (7.5%, 0.6) | |
| Mexican American | 126 (2.5%, 0.1) | 91 (2.1%, 0.2) | |
| Other | 20 (7.4%, 1.2) | 26 (7.1%, 0.8) | |
| Marital status | |||
| Married | 400 (81.0%, 0.3) | 234 (46.9%, 1.0) | <0.0001 |
| Not married | 112 (19.0%, 0.3) | 317 (53.1%, 1.0) | |
| Educational attainment | |||
| Less than high school | 307 (43.6%, 1.0) | 293 (40.5%, 1.2) | 0.004 |
| High school or more | 205 (56.4%, 1.0) | 258 (59.5%, 1.2) | |
| Smoking status | |||
| Current | 96 (17.1%, 0.8) | 68 (13.1%, 0.6) | <0.0001 |
| Former | 258 (51.3%, 0.7) | 127 (26.4%, 0.5) | |
| Never | 158 (31.5%, 0.7) | 359 (60.6%, 0.8) | |
| BMI category | |||
| BMI < 25 kg/m2 | 160 (31.6%, 0.8) | 181 (38.0%, 0.7) | <0.0001 |
| BMI 25–29.9 kg/m2 | 239 (44.5%, 1.0) | 204 (36.0%, 0.8) | |
| BMI ≥ 30 kg/m2 | 113 (24.0%, 0.9) | 167 (26.0%, 0.8) | |
| Mean (SE) | Mean (SE) | ||
| Age | 69.9 (0.16) | 71.0 (0.16) | <0.0001 |
| BMI, kg/m2 | 27.2 (0.05) | 27.3 (0.11) | 0.49 |
| Cardiometabolic factors | |||
| Glucose (mg/dL) | 115.3 (0.90) | 108.2 (0.23) | <0.0001 |
| Insulin (uIU/mL) | 11.4 (0.14) | 12.0 (0.16) | 0.68 |
| HOMA-IR | 3.6 (0.07) | 3.4 (0.04) | 0.06 |
| HDL-cholesterol (mg/dL) | 46.5 (0.65) | 56.5 (0.28) | <0.0001 |
| LDL-cholesterol (mg/dL) | 135.1 (0.88) | 142.7 (0.76) | <0.0001 |
| Triglycerides (mg/dL) | 160.5 (1.59) | 158.9 (1.66) | 0.39 |
| Waist:hip ratio | 1.006 (0.001) | 0.92 (0.001) | <0.0001 |
| SBP | 137.1 (0.46) | 139.7 (0.32) | <0.0001 |
| DBP | 75.7 (0.17) | 72.6 (0.04) | <0.0001 |
| Leptin (µg/L) | 7.6 (0.08) | 18.9 (0.29) | <0.0001 |
All data are design-adjusted (using weights, cluster and stratum variables) to account for the survey sample design.
Bold values indicate findings that are statistically significant.
Body size, cardiometabolic characteristics and knee OA
The design-adjusted average BMI among those with osteophytes-defined radiographic knee OA was 29.4 kg/m2 compared to 26.0 kg/m2 among those without knee OA (P < 0.0001). While one-fourth of the total sample (25.3%) was classified as obese, 40% of those with osteophytes-defined radiographic knee OA were classified as obese.
With the exception of waist:hip ratio, all of the cardiometabolic factors were significantly associated with osteophytes-defined radiographic knee OA status. Those with knee OA had 35% higher HOMA-IR values and 52% higher leptin levels as compared to those without knee OA. While statistically significant, lipids were only 2–7% worse in those with knee OA while blood pressure levels were 1–3% higher compared to those without knee OA.
Sex dimorphism with respect to cardiometabolic characteristics and OA
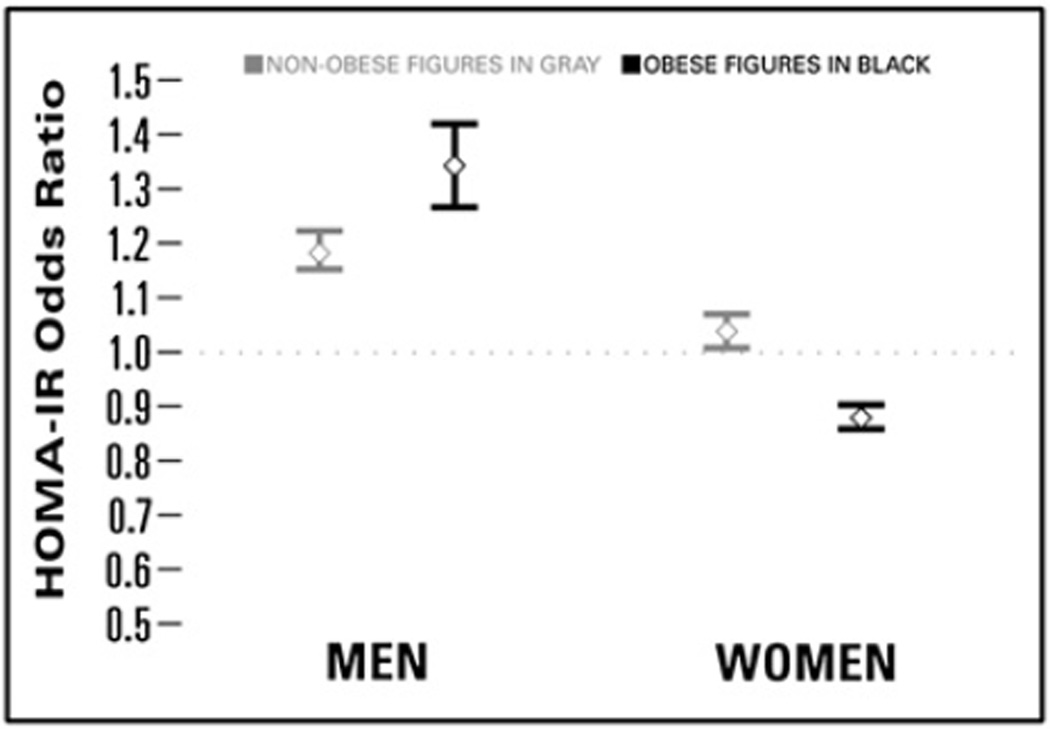
After adjustment for BMI, HOMA-IR and leptin levels had the strongest relationships with osteophytes-defined radiographic knee OA in multivariable models that included all of the cardiometabolic measures (leptin, HOMA-IR, LDL-c, SBP, triglycerides) and adjustment for age, race/ethnicity, marital status, educational attainment and smoking status (Table III). The magnitude of the association between HOMA-IR and knee OA was much greater among men than women. Among non-obese and obese men, respectively, each higher unit in HOMA-IR was associated with 18–34% greater odds (OR = 1.18, 95% confidence interval (CI) 1.15, 1.22 and OR = 1.34, 95% CI 1.27, 1.42) of having osteophytes-defined radiographic knee OA. Among non-obese women, a one-unit higher HOMA-IR was associated with only 4% greater odds of knee OA (95% CI 1.01, 1.07) while HOMA-IR was actually associated with decreased odds of knee OA among obese women (Fig. 2).
Table III.
Adjusted ORs (95% CIs) for the association between cardiometabolic factors and osteophytes-defined radiographic knee OA modeled separately by gender and obesity status, NHANES III*
| Models in the following stratum | Leptin† | HOMA-IR | BMI | LDL-c‡ | SBP§ |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Non-obese men (N = 372) | 0.61 (0.56, 0.67) | 1.18 (1.15, 1.22) | 1.33 (1.29, 1.36) | 1.05 (1.03, 1.06) | 0.97 (0.96, 0.99) |
| Obese men (N = 102) | 0.73 (0.70, 0.77) | 1.34 (1.27, 1.42) | 1.01 (0.96, 1.05) | 1.06 (1.04, 1.08) | 0.88 (0.86, 0.90) |
| Non-obese women (N = 356) | 1.04 (1.01, 1.07) | 1.04 (1.01, 1.07) | 1.15 (1.12, 1.18) | 1.00 (0.99, 1.01) | 1.10 (1.09, 1.11) |
| Obese women (N = 148) | 1.28 (1.26, 1.31) | 0.88 (0.86, 0.89) | 1.18 (1.17, 1.20) | 0.94 (0.93, 0.95) | 1.09 (1.05, 1.13) |
All models are adjusted for age, ethnicity, marital status, educational attainment, logtriglycerides and smoking status (current, former, never).
Estimates for leptin reflect a 5 µg/L increase in leptin.
Estimates for LDL-c represent a 5 mg/dL increase in LDL-c.
Estimates for SBP represent a 5 mmHg increase in SBP. Bold values indicate findings that are statistically significant.
Fig. 2.
OR (95% CI) of osteophytes-defined radiographic knee OA associated with HOMA-IR by gender and obesity status, NHANES III. Estimates from each of the four obesity by sex models were adjusted for age, ethnicity, marital status, educational attainment, smoking status, leptin, BMI, logtriglycerides, LDL-c, and SBP.
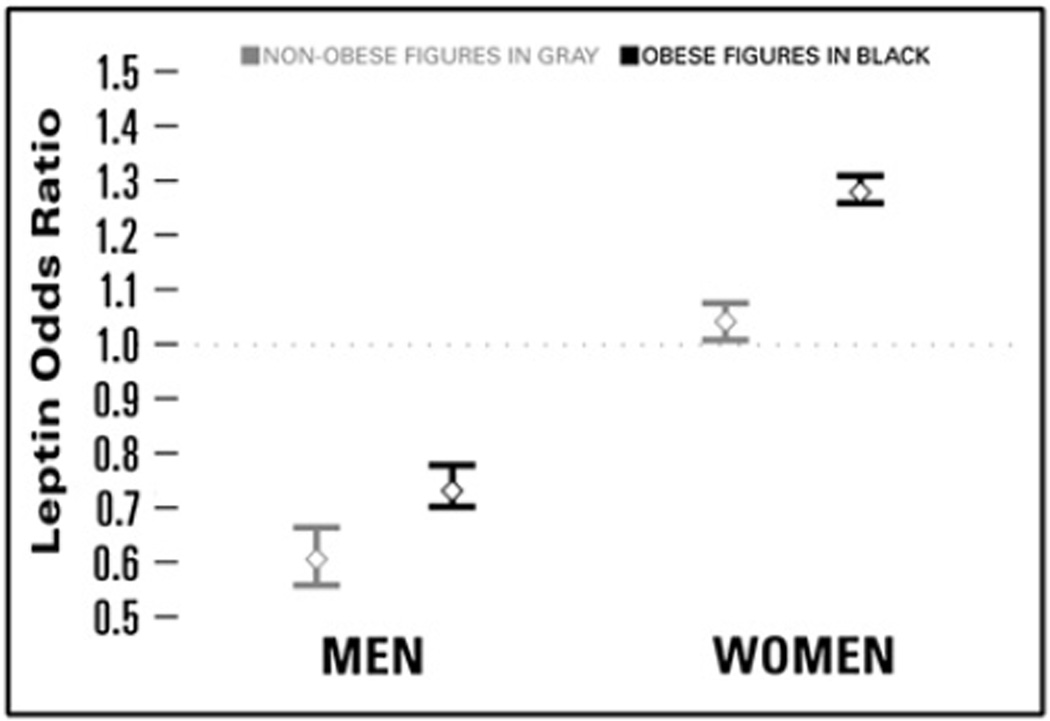
Leptin levels were strongly associated with osteophytes-defined radiographic knee OA. Among obese women, a 5 µg/L higher leptin was associated with 28% greater odds of having knee OA (OR = 1.28, 95% CI 1.26, 1.31). Among non-obese women, the magnitude of the association between leptin levels and knee OA was less although statistically significant (OR = 1.04, 95% CI 1.01, 1.07). The relationship of leptin and knee OA was reversed in men compared to women. A 5 µg/L higher level of leptin was associated with 27% decreased odds of having knee OA among obese men, and 39% decreased odds of having knee OA among non-obese men (Fig. 3).
Fig. 3.
OR (95% CI) of osteophytes-defined radiographic knee OA associated with leptin by gender and obesity status, NHANES III. Estimates from each of the four obesity by sex models were adjusted for age, ethnicity, marital status, educational attainment, smoking status, HOMA-IR, BMI, logtriglycerides, LDL-c, and SBP.
LDL-c was significantly and positively associated with osteophytes-defined radiographic knee OA among obese and non-obese men (P < 0.0001) and negatively associated with knee OA among obese women (P < 0.0001) (Table III). For both obese and non-obese men, a 5 mg/dL higher LDL-c was associated with 5–6% greater odds of knee OA. Conversely, for obese women, a 5 mg/dL increase in LDL-c was associated with 6% decreased odds of having knee OA. LDL-c was not associated with knee OA among non-obese women (Table III).
The relationship of SBP and osteophytes-defined radiographic knee OA was also different among men and women, regardless of obesity status. A 5 mmHg increase in SBP was associated with 3–12% decreased odds of having knee OA among men but with 9–10% increased odds of having knee OA among women (Table III).
BMI was associated with having osteophytes-defined radiographic knee OA among women. A 1 kg/m2 increase in BMI was associated with 15–18% increased odds of having knee OA among women. However, among men, the relationship of BMI and knee OA differed based on obesity status. In non-obese men, a 1 kg/m2 increase in BMI was associated with 30% increased odds of having knee OA (OR = 1.33, 95% CI 1.29, 1.36). Among obese men, however, BMI was not associated with having knee OA.
When multivariable analyses were adjusted for waist:hip ratio instead of BMI as a sensitivity analysis, estimates for LDL-c and SBP were virtually unchanged in terms of magnitude or direction (data not shown). Odds ratios (ORs) for HOMA-IR changed very little (<5%) and ORs for leptin changed by 3–12% among women and obese men in models adjusted for waist:hip ratio instead of BMI. Changes were more pronounced among non-obese men (+16% change for HOMA-IR and +26% change for leptin).
Discussion
Using data from a nationally-representative sample of the US elderly population, the importance of obesity as a risk factor for osteophytes-defined radiographic knee OA was confirmed. The prevalence of obesity was twice as great among those having knee OA as compared to those without knee OA. There were also very strong associations of having osteophytes-defined radiographic knee OA with cardiometabolic characteristics, even following adjustment for body size using BMI. Most importantly, we report compelling and consistent relationships of HOMA-IR and leptin, two obesity-related biomarkers, with knee OA. Notably, the overwhelming difference in the magnitude and direction of these associations had to be considered within the context of being male or female. Striking sex differences were observed for HOMA-IR and leptin in relation to having osteophytes-defined radiographic knee OA. ORs for HOMA-IR were 14% greater among non-obese men vs non-obese women. For those who were obese, the odds of having knee OA increase substantially with increasing HOMA-IR among men; however, among obese women, the ORs for HOMA-IR were in the opposite direction. Conversely, leptin levels were most important with respect to osteophytes-defined radiographic knee OA among women compared to men. We found that higher leptin is associated with increased odds of knee OA among women but the opposite was observed in men. These relationships with cardiometabolic risk factors were observed in multivariable models also adjusting for BMI. Our findings suggest that the impact of leptin has an independent effect on knee OA prevalence among women, over-and-above the effect of body size alone.
Sex dimorphism with respect to body composition is well-reported in the literature, suggesting that fatness means something different for men vs women. For a given level of BMI, women generally have a larger proportion of body mass that is fat as compared to men and women are more likely to deposit adipose tissue subcutaneously whereas men are more likely to deposit fat viscerally35. This is particularly relevant to our findings because, although circulating levels of both leptin and insulin are proportional to fat mass36, the levels of each reflect different fat depots. Leptin levels correlate better with subcutaneous adipose tissue37–40, which is proportionally larger in women whereas insulin is better correlated with visceral adipose tissue41, which is proportionally larger in men. Our findings of stronger relationships between osteophytes-defined radiographic knee OA and leptin among women and between osteophytes-defined radiographic knee OA and an index of insulin resistance among men may reflect the predominance of each as the more important obesity-related hormone and fat depot for that sex. This hypothesis is supported in studies using animal models where the brains of male rats are more sensitive to insulin but female rats are more sensitive to leptin42. Furthermore, for a given amount of fat mass, women have higher circulating leptin levels37–40 but increases in body fat among women are associated with smaller decreases in insulin sensitivity as compared to men43.
The sex dimorphism with respect to leptin and insulin may also be related to differences in sex steroid metabolism and levels among men and women. Estrogen levels are higher in women, even postmenopausal women, and estrogen has been shown to induce leptin secretion in women44. Further, male rats exposed to exogenous estrogen have increased sensitivity to leptin42. Excess androgens in men45 and women46 are associated with increased insulin resistance but androgens have a negative association with leptin levels in men47,48.
There have been very few studies of OA and cardiometabolic measures that have included both men and women24,49–52, and few have analyzed their data stratifying by gender. In the AGES Reykjavik Study, carotid plaque severity and coronary calcifications were associated with hand OA52 and marginally associated with total knee or hip replacements in women53 but not men. Our finding of a “protective” effect for leptin among men is interesting and has not been reported in any other studies. We hypothesize that our findings may be reflective of the reportedly higher synovial fluid leptin levels within the joint among women54,55.
Our finding of no association of BMI and osteophytes-defined radiographic knee OA among obese men is interesting, especially given its importance among women and non-obese men. Given our findings of a stronger relationship of HOMA-IR and knee OA among obese men as compared to non-obese men, we hypothesize that obese men may have proportionally greater depots of visceral fat, which is associated with insulin resistance41, as compared to non-obese men. This hypothesis is supported by our sensitivity analyses in which waist:hip ratio, a proxy for central adiposity, was associated with greater odds of knee OA among obese men but not among non-obese men (data not shown). However, we do not have direct measures of visceral fat to test this hypothesis.
This analysis extends previous work by Sowers et al.21 with regard to cardiometabolic obesity and knee OA. That study reported that the odds of knee OA were greatest among women who were both obese and had cardiometabolic dysfunction; however, the cardiometabolic measures were not considered individually. We now report that leptin levels and a proxy indicator of insulin resistance, in particular, are strongly and significantly related to knee OA status.
This investigation has substantial strengths. Notably, our analytical approach considered obesity and gender-stratified models of the cardiometabolic measures relative to osteophytes-defined radiographic knee OA status. Because many of the cardiometabolic measures vary with respect to obesity, this approach allowed us to examine their relationship with OA, independent of obesity. In doing so, it was observed that, regardless of obesity status, and after adjustment for BMI, cardiometabolic risk factors are associated with OA prevalence. The decision to utilize gender-stratified models allowed us to reveal important patterns in the relationship of osteophytes-defined radiographic knee OA status and cardiometabolic measures. The nationally-representative sample utilized in this analysis is a strength in that it provides sufficient data to address variation in age, sex, and race/ethnic status.
Limitations of this report are that non-weight bearing radiographs were used to determine OA status in the NHANES III population and so our measure is truly reflective of osteophytes-defined radiographic knee OA status. Usage of non-weight bearing radiographs means that measures of joint space narrowing, a proxy measure of cartilage loss, cannot be ascertained. The impact of cardiometabolic dysfunction may have a different relationship with cartilage loss as compared to measures of bone overgrowth which were described by the KL scoring system. However, utilization of the non-weight bearing radiographs should not affect the measures of osteophytes and bone sclerosis, reflected in the KL scoring we report here. The cross-sectional design of NHANES is a limitation of this study so causality between cardiometabolic measures and OA cannot be determined. We used HOMA-IR as a proxy for insulin resistance because more sophisticated estimates such as glucose clamp measures were not available in this large epidemiologic dataset. While some studies have suggested that HOMA-IR may not be a good marker of insulin resistance in older adults56, a larger study demonstrated high correlation between HOMA-IR and insulin sensitivity among older individuals with and without impaired glucose tolerance57. Additionally, although the hypothesized relationship between cardiometabolic measures and knee OA operates under a paradigm of increased systemic inflammation, we did not include the measure of C-reactive protein (CRP), an inflammatory biomarker, due to the known technical limitations of the available CRP assay in this population.
The observations warrant further investigation in other populations with data available on men and women. The NHANES dataset only includes information about OA in the knee joints. However, because knee joints may be vulnerable to damage associated with increased mechanical loading due to obesity, confirmation of these findings in populations with information about OA in non-weight bearing joints is of high interest. Reproducibility of these findings in other populations and across joint sites would have important implications for intervention and treatment and possibly indicate that effective therapies for OA prevention may differ by sex.
Conclusion
We have reported consistent, statistically significant associations of cardiometabolic biomarkers and osteophytes-defined radiographic knee OA among a nationally-representative sample of US adults and conclude that cardiometabolic dysfunction is related to knee OA prevalence over-and-above the effect imparted by obesity. Furthermore, we describe a striking sex dimorphism in the pattern (direction and magnitude) of cardiometabolic risk factors relative to osteophytes-defined radiographic knee OA. A proxy measure of insulin resistance is associated with OA prevalence among men, whereas among women, leptin levels appear to be more important. Considering the substantial implications for primary and secondary interventions, replication of these findings should be pursued in other populations with OA and cardiometabolic data, especially in those populations including both men and women.
Supplementary Material
Acknowledgments
Role of the funding source
Carrie Karvonen-Gutierrez is a trainee on the T32 Public Health and Aging Training Grant (AG-027708). This work was additionally supported by AR051384. The funding sponsors had no role in the design/conduct of the study; in the collection, analysis or interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Author contributions
Carrie Karvonen-Gutierrez (ckarvone@umich.edu) and MaryFran Sowers (mfsowers@umich.edu) take responsibility for the integrity of the work as a whole, from inception to finished article. Author contributions are as follows:
Carrie Karvonen-Gutierrez: conception and design of study, analysis and interpretation of data, drafting of the article, critical revision of the article for important intellectual content, final approval of the article, statistical expertise, obtaining funding.
MaryFran Sowers: conception and design of study, drafting of the article, critical revision of the article for important intellectual content, final approval of the article, obtaining funding.
Steven Heeringa: analysis and interpretation of data, critical revision of the article for important intellectual content, final approval of the article, statistical expertise.
Conflict of interest
None of the authors have any financial conflicts of interest to report.
Supplementary material
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.joca.2012.02.644.
References
- 1.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. for the National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maetzel A, Li LC, Pencharz J, Tomlinson G, Bombardier C for the Community Hypertension and Arthritis Project Study Team. The economic burden associated with osteoarthritis, rheumatoid arthritis and hypertension: a comparative study. Ann Rheum Dis. 2004;63:395–401. doi: 10.1136/ard.2003.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sowers M, Jannausch ML, Gross M, Karvonen-Gutierrez CA, Palmieri RM, Crutchfield M, et al. Performance-based physical functioning in African-American and Caucasian women at midlife: considering body composition, quadriceps strength, and knee osteoarthritis. Am J Epidemiol. 2006;163:950–958. doi: 10.1093/aje/kwj109. [DOI] [PubMed] [Google Scholar]
- 5.Ling SM, Fried LP, Garrett ES, Fan MY, Rantanen T, Bathon JM. Knee osteoarthritis comprises early mobility function: the Women’s Health and Aging Study II. J Rheumatol. 2003;30:114–120. [PubMed] [Google Scholar]
- 6.Kauppila AM, Kyllonen E, Mikkonen P, Ohtonen P, Laine V, Siira P, et al. Disability in end-stage knee osteoarthritis. Disabil Rehabil. 2009;31:370–380. doi: 10.1080/09638280801976159. [DOI] [PubMed] [Google Scholar]
- 7.Hart DJ, Spector TD. The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. J Rheumatol. 1993;20:331–335. [PubMed] [Google Scholar]
- 8.Cicuttini FM, Baker JR, Spector TD. The association of obesity with osteoarthritis of the hand and knee in women: a twin study. J Rheumatol. 1996;23:1221–1226. [PubMed] [Google Scholar]
- 9.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman B, Aliabadi P, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728–733. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 10.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 11.Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–66. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 12.Pearle AD, Scanzello CR, George S, Mandl LA, DiCarlo EF, Peterson M, et al. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15:516–523. doi: 10.1016/j.joca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Richette P, Poitou C, Garnero P, Vicaut E, Bouillot JL, Lacorte JM, et al. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis. 2011;70:139–144. doi: 10.1136/ard.2010.134015. [DOI] [PubMed] [Google Scholar]
- 14.Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol. 2010;22:533–537. doi: 10.1097/BOR.0b013e32833b4682. (Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mundermann A, Dyrby CO, Andriacchi TP. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis Rheum. 2005;52:2835–2844. doi: 10.1002/art.21262. [DOI] [PubMed] [Google Scholar]
- 16.Maly MR, Costigan PA, Olney SJ. Contribution of psychosocial and mechanical variables to physical performance measures in knee osteoarthritis. Phys Ther. 2005;85:1318–1328. [PubMed] [Google Scholar]
- 17.Carman WJ, Sowers M, Hawthorne VM, Weissfeld LA. Obesity as a risk factor for osteoarthritis of the hand and wrist: a prospective study. Am J Epidemiol. 1994;139:119–129. doi: 10.1093/oxfordjournals.aje.a116974. [DOI] [PubMed] [Google Scholar]
- 18.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69:761–765. doi: 10.1136/ard.2008.106930. (Review). [DOI] [PubMed] [Google Scholar]
- 20.Katz JD, Agrawal S, Velasquez M. Getting to the heart of the matter: osteoarthritis takes its place as part of the metabolic syndrome. Curr Opin Rheumatol. 2010;22:512–519. doi: 10.1097/BOR.0b013e32833bfb4b. (Review). [DOI] [PubMed] [Google Scholar]
- 21.Sowers M, Karvonen-Gutierrez CA, Palmieri-Smith R, Jacobson JA, Jiang Y, Ashton-Miller JA. Knee osteoarthritis in obese women with cardiometabolic clustering. Arthritis Rheum. 2009;61:1328–1336. doi: 10.1002/art.24739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart DJ, Doyle DV, Spector TD. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. J Rheumatol. 1995;22:1118–1123. [PubMed] [Google Scholar]
- 23.Acheson RM, Collart AB. New Haven survey of joint diseases. XVII: relationship between some systemic characteristics and osteoarthrosis in a general population. Ann Rheum Dis. 1975;34:379–387. doi: 10.1136/ard.34.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis MA, Ettinger WH, Neuhaus JM. The role of metabolic factors and blood pressure in the association of obesity with osteoarthritis of the knee. J Rheumatol. 1988;15:1827–1832. [PubMed] [Google Scholar]
- 25.Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I). Evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol. 1988;128:179–189. doi: 10.1093/oxfordjournals.aje.a114939. [DOI] [PubMed] [Google Scholar]
- 26.Vuolteenaho K, Koskinen A, Kukkonen M, Nieminen R, Paivarinta U, Moilanen T, et al. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage-mediator role of NO in leptin-induced PGE2, IL-6 and IL-8 production. Mediators Inflamm. 2009;2009:345838. doi: 10.1155/2009/345838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, et al. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003;48:3118–3129. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- 28.Kume K, Satomura K, Nishisho S, Kitaoka E, Yamanouchi K, Tobiume S, et al. Potential role of leptin in endochondral ossification. J Histochem Cytochem. 2002;50:159–169. doi: 10.1177/002215540205000204. [DOI] [PubMed] [Google Scholar]
- 29.Figenschau Y, Knutsen G, Shahazeydi S, Johansen O, Sveinbjornson B. Human articular chondrocytes express functional leptin receptors. Biochem Biophys Res Commun. 2001;287:190–197. doi: 10.1006/bbrc.2001.5543. [DOI] [PubMed] [Google Scholar]
- 30.Berry PA, Jones SW, Cicuttini FM, Wluka AE, Maciewicz RA. Temporal relationship between serum adipokines, biomarkers of bone and cartilage turnover, and cartilage volume loss in a population with clinical knee osteoarthritis. Arthritis Rheum. 2011;63:700–707. doi: 10.1002/art.30182. [DOI] [PubMed] [Google Scholar]
- 31.Kellgren JH, Lawrence JS. Atlas of Standard Radiographs of Arthritis. Volume II. Philadelphia: FA Davis; 1963. The epidemiology of chronic rheumatism. 1963;t:/pgn/ELSEVIER/YJOCA/20(7)/web/00002637/. [Google Scholar]
- 32.U.S. Department of Health and Human Services (DHHS), National Center for Health Statistics. Knee Osteoarthritis X-ray Data and Documentation Data Release. Hyattsville, MD: 2001. The Third National Health and Nutrition Examination Survey (NHANES III), 1988–94. Series 11, No. 11A. [Google Scholar]
- 33.U.S Department of Health and Human Services (DHHS), National Center for Health Statistics. Serum Leptin Data and Documentation Data Release. Hyattsville, MD: 2001. The Third National Health and Nutrition Examination Survey (NHANES III), 1988–94. Series 11, No. 12A. [Google Scholar]
- 34.U.S. Department of Health and Human Services (DHHS), National Center for Health Statistics. NHANES III Laboratory Data File and Documentation, Ages one year and older. Hyattsville, MD: 1996. Third National Health and Nutrition Examination Survey (NHANES III), 1988–94. Catalog Number 76300. (revised 2006). [Google Scholar]
- 35.Garaulet M, Perex-Llamas F, Fuente T, Zamora S, Tebar FJ. Anthropometric, computed tomography and fat cell data in an obese population: relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone binding globulin, and sex hormones. Eur J Endocrinol. 2000;143:657–666. doi: 10.1530/eje.0.1430657. [DOI] [PubMed] [Google Scholar]
- 36.Woods SC, Gotoh K, Clegg DJ. Gender differences in the control of energy homeostasis. Exp Biol Med. 2003;228:1175–1180. doi: 10.1177/153537020322801012. [DOI] [PubMed] [Google Scholar]
- 37.Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, et al. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81:3424–3427. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 38.Ostlund RE, Yang JW, Klein S, Gingerich R. Relation between plasma leptin concentration and body, fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab. 1996;81:3909–3913. doi: 10.1210/jcem.81.11.8923837. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, Pan Q, et al. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82:1293–1300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
- 40.Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadiian R, et al. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab. 1997;82:579–584. doi: 10.1210/jcem.82.2.3739. [DOI] [PubMed] [Google Scholar]
- 41.Racette SB, Hagberg JM, Evans EM, Holloszy JO, Weiss EP. Abdominal obesity is a stronger predictor of insulin resistance than fitness among 50–95 year olds. Diabetes Care. 2006;29:673–678. doi: 10.2337/diacare.29.03.06.dc05-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 43.Sierra-Johnson J, Johnson BD, Bailey KR, Turner ST. Relationships between insulin sensitivity and measures of body fat in asymptomatic men and women. Obes Res. 2004;12:2070–2077. doi: 10.1038/oby.2004.258. [DOI] [PubMed] [Google Scholar]
- 44.Casabiell X, Pineiro V, Peino R, Lage M, Camina J, Gallego R, et al. Gender differences in both spontaneous and stimulated leptin secretion by human omental adipose tissue in vitro: dexamethasone and estradiol stimulate leptin release in women, but not in men. J Clin Endocrinol Metab. 1988;83:2149–2155. doi: 10.1210/jcem.83.6.4849. [DOI] [PubMed] [Google Scholar]
- 45.Cohen JC, Hickman R. Insulin resistance and diminished glucose tolerance in powerlifters ingesting anabolic steroids. J Clin Endocrinol Metab. 1987;64:960–963. doi: 10.1210/jcem-64-5-960. [DOI] [PubMed] [Google Scholar]
- 46.Moghetti P, Tosi F, Castello R, Magnani CM, Negri C, Brun E, et al. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J Clin Endocrinol Metab. 1996;81:952–960. doi: 10.1210/jcem.81.3.8772557. [DOI] [PubMed] [Google Scholar]
- 47.Luukkaa V, Pesonen U, Huhtaniemi I, Lehtonen A, Tilvis R, Tuomilehto J, et al. Inverse correlation between serum testosterone and leptin in men. J Clin Endocrinol Metab. 1998;83:3243–3246. doi: 10.1210/jcem.83.9.5134. [DOI] [PubMed] [Google Scholar]
- 48.Haffner SM, Miettinen H, Karhapaa P, Mykkanen L, Laakso M. Leptin concentrations, sex hormones, and cortisol in nondiabetic men. J Clin Endocrinol Metab. 1997;82:1807–1809. doi: 10.1210/jcem.82.6.3978. [DOI] [PubMed] [Google Scholar]
- 49.Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med. 2009;121:9–20. doi: 10.3810/pgm.2009.11.2073. [DOI] [PubMed] [Google Scholar]
- 50.Sturmer T, Sun Y, Sauerland S, Zeissig I, Gunther KP, Puhl W, et al. Serum cholesterol and osteoarthritis. The baseline examination of the Ulm Osteoarthritis Study. J Rheumatol. 1998;25:1827–1832. [PubMed] [Google Scholar]
- 51.Ku JH, Lee CK, Joo BS, An BM, Choi SH, Wang TH, et al. Correlation of synovial fluid leptin concentrations with the severity of osteoarthritis. Clin Rheumatol. 2009;28:1431–1435. doi: 10.1007/s10067-009-1242-8. [DOI] [PubMed] [Google Scholar]
- 52.Jonsson H, Helgadottir GP, Aspelund T, Eiriksdottir G, Sigurdsson S, Ingvarsson T, et al. Hand osteoarthritis in older women is associated with carotid and coronary atherosclerosis: the AGES Reykjavik study. Ann Rheum Dis. 2009;68:1696–1700. doi: 10.1136/ard.2008.096289. [DOI] [PubMed] [Google Scholar]
- 53.Jonsson H, Helgadottir GP, Aspelund T, Eiriksdottir G, Sigurdsson S, Siggeirdottir K, et al. The presence of total knee or hip replacements due to osteoarthritis enhances the positive association between hand osteoarthritis and atherosclerosis in women: the AGES-Reykjavik study. Ann Rheum Dis. 2011;70:1087–1090. doi: 10.1136/ard.2010.144980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gandhi R, Takahashi M, Syed K, Davey JR, Mahomed NN. Relationship between body habitus and joint leptin levels in a knee osteoarthritis population. J Orthop Res. 2010;28:329–333. doi: 10.1002/jor.21000. [DOI] [PubMed] [Google Scholar]
- 55.Presle N, Pottie P, Dumond H, Guillaume C, Lapicque F, Pallu S, et al. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthritis Cartilage. 2006;14:690–695. doi: 10.1016/j.joca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Ferrara CM, Goldberg AP. Limited value of the Homeostasis Model Assessment to predict insulin resistance in older men with impaired glucose tolerance. Diabetes Care. 2001;24:245–249. doi: 10.2337/diacare.24.2.245. [DOI] [PubMed] [Google Scholar]
- 57.Chang AM, Smith MJ, Bloem CJ, Galecki AT, Halter JB, Supiano MA. Limitation of the Homeostasis Model Assessment to predict insulin resistance and β-cell dysfunction in older people. J Clin Endocrinol Metab. 2006;91:629–634. doi: 10.1210/jc.2005-1803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.