Abstract
Purpose
The Charlson comorbidity index (CCI) is a commonly used scale for assessing morbidity, but its role in assessing mortality in hemodialysis patients is not clear. Age, a component of CCI, is a strong risk factor for morbidity and mortality in chronic diseases and correlates with comorbidities. We hypothesized that the Charlson comorbidity index without age is a strong predictor of mortality in hemodialysis patients.
Methods
A 6-year cohort of 893 hemodialysis patients was examined for an association between a modified CCI (without age and kidney disease) (mCCI) and mortality.
Results
Patients were 53 ± 15 years old (mean ± SD), had a median mCCI score of 2, and included 47% women, 31% African Americans and 55% diabetics. After adjusting for case-mix and nutritional and inflammatory markers including C-reactive protein and interleukin-6, 2nd (mCCI: 1–2), 3rd (mCCI = 3), and 4th (mCCI: 4–9) quartiles compared to 1st (mCCI = 0) quartiles showed death hazard ratios (95% confidence intervals) of 1.43 (0.92–2.23), 1.70 (1.06–2.72), and 2.33 (1.43–3.78), respectively. The mCCI-death association was robust in non-African Americans. The CCI-death association linearity was verified in cubic splines. Each 1 unit higher mCCI score was associated with a death hazard ratio of 1.16 (1.07–1.27).
Conclusions
CCI independent of age is a robust and linear predictor of mortality in hemodialysis patients, in particular in non-African Americans.
Keywords: Chronic Kidney Disease (CKD), Hemodialysis, Charlson Comorbidity Index, Inflammation, Survival
Introduction
The mortality rate of patients with advanced chronic kidney disease (CKD) is unacceptably high [1]. These patients have a high prevalence of comorbid conditions, which in itself is one of the major risk factors for high mortality [1]. Khan defined comorbidity in kidney failure patients as a significant concurrent or past disease in addition to CKD, which involved organs other than the kidneys but which may also be responsible for the renal failure, for example, diabetes mellitus and myeloma [2]. The higher number of comorbid conditions in chronic dialysis patients is associated with an increased risk of mortality, which may range from 20% to almost 60% as compared to chronic dialysis patients without comorbidity [3]. Apart from being a prognostic factor, comorbidity is also an important confounding factor so it is essential for epidemiological studies of survival that it should be assessed in dialysis patients [4, 5].
Several scales have been devised to assess and quantify comorbidity in patients with chronic diseases. These include the Charlson comorbidity index (CCI) [6], index of co-existent diseases (ICED) [7] and Davies [8] and Wright-Khan indices [4]. CCI was developed for mortality analysis and was based on an assessment of survival in the general population. Since its inception, it has been used for assessment of the prognostic value of comorbidity in longitudinal studies of patients with a variety of disease states [6]. Age, which is per se a strong predictor of mortality, is one of the components of the original CCI. Hence, it may be speculated that the outcome predictability of CCI is driven by age especially in populations with exceptionally advanced age such as hemodialysis patients.
Few studies have examined the validity of CCI in ESRD patients [3, 9]. Fried et al. [10] studied CCI in peritoneal dialysis patients, whereas Di Iorio et al. [11] examined its outcome predictability in maintenance hemodialysis dialysis (MHD) patients. These studies examined incident (new) dialysis population or had small sample size or other limitations [10]. We examined the predictive value of modified CCI (mCCI) by excluding age as a CCI component and hypothesized that the utility and robustness of mCCI without age maintains as a predictor of mortality in MHD patients.
Subjects and methods
Patient population
We studied MHD patients who participated in the Nutritional and Inflammatory Evaluation in Dialysis (NIED) study [12]. The original NIED cohort consisted of 893 patients who were recruited from a population base of more than 3,000 MHD outpatients treated in eight DaVita maintenance dialysis clinics in Southern California during a period of 6 years (see the NIED study Website at http://www.Niedstudy.org for more details as well as previous publications [13–17]). To be included in the study, patients had to be at least 18 years old and receiving outpatient hemodialysis for at least 8 weeks. Patients were excluded if they had an acute infection or had a life expectancy of <6 months. The study was approved by the IRB, and all subjects gave informed consent prior to being enrolled in the study. The medical records for each subject were thoroughly reviewed by a collaborating physician in the study. Such information as underlying kidney disease, cardiovascular disease history and other illnesses was abstracted.
Modified Charlson co morbidity score
A modified version of the Charlson comorbidity index (CCI), i.e., by excluding subject’s age and presence or absence of kidney disease, was used to assess severity of comorbidities [18, 19]. Because all MHD patients would have positive “kidney disease” component, the only material difference between our mCCI and the conventional CCI is the lack of age component in the former. The CCI is based on the following components: 1 point is assigned for history of myocardial infarction, congestive heart failure, peripheral vascular disease, cerberovascular disease (transient ischemic attack or cerbrovascular accident with minor or no residua), dementia, chronic pulmonary disease, connective tissue disorder, peptic ulcer disease, mild liver disease and diabetes without end organ damage; 2 points for hemiplegia, moderate to severe renal disease (excluded in our scale) diabetes with end organ damage, tumor without metastases, leukemia, lymphoma and myeloma; 3 points for moderate or severe liver disease; and 6 points for metastatic solid tumor or full-blown AIDS.
Anthropometric and body composition measures
Body weight and anthropometric measurements were performed while patients were receiving MHD or within 5–20 min after termination of their hemodialysis treatment. Biceps and triceps skin fold thickness was measured by standard techniques using a conventional skin fold caliper [20, 21].
Near infrared interactance
To estimate percentage body fat and fat free body mass, near infrared (NIR) interactance was measured at the same time as the anthropometric measurements [22]. A commercial NIR interactance sensor with a coefficient of variation of 0.5% for total body fat measurements (portable Furtex 6100; Furtex, Inc, Rockville, VA; www.furtex.com) was used. NIR measurements were performed by placing a Furtex sensor on the upper arm (free of vascular access) for several seconds and entering the required data (data of birth, sex, weight, and height) for each patient. NIR measurements of body fat correlate significantly with other standard measures of body fat such as via DEXA in MHD patients [23].
Other laboratory tests
Predialysis and post-dialysis blood samples were obtained on a mid-week day that coincided with the day that the required quarterly blood drawings were obtained for testing at the DaVita dialysis facilities. Single pooled Kt/V was used to represent the weekly dialysis dose. All laboratory studies were performed by DaVita Laboratories (Deland, FL) using automated methods. Serum high sensitivity C-reactive protein (CRP) was measured using a turbidometric immunoassay (WPCI, Osaka, Japan; normal range <3.0 mg/l) [24, 25]. Interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) levels were measured with using immunoassay kits (R&D Systems, Minneapolis, Minn., USA; units: pg/ml; normal range: IL-6: <9.9 pg/ml, TNF-α: <4.7 pg/ml) [26, 27]. The C-reactive protein (CRP), TNF-α and IL-6 levels were measured in the General Clinical Research Center Laboratories at Harbor UCLA. Serum transthyretin (prealbumin) was measured by immunoprecipitation and plasma homocysteine concentration was measured by high performance liquid chromatography (HPLC) in the Harbor-UCLA Clinical Laboratories.
Statistical methods
Data were summarized using proportions, means (±standard deviation [SD]) or medians (interquartile range [IQR]) as appropriate. Categorical variables were compared using χ2 tests, and continuous variables were compared using χ2 tests or Mann–Whitney U tests, Kruskal–Wallis H tests, or analyses of variance, as appropriate. Pearson’s correlation coefficient (r) was used for analyses of linear associations. Logistic regression models were employed to estimate the odds ratio (OR) and 95% CI of mCCI ≥2 (median). The association between mCCI and mortality was assessed using Cox regression analysis and Kaplan–Meier plots with log rank tests. Non-linear associations were assessed using fractional polynomials and restricted cubic splines. Death hazard ratios (HRs) were obtained using Cox proportional hazard models controlling for the relevant covariates. In the mortality analyses, the patients were followed until event (death) or censoring (lost to follow-up or end of follow-up period), whichever happened first.
We performed incremental levels of multivariate adjustment where (A) case-mix variables including age, gender, race (African American vs. all other races), and duration of dialysis were included. (B) Malnutrition-inflammation complex syndrome (MICS) variables included serum albumin, creatinine and total iron-binding capacity, hemoglobin, white blood count, normalized protein nitrogen appearance (nPNA) [also known as normalized protein catabolic rate (nPCR)], and body mass index. (C) Additional adjustment was done for three inflammatory markers (serum CRP, IL-6, and TNFα) in a fully adjusted Cox regression model.
We expected significant confounding in the unadjusted models where relevant confounders such as age and gender were not taken into account. In fact, while the results from the adjusted models may have been over-adjusted (possibly due to inclusion of biological intermediates that are along the causal pathway from predictor to outcome variable), we make our inferences based on models adjusted for case-mix. Because of uncertainty regarding which final model is in fact the most parsimonious, we include three levels of adjustment in the presented data so that the full spectrum of results can be appreciated. The data analysis was done using STATA version 11.1 (STATA Corporation, College Station, TX).
Results
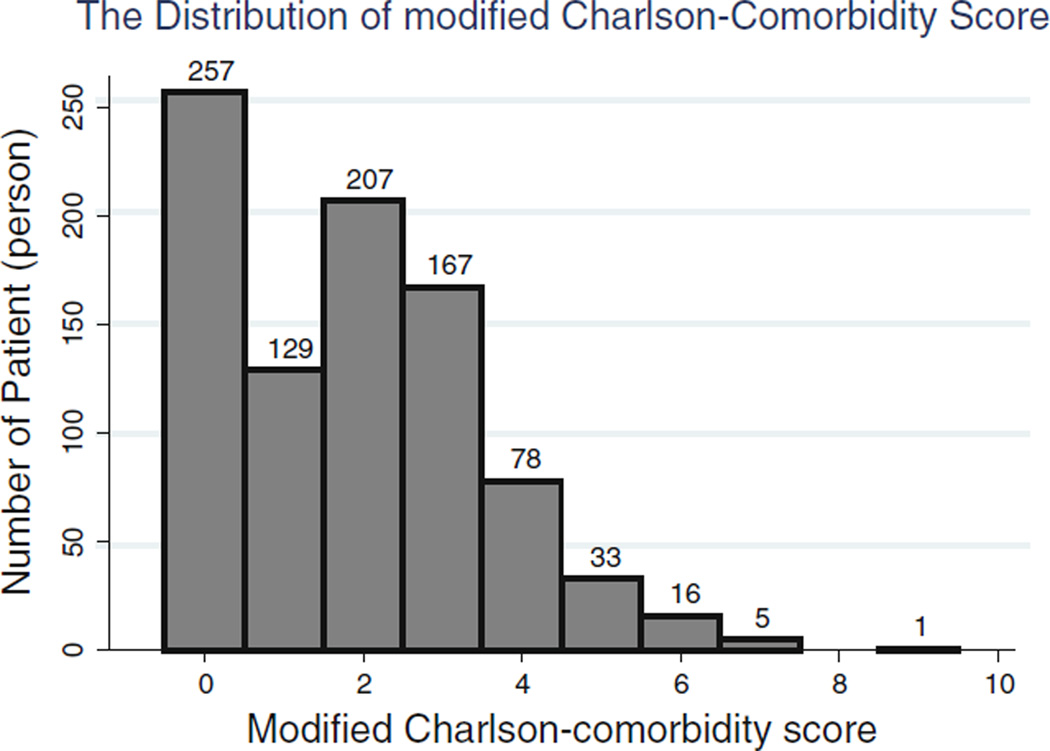
Data were available for the Charlson comorbidity index (CCI) of all enrolled patients at enrollment. The patients’ mean age (± SD) was 53 ± 15 years; 47% (n = 419) of patients were women, 31% (n = 279) were African American and 55% (n = 477) were diabetic. The mean duration of dialysis was 30 ± 34 months (median; interquartile range: 19; 37 months). The median mCCI obtained in individual patients was 2 (min = 0 and max = 9, IQR: 0–4). Figure 1 shows the distribution of mCCI score. After ranking subjects according to mCCI scores, we categorized them into score quartiles with 257, 336, 167 and 133 numbers of patients in the four groups, respectively. Table 1 lists relevant demographic, clinical and laboratory measures across the quartiles of mCCI scores of the 893 enrolled patients. Older patients were more likely to be in the higher quartiles of mCCI scores. Patients in the group of scores ranging from 4 to 9 showed the highest percentages of mortality rates over 5 years. Serum albumin, pre -albumin, creatinine, calcium and IL-6 showed significant decreasing trends as mCCI increased across the four quartiles.
Fig. 1.
Histogram showing distribution of modified Charlson comorbidity score across 893 patients
Table 1.
Baseline demographic, clinical and laboratory values in total and according to quartiles of Charlson comorbidity score in 893 maintenance hemodialysis patients
| Variable | Quartile1 N = 257 (0) |
Quartile2 N = 336 (1–2) |
Quartile3 N = 167 (3) |
Quartile4 N = 133 (4–9) |
P for trend |
|---|---|---|---|---|---|
| Demographic | |||||
| CCI score (median, IQR) | 0, 0 | 2, 1–2 | 3, 3–3 | 4, 4–5 | <0.001 |
| Age (year) | 45 ± 16 | 55 ± 14 | 58 ± 10 | 60 ± 11 | <0.001 |
| Women (%) | 119 (46) | 160 (48) | 81 (48) | 59 (44) | 0.89 |
| Marital status (% Married) | 65 (38) | 129 (51) | 65 (47) | 52 (49) | 0.08 |
| Race (% African American) | 72 (28) | 115 (34) | 49 (29) | 43 (32) | 0.39 |
| Insurance (% medicare) | 66 (43) | 123 (57) | 58 (55) | 40 (51) | 0.06 |
| Diabetes mellitus (%) | 27 (11) | 185 (57) | 152 (92) | 113 (86) | <0.001 |
| Mortality in 5 year (%)a | 55 (21) | 131 (39) | 86 (51) | 82 (62) | <0.001 |
| Body composition | |||||
| Body mass index (kg/m2) | 25.47 ± 5.83 | 26.60 ± 5.82 | 26.73 ± 6.40 | 27.77 ± 6.91 | 0.09 |
| Triceps skinfold (mm) | 16.34 ± 9.80 | 17.69 ± 10.19 | 17.50 ± 8.74 | 19.34 ± 10.03 | 0.2 |
| Biceps skinfold (mm) | 9.11 ± 7.34 | 10.47 ± 8.73 | 8.72 ± 6.16 | 11.02 ± 7.46 | <0.001 |
| Midarm muscle circumference (cm) | 30.64 ± 5.35 | 31.36 ± 5.94 | 31.40 ± 5.67 | 32.45 ± 6.36 | 0.14 |
| Near infrared measured body fat (%) | 37.81 ± 25.10 | 45.38 ± 5.69 | 47.86 ± 27.10 | 50.79 ± 29.53 | 0.2 |
| Hemodialysis treatment measures | |||||
| Duration of dialysis (mo) | 32.72 ± 33.51 | 34.11 ± 38.71 | 26.56 ± 26.67 | 25.58 ± 27.08 | <0.001 |
| Dialysis dose (Kt/V single pool) | 1.62 ± 0.28 | 1.63 ± 0.29 | 1.60 ± 0.29 | 1.55 ± 0.28 | 0.83 |
| nPNA (nPCR) (g/kg/d) | 1.07 ± 0.24 | 1.07 ± 0.24 | 1.07 ± 0.24 | 1.04 ± 0.23 | 0.85 |
| Erythropoietin dose (1,000 U/week) | 13.27 ± 10.62 | 13.96 ± 11.55 | 12.82 ± 90.7 | 16.90 ± 15.16 | <0.001 |
| Active vitamin D dose (µg/mo) | 40.77 ± 35.87 | 42.64 ± 36.64 | 40.54 ± 28.86 | 41.47 ± 39.32 | 0.90 |
| Biochemical measurements | |||||
| Serum albumin (g/dl) | 4.0 ± 0.34 | 3.89 ± 0.32 | 3.81 ± 0.36 | 3.76 ± 0.44 | <0.001 |
| Prealbumin (transthyretin) (mg/dl) | 30.61 ± 11.00 | 28.00 ± 8.93 | 26.94 ± 8.26 | 26.17 ± 9.22 | <0.001 |
| Creatinine (mg/dl) | 11.34 ± 3.06 | 10.29 ± 3.18 | 9.27 ± 2.73 | 9.02 ± 2.68 | 0.03 |
| Total iron-binding capacity (mg/dl) | 207.62 ± 38.79 | 210.85 ± 40.44 | 202.17 ± 35.28 | 206.54 ± 43.71 | 0.08 |
| Calcium (mg/dl) | 9.47 ± 0.65 | 9.35 ± 0.68 | 9.28 ± 0.53 | 9.28 ± 0.70 | <0.001 |
| Iron (mg/dl) | 68.73 ± 28.42 | 65.41 ± 25.79 | 66.87 ± 23.33 | 65.23 ± 30.89 | <0.001 |
| Iron saturation ratio | 33.33 ± 12.96 | 30.9 ± 10.33 | 32.87 ± 10.43 | 30.51 ± 9.83 | <0.001 |
| Phosphorus (mg/dl) | 6.13 ± 1.58 | 5.75 ± 1.44 | 5.55 ± 1.35 | 5.54 ± 1.4 | 0.17 |
| Ferritin (ng/ml) | 508.20 ± 392.31 | 539.74 ± 393.02 | 628.67 ± 447.58 | 682.63 ± 421.25 | 0.17 |
| Total homocysteine (µmol/l) | 23.14 ± 9.98 | 24.42 ± 11.85 | 22.40 ± 8.52 | 22.67 ± 8.56 | <0.001 |
| C-reactive protein (mg/l) | 5.07 ± 8.36 | 5.45 ± 5.98 | 6.33 ± 5.80 | 6.22 ± 5.76 | <0.001 |
| Interleukin 6 (pg/ml) | 11.3 ± 20.7 | 15.5 ± 44.5 | 24.3 ± 71.7 | 24.3 ± 56.9 | <0.001 |
| Tumor necrosis factor (pg/ml) | 9.03 ± 12.89 | 8.5 ± 12.65 | 8.29 ± 9.27 | 8.18 ± 7.52 | <0.001 |
| White blood cells (cmm) | 6.70 ± 1.85 | 6.70 ± 1.83 | 7.69 ± 2.07 | 7.47 ± 2.05 | 0.14 |
| Lymphocytes (cmm) | 24.29 ± 8.13 | 23.36 ± 7.88 | 20.49 ± 7.04 | 20.97 ± 7.34 | 0.21 |
| Blood hemoglobin (g/dl) | 12.03 ± 0.98 | 12.1 ± 0.89 | 12.20 ± 1.06 | 11.89 ± 1.14 | <0.001 |
Values expressed as mean SD or percentage. P values for dialysis dose (duration of dialysis), ferritin level, C-reactive protein level, interleukin 6 level, and tumor necrosis factor α level are based on the logarithmic values of these measures. Conversion factors for units: albumin in g/dl to g/l × 10; creatinine in mg/dl to umol/l × 88.4; calcium in mg/dl to mmol/l × 0.2495; phosphorus in mg/dl to mmol/l × 0.3229; homocysteine in umol to mg/l × 0.1352; total, low density lipoprotein, and high-density lipoprotein cholesterol in mg/dl to mmol/l × 0.2586; triglycerides in mg/dl to mmol/l × 0.01129; hemoglobin in g/dl to g/l × 10. Ferritin in ng/ml and µg/l and white blood cell count in 103/µl and 109/l require no conversion
Kt/V dialysis dose, nPCR normalized protein catabolic rate
Mortality pertains to a maximum of 33 months
The mCCI and survival
During the 72 months of follow-up, 354 (40%) subjects died. The crude all-cause mortality rate was 3.6/10,000 patient-days (95% confidence interval [CI]: 3.3–4.0). The associations of severity of mCCI with all-cause mortality are shown in Table 2. Hazard ratio for mortality was significant across the quartiles of increasing mCCI both for unadjusted and for adjusted models. Compared with the low comorbidity group (reference group), fully adjusted models of 2nd, 3rd and 4th quartiles showed hazard ratios for mortality of 1.43 (95% CI 0.92–2.23), 1.70 (95% CI 1.06–2.72), 2.33 (95% CI 1.43–3.78), respectively.
Table 2.
Hazard ratios for mortality across quartiles of charlson scores in 893 maintenance hemodialysis patients
| Charlson score quartiles | 1st quartile (n = 257) |
2nd quartile (n = 336) |
3rd quartile (n = 167) |
4th quartile (n = 133) |
Trend P |
|---|---|---|---|---|---|
| Unadjusted | 1.00 (Reference) | 1.72 (1.26–2.36) | 2.60 (1.13–2.26) | 3.40 (2.41–4.79) | <0.001 |
| P = 0.001 | P = 0.008 | P < 0.001 | |||
| Case mixa | 1.00 (Reference) | 1.22 (0.85–1.75) | 1.87 (1.28–2.73) | 2.31 (1.56–3.42) | <0.001 |
| P = 0.275 | P = 0.001 | P < 0.001 | |||
| Case mix + MICSb | 1.00 (Reference) | 1.46 (0.94–2.27) | 2.01 (1.26–3.20) | 2.41 (1.49–3.91) | <0.001 |
| P = 0.092 | P = 0.003 | P < 0.001 | |||
| Previous + inflammationc (full) | 1.00 (Reference) | 1.43 (0.92–2.23) | 1.70 (1.06–2.72) | 2.33 (1.43–3.78) | <0.001 |
| P = 0.109 | P = 0.029 | P = 0.001 |
CI confidence interval, HR hazard ratio, MICS malnutrition-inflammation-cachexia syndrome
Case-mix variables includes age, sex, race/ethnicity, and log duration of dialysis
MICS variables includes values for albumin, creatinine, hemoglobin, total iron-binding capacity, normalized protein catabolic rate, lymphocyte percentage, and body mass index
Full model consists of case mix and MICS and logarithm of 3 inflammatory markers: C-reactive protein, interleukin 6, and tumor necrosis factor α
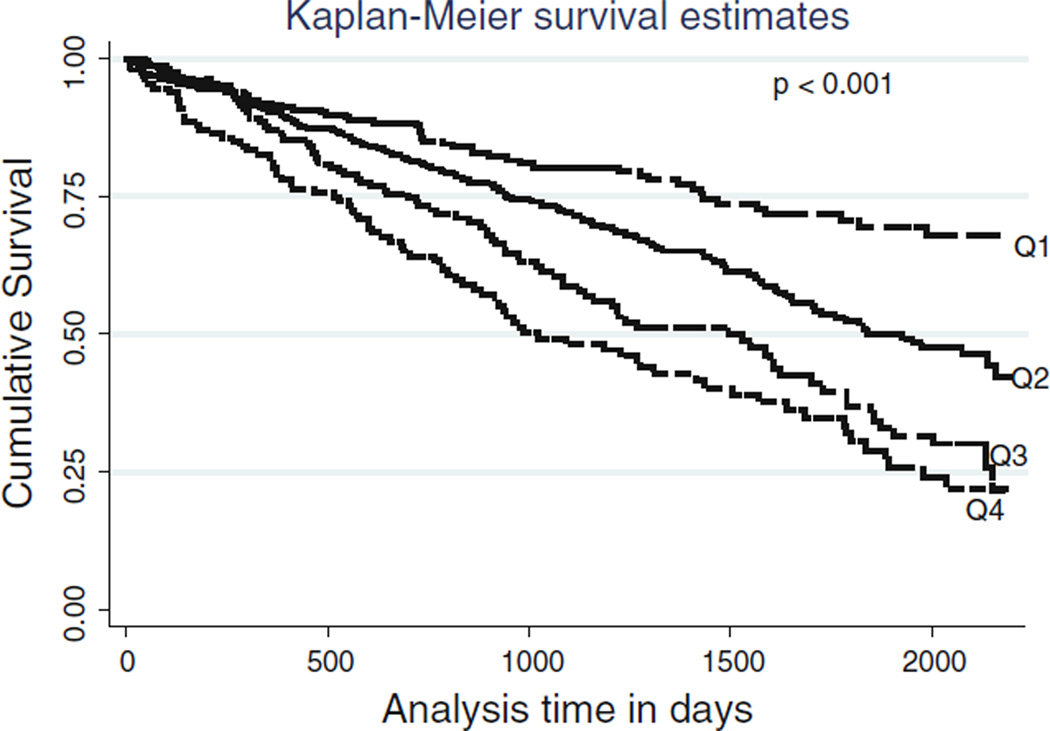
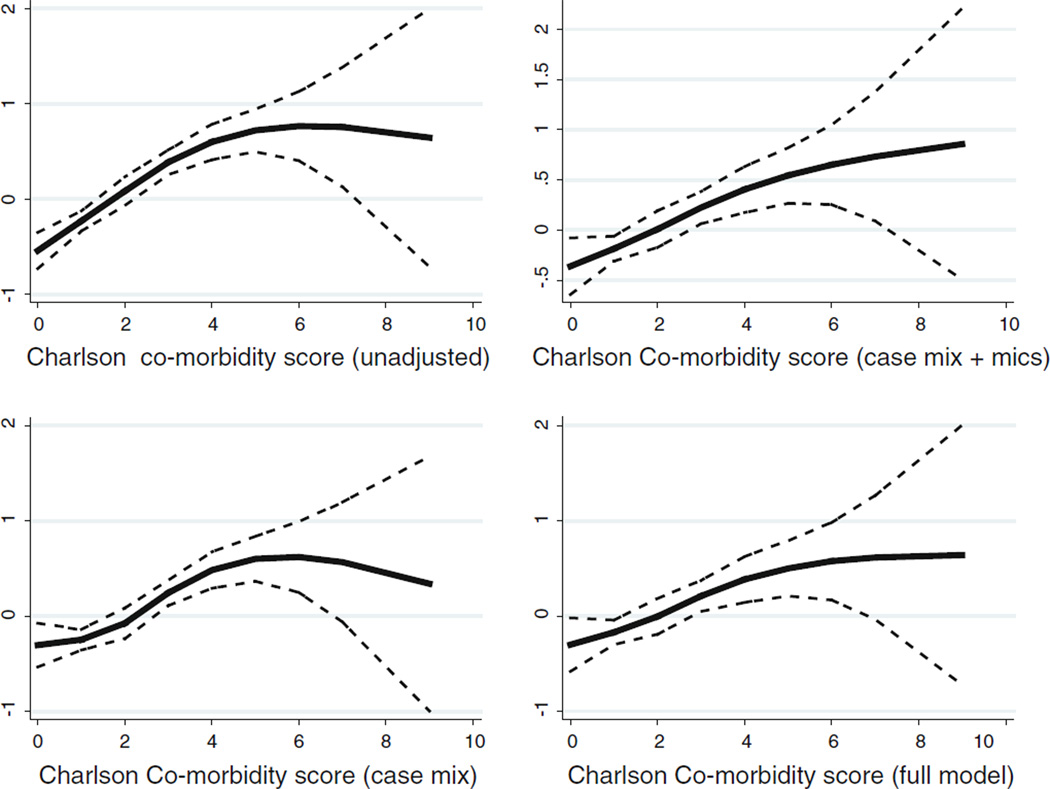
We further evaluated the hazard ratio by defining different characteristics of the population in Table 3. Patients who were male, age >65 years, non-African American and non-diabetic showed higher risks of mortality. Patients with the lowest mCCI had lower (1.9/10,000 patient-days (95% confidence interval [CI]: 1.5–2.5) crude all-cause mortality rates than did patients in the 2nd (3.3/10,000 patient-days (95% confidence interval [CI]: 2.8–4.0)), 3rd (5.0/10,000 patient-days (95% confidence interval [CI]: 4.1–6.2)) and 4th quartiles (6.5/10,000 patient-days (95% confidence interval [CI]: 5.2–8.1)) as is clearly shown in Kaplan–Meier figure (Fig. 2) (P < 0.001). Moreover, Fig. 3 shows cubic spline plots with unadjusted and adjusted models. Both graphical analyses further strengthen the findings of the hazard ratio analyses that higher mCCIs are related to a higher mortality rate.
Table 3.
Hazard ratios for mortality with regard to different variables
| Unadjusted | Case mixa | Case mix + MICSb | Full modelc | |
|---|---|---|---|---|
| All patients (n = 893) | 1.27 (1.19–1.35) | 1.20 (1.12–1.28) | 1.19 (1.09–1.29) | 1.16 (1.07–1.27) |
| (<0.001) | (<0.001) | (<0.001) | (<0.001) | |
| African Americans (n = 279) | 1.12 (1.01–1.23) | 1.06 (0.95–1.19) | 1.13 (1.0–1.28) | 1.07 (0.94–1.21) |
| (0.02) | (0.28) | (0.04) | (0.30) | |
| Non-African Americans (n = 614) | 1.40 (1.29–1.51) | 1.32 (1.20–1.45) | 1.26 (1.12–1.43) | 1.27 (1.12–1.44) |
| (<0.001) | (<0.001) | (<0.001) | (<0.001) | |
| Age <65 (n = 673) | 1.26 (1.17–1.36) | 1.20 (1.1–1.32) | 1.20 (1.08–1.34) | 1.16 (1.04–1.30) |
| (<0.001) | (<0.001) | (0.001) | (0.006) | |
| Age >65 (n = 136) | 1.19 (1.08–1.33) | 1.22 (1.09–1.36) | 1.24 (1.08–1.43) | 1.24 (1.07–1.44) |
| (<0.001) | (<0.001) | (0.003) | (0.004) | |
| Gender female (n = 419) | 1.27 (1.17–1.38) | 1.18 (1.07–1.29) | 1.17 (1.06–1.37) | 1.16 (1.04–1.30) |
| (<0.001) | (<0.001) | (0.003) | (0.008) | |
| Male (n = 479) | 1.28 (1.17–1.39) | 1.22 (1.10–1.35) | 1.20 (1.06–1.37) | 1.20 (1.04–1.38) |
| (<0.001) | (<0.001) | (0.005) | (0.01) | |
| Non-diabetic (n = 386) | 1.32 (1.17–1.49) | 1.20 (1.03–1.40) | 1.31 (1.08–1.59) | 1.29 (1.06–1.57) |
| (<0.001) | (0.02) | (0.006) | (0.012) | |
| Diabetic (n = 477) | 1.19 (1.09–1.31) | 1.18 (1.07–1.30) | 1.13 (1.01–1.26) | 1.11 (0.98–1.24) |
| (<0.001) | (0.001) | (0.034) | (0.089) |
CI confidence interval, HR hazard ratio, MICS malnutrition-inflammation-cachexia syndrome
Case-mix variables includes age, sex, race/ethnicity, and log duration of dialysis
MICS variables includes values for albumin, creatinine, hemoglobin, total iron-binding capacity, normalized protein catabolic rate, lymphocyte percentage, and body mass index
Full model consists of case mix and MICS and logarithm of 3 inflammatory markers: C-reactive protein, interleukin 6, and tumor necrosis factor α
Fig. 2.
Kaplan–Meier proportion of surviving patients comparing four quartiles of the Charlson score in 893 MHD patients
Fig. 3.
Cubic spline exhibiting the association between Charlson comorbidity score and mortality level in 893 MHD patients
Factors correlated with mCCI
Table 4 shows the unadjusted and adjusted correlations between mCCIs and relevant nutritional, inflammatory and biochemical variables. Apart from age and serum creatinine, no other strong positive or negative correlations were found between the mCCI and different variables. We also performed univariate (unadjusted) and multivariate logistic regression analyses of the association between the variables of interest and the mCCI as shown in Table 5. Age, NIR, Kt/V, erythropoiesis stimulating agent (ESA) dose, serum creatinine, CRP and IL-6 were significant and independent predictors of mCCIs that were higher or equal to the median. Each g/dl increase in serum albumin was associated with a 62% lower risk of a high mCCI score in our fully adjusted model [OR: 0.38, 95% CI: (0.24–0.60)].
Table 4.
Unadjusted and adjusted partial correlation coefficients r for the Charlson score against pertinent clinical, laboratory and demographic values
| Correlation | Case mixa | Case mix + MICSb | Full modecl | |
|---|---|---|---|---|
| Age | 0.36 (<0.001) | 0.35 (<0.001) | 0.22 (<0.001) | 0.23 (<0.001) |
| NIR body fat % | 0.17 (<0.001) | 0.14 (<0.001) | 0.06 (0.1) | 0.06 (0.14) |
| Kt/V | −0.09 (0.01) | −0.08 (0.02) | −0.13 (<0.001) | −0.14 (<0.001) |
| Dialysis month | −0.10 (<0.001) | −0.08 (0.04) | 0.06 (0.15) | 0.05 (0.26) |
| BMI | 0.11 (0.001) | 0.12 (0.002) | 0.13 (<0.001) | 0.1 (0.01) |
| Biceps | 0.05 (0.19) | 0.04 (0.24) | −0.03 (0.41) | −0.03 (0.44) |
| Triceps | 0.1 (0.01) | 0.12 (0.001) | 0.07 (0.078) | 0.06 (0.12) |
| MAMC | 0.09 (0.01) | 0.08 (0.03) | 0.06 (0.14) | 0.05 (0.21) |
| Erythropoietin dose | 0.06 (0.09) | 0.1 (0.006) | 0.05 (0.17) | 0.08 (0.06) |
| Serum albumin | −0.24 (<0.001) | −0.16 (<0.001) | −0.11 (0.005) | −0.08 (0.04) |
| Creatinine | −0.30 (<0.001) | −0.24 (<0.001) | −0.25 (<0.001) | −0.24 (<0.001) |
| Hemoglobin | −0.003 (0.94) | −0.03 (0.49) | −0.03 (0.48) | −0.001 (0.98) |
| Ferritin | 0.14 (<0.001) | 0.15 (<0.001) | 0.13 (0.002) | 0.13 (0.002) |
| TIBC | −0.01 (0.76) | −0.008 (0.83) | 0.02 (0.68) | 0.01 (0.75) |
| Log (CRP) | 0.17 (<0.001) | 0.1 (<0.001) | 0.1 (0.02) | 0.09 (0.03) |
| Log (IL-6) | 0.19 (<0.001) | 0.12 (<0.001) | 0.04 (0.29) | 0.02 (0.64) |
| Log (TNF-a) | 0.015 (0.66) | 0.02 (0.54) | 0.02 (0.7) | 0.002 (0.95) |
Case-mix variables includes age, sex, race/ethnicity, and log duration of dialysis
MICS variables includes values for albumin, creatinine, hemoglobin, total iron-binding capacity, normalized protein catabolic rate, lymphocyte percentage, and body mass index
Full model consists of case mix and MICS and logarithm of 3 inflammatory markers: C-reactive protein, interleukin 6, and tumor necrosis factor α
Table 5.
Odds ratios and 95% confidence interval for Charlson score (Score <2 vs. ≥2) in 893 maintenance hemodialysis patients using logistic regression model
| Variable | OR and 95% CI (unadjusted model) |
P value | OR and 95% CI (adjusted model)a |
P value |
|---|---|---|---|---|
| Age (each 10 year increase in age) | 1.04 (1.03–1.05) | <0.001 | 1.04 (1.03–1.05) | <0.001 |
| NIR total body fat % (each 10 g/kg/day unit increase) | 1.09 (1.04–1.16) | 0.001 | 1.09 (1.02–1.17) | 0.007 |
| Kt/V (single pool) | 0.57 (0.35–0.93) | 0.024 | 0.52 (0.30–0.91) | 0.021 |
| Duration of dialysis in month (log scale) (each 12 months per unit increase) | 0.84 (0.71–1.0) | 0.05 | 0.89 (0.74–1.06) | 0.19 |
| Erythropoietin dose (each 10,000 U/week increase) | 1.07 (0.95–1.2) | 0.24 | 1.16 (1.02–1.32) | 0.02 |
| Serum albumin (each 1 mg/dl unit increase) | 0.32 (0.21–0.47) | <0.001 | 0.38 (0.24–0.60) | <0.001 |
| Creatinine (each 1 mg/dl unit increase) | 0.83 (0.78–0.88) | <0.001 | 0.83 (0.78–0.88) | <0.001 |
| Hemoglobin (each 10 mg/dl unit increase) | 1.06 (0.24–4.5) | 0.94 | 1.53 (0.32–7.22) | 0.59 |
| TIBC (each 1 mg/dl unit increase) | 1.0 (0.99–1.0) | 0.07 | 1.0 (0.99–1.0) | 0.1 |
| CRP (log scale) (each 1 mg/dl unit increase) | 1.39 (1.19–1.62) | <0.001 | 1.39 (1.21–1.62) | <0.001 |
| IL-6 (log scale) (each1 mg/dl unit increase) | 1.44 (1.26–1.67) | <0.001 | 1.37 (1.17–1.61) | <0.001 |
| TNF (log scale) (each 1 mg/dl unit increase) | 1.09 (0.91–1.31) | 0.37 | 1.37 (0.92–1.39) | 0.23 |
| Race (African American) | 0.96 (0.71–1.30) | 0.79 | 0.93 (0.66–1.30) | 0.65 |
| Gender (women vs. men) | 1.02 (0.77–1.34) | 0.91 | 1.13 (0.83–1.55) | 0.42 |
In these models we adjusted for all variables which were listed in our fully adjusted model
Discussion
In our prevalent cohort study of 893 MHD patients with a mean age of 53 years, the mCCI without the age component was a strong and independent predictor of mortality. After dividing patients into four subgroups depending on their mCCI scores, patients in the highest scoring quartile with a score ranging from 4 to 9 showed mortality risk of 133% higher than the lowest quartile with a mean score range of 0. These associations persisted despite exclusion of age and additional multivariate adjustment for age and other potential confounders. These findings can have important clinical implications in risk-stratification of such multi-morbid persons as dialysis patients in whom comorbid states may have outcome predictability independent of advanced age.
Several risk factors are associated with increased mortality in ESRD patients. Age, diabetes mellitus, hypertension, coronary disease, smoking and low serum albumin are each important risk factors with a demonstrated adverse effect on mortality [28]. Several scales have been used to assess the degree of morbidity of ESRD patients. The index of co-existent diseases (ICED) [7], the Davies [8] and Wright-Khan indices [4, 29] and the CCI are the most commonly used instruments for assessment of comorbidity.
The CCI was originally developed in the general population in the United States. The CCI has been used to assess the survival of patients with a variety of disease states [6, 30–33]. CCI has also been used to measure comorbidity in ESRD patients [3, 9]. Some studies that compared CCI with the other above mentioned scales reported that CCI is more accurate and reliable predictor of mortality [3, 10]. However, Miskulin et al. [34] did not show any differences among different comorbidity scales in their power to predict one-year mortality rates in MHD patients when the scale scores were adjusted for serum albumin, race and cause of ESRD. Our study also shows that CCI is a strong predictor of mortality. We found that MHD patients who had an age-free-mCCI score in the highest quartile (quartiles 4: score range 4–9) had a 60% higher mortality risk than patients who were in the quartile 3 with median score of 3. The HRs were 2.33 (1.43–3.78) and 1.70 (1.06–2.72), respectively, for quartiles 4 and 3 in fully adjusted models. Our study confirms and extended the results of previous studies [10, 11].
Age is an important factor for consideration in ESRD patients when they are assessed for morbidity and mortality. Older patients may or may not adapt to their preexisting illness and appear less likely to further reduce their health perceptions in case their comorbid conditions aggravate [30]. A study showed that increase in age was associated with better mental health component but poorer physical component of health-related quality of life in patients suffering from chronic diseases [35]. Hence, age may confound the role of comorbid states predicting outcomes of such multi-morbid patients as MHD patients. On the other hand, it may be argued that the main driver of CCI is age and that comorbid states without age would not strongly associate with outcomes.
In this study, fully adjusted model for the hazard ratio in non-African American patients was 1.27 (1.12–1.44) (P < 0.001) as compared to 1.07 (0.94–1.21) (P = 0.30) in African Americans. These observations may indicate that the mCCI is more robust in non-African American patients, although we might have not enough statistical power in the subgroup of African Americans. Over the past several decades, African American and Hispanic patients with end-stage renal disease (ESRD) have been shown to have consistently greater survival as compared to non-Hispanic Whites [36, 37]. The death rates in the former two groups were 187 and 180 per 1,000 patient-years at risk, respectively, as compared to 207 per 1,000 patient-years at risk for non-Hispanic Whites with ESRD [36, 37]. The causes for these disparities are largely unknown. Similarly, for the healthier MHD patients who are on transplant wait lists, the annual mortality rate is higher for non-Hispanics whites as compared with African Americans [38–40].
Di Iorio et al. [11] reported that the crude mortality rate increased by approximately 60% of patient-years across incident hemodialysis patients when the CCI score was 3 in contrast to when the CCI score was 6. They also found that in addition to CCI, days of hospitalization were an important independent predictor of mortality, and the predictive capacity of CCI for survival improved when it was adjusted for days of hospitalization [11]. Our multivariate analysis did not include the effect of days of hospitalization on mortality as done by Iorio et al. [11], because we do not have data about hospitalization. Fried et al. [10] studied incident peritoneal dialysis patients and reported a 54% higher risk of death for every increase of 1 in CCI score. Our study results have shown a similar strength of CCI in predicting mortality in prevalent MHD patients when compared to previously reported studies. Kalantar et al. [41] showed that an increase in serum albumin levels in MHD patients is associated with increased survival, consistent with our correlation analyses, demonstrating serum albumin correlated significantly with CCI. When we further explored this relation by logistic regression, and the data showed in our fully adjusted model that each mg/dl increase in serum albumin level was associated with a 62% lower risk of a high CCI score [OR: 0.38, 95% CI: (0.24–0.60)].
A potential limitation of the present study is selection bias during enrollment. However, since mortality in our cohort was less than in the general MHD population in the United States [12], it might be argued that such a selection bias would lead to a bias toward the null. Hence, without such a bias, our results may have been even stronger. Moreover, our cohort was younger and had shorter duration of dialysis than the national average MHD patient as described in the USRDS. Other limitations include lack of information on vascular access for dialysis, dialysis membranes used and other unknown confounders. Another limitation is the mCCI scores were only obtained at baseline. Since comorbidity must have changed in some patients over the time, this might have affected our results. The strengths of our study include the sample size, which was moderately large, the comprehensive clinical and laboratory evaluations with concomitant assessment of quality of life and body composition measures, and detailed evaluation of comorbid states by study physicians. Unlike previous cohorts that have been studied, ours was rather well characterized for important emerging mediators for mortality, specifically markers of inflammation and nutritional status, including direct total body fat measurements. Also the subjects were selected randomly without prior knowledge of their status. Moreover, this was the first attempt to study the strength of mCCI to predict mortality according to race.
Conclusions
Our study has further confirmed the fact that mCCI even without the age component is still a strong predictor of mortality in MHD patients. The mCCI score was a more robust predictor of mortality in non-African Americans MHD patients. Additional studies are needed to assess mortality predictors in different subgroups of dialysis patients, including whether there are other factors such a socio-cultural, bio-nutritional or others that override traditional co-existing medical conditions as significant mortality predictors in this population. Such studies could help extend our understanding of high dialysis mortality and ultimately lead to improved outcomes for all populations with chronic disease states.
Acknowledgments
The study was supported by Dr. Kalantar-Zadeh’s research grants from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (R01 DK078106, R21 DK078012, and K23 DK61162), a research grant from DaVita Clinical Research and a philanthropic grant from Mr. Harold Simmons. MZM received grants from the National Developmental Agency (KTIAOTKA-EU 7KP-HUMAN-MB08-A-81231) from the Research and Technological Innovation Fund, was also supported by Hungarian Kidney Foundation.
Footnotes
Conflict of interest Dr. Nissenson is an employee of DaVita. Dr. Kalantar-Zadeh is the medical director of DaVita Harbor-UCLA/MFI in Long Beach, CA. Other authors have not declared any conflict of interest.
Contributor Information
Manoch Rattanasompattikul, Harold Simmons Center for Chronic Disease Research and Epidemiology, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, 1124 West Carson Street, C1-Annex, Torrance, CA 90502, USA; Division of Nephrology and Hypertension, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA, USA.
Usama Feroze, Harold Simmons Center for Chronic Disease Research and Epidemiology, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, 1124 West Carson Street, C1-Annex, Torrance, CA 90502, USA; Division of Nephrology and Hypertension, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA, USA.
Miklos Z. Molnar, Harold Simmons Center for Chronic Disease Research and Epidemiology, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, 1124 West Carson Street, C1-Annex, Torrance, CA 90502, USA Institute of Pathophysiology, Semmelweis University, Budapest, Hungary.
Ramanath Dukkipati, Division of Nephrology and Hypertension, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA, USA; David Geffen School of Medicine at UCLA and the UCLA School of Public Health, Los Angeles, CA, USA.
Csaba P. Kovesdy, Division of Nephrology, University of Virginia, Charlottesville, VA, USA Division of Nephrology, Salem VA Medical Center, Salem, VA, USA.
Allen R. Nissenson, Email: Allen.nissenson@davita.com, David Geffen School of Medicine at UCLA and the UCLA School of Public Health, Los Angeles, CA, USA; DaVita, Inc., Denver, CO, USA.
Keith C. Norris, David Geffen School of Medicine at UCLA and the UCLA School of Public Health, Los Angeles, CA, USA
Joel D. Kopple, Division of Nephrology and Hypertension, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA, USA David Geffen School of Medicine at UCLA and the UCLA School of Public Health, Los Angeles, CA, USA.
Kamyar Kalantar-Zadeh, Email: kamkal@ucla.edu, Harold Simmons Center for Chronic Disease Research and Epidemiology, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, 1124 West Carson Street, C1-Annex, Torrance, CA 90502, USA; Division of Nephrology and Hypertension, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA, USA; David Geffen School of Medicine at UCLA and the UCLA School of Public Health, Los Angeles, CA, USA.
References
- 1.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, Kasiske B, Liu J, Mau LW, McBean M, Murray A, St Peter W, Guo H, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. United States Renal Data System 2008 annual data report. Am J Kidney Dis. 2009;53:S1–S374. doi: 10.1053/j.ajkd.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Khan IH. Comorbidity: the major challenge for survival and quality of life in end-stage renal disease. Nephrol Dial Transpl. 1998;13(suppl 1):76–79. doi: 10.1093/ndt/13.suppl_1.76. [DOI] [PubMed] [Google Scholar]
- 3.van Manen JG, Korevaar JC, Dekker FW, Boeschoten EW, Bossuyt PM, Krediet RT. How to adjust for comorbidity in survival studies in ESRD patients: a comparison of different indices. Am J Kidney Dis. 2002;40:82–89. doi: 10.1053/ajkd.2002.33916. [DOI] [PubMed] [Google Scholar]
- 4.Khan IH, Catto GR, Edward N, Fleming LW, Henderson IS, MacLeod AM. Influence of coexisting disease on survival on renal-replacement therapy. Lancet. 1993;341:415–418. doi: 10.1016/0140-6736(93)93003-j. [DOI] [PubMed] [Google Scholar]
- 5.Chandna SM, Schulz J, Lawrence C, Greenwood RN, Farrington K. Is there a rationale for rationing chronic dialysis? A hospital based cohort study of factors affecting survival and morbidity. BMJ (Clin Res Ed) 1999;318:217–223. doi: 10.1136/bmj.318.7178.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Athienites NV, Miskulin DC, Fernandez G, Bunnapradist S, Simon G, Landa M, Schmid CH, Greenfield S, Levey AS, Meyer KB. Comorbidity assessment in hemodialysis and peritoneal dialysis using the index of coexistent disease. Semin Dial. 2000;13:320–326. doi: 10.1046/j.1525-139x.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- 8.Davies SJ, Russell L, Bryan J, Phillips L, Russell GI. Comorbidity, urea kinetics, and appetite in continuous ambulatory peritoneal dialysis patients: their interrelationship and prediction of survival. Am J Kidney Dis. 1995;26:353–361. doi: 10.1016/0272-6386(95)90657-6. [DOI] [PubMed] [Google Scholar]
- 9.Hall SF. A user’s guide to selecting a comorbidity index for clinical research. J Clin Epidemiol. 2006;59:849–855. doi: 10.1016/j.jclinepi.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Fried L, Bernardini J, Piraino B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis. 2001;37:337–342. doi: 10.1053/ajkd.2001.21300. [DOI] [PubMed] [Google Scholar]
- 11.Di Iorio B, Cillo N, Cirillo M, De Santo NG. Charlson comorbidity index is a predictor of outcomes in incident hemodialysis patients and correlates with phase angle and hospitalization. Int J Artif Organs. 2004;27:330–336. doi: 10.1177/039139880402700409. [DOI] [PubMed] [Google Scholar]
- 12.Colman S, Bross R, Benner D, Chow J, Braglia A, Arzaghi J, Dennis J, Martinez L, Baldo DB, Agarwal V, Trundnowski T, Zitterkoph J, Martinez B, Khawar OS, Kalantar-Zadeh K. The nutritional and inflammatory evaluation in dialysis patients (NIED) study: overview of the NIED study and the role of dietitians. J Ren Nutr. 2005;15:231–243. doi: 10.1053/j.jrn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Shantouf RS, Budoff MJ, Ahmadi N, Ghaffari A, Flores F, Gopal A, Noori N, Jing J, Kovesdy CP, Kalantar-Zadeh K. Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am J Nephrol. 2010;31:419–425. doi: 10.1159/000294405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noori N, Kopple JD, Kovesdy CP, Feroze U, Sim JJ, Murali SB, Luna A, Gomez M, Luna C, Bross R, Nissenson AR, Kalantar-Zadeh K. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:2258–2268. doi: 10.2215/CJN.02080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noori N, Kalantar-Zadeh K, Kovesdy CP, Bross R, Benner D, Kopple JD. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:683–692. doi: 10.2215/CJN.08601209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noori N, Kalantar-Zadeh K, Kovesdy CP, Murali SB, Bross R, Nissenson AR, Kopple JD. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010;56:338–347. doi: 10.1053/j.ajkd.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raj DS, Shah VO, Rambod M, Kovesdy CP, Kalantar-Zadeh K. Association of soluble endotoxin receptor CD14 and mortality among patients undergoing hemodialysis. Am J Kidney Dis. 2009;54:1062–1071. doi: 10.1053/j.ajkd.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehrotra R, Kermah D, Fried L, Kalantar-Zadeh K, Khawar O, Norris K, Nissenson A. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J Am Soc Nephrol. 2007;18:2781–2788. doi: 10.1681/ASN.2006101130. [DOI] [PubMed] [Google Scholar]
- 19.Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med. 2000;108:609–613. doi: 10.1016/s0002-9343(00)00371-5. [DOI] [PubMed] [Google Scholar]
- 20.Nelson EE, Hong CD, Pesce AL, Peterson DW, Singh S, Pollak VE. Anthropometric norms for the dialysis population. Am J Kidney Dis. 1990;16:32–37. doi: 10.1016/s0272-6386(12)80782-7. [DOI] [PubMed] [Google Scholar]
- 21.Williams AJ, McArley A. Body composition, treatment time, and outcome in hemodialysis patients. J Ren Nutr. 1999;9:157–162. doi: 10.1016/s1051-2276(99)90056-0. [DOI] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Dunne E, Nixon K, Kahn K, Lee GH, Kleiner M, Luft FC. Near infra-red interactance for nutritional assessment of dialysis patients. Nephrol Dial Transpl. 1999;14:169–175. doi: 10.1093/ndt/14.1.169. [DOI] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Kuwae N, Wu DY, Shantouf RS, Fouque D, Anker SD, Block G, Kopple JD. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr. 2006;83:202–210. doi: 10.1093/ajcn/83.2.202. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 25.Erbagci AB, Tarakcioglu M, Aksoy M, Kocabas R, Nacak M, Aynacioglu AS, Sivrikoz C. Diagnostic value of CRP and Lp(a) in coronary heart disease. Acta Cardiol. 2002;57:197–204. doi: 10.2143/AC.57.3.2005389. [DOI] [PubMed] [Google Scholar]
- 26.Pecoits-Filho R, Barany P, Lindholm B, Heimburger O, Stenvinkel P. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transpl. 2002;17:1684–1688. doi: 10.1093/ndt/17.9.1684. [DOI] [PubMed] [Google Scholar]
- 27.Beutler B, Cerami A. The biology of cachectin/TNF–a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 28.Keane WF, Collins AJ. Influence of co-morbidity on mortality and morbidity in patients treated with hemodialysis. Am J Kidney Dis. 1994;24:1010–1018. doi: 10.1016/s0272-6386(12)81076-6. [DOI] [PubMed] [Google Scholar]
- 29.Wright LF. Survival in patients with end-stage renal disease. Am J Kidney Dis. 1991;17:25–28. doi: 10.1016/s0272-6386(12)80245-9. [DOI] [PubMed] [Google Scholar]
- 30.Heller DA, Ahern FM, Pringle KE, Brown TV. Among older adults, the responsiveness of self-rated health to changes in Charlson comorbidity was moderated by age and baseline comorbidity. J Clin Epidemiol. 2009;62:177–187. doi: 10.1016/j.jclinepi.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61:1234–1240. doi: 10.1016/j.jclinepi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Zavascki AP, Fuchs SC. The need for reappraisal of AIDS score weight of Charlson comorbidity index. J Clin Epidemiol. 2007;60:867–868. doi: 10.1016/j.jclinepi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Nuttall M, van der Meulen J, Emberton M. Charlson scores based on ICD-10 administrative data were valid in assessing comorbidity in patients undergoing urological cancer surgery. J Clin Epidemiol. 2006;59:265–273. doi: 10.1016/j.jclinepi.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Miskulin DC, Martin AA, Brown R, Fink NE, Coresh J, Powe NR, Zager PG, Meyer KB, Levey AS. Predicting 1 year mortality in an outpatient haemodialysis population: a comparison of comorbidity instruments. Nephrol Dial Transpl. 2004;19:413–420. doi: 10.1093/ndt/gfg571. [DOI] [PubMed] [Google Scholar]
- 35.Hopman WM, Harrison MB, Coo H, Friedberg E, Buchanan M, VanDenKerkhof EG. Associations between chronic disease, age and physical and mental health status. Chronic Dis Can. 2009;29:108–116. [PubMed] [Google Scholar]
- 36.System United States Renal Data. United States renal data system 2006 annual data report atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2007;49:1–296. doi: 10.1053/j.ajkd.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 37.United States Renal Data System. Excerpts from the USRDS 2005 annual data report: atlas of end-stage renal disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Am J Kid Dis. 2006;47(suppl 1):1–286. [Google Scholar]
- 38.Agodoa L, Norris K, Pugsley D. The disproportionate burden of kidney disease in those who can least afford it. Kidney Int Suppl. 2005;97:S1–S3. doi: 10.1111/j.1523-1755.2005.09700.x. [DOI] [PubMed] [Google Scholar]
- 39.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14:2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 40.Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ. Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged medicare beneficiaries. J Am Soc Nephrol. 2007;18:1299–1306. doi: 10.1681/ASN.2006050524. [DOI] [PubMed] [Google Scholar]
- 41.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, McAllister CJ, Alcorn H, Jr, Kopple JD, Greenland S. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transpl. 2005;20:1880–1888. doi: 10.1093/ndt/gfh941. [DOI] [PubMed] [Google Scholar]