Abstract
Objective
In severe stenosis, von Willebrand Factor (vWF) experiences millisecond exposures to pathological wall shear rates (γw). We sought to evaluate the deposition of vWF onto collagen surfaces under flow in these environments.
Methods and Results
Distinct from shear experiments that last many seconds, we deployed microfluidic devices for single-pass perfusion of whole blood or platelet free plasma (PFP) over fibrillar type 1 collagen (< 50 msec transit time) at pathological γw or spatial wall shear rate gradient (grad γw). Using fluorescent anti-vWF, long thick vWF fibers (>20 μm) bound to collagen were visualized at constant γw > 30,000 s−1 during perfusion of PFP, a process enhanced by EDTA. Rapid acceleration or deceleration of EDTA-PFP at grad γw = ± 5.5 × 105 to 4.3 × 107 s−1/cm did not promote vWF deposition. At 19,400 s−1, EDTA-blood perfusion resulted in rolling vWF-platelet nets, while blood perfusion (normal Ca2+) generated large vWF/platelet deposits that repeatedly embolized and were blocked by anti-GPIb or the α IIbβ3 inhibitor GR144053 and did not require grad γw. Blood perfusion at venous shear rate (200 s−1) produced a stable platelet deposit that was a substrate for massive but unstable vWF-platelet aggregates when flow was increased to 7800 s−1.
Conclusion
Triggered by collagen and enhanced by platelet GPIb and α IIbβ3, vWF fiber formation occurred during acute exposures to pathological γw and did not require gradients in wall shear rate.
Keywords: hemodynamics, stenosis, von Willebrand Factor, platelet, glycoprotein Ib
Introduction
Severe arterial stenoses are sites of extreme flow acceleration and deceleration where pathological wall shear rates (γw) can range from 5,000 to >100,000 s−1 (wall shear stresses of 150 to >3000 dyne/cm2).1-5 Greater than 75% stenosis is required before a significant flow resistance causes symptoms of angina.6 Severe stenosis or coarctation can result in a flow recirculation zone in the post stenotic region with a transition to turbulence and vortex shedding during the deceleration phase of diastoli.5,7,8
Sites of flow disturbance due to complex secondary flows, oscillatory slow flow, reversing flows, or extreme pathological shear rates and shear rate gradients are correlated with endothelial dysfunction.9 These sites are also predisposed to atherosclerosis, plaque rupture, platelet activation and thrombosis. Peak spatial gradients in γw, grad γw = + 600,000 s−1/cm (grad wall shear stress, grad τw = 20,000 dyne/cm2 per cm) are predicted for the inlet to a human coronary artery stenosis.3 At the exit of a human coronary stenosis, maximal grad γw approaches -3 × 106 s−1/cm.3 Similarly, Schirmer4 report grad τw = ± 20,000 dyne/cm2 per cm in human carotid stenosis (grad γw = ± 570,000 s−1/cm).
At sites of arterial injury, von Willebrand factor (vWF) greatly facilitates platelet capture via tethering through GPIb binding.10 After binding collagen type I or type III, vWF can undergo a conformational change which enhances exposure of the A1 domain to promote GPIb interaction with platelets.11 The binding of vWF A1 domain to platelet GPIb is essential for platelet adhesion at physiological arterial wall shear rates (>1500 s−1). In patients with von Willebrand disease (vWD) type 3, a condition in which almost no detectable vWF is present, a severe bleeding phenotype is observed.12
When sheared continuously in a cone-and-plate viscometer, high concentrations of soluble vWF (100 μg/ml) will undergo aggregation into enormous fibrous aggregates of 32 × 106 MW (2155 s−1 for 30 sec) to 847 × 106 MW (6000 s−1 for 120 sec).13 Similarly, shearing in a cone-and-plate viscometer at > 2200 s−1 for ∼ 3 min will result in shear induced platelet activation (SIPA), a phenomena that requires vWF and platelet release of ADP.14-17 Ultralarge vWF (uLvWF) is particularly potent in mediating SIPA as observed in a cone-and-plate viscometer.18 Recirculation of a vWF solution in an air-exposed, piezo-driven recirculation results in vWF unfolding and extension at a critical shear rate > 5000 s−1, but the time of exposure needed to observe this response is unknown and the effect of the air-liquid interface is potentially problematic in the study of proteins.19 Steppich et al. used 10 to 20× physiological levels of vWF (200 μg/mL) exposed to “high shear” for a “few minutes” to create vWF fibril-aggregates.20 Similarly, Barg et al. detected vWF fiber formation on collagen following 1-minute perfusion of 10× physiological levels (50 μg/mL) VWF at 35 dyne/cm2 (∼5000 s−1) which was not observed at physiological levels of 5 μg/ml vWF.21
In contrast to a viscometer or closed-loop recirculation systems, blood is never continually experiencing pathological shear stresses in vivo for many seconds or minutes. Rather, the exposures are extremely brief and the unfolding/fiber forming behavior of vWF during exposure to pathological flows is not well studied under such conditions. Using a stenosis microfluidic device, Nesbitt et al. reported a role for shear rate gradients as a causative mechanisms in local platelet accumulation which was GPIb-dependent,22 however the underlying role of vWF structural changes were not explored. Interestingly, platelet display of P-selectin downstream of a human stenosis can increase relative to the upstream position.23 Also patients with severe aortic stenosis (wall shear stress of 118 dyne/cm2 or ∼4000 s−1 shear rate) display a depletion of the largest vWF multimers to yield an acquired von Willebrand syndrome.24 Similarly, left ventricular assist device (LVAD) patients can experience an acquired von Willebrand disease.25,26 As a shear sensitive molecule, vWF undergoes a conformational change in these extreme hemodynamic environments enhancing exposure of a concealed site, the A2 domain, which is susceptible to cleavage by the metalloprotease, ADAMTS13.27 At shear rates exceeding 15,000 s−1, rolling aggregates of discoid platelets have been observed in flow chambers on surfaces of vWF and collagen.28 These aggregates were independent of integrin function and required soluble vWF.28 In vivo, aggregates of discoid platelets have also been generated downstream of an artificial stenosis in the mouse mesenteric arteriole.22 In addition to GPIb/vWF interactions, these thrombi were dependent on αIIb β3 interactions, suggesting a role for integrin activation. These prior studies however did not investigate the structure of vWF during the creation of thrombotic entities.
The present study explores platelet free plasma and whole blood exposure to pathological shear rates during millisecond exposures in a microfluidic injury model of surface fibrillar collagen. We observed that vWF, detected via epifluorescence microscopy, forms massive fiber aggregates on collagen type 1 surfaces under pathological wall shear rate conditions in plasma and whole blood. When platelets are present, platelet-vWF deposits with elongated and aggregated vWF can be achieved at pathological shear rates even in the absence of Ca2+. Unstable and rolling aggregates that contain significant amounts of vWF as fibrous aggregates were also observed. We demonstrate that high shear rate environments lead to vWF deposition as well as massive and unstable platelet aggregation on platelet surfaces in the presence of Ca2+. These results represent the first real time visualization of massive vWF fiber deposition under pathophysiologically relevant conditions supported by collagen. Platelet interactions with these vWF aggregates range from firmly adhered monolayers to massive thrombi with embolic potential.
Materials and Methods
Blood collection and preparation
Blood was drawn via venipuncture from healthy males, who were self-reported as free of any disease or bleeding disorders, as well as any oral medication for at least 10 days. All blood donors provided informed written consent in accordance with the Internal Review Board of the University of Pennsylvania. Blood was anticoagulated with sodium citrate (Sigma, St. Louis, MO, USA) or D-Phe-Pro-Arg chloromethylketone (PPACK, 100 μM, Haematologic Industries, Essex, VT, USA) to inhibit thrombin. Platelet free plasma (PFP) was generated by centrifugation of whole blood at 1000g for 10 min. Severe von Willebrand Disease (vWD) plasma was from George King Biomedical (Overland Park, KS, USA) and contained less than 1% detectable vWF. For fluorescent detection of von Willebrand factor all samples were treated with a FITC-conjugated polyclonal anti-vWF antibody (1 μg/mL, AbD Serotec, Kidlington, UK). Some samples were treated with 5 mM EDTA (Sigma) to chelate calcium or 40 μg/mL of function-blocking anti-GPIb antibody AK2 (abcam, Cambridge, MA, USA).29 When platelet deposition was measured in real time epifluorescent microscopy, 0.125 μg/mL of fluorescently conjugated monoclonal antibody HIP8 (BD biosciences, San Jose, CA, USA) was added.30 In some experiments the αIIbβ3 antagonist, GR144053 (Tocris Biosciences, Bristol, UK) was added (final concentration, 2 μM) to block αIIbβ3 function.
Microfluidic flow experiments
Two types of microfluidic devices were used in this study. First, the straight channel microfluidic device had a 100 μm wide × 60 μm high cross section generated in poly(dimethylsiloxane) (Sylgard, Dow Corning, Midland, MI, USA) as previously described31 (Supplemental Fig. SIA). The second type of microfluidic device, the stenosis channel, consisted of a 500 μm wide × 60 μm high channel that rapidly constricted to a 15 μm wide × 1000 μm long channel (Supplemental Fig. SIB,C) before expanding again to a 500 μm wide channel. The entrance and exits to the stenosis region tapered and expanded over a 100 μm distance in an identical nonlinear fashion to prevent boundary layer separation. Whole blood or PFP samples were perfused through either device via withdraw using a syringe pump (Harvard Apparatus, Holliston, MA, USA). Surfaces of equine collagen type 1 (Chronopar, Chronolog, Havertown, PA, USA) were presented to the flow by using a previously described surface patterning technique (Supplemental Fig. SII).30,32 Briefly, a microchannel 250 μm wide by 5 cm long (which runs perpendicular to flow channels) was affixed to a glass substrate and was filled with a 1 mg/mL collagen solution and immediately rinsed with 0.5% bovine serum albumin (BSA, Sigma) in HEPES Buffered Saline (HBS, 20 mM HEPES, 150 mM NaCl, pH 7.4, Sigma). The patterning device was removed and the microfluidic device for blood or plasma perfusion was were placed on top of the collagen strip. The flow channels were blocked with BSA for at least 30 min prior to sample perfusion. Real-time deposition of vWF fibers was visualized using epifluorescence microscopy. In plasma experiments, vWF fibers were deposited and subsequently washed in BSA, stained with the fluorescently labeled anti-vWF, and visualized. In whole blood experiments, vWF fiber formation was visualized in real time by direct addition of the fluorescent antibody to the perfusion sample. Microfluidic chambers were mounted on an Olympus IX81 inverted microscope (Olympus America, Center Valley, PA) equipped with a CCD camera (Hamamatsu, Bridgewater, NJ).
Wall shear rates were calculated by evaluating the analytical solution to flow in a rectangular channel31 using a custom MATLAB (Mathworks, Nattick, MA, USA) script. Furthermore, a 3-dimensional model of the stenosis channel was generated in COMSOL (Burlington, MA) in order to determine the flow field and wall shear rate in the more complicated entrance and exit regions. COMSOL simulations were confirmed to reproduce the analytical results for steady flow in the straight rectangular regions of the stenosis microfluidic device. The spatial gradient of wall shear rate along the centerline is defined as grad γw = ∂γw/∂x|z=centerline, where the centerline wall shear rate is γw = ∂vx(y)/∂y|y=0.
Quantification of vWF fiber density and length
Density of vWF fibers on patterned collagen surfaces was assessed by measuring the number of fibers intersecting a line drawn perpendicular to the flow direction (we refer to this as a line scan). Unless noted, vWF density represents the average of three line scans drawn at 10, 50 and 90% of the height of the image. Fiber length was assessed by measuring the length of all fibers which intersect the line scan drawn at 50% of the image height.
Results
vWF fibers deposit on collagen type 1 surfaces under plasma flow: effect of spatial gradients
Fibrillar collagen type 1 (250 μm wide) was patterned across the inlet to the 15 μm region of the stenosis channel or across the outlet from the 15 μm region of the stenosis channel. A total of 20 μL of platelet free plasma (PFP) treated with 5 mM EDTA and perfused through the channels at three flow rates: 0.5, 2 or 20 μL/min. These plasma flow rates resulted in maximum centerline wall shear rates of 3,000, 12,500 and 125,000 s−1, respectively. Based on the velocity at a height of 150 nm above the surface, plasma transit time across the 250 μm collagen patch was 231 ms for a channel cross section of 500 μm and 38 ms at a 15 μm cross section for a 20 μL/min perfusion. After the 20 μL of plasma was perfused, the surface was rinsed with 0.5% BSA in HBS and treated with fluorescently labeled anti-vWF and rinsed again for visualization.
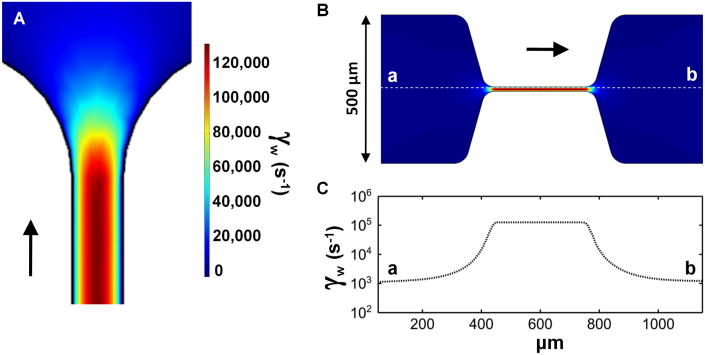
Computational fluid dynamics revealed a very steep center line wall shear rate gradient (grad γw) into the constricting and out of the expanding regions of the stenosis microfluidic device (Fig. 1A-C). For a 20 μL/min perfusion this gradient (grad γw) was calculated to be ± 4.3 × 107 s−1/cm. At 2 μL/min perfusion, the centerline grad γw was ± 4.3 × 106 s−1/cm and at 0.5 μL/min, grad γw was ± 110,000 s−1/cm.
Figure 1. Large wall shear rate gradients are generated in the inlet and outlet of a novel microfluidic model of stenosis.
A, Computational fluid dynamics defined the wall shear rates in the outlet of the stenosis channel. B, A representation of the stenosis channel in COMSOL. The colors indicate local wall shear rate and are equivalent to the scale bar in A. C, The local wall shear rate along the central line of the stenosis channel (dotted white line in B) indicates a steep gradient in centerline wall shear rate (1,000 s−1 to 125,000 s−1) at the inlet and outlet to the stenosis (grad γw of ± 4.3 × 107 s−1/cm).
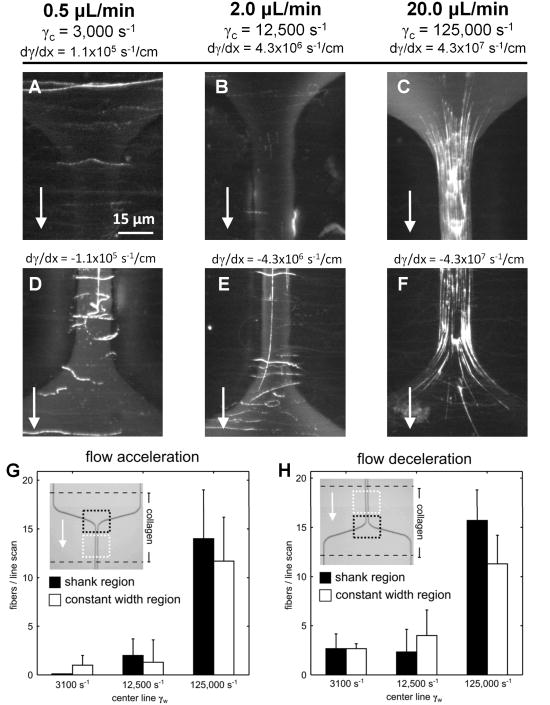
For centerline wall shear rates of 3,000 (Fig. 2A and 2D) and 12,500 s−1 (Fig. 2B and 2E) less than 5 fibers per line scan were observed and grad γw of ± 110,000 to ± 4.3 × 106 s−1/cm did not enhance fiber formation in the entrance or exit regions of the stenosis (Fig. 2G and 2H). At 20 μL/min, the maximum centerline gradient in wall shear rate was ±4.3 × 107 s−1/cm (Fig. 2C and 2F). In the shear gradient region, fiber generation was restricted to regions where the local wall shear rate was greater than ∼30,000 s−1 (Fig. 2C and 2F, Fig. 1A). There was no marked difference (Fig. 2G and 2H) between vWF deposition in the inlet region, the outlet region, or the constant width region (distal to a narrowing or distal to an expansion). The elevated shear rate environment produced long vWF fibers (>100 μm). A constant wall shear rate of 125,000 s−1 was sufficient to cause vWF elongation and fiber formation on the surface collagen. An extreme wall shear rate gradient of -4.3×107 s−1/cm for a rapidly decelerating flow actually quenched vWF deposition (Fig. 2F).
Figure 2. Von Willebrand Factor fibers deposit on surfaces of collagen type 1 in a microfluidic model of coronary stenosis.
A, 20 μL of citrated platelet free plasma (plus 5 mM EDTA) was perfused through a microfluidic channel over a collagen type 1 surface at the indicated wall shear rate (arrow, flow direction). The collagen surface was localized to the inlet of the stenosis where a gradient in wall shear rate was present (grad γw of 1.1 × 105 s−1/cm). Subsequently, the channel was treated with a fluorescently labeled antibody against vWF and washed. B-C, The same volume of PFP was perfused through an identical channel at 12,500 s−1 and 125,000 s−1, respectively. Few fibers were observed at 12,500 s−1 while many fibers were present at 125,000 s−1. D-F, The collagen strip was localized to the outlet region of the stenosis and similar flow conditions were imposed. Few vWF fibers were present at shear rates of 12,500 s−1 or less, while many fibers were observed at 125,000 s−1. G-H, The number of fibers intersecting a line drawn horizontally through the middle of the regions bound by dotted lines (black, shank region; white, constant width region) was counted at each centerline wall shear rate. The results indicate that non-zero grad γw in either the inlet or outlet region did not enhance the number of vWF fibers observed as compared to the constant width region distal to the shank region. We conclude that acute exposure to an extreme wall shear rates above 30,000 s−1 is a sufficient criteria for vWF fiber formation on collagen, independent of elongational forces in accelerating or decelerating flows.
vWF fibers deposit on collagen type 1 surfaces under plasma flow: effect of calcium
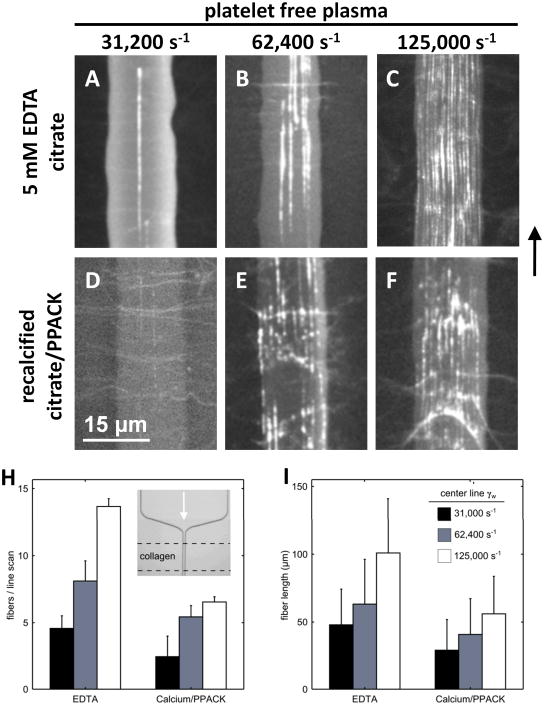
Since vWF and its cleaving protease, ADAMTS13, have calcium sensitive domains,35,36 we sought to understand the role of Ca2+ in the fiber deposition process. We compared citrated plasma treated with an additional 5 mM EDTA (∼2 nM free Ca2+) to recalcified citrated PFP treated with PPACK to prevent thrombin generation. In these experiments collagen patterning was restricted to the region of 15 μm channel width (Fig. 3H, inset). We observed that vWF fibers formed under EDTA conditions were continuous strands greater than 300 nm in width and 100 μm in length at the highest shear rate tested (125,000 s−1, Fig. 3C, Fig. 3H and 3I). At lower shear rates (31,200 s−1 and 62,400 s−1) the deposition of vWF was significantly reduced, as was fiber length (Fig. 3A,B, Fig. 3H and 3I). In recalcified citrated PFP with PPACK, we observed a significant increase in the number of deposited vWF fibers between the lowest and highest shears tested (Fig. 3D-F, Fig. 3H and 3I). At the highest shear rate tested (125,000 s−1) the fiber morphology was disjointed as compared to EDTA-treated samples, consisting of many strings of different lengths resulting in an average fiber length of 50 μm (Fig. 3F, Fig. 3I). Citrated PFP (∼40 μm Ca2+) performed identically to PPACK-plasma in this experiment (not shown). These results demonstrate that vWF elongated and formed fibers on collagen in a platelet-independent manner, but very high wall shear rates (those above 30,000 s−1) were required. Removal of calcium enhanced the deposition process. As expected, no deposition of vWF fibers were detected at 125,000 s−1 in vWD plasma (Supplemental Fig. SIII).
Figure 3. von Willebrand factor fibers deposit on collagen type 1 surfaces in the presence and absence of calcium and were dependent on the wall shear rate.
A-C, Equal quantities of platelet free plasma treated with EDTA (to chelate calcium) were perfused through the stenosis channel at the indicated wall shear rates over a collagen surface restricted to the constant width region. Representative images demonstrate the increase in vWF deposition as the wall shear rate was increased. D-F, The identical experiment performed in recalcified citrated PFP treated with PPACK to prevent thrombin generation. We observed vWF fibers under these conditions as well which showed a shear-dependent increase in fiber deposition. However, at the highest shear rate tested, the vWF fibers appeared disjointed. H, The number of fibers intersecting a horizontal line drawn across the channel (a line scan) were counted and averaged over 3 positions in the channel with 3 replicates per condition. A shear-dependent increase in the number of fibers was observed for all shear rates in the absence of Ca2+ (p<0.02) and between the lowest and highest shears tested in the presence of Ca2+ (p<0.01). There was also a significant increase in the number of fibers observed with chelation of Ca2+ at the highest and intermediate shear rates (p<0.03). I, The length of the fibers intersecting a line drawn through the center of the image were averaged for 3 replicates per condition. In EDTA, we observed significant reductions in fiber length between the highest shear rate tested and the intermediate and low shear rate (p<0.01). In the presence of Ca2+ a significant increase in fiber length was observed between the lowest and highest shear rates tested (p<0.01). Chelation of Ca2+ caused a significant increase in fiber length for all shear rates tested (p<0.03).
In order to confirm that accelerating flows at the stenosis inlet were not strictly necessary for vWF fiber deposition, these experiments were repeated using a constant width device lacking a stenotic region. For both EDTA-treated plasma as well as EDTA/anti-GPIb treated whole blood, vWF elongation on collagen was observed (Supplemental Fig. SIV-SV), demonstrating that exposure to an elongational flow was not strictly required for vWF fiber formation on collagen.
vWF fibers deposit on collagen type 1 surfaces under whole blood flow
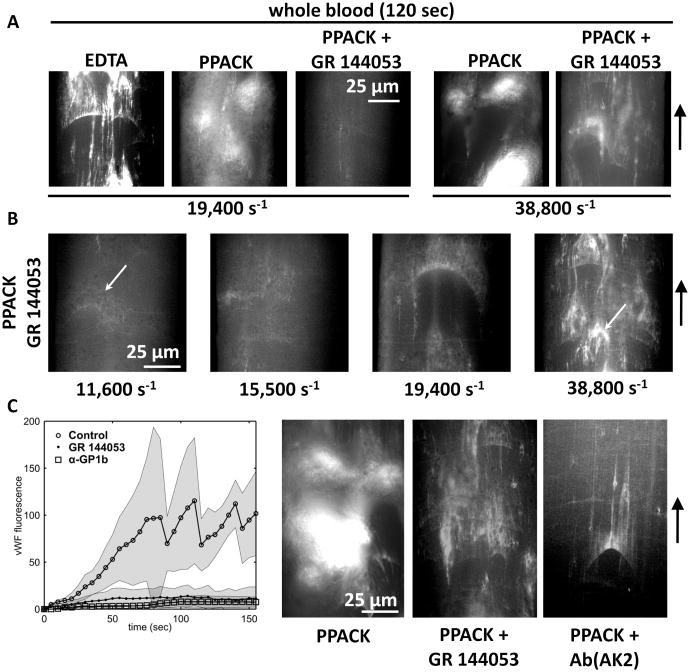
In order to avoid spatial wall shear rate gradients in the direction of flow, a larger microchannel with a constant cross section of 100 μm wide × 60 μm high was used for the study of whole blood flow. Transit times across the 250 μm long collagen stripe at 150 nm (a near wall vWF distance) and 3 μm (a near wall platelet distance) were 61.5 msec and 3.4 msec, respectively, at a centerline wall shear rate of 19,400 s−1. As was seen with EDTA-PFP, the deposition of long vWF fibers was observed at constant wall shear rate (grad γw = 0) for perfusion of EDTA-whole blood at 19,400 s−1 (Fig. 4A, EDTA). With EDTA present, large vWF fibers and nets were observed forming on the surface (Fig. 4A, PPACK). Even without functional integrins (due to chelation of Ca2+), these long fibers had adherent platelets present, while nets appeared to roll on the surface with trapped platelets (Supplemental Movie M1). Supplemental Supplemental Fig. SVI provides a montage of all of the conditions tested in Fig. 4A with 5 time points per condition.
Figure 4. vWF fibers deposit on collagen type 1 surfaces during whole blood flow perfusion in a straight channel geometry.
A, Whole blood was perfused in the presence of EDTA or normal Ca2+ with or without GR 144053 to block integrin function at the indicated wall shear rates. At 120 sec in the absence of Ca2+, long fibers of vWF are detected on the surface as well as rolling aggreagates of vWF and platelets. In the presence of Ca2+ large platelet aggregates form which embolized rapidly and incorporated vWF. In the absence of integrin function large aggregates no longer formed but faint fibers of vWF were seen on the surface, consistent with our previous results indicating that normal Ca2+ led to less vWF than EDTA conditions. At 2-fold elevated shear rates vWF was observed both in platelet aggregates (functioning integrins) and on the surface (blocked integrins). B, vWF fiber deposition was a shear-dependent process under normal Ca2+ in the absence of integrin function. At the lowest shear tested, firmly adherent platelets were observed (arrow) although vWF was not detected. With increasing shear more vWF was observed on the collagen surface. At the highest shear rate tested, rolling aggregates of vwF and platelets were observed (arrow). C, vWF fluorescence was measured over a 150 sec perfusion of untreated WB (PPACK) at 38,800 s−1 or samples treated with GR 144053 and a GPIb function blocking antibody (AK2). The results indicate that the large platelet masses formed on the collagen surface enhance vWF capture and require both active aIIbβ3 integrin as well as GPIb. The representative images (right) demonstrate the vWF fluorescence at the 120 sec time point for all conditions. The large platelet masses seen with PPACK vanish with addition of either inhibitor.
In the presence of Ca2+, functional integrins led to rapid platelet adhesion and embolization (Fig. 4A, PPACK). Fibers were observed, while large masses of platelets also stained positive for vWF (Supplemental Movie M2). To block αIIbβ3 platelet-platelet interactions via fibrinogen, a small molecule inhibitor of αIIbβ3 was used (2 μM, GR 144053, Supplemental Fig. SVII). This allowed us to replicate the integrin blocking effects of EDTA without chelating Ca2+. When αIIbβ3 was inhibited in the presence of Ca2+, large platelet masses were no longer present and faint fibers were seen adhering to the surface at 19,400 s−1 (Fig. 4A, PPACK + GR144053). VWF fiber formation (normal Ca2+) was more evident when the wall shear rate was increased to 38,800 s−1 (Fig. 4A). The dependency on γw of vWF deposition in the presence of PPACK and GR 144053 is shown in Fig. 4B. At the lowest shear rate tested (11,600 s−1), few fibers were observed, although several collagen fibers did stain positively for vWF. Increasing the shear rate to 15,500 s−1 revealed a modest increase in vWF fibers, while fibers longer than 50 μm required shear rates of 19,400 s−1. When the wall shear rate was increased to 38,800 s−1, many vWF fibers were generated on the surface as well as rolling clumps of vWF and platelets.
Fig. 4C presents time course data of vWF fluoescence on the collagen surface under perfusion of WB with normal Ca2+ (PPACK), PPACK plus GR 144053, and PPACK plus 40 μg/mL of a function-blocking antibody against GPIb (AK2). In the absence of inhibitors, large platelet aggregates that were highly vWF-positive (Fig. 4C, PPACK) were repeatedly generated and embolized rapidly. With αIIbβ3 antagonism (2 μM GR 144053), larger fibers as well as nets of vWF and platelets were detected (Fig. 4C, PPACK + GR 144053), however, large thrombi were not, resulting in a significant reduction in vWF signal (Fig. 4C). In the presence of AK2, formation of vWF fibers was not blocked, however, the large platelet masses and rolling nets of vWF and platelets were completely abolished. This resulted in a significant reduction in vWF signal, as well (Fig. 4C, PPACK + ab(AK2) and Supplemental Fig. SVIII). The presence of platelets in whole blood enhanced the formation vWF fibers on collagen surfaces under conditions of normal Ca2+ (Fig. 3B and 3D) for single pass transit time exposures of 3.4 to 61.5 msec.
vWF mediates rapid and embolic platelet adhesion at pathologic shear rates
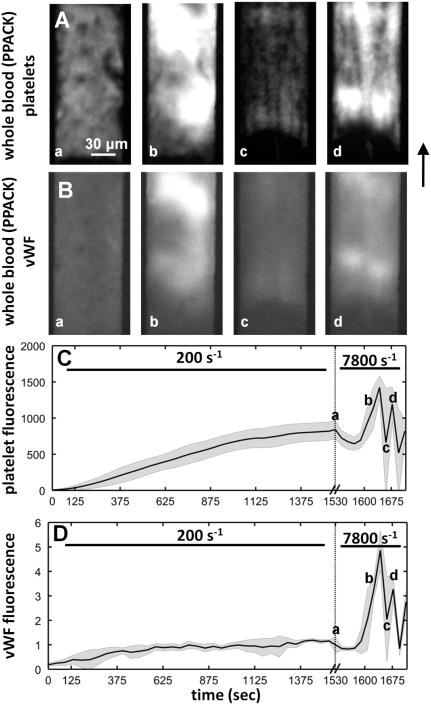
Whole blood anticoagulated with PPACK (normal Ca2+) and treated with fluorescently conjugated anti-vWF and anti-CD41a to label platelets was perfused over a collagen type 1 surface at a venous wall shear of 200 s−1. Platelet adhesion after 1500 seconds of perfusion had reached a steady state (Fig. 5A(a) and C). Very little vWF immunofluorescece and no elongated vWF fibers were observed in the platelet mass formed at venous shear rate (Fig. 5B(a) and D). At ∼1600 sec, the flow rate was suddenly increased such that the nominal wall shear rate would have been 7,800 s−1 in an empty channel, but was several fold-higher here because of channel obstruction due to the platelet mass.32 Within 100 sec of the shear rate increase, massive platelet accumulation had occurred, embolized, and rebounded (Fig. 5A(b-d) and C). However, upon shear increase the large platelet masses formed on top of the steady state surface incorporated vWF (Fig. 5B(b and d)). The vWF fluorescence data presented in Fig. 5d were normalized to the 1530 sec time point. When platelet deposition was reinitiated after the flow increase, a 5-fold increase in vWF was measured. Compared to the 2-fold increase in platelet adhesion, the vWF fluorescence indicated that the high shear rate conditions facilitated the incorporation and accumulation of plasma vWF into the growing but fragile deposit (Fig. 5C-D).
Figure 5. Onset of pathological shear rates triggered formation of massive aggregates that were unstable.
A, Platelet deposition from whole blood (PPACK) onto a collagen type 1 surface was monitored over a ∼1530 sec time period at an intial venous wall shear rate of 200 s−1. At the 1530 s, a stable non-growing platelet deposit had formed (a). The flow rate was suddenly increased such that in an empty channel the local wall shear rate would have been 7,800 s−1. Within 100 sec, massive platelet adhesion had initiated which resulted in a cycle of thrombus growth and embolization (b-d). The images are representative of multiple experiments. B, vWF content was also measured in the growing platelet aggregates. After 1530 sec of perfusion no detectable vWF was present. After the shear rate increase, the massive platelet aggregates that form were highly vWF-positive. C, Platelet accumulation was measured by fluorescence at the microfluidic injury site. The gray shading indicates the standard deviation of 4 experiments. Upon shear rate increase (a) the fluorescence intensity briefly drops as platelets were torn from the surface, and then recovered in a rapid burst of adhesion. Note the difference in time scales. D, vWF accumulation in the growing thrombus as flow was increased. The gray shading indicates the standard deviation of 4 experiments. The fluorescent intensity was normalized to the 1530 sec time point. Upon shear rate increase (a) vWF was incorporated into the rapidly growing thrombus.
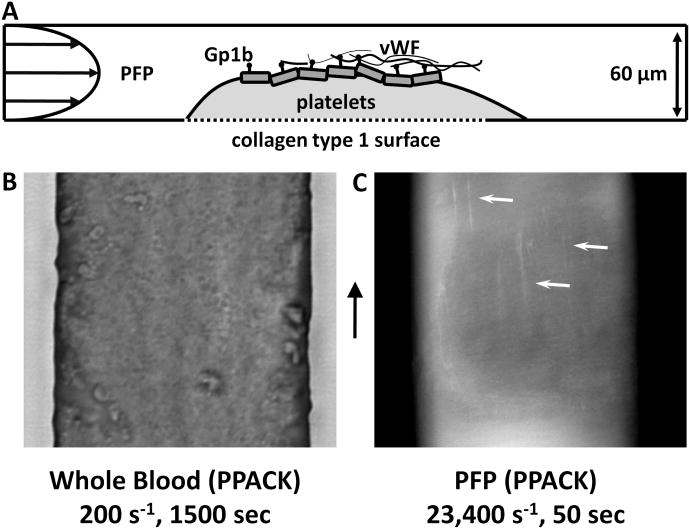
In a parallel experiment (Fig. 6A), a steady state surface of platelets was generated in an identical manner to that in Fig. 5A(a) and was briefly rinsed with HBS containing 5 mM Ca2+(Fig. 6B). PPACK-treated-PFP (normal Ca2+) was perfused over the surface at a wall shear rate of 23,400 s−1. Fig. 6C demonstrates vWF fibers forming on the surface of the platelet mass. These elongated fibers formed from PFP at pathogical shear conditions were generated on the platelet surface (no collagen present) and are likely the molecular unit responsible for nucleating the massive platelet adhesion events that occur under the pathological shear rate environments where whole blood was present (Fig. 5A(a)) instead of PFP.
Figure 6. vWF fibers deposit on platelet surfaces at pathological shear rates.
A, A schematic representation of the experimental setup. First, a steady state platelet surface was deposited on a collagen type 1 surface under whole blood (PPACK) flow for 1500 sec at 200 s−1. The flow was then switched to PFP, which was perfused at 23,400 s−1. B, A bright field image of the steady state platelet surface before the perfusion was switched to plasma. C, Plasma (PPACK, 1 μg/mL anti-vWF) was perfused over the surface in B at 23,400 s−1. After 50 seconds of plasma perfusion, vWF fibers were detected on the platelet surface (arrows).
Discussion
Long and thick fibers of von Willebrand factor were visualized while they formed on collagen type 1 surfaces under pathological shear rate conditions. These fibers were present regardless of the use of fluorescent antibody during the experiment or after perfusion. The observed fibers were collagen bound. Given their fluorescence width of several pixels (> 300 nm), the observed elongated vWF fiber bundles were highly unlikely to be individual extended vWF molecules (30 nm width < 1 pixel). Importantly, we observed that these vWF fibers were in a quenched state (i.e. bound to collagen), such that they did not relax when flow was stopped. We report that the fiber deposition process was not sensitive to the elongational flows present at the inlet or the outlet of the stenosis microfluidic device. We conclude that acute exposure to an extreme wall shear rates above 30,000 s−1 is a sufficient criteria for vWF fiber formation on collagen, independent of elongational forces in accelerating or decelerating flows. High wall shear rate and elongational flows are not mutually exclusive, however, and either or both may be operative in various situations. For example, in cases of borderline high or pathological arterial wall shear rates (3000-10,000 s−1), elongational flows at sites of stenosis may be relevant to vWF elongation as well as a driver of enhanced wall directed platelet fluxes. Furthermore, the process of vWF fiber deposition is distinct from existing literature focused on coil-stretched transitions33,34 in bulk flow as the fiber generation process described here occurs on the channel wall via collagen (see Supplemental Discussion).
Formation of these fibrils was evident in PPACK-treated whole blood at shear rates of 19,400 s−1, but was best visualized at 38,800 s−1. In PFP, the phenomenon had a threshold of ∼30,000 s−1. The absence of Ca2+ greatly enhanced the visualization of the fibers. The role of Ca2+ may be two fold in preventing the aggregation of vWF, as it supports refolding of the vWF A2 domain and is required for ADAMTS13 function.35,36 We have demonstrated that platelets rapidly accumulate and embolize under conditions of high pathological shear with a mechanism likely related to vWF unfolding and did not require a shear rate gradient. The large and unstable masses incorporate vWF and appear to be nucleated by vWF fibers aggregating on collagen or platelet surfaces. Fig. 4C demonstrated that VWF fiber formation and deposition during high shear whole blood flow was dependent on GPIb and αIIbβ3. However, platelet aggregation also requires these receptors and it is difficult to delineate collagen-vWF-receptor mechanisms from platelet aggregation-vWF mechanisms under these flow conditions. Since significant VWF fluorescence is associated with the platelet aggregates beyond that formed on the collagen surface, multiple flow-dependent processes may coexist (platelet aggregation, wall directed platelet fluxes, etc.) beyond the direct effect of flow on vWF fiber formation on collagen. The experiment shown in Fig. 6 was designed to understand if high shear rate can cause vWF fiber extension as a platelet mass accumulates. Indeed, elongated fibers were detected at high wall shear rate (Fig. 6C) when plasma was perfused over a preexisting platelet deposit. However, this result does not address if platelet receptors on flowing platelets potentiate vWF elongation and fiber bundle formation. Interestingly, the vWF:platelet ratio increased by a factor of about 3.3-fold for deposits formed at inlet wall shear rate of 7800 s−1 compared to those formed at 200 s−1 (Fig. 5C-D), suggestive of a direct enhancing effect of platelets on vWF deposition at pathological shear rates.
Pathological wall shear rates exceeding 30,000 s−1 are predicted in severe stenoses as well as in left ventricular assist devices (LVAD).1-5 Patients with severe aortic stenosis or an LVAD often present with a bleeding tendency that is linked to loss of the largest vWF multimers. Upon valve replacement or heart transplant the distribution of vWF multimers is corrected.25,37 Recently, Yong et al. have demonstrated that platelets respond to the acute exposure of elevated shear, as well.23 By sampling blood proximal and distal to a variety of coronary stenoses, the authors reported a significant increase in P-selectin exposure downstream of the occlusion, a result that correlated with stenosis severity. In contrast to soluble and globular plasma vWF in physiological hemodynamic conditions, elongated and aggregated vWF fibers formed under the flow conditions in this study may be particularly well suited for multivalent engagement of platelet GPIb, a receptor whose clustering by closely situated A1 domains in the vWF fiber may help drive GPIb-mediated signaling. Generation of vWF fibers may participate in this P-selectin display.
In a recent study, Nesbitt et al. suggested that shear gradients drove the formation of a loosely packed discoid platelet aggregate in a microfluidic model of stenosis lacking an adhesive protein surface. The authors demonstrated that no aggregates formed in a channel of constant cross section under the same hemodynamic conditions. They reported that Gp1b was required for initial platelet adhesion at the stenosis apex, while Gp1b and αIIbβ3 were required for thrombus propagation. In this study we have found that rapid formation of loosely packed aggregates did form in channels of constant cross section but required a surface of vWF-fiber coated collagen or platelets. In agreement with the authors, we demonstrate that Gp1b can support platelet firm adhesion on these surfaces in the absence of active integrin, but that both are necessary for thrombus propagation. We suggest that the magnitude of γw can serve as the driving force behind loosely packed platelet aggregates, a process which requires the elongation and aggregation of vWF bundles on a suitable surface. We have demonstrated that shear rate gradients of ± 1.1 × 105 - 4.3 × 107 s−1/cm do not substantially enhance the process of vWF deposition using a microfluidic stenosis model when perfusing EDTA-PFP over collagen.
Lastly, we present a role for platelet bound vWF in thrombogenesis at pathological shear rates. Using a microfluidic channel of constant 100 μm cross section, we generated steady state thrombi whose ability to capture platelets was restored by a sudden increase in shear rate. We suggest that platelet recruitment was re-established by enhanced GPIb/vWF tethering due to increased vWF self-aggregation at high shear on the thrombus surface. Before the sudden increase in shear rate (Fig. 5), the thrombus had grown to create hemodynamic conditions which prevented further platelet attachment and was insufficient to cause vWF fiber formation. After the flow rate increase, vWF fibers formed and were able to support platelet tethering and subsequent activation required for stable adhesion. A similar process was proposed by Kulkarni et al. who illustrated the importance of soluble vWF in platelet adhesion to an immobilized platelet monolayer in the absence of an activating surface, even at venous shear rates.38 They suggest that without soluble agonists tethering platelets have less active αIIbβ3 and require more GPIb/vWF interactions to achieve stable adhesion. Our results are consistent with these earlier findings and we have visualized the fundamental event of vWF fiber formation that may underlie these prior observations.
During arterial thrombosis at physiological wall shear rates (for example in mild stenosis with plaque rupture), vWF elongation and fiber formation may initially be minimal. However, as the thrombus growth begins to occlude the vessel and before a significant pressure drop is created by the nonocclusive thrombus (<75% stenosis), the wall shear rates will increase dramatically with a consequent transition to elongated and fibrous vWF mediating platelet capture. At high pathological shear rates, these nets of vWF and platelets may be less stable and subject to embolism, consistent with a condition of unstable angina or cyclic thrombosis. This study defines precise experimental conditions in which to detect and measure the dynamics of vWF unfolding, vWF fiber elongation, and aggregate formation at pathological shear rates on collagen.
Supplementary Material
Acknowledgments
T.V. Colace performed the experiments, analyzed data, and contributed to the manuscript. S.L. Diamond designed the research, analyzed data, and contributed to the manuscript.
Sources of Funding: This work was supported by the National Institutes of Health NIH R01 HL103419 (S.L.D.) and a predoctoral training grant (5T32HL007439-33) to T. Colace from the NIH.
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li MX, Beech-Brandt JJ, John LR, Hoskins PR, Easson WJ. Numerical analysis of pulsatile blood flow and vessel wall mechanics in different degrees of stenoses. Journal of biomechanics. 2007;40(16):3715–3724. doi: 10.1016/j.jbiomech.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Bark DL, Ku DN. Wall shear over high degree stenoses pertinent to atherothrombosis. J Biomech. 2010;43:2970–2977. doi: 10.1016/j.jbiomech.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee RK, Back LH, Back MR, Cho YI. Physiological flow analysis in significant human coronary artery stenoses. Biorheology. 2003;40:451–476. [PubMed] [Google Scholar]
- 4.Schirmer CM, Malek AM. Computational fluid dynamic characterization of carotid bifurcation stenosis in patient-based geometries. Brain Behav. 2012;2:45–52. doi: 10.1002/brb3.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stroud JS, Berger SA, Saloner D. Numerical analysis of flow through a severely stenotic carotid artery bifurcation. J Biomech Eng. 2002;124:9–20. doi: 10.1115/1.1427042. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Vignon-Clementel IE, Coogan JS, Figueroa CA, Jansen KE, Taylor CA. Patient-specific modeling of blood flow and pressure in human coronary arteries. Ann Biomed Eng. 2010;38(10):3195–3209. doi: 10.1007/s10439-010-0083-6. [DOI] [PubMed] [Google Scholar]
- 7.Long Q, Xu XY, Ramnarine KV, Hoskins P. Numerical investigation of physiologically realistic pulsatile flow through arterial stenosis. J Biomech. 2001;34:1229–1242. doi: 10.1016/s0021-9290(01)00100-2. [DOI] [PubMed] [Google Scholar]
- 8.Ojha M, Cobbold RS, Johnston KW, Hummel RL. Detailed visualization of pulsatile flow fields produced by modeled arterial stenoses. J Biomed Eng. 1990;12:463–469. doi: 10.1016/0141-5425(90)90055-r. [DOI] [PubMed] [Google Scholar]
- 9.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. J Am Med Assoc. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 10.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand Factor. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 11.Goto S, Salomon DR, Ikeda Y, Ruggeri ZM. Characterization of the unique mechanism mediating the shear-dependent binding of soluble von Willebrand factor to platelets. J Biol Chem. 1995;270:23352–23361. doi: 10.1074/jbc.270.40.23352. [DOI] [PubMed] [Google Scholar]
- 12.Sadler JE, Budde U, Eikenboom J, Favaloro EJ, Hill FGH, Holmberg L, Ingersleve J, Lee CA, Lillicrap D, Mannucci PM, Mazurier C, Meyer D, Nichols WL, Nishino M, Peake IR, Rodeghiero F, Schneppenheim R, Ruggeri ZM, Srivastava A, Montgomery RR, Federici AB. Update on the pathophysiology and classification of von Willebrand disease: a report of the SUbcommitte on von Willebrand factor. J Thromb Haemost. 2006;4:2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 13.Shankaran H, Alexandridis P, Neelamegham S. Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood. 2003;101:2637–2645. doi: 10.1182/blood-2002-05-1550. [DOI] [PubMed] [Google Scholar]
- 14.Dayananda KM, Singh I, Mondal N, Neelamegam S. Von Willebrand factor self-association on platelet GpIba under hydrodynamic shear: effect on shear-induced platelet activation. Blood. 2010;119(19):3990–3998. doi: 10.1182/blood-2010-02-269266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson DM, Stathopoulos NA, Giorgio TD, Hellums JD, Moake JL. Shear-induced platelet aggregation requires von Willebrand factor and platelet membrane glycoproteins Ib and IIb-IIIa. Blood. 1987;69:625–628. [PubMed] [Google Scholar]
- 16.Moake JL, Turner NA, Stathopoulos NA, Nolasco L, Hellums JD. Shear-induced platelet aggregation can be mediated by vWF released from platelets, as well as by exogenous large or unusually large vWF multimers, requires adenosine diphosphate, and is resistant to aspirin. Blood. 1988;71:1366–1374. [PubMed] [Google Scholar]
- 17.Li F, Li CQ, Moake JL, Lopez JA, McIntire LV. Shear stress-induced binding of large and unusually large von Willebrand factor to human platelet glycoprotein Ibalpha. Ann Biomed Eng. 2004;32:961–969. doi: 10.1023/b:abme.0000032458.88212.54. [DOI] [PubMed] [Google Scholar]
- 18.Kumar RA, Moake JL, Nolasco L, Bergeron AL, Sun C, Dong JF, McIntire LV. Enhanced platelet adhesion and aggregation by endothelial cell-derived unusually large multimers of von Willebrand factor. Biorheology. 2006;43:681–691. [PubMed] [Google Scholar]
- 19.Schneider SW, Nuschele S, Wixforth A, Gorzelanny C, Alexander-Katz A, Netz RR, Schneider MF. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci U S A. 2007;104:7899–7903. doi: 10.1073/pnas.0608422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steppich DM, Angerer JI, Sritharan K, Schneider SW, Thalhammer S, Wixforth A, Alexander-Katz A, Schneider MF. Relaxation of ultralarge VWF bundles in a microfluidic-AFM hybrid reactor. Biochem Biophys Res Commun. 2008;369:507–512. doi: 10.1016/j.bbrc.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 21.Barg A, Ossig R, Goerge T, Schneider MF, Schillers H, Oberleithner H, Schneider SW. Soluble plasma-derived von Willebrand factor assembles to a haemostatically active filamentous network. Thromb Haemost. 2007;97:514–526. [PubMed] [Google Scholar]
- 22.Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, Carberry J, Fouras A, Jackson SP. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 23.Yong ASC, Pennings GJ, Chang M, Hamzah A, Chung T, Qi M, Brieger D, Behnia M, Krilis SA, Ng MKC, Lowe HC, Kritharides L. Intracoronary shear-related up-regulation of platelet P-selectin and platelet-monocyte aggregation despite the use of aspirin and clopidogrel. Blood. 2011;117:11–20. doi: 10.1182/blood-2010-04-278812. [DOI] [PubMed] [Google Scholar]
- 24.Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F, Bauters A, Decoene C, Goudemand J, Prat A, Jude B. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349:343–349. doi: 10.1056/NEJMoa022831. [DOI] [PubMed] [Google Scholar]
- 25.Uriel N, Pak SW, Jorde UP, Jude B, Susen S, Vincentelli A, Ennezat PV, Cappleman S, Naka Y, Mancini D. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–1213. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Geisen U, Heilmann C, Beyersdorf F, Benk C, Berchtol-Herz M, Schlensak C, Budde U, Zieger B. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willedbrand disease. Eur J Cardiothorac Surg. 2008;33:679–684. doi: 10.1016/j.ejcts.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Halvorsen K, Zhang CZ, Wong WP, Springer TA. Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand factor. Science. 2009;324:1330–1334. doi: 10.1126/science.1170905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood. 2006;108:1903–1910. doi: 10.1182/blood-2006-04-011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong JF, Berndt MC, Schade A, McIntire LV, Andres RK, Lopez JA. Ristocetin-dependent, but not botrocetin-dependent, binding of von Willebrand factor to the platelet glycoprotein Ib-IX-V complex correlates with shear-dependent interactions. Blood. 2001;97:162–168. doi: 10.1182/blood.v97.1.162. [DOI] [PubMed] [Google Scholar]
- 30.Flamm MF, Colace TV, Chatterjee MS, Jing H, Zhou S, Jaeger D, Brass LF, Sinno T, Diamond SL. Multiscale prediction of patient-specific platelet function under flow. Blood. 2012;120:190–198. doi: 10.1182/blood-2011-10-388140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neeves KB, Maloney SF, Fong KP, Schmaier AA, Kahn ML, Brass LF, Diamond SL. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear-resistance of platelet aggregates. Blood. 2008;12:2193–201. doi: 10.1111/j.1538-7836.2008.03188.x. [DOI] [PubMed] [Google Scholar]
- 32.Colace TV, Muthard R, Diamond SL. Thrombus growth and embolism on tissue factor-bearing surfaces under flow: role of thrombin with and without fibrin. Arteroscler Thromb and Vasc Biol. 2012;32:1466–1476. doi: 10.1161/ATVBAHA.112.249789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sing CE, Alexander-Katz A. Globule-stretch transitions of collapsed polymers in elongational flow fields. Macromolecules. 2010;43:3532–3541. [Google Scholar]
- 34.Sing CE, Alexander-Katz A. Elongational flow induces the unfolding of von Willebrand factor at physiological flow rates. Biophys J. 2010;98:L35–L37. doi: 10.1016/j.bpj.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson PJ, Kokame K, Sadler JE. Zinc and calcium ions cooperatively modulate ADAMTS13 activity. J Biol Chem. 2006;281:850–857. doi: 10.1074/jbc.M504540200. [DOI] [PubMed] [Google Scholar]
- 36.Xu AJ, Springer TA. Calcium stabilizes the von Willebrand factor A2 domain by promoting refolding. Proc Natl Acad Sci. 2012;109:3742–3747. doi: 10.1073/pnas.1121261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pareti FI, Lattuada A, Bressi C, Zanobini M, Sala A, Steffan A, Ruggeri ZM. Proteolysis of von Willebrand factor and shear stress-induced platelet aggregation in patients with aortic valve stenosis. Circulation. 2000;102:1290–1295. doi: 10.1161/01.cir.102.11.1290. [DOI] [PubMed] [Google Scholar]
- 38.Kulkarni S, Dopheide SM, Yap CL, Ravant C, Freund M, Mangin P, Heel KA, Street A, Harper IS, Lanza F, Jackson SP. A revised model of platelet aggregation. J Clin Invest. 2000;105:783–791. doi: 10.1172/JCI7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.