Abstract
Objectives:
To examine the rates of behavioral and adaptive functioning difficulties among youth who never had sleep disordered breathing (SDB), had remitted SDB, had incident SDB, or had persistent SDB; and to determine if there were increased odds of behavioral difficulties among youth with varying SDB histories relative to those who never had SDB.
Methods:
263 youth had valid polysomnography and neurobehavioral data at two time points approximately 5 years apart from the prospective Tucson Children's Assessment of Sleep Apnea study. Primary outcomes were the Behavior Assessment Scale for Children-2nd Edition Parent Report Form (BASC-PRF) and Self-Report of Personality (SRP), and the Adaptive Behavior Assessment System-2nd Edition (ABAS-2).
Results:
Compared to those who never had SDB, individuals with persistent SDB had significant odds and met more cutoff scores on the BASC-2-PRF Externalizing Problems Composite (odds ratio [OR] 3.29; 8.92% vs. 35.3%), Behavioral Symptoms Index (OR 6.82; 7.4% vs. 35.3%) and Hyperactivity subscale (OR 6.82; 11.1% vs. 41.2%). Similarly, greater difficulties was seen for the group with persistent SDB (relative to never) on the ABAS-2 Social Domain (OR 3.39; 22% vs. 50%), and Communication (OR 4.26; 15% vs. 42.9%) and Self-Care subscales (OR = 2.97; 25.2% vs. 50%). Relative to youth who never had SDB, youth who developed SDB at Time 2 had compromised adaptive skills as evidenced by the BASC-2 PRF Adaptive Behavior Composite (OR 3.34; 15.6% vs. 38.1%) and the ABAS-2 General Adaptive Composite (OR 2.83; 20.5% vs. 42.1%).
Conclusions:
Youth with current SDB exhibited hyperactivity, attention problems, aggressivity, lower social competency, poorer communication, and/or diminished adaptive skills.
Citation:
Perfect MM; Archbold K; Goodwin JL; Levine-Donnerstein D; Quan SF. Risk of behavioral and adaptive functioning difficulties in youth with previous and current sleep disordered breathing. SLEEP 2013;36(4):517-525.
Keywords: Sleep disordered breathing, social-emotional behavior, adaptive functioning
INTRODUCTION
Youth with sleep disordered breathing (SDB) often have parental behavior ratings indicative of attention difficulties;1–3 externalizing symptoms2–4 (such as aggressiveness,1–5 oppositional behaviors,2–5 and hyperactivity1,3,4,6); internalizing symptoms2,4,5 (such as anxiety and depression1); social problems;3–5,7 learning problems;1,8–10 and somatization.4,5,9 Despite the fact that many studies have found an association with SDB and behaviors, there has been inconsistency across studies in regard to which behaviors are affected by SDB. Additionally, it is unclear whether any amount of SDB confers risk or if a minimum degree of severity is required.3,6,9 Further, all of the aforementioned studies were cross-sectional, and only one1 focused on adolescents. Participants in this latter investigation were obese preadolescents and adolescents recruited from a weight management clinic or a sleep clinic rather than a population-based cohort from local schools.
Adaptive functioning refers to a series of behaviors an individual requires to negotiate social situations, engage in self-care to meet his or her own needs, and apply skills learned in school, based on their development level.11 Some examples include “skills needed to independently care for one's personal health and safety, dress and bathe, communicate, display socially appropriate behaviors and academic skills, effectively engage in recreation and work, and to engage in community life.”11 Adaptive functioning is often examined in the context of an intellectual disability, such as mental retardation or autism, but Ditterline and colleagues proposed that assessment of adaptive functioning might also be beneficial to identify problems with daily living skills in groups with other types of conditions.11 In the case of the current study, the relevant question is whether SDB interferes with the development or execution of adaptive behavior skills in otherwise healthy youth.
The connections between sleep and adaptive functioning have only recently begun to be made.12–14 However, none of these studies considered SDB. One study with overweight youth examined the Adaptive Behavior composite and the Social Skills and Leadership subscales from the Behavior Assessment System for Children-2nd Edition (BASC-2)15 in groups of those with moderate SDB, mild SDB, snorers, and those without SDB.1 Youth with moderate SDB had lower scores on the Leadership subscale. However, after using a conservative P-value and adjusting for covariates, the authors concluded the groups were not significantly different in scores on this subscale.
Even for studies that have found differences based on SDB status, scores on the behavioral ratings scales remain in the average range.1,3 However, another way of considering the impact of SDB is to examine if youth with SDB are more likely to have scores above or below established cutoffs on behavioral rating scales (signifying at-risk for or clinically significant problems) than youth without SDB. Such scores in the at-risk or clinical range for an individual child would prompt professionals in clinics and schools to do further evaluation and assess the need for intervention. Such an approach was utilized by Mulvaney and colleagues,3 who found that relative to youth in the lower 85% of respiratory disturbance index (RDI) values, children with RDI values in the highest 15% exhibited higher rates of parent-reported problems with aggression, social development, oppositionality, and attention. Although they used continuous scores to compare group differences in behaviors based on SDB status in their primary analyses, Beebe et al.1 noted that 11% of youth without SDB had scores on a parent rating of attention in the at-risk range, whereas 44% with moderate or worse OSA evidenced attention problems. In both of these studies,1,3 mean scores on the scales were within the average range for the SDB group, albeit the scores were higher than non-SDB counterparts. Some studies have looked at increased odds of being at-risk for or having behavioral difficulties using different definitions of SDB, but they were in younger samples.4,5 Additionally, even though these aforementioned studies1,3–5 considered the concept of impairment, all were cross-sectional and only one included adolescents,1 limiting conclusions about longitudinal associations in older youth.
Given the lack of information about the long-term impact of SDB into adolescence, the current study utilized data from a longitudinal cohort, the Tucson Children's Assessment of Sleep Apnea Study (TuCASA), to determine if there were increased odds of being at-risk for or impaired on measures of behavioral and adaptive functioning among youth who had current (incident or persistent) SDB relative to those who never had SDB. Further, we sought to examine the rates of difficulties in behavioral and adaptive functioning among youth who never had SDB, had remitted SDB, had incident SDB, or had persistent SDB over an approximate 5-year time span.16 We hypothesized that youth with current, particularly persistent, SDB would have increased risk of behavioral problems in comparison to those who never had SDB. Further, we believed that the issues identified on behavioral rating scales would translate into problems in the classroom.1 Therefore we examined parent-reported learning problems and grades across the four SDB groups to determine if those with persistent SDB also would have worse school performance.
METHODS
Participants and Procedures
The TuCASA study prospectively examined Hispanic and Caucasian children between 6 and 11 years of age to determine the prevalence and incidence of SDB and its effects on neurobehavioral functioning. Recruitment began after approval by the university and local school district research committees. As previously described,17 during the initial recruitment, parents at elementary schools with the highest enrollments of children who identified as Hispanic completed a screening questionnaire asking about their child's sleep habits that was contained in their “notes home folder.” The questionnaire also asked permission to initiate further contact for the child to have a polysomnogram (PSG) and other study procedures. The exclusion criteria for subsequent enrollment in the PSG portion were having a neuropsychological, health, cognitive (e.g., mental retardation), behavioral (e.g., attention deficit hyperactivity disorder), or psychological impairment that would affect performance on neurobehavioral measures or interfere with sleep. Children who were eligible and had parental consent to have a PSG performed were scheduled for an evening home visit. Children were prepared for their PSG approximately 1 h before their typical bedtime and were instructed to follow their usual bedtime routine. During this time, parents completed a Sleep Habit Questionnaire (SHQ) asking about their child's sleep. Within approximately one month after the PSG portion was completed, parents and children completed a neurobehavioral battery of assessments that included the parent and self-reported rating measures. A total of 503 participated in the initial examination (time 1). Approximately 5 years later (mean 56.4 months, time 2), 348 children (9.9 to 17.4 years old) participated in a second examination virtually identical to the first, of whom 263 completed both the PSG and the self-reported rating scales at both time points. We include all these participants when self-reported behavioral ratings were the outcome. Within this subgroup, fewer parents completed the parent rating forms, and thus for these analyses, the number of participants is smaller.
Measures
Measures of sleep and neurobehavioral performance were administered to participants at both examinations. We describe the ones that are relevant to these analyses.
Home-Based Polysomnography (PSG)
Unattended overnight PSGs were completed with the Compumedics PS-2 system (Abbotsford, Victoria, Australia). Participants were allowed to sleep for as long as they wanted. The following channels were recorded: electroencephalogram (EEG; C3/A2 and C4/A1), bilateral electrooculogram (EOG), a bipolar submental electromyogram (EMG), electrocardiogram (ECG), thoracic and abdomen movement (inductive plethysmography bands), nasal pressure, airflow (nasal/oral thermistor), and pulse oximetry. The sleep studies were scored by a trained technician in accordance with Rechtschaffen and Kales criteria.18 For SDB, apneas were scored if the amplitude (peak to trough) of the thermistor airflow signal decreased by ≥ 25% of the amplitude of baseline breathing and if this change lasted > 6 sec or 2 breath cycles. Hypopneas were scored for events that were not deemed apneas if the amplitude of any respiratory signal decreased below 70% or more of the baseline amplitude. The respiratory disturbance index (RDI) was computed by averaging the number of apneas and hypopneas per hour of sleep. In the current study the definition of SDB was RDI ≥ 1 per hour with an associated oxygen desaturation ≥ 3%; this definition has been found to be clinically relevant in previous analyses of the TuCASA cohort.8,16,17,19
Sleep Screening Questionnaire
At both time points, parents were asked to report the frequency their child's loud snoring (5-point Likert scale ranging from never to almost always). Youth were considered to engage in habitual snoring when the parents endorsed frequently or almost always.
The Behavior Assessment System for Children-Second Edition (BASC-2)15
The BASC-2 Parent Rating Scale (PRS) and Self-Report of Personality (SRP) were completed at the second time point to assess social-emotional behaviors. Parents rated their child's behavior on a 4-point Likert scale (1 = never to 4 = almost always). The SRP uses a combination of true-false and 4-point Likert scale responses. The PRS yielded the following composite scores: Behavioral Symptoms Index (BSI), Adaptive Skills, Internalizing Behaviors, and Externalizing Behaviors. The following subscales were also examined: Social Skills, Leadership, Hyperactivity, Aggression, Conduct Problems, Anxiety, Depression, Somatization, Atypicality, Withdrawal, and Attention Problems. The SRP yielded the following composites: School Problems, Internalizing Problems, Inattention/Hyperactivity, Personal Adjustment, and Emotional Symptoms Index (ESI). These subscales were also derived: Attitude to School, Attitude to Teachers, and Sensation Seeking (Adolescent version only), Atypicality, Locus of Control, Social Stress, Anxiety, Depression, Attention Problems, Hyperactivity, Sense of Inadequacy, Self-Esteem, and Self-Reliance.
All scores are reported as T-scores (M = 50, SD = 10). On the clinical scales, the at-risk range includes T-scores between 60 and 70, whereas the clinically significant range includes scores ≥ 70. Thus, risk for impairment was defined as ≥ 60, a threshold suggestive of youth at-risk for significant problems.1,14 For the Adaptive Scales, the at-risk range includes scores between 40 and 30, and the clinically significant range consists of scores ≤ 30. Thus, risk for impairment was defined as ≤ 40.
Adaptive Behavior Assessment System-2nd Edition (ABAS-II)20
The ABAS-II evaluates an individual's ability to engage in skills of daily living in an autonomous fashion as well as whether he or she interacts with others in an effective manner. The ABAS-II comprises 4 composite scores that are made up of different domain areas (referred to as skill areas): Conceptual (Communication, Functional Academics, and Self-Direction), Social (Social and Leisure), Practical (Self-Care, Home Living, Community Use, and Health and Safety), and General Adaptive Composite (GAC). Responses to questions are rated on a 4-point Likert scale (0 = is not able; 1 = never or almost never; 2 = sometimes when needed; and 3 = always or almost always). Composite scores are standard scores (M = 100, SD = 15), and the subtests are reported as scaled scores (M = 10, SD = 3). Thus, standard scores (≤ 85) and scaled scores (≤ 7) with ≥ 1 SD were considered to be problematic.
Sociodemographics
Parents were asked about their child's race/ethnicity, date of birth, income level, highest level of education (time 2), and type of employment (time 2). Measurements for height and weight were taken on the night of the PSG at both time points; body mass index (BMI) was computed using the algorithm of the Center for Disease Control21 and are reported as z-scores and percentiles.
School Problems
Parents were also asked about whether they believed their child to have a learning problem (5-point Likert scale ranging from never to always) and their child's typical grades on a 6-point scale (1 = Gets mostly A's; 2 = Gets mostly A's and B's; 3 = Gets mostly Bs; 4 = Gets mostly B's and C's; 5 = Gets mostly C's; or 6 = Gets mostly grades worse than C's). Grades were categorized based on whether the youth typically received A's or B's or C's and lower.22
Data Analyses
Groups were classified based on whether they had never had SDB, previously had SDB but no longer did (remitted), new onset SDB (incident), or SDB both times (persistent). We first conducted χ2 (sex, race/ethnicity) or one-way between-subjects analysis of variance models (age, body mass index, RDI) to determine if SDB grouping varied according to the sociodemo-graphics. We then calculated the percentage of youth who had scores on the behavioral rating measures above (or below when relevant) our established cutoff scores to indicate impairment. Second, since the outcome of impairment on various measures (based on their cutoff scores) was dichotomous, we used binary logistic regression to model impairment as a function of SDB grouping (with never SDB as the reference group). We ran a second set of logistic regression models to control for any demographic variables (i.e., age, sex, race/ethnicity, and body mass index) that were related to the impairment status of a particular scale or subscale at α ≤ 0.05 in bivariate analyses; we report these adjusted odds. Given the potential but unknown impact of snoring on daily functioning,4 youth who did not meet current criteria for SDB but who were reported to snore frequently by their parents were removed from the primary analyses. However, we did a comparison of logistic regression models with the non-SDB snorers integrated into their respective groups and noted any differences in outcome.
RESULTS
Sample Characteristics
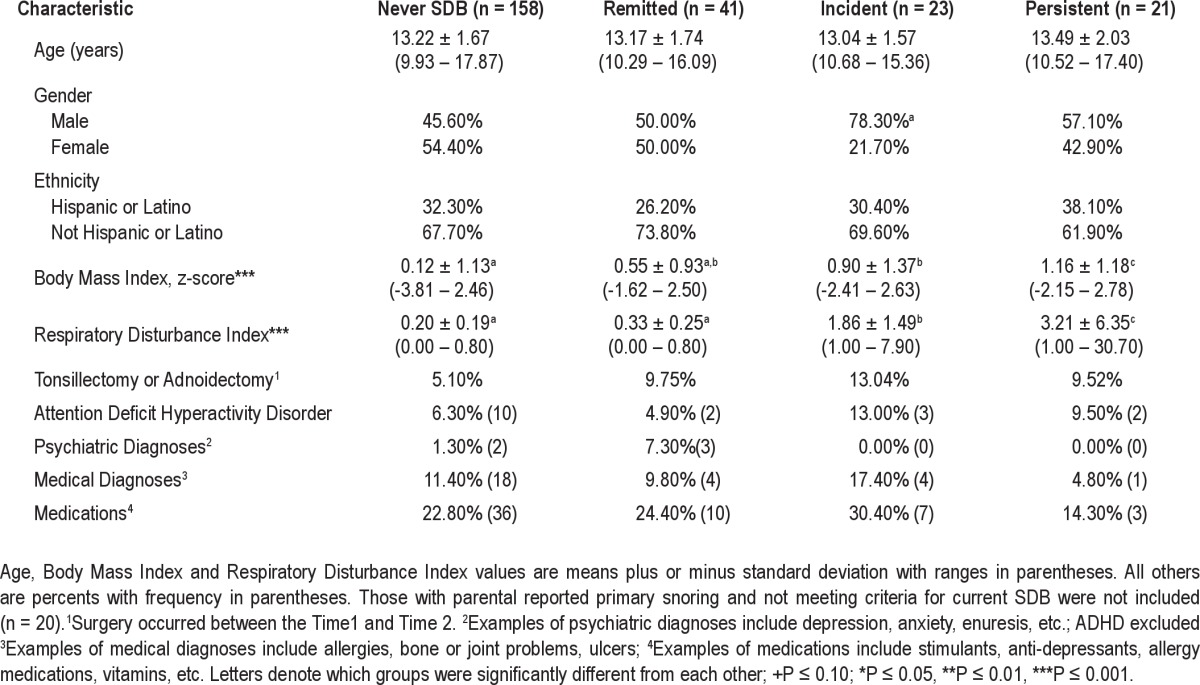
Of the 263 participants with completed PSG and self-reported data, 25 were reported by their parents to snore. Five of those 25 also met the criteria for SDB at time 2. The remaining youth with parent-reported snoring were divided between the never SDB (n = 11) and the remitted SDB (n = 9) groups. Thus, snoring was not indicative of one specific SDB category. Table 1 displays the sample characteristics of the 4 groups. Youth did not differ on race/ethnicity or age based on whether they had previous or current SDB. There were equal rates of boys and girls who never had SDB or had remitted SDB, but there was a higher prevalence of boys with incident SDB than would be expected by chance. Youth without current SDB (never and remitted groups) had significantly lower RDI values than both current groups; however, youth with persistent SDB had significantly higher RDI values than youth with incident SDB. Youth who never met the criteria for SDB weighed significantly less than youth with incident and persistent SDB. Youth with remitted SDB also weighed significantly less than youth with persistent SDB, but the remitted group was not different than the never or incident SDB groups.
Table 1.
Sample characteristics of participants based on SDB history at time 2
Risk of Behavioral Difficulties
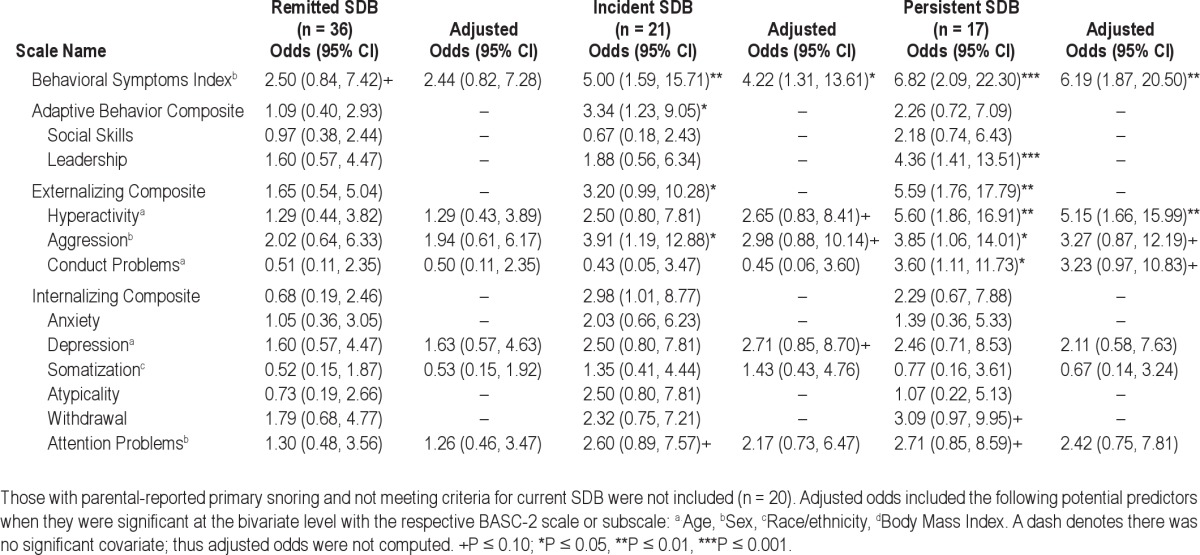
The means and standard deviations for the BASC-2 PRF, ABAS-II, and BASC-2 SRP are available (Tables S1–S3). The raw and adjusted odds ratios for the groups with remitted, incident, and persistent SDB relative to those who never had SDB are depicted in Table 2 for the BASC-2 PRF. Consistent with the visual inspection for rates of impairment, the odds of scores in the at-risk or clinical range on the BSI were 4-5 times greater for the incident SDB group and > 6 times greater for the group with persistent SDB. The odds of having at-risk or higher scores on the Externalizing Composite relative to the group who never had SDB were 3.20 (P = 0.05, CI [1.00, 10.28]) and 5.59 (P = 0.004, CI [1.76, 17.79]) for youth with incident or persistent SDB, respectively. Having persistent SDB significantly increased the odds of having difficulties in the areas of hyperactivity, aggression, and conduct problems. There were trends for the Withdrawal and Attention Problem subscales to be affected if youth had persistent SDB. Although slightly attenuated, there was still a trend for greater odds of impairment on the Aggression and Conduct Problems subscales among the group with persistent SDB, after adjusting for demographics.
Table 2.
Odds of impairment on the Behavior Assessment Scale for Children Parent Report Form-2nd Edition (BASC-2 PRF) relative to never having SDB
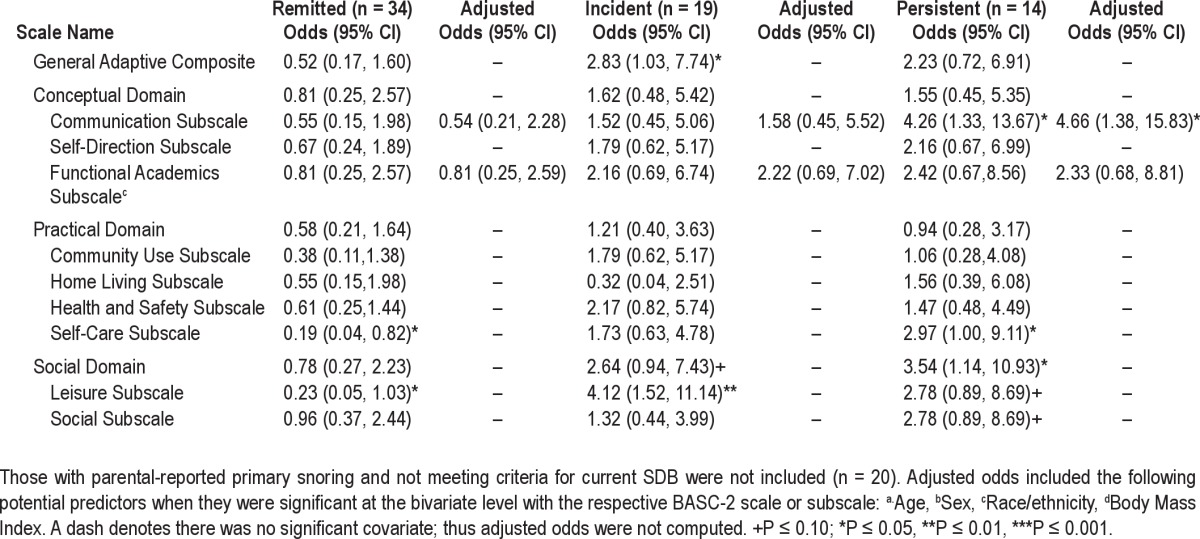
Raw and adjusted odds ratios for the ABAS-2 according to SDB group are shown in Table 3. Once again, relative to youth who never had SDB, those with persistent SDB had significantly greater risk of impairment on the Communication (odds ratio [OR] = 4.26, P = 0.02, CI [1.33, 13.67]) and Self-Care (OR = 2.97, P = 0.05, CI [1.00, 9.11]) subscales, and the Social Domain (OR = 3.39, P = 0.03, CI [1.07, 10.72]). The incident group also had increased odds for the Leisure subscale (OR = 4.12, P = 0.005, CI [1.52, 11.14]) and the General Adaptive Composite (OR = 2.83, P = 0.04, CI [1.03, 7.74]).
Table 3.
Odds of impairment on the Adaptive Behavior Assessment System-2nd Edition (ABAS-2) relative to never having SDB
Most of the logistic regression models were not significant for the BASC-2 SRP (see Table S4), particularly when adjustments were made for sociodemographics. However, the remitted group had higher odds of at least an at-risk score on the Hyperactivity (odds ratio = 2.20, P = 0.05, CI [1.00, 4.88]) and Social Stress subscales (odds ratio = 3.86, P = 0.01, CI [1.31, 11.37]). In addition, sensitivity analyses showed no substantial changes in our results when snorers were included in predicting impairment on the BASC-2 PRF, ABAS-2, or BASC-2 SRP (data not shown).
Compared to youth who never had SDB, youth with persistent SDB had significantly increased odds (OR = 7.4, P = 0.001, CI [2.26, 24.19]) of having learning problems as reported by the parents and were 3 times (OR = 3.4, P = 0.01, CI [1.33, 8.66]) more likely to have grades of C or lower.
Rates of Behavioral Difficulties
Tables 4–6 present the rates of impairment on each of the composites and subscales for the BASC-2 PRF, ABAS-2, and BASC-2 SRP. As shown in Table 4, in regard to the BASC-2 PRF, the percent of those meeting the cutoff score for youth who never had SDB ranged from 7.4% to 20.0%. Rates of impairment for those with remitted SDB ranged from 5.6% to 19.4%. In general, these rates were consistent with youth who never had SDB. In contrast, the range of impairment rates those with incident SDB was much higher (4.8% to 38.1%), with most composites and subscales, reflecting that more than one-fifth of the youth were at-risk for or had impairment. Relative to youth who did not have current SDB (never and remitted), those with incident SDB had higher rates on the BSI (28.6% vs. 7.4% vs. 16.7%), Externalizing Composite (23.8% vs. 13.9%vs. 8.9%), Internalizing Composite (28.6% vs. 11.9% vs. 8.3%); they had the highest cutoff rates on the Adaptive Behavior Composite (38.1%) as well. Youth with persistent SDB clearly had the highest rates of impairment, with many scales having rates 2-4 times that of the never SDB group (11.8% to 41.2%). The Externalizing Composite demonstrated increased rates above the cutoff (35.3%), with the Hyperactivity and Aggression subscales showing incremental increases among the SDB groups relative to those who never had SDB. Similarly, high rates of difficulties in those with persistent SDB were observed for the Conduct Problems and Attention Problem subscales.
Table 4.
Rates of impairment on the Behavior Assessment Scale for Children (BASC)-Parent Report Form based on SDB history
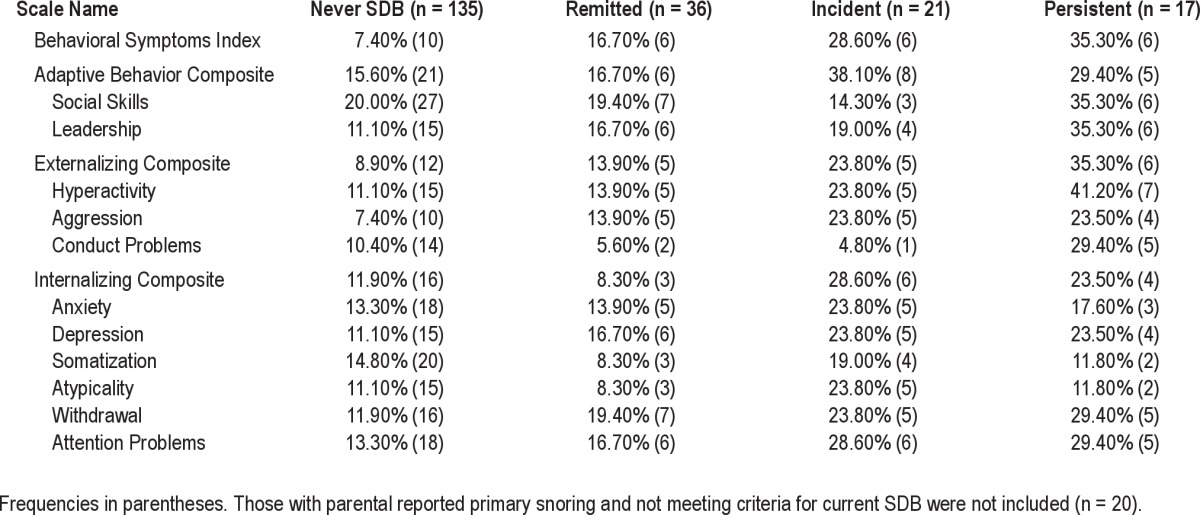
Table 5.
Rates of impairment on the Adaptive Behavior Assessment System-2nd Edition (ABAS-2) based on SDB history
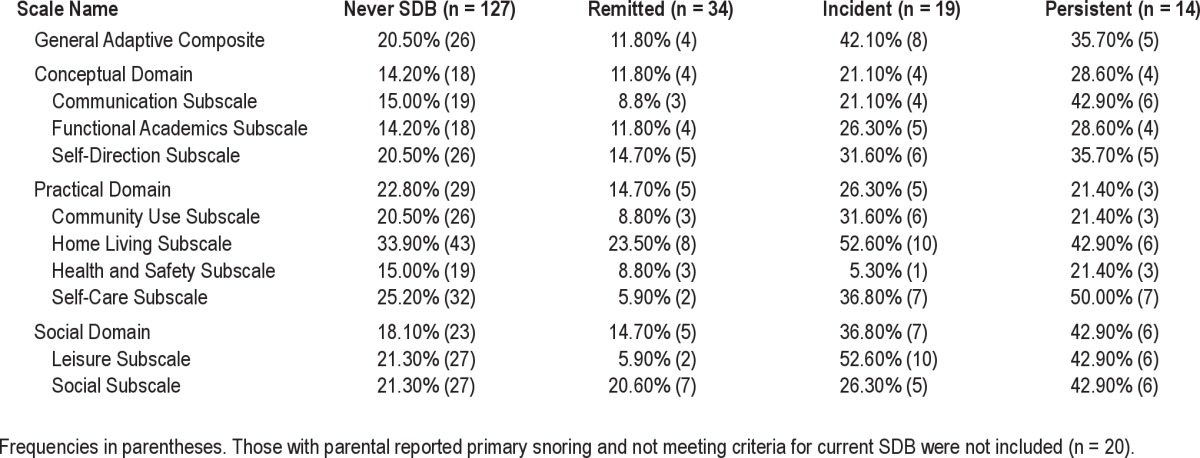
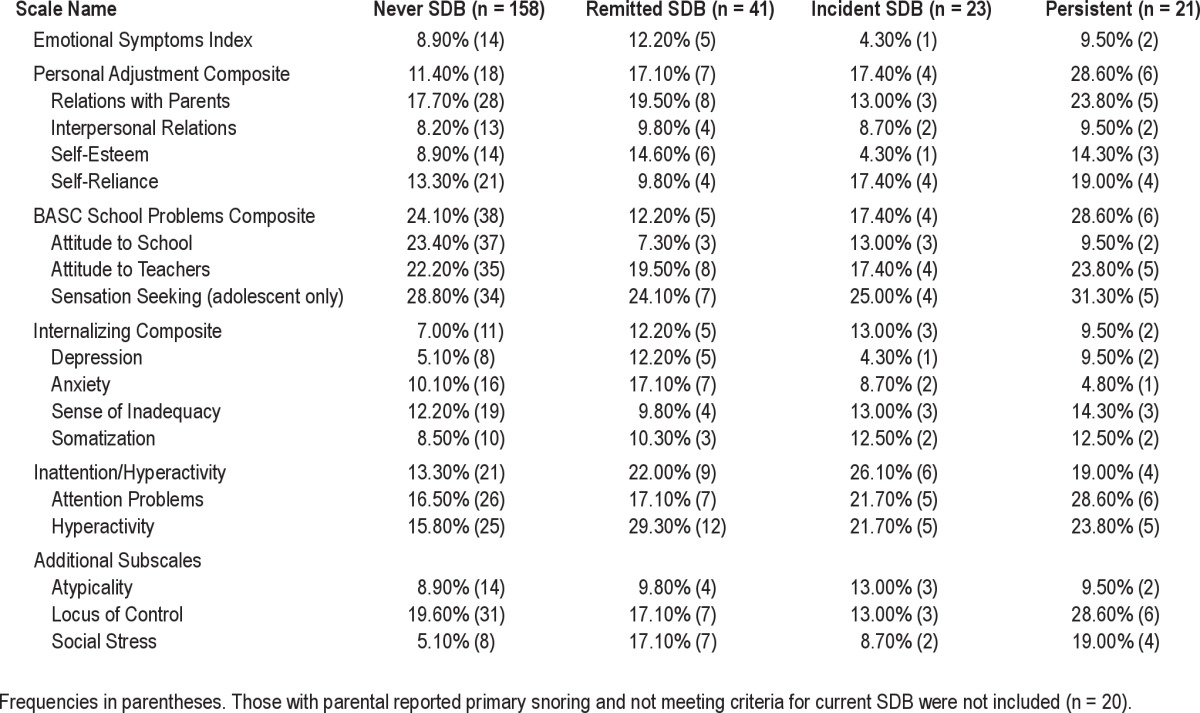
Table 6.
Rates of impairment on the Behavior Assessment Scale for Children-Self Report-2nd Edition (BASC-2) Based on SDB history
As shown in Table 5, the ABAS-2 showed similar patterns as the BASC-PRF. Those with SDB both times demonstrated the highest impairment on the Social Domain (42.9%) relative to the never (18.1%), remitted (14.7%), and incident (36.8%) groups. Furthermore, 42.1% of those with incident SDB met the criteria for at-risk or impaired functioning on the General Adaptive Composite. The group with persistent SDB (42.9%) had the highest rates of impairment on the Communication subscale compared to the never (15.0%), remitted (8.8%), and incident (21.1%) SDB groups. Youth with current SDB also evidenced a greater percentage of at-risk behaviors on several ABAS-2 subscales. Both current SDB groups had double the rates of scores above the cutoff on the Functional Academics subscale (26.3% and 28.6% vs. 14.2% and 11.8%). More than half of the youth with incident SDB exhibited difficulties on the Leisure subscale, and half of the youth with persistent SDB showed difficulties on the Self-Care subscale.
The rates of behavioral difficulties on the BASC-SRP as shown in Table 6 were relatively consistent across SDB groups, suggesting that youth were not reporting differently based on their SDB status. However, there were a few exceptions. The Personal Adjustment Composite was more likely to show impairment for the persistent SDB group (28.6%) relative to never SDB (11.4%) and other SDB groups (17%). Interestingly, those with persistent (19%) and remitted (17.1%) SDB had higher rates of meeting the criterion for the cutoff on the Social Stress subscale than the other 2 SDB groups.
DISCUSSION
The current study examined the rates of impairment of parent and youth reported social-emotional behaviors and parent-reported adaptive behaviors across four SDB groups: never, remitted, incident, and persistent over an approximate 5-year interval from preadolescence to adolescence. We also examined if there was an increased risk of difficulties with behaviors and adaptive skills among groups who previously had or currently have SDB relative to those who never had SDB. The highest rates of impairment existed among youth with current, particularly persistent SDB. Parents were most likely to endorse symptoms above the cutoffs in the areas of hyper-activity, attention, aggressivity, communication, social competency, and self-care. Although with some behaviors (e.g., aggression and conduct problems), the odds were attenuated after controlling for sociodemographic variables, there were still trends for certain disruptive behaviors to be more problematic for those with persistent SDB. Further, these problems may translate into challenges with school functioning, as the youth with persistent SDB also had 3 to 7 times the odds of learning problems and lower grades, respectively, than youth who never had SDB.
Social-Emotional Behaviors
Cross-sectional studies with a focus on younger children with SDB have often found them to have more difficulties in the areas of attention, learning, social skills, externalizing behaviors, and internalizing symptoms.1–10 Beebe et al.1 verified such findings in a sample of overweight youth ages 10 through 16 years; those with mild and/or moderate SDB evidenced significantly higher scores on parents' ratings of depression, anxiety, hyper-activity, aggression, and attention, as well as teacher ratings of attention and learning problems. Extending these findings, our study supported that if left untreated, preadolescents and adolescents with SDB may exhibit symptoms commensurate with attention deficit hyperactivity disorder (ADHD)-like symptoms and disruptive behaviors. Therefore, it is possible that ADHD is misdiagnosed or exacerbated in youth with SDB.23
In contrast to some previous research,1 we did not observe significant findings supporting a higher prevalence of anxiety and depression in youth with any SDB history. While the potential for SDB to impact internalizing symptoms should not be discounted, it is possible that the sadness and worries do not rise to the level of functional impairment. For instance, in one study1 overweight youth with mild and moderate SDB had significantly higher scores on the BASC-PRF Depression and Anxiety subscales, but the scores were still in the average range. Further research is needed to elucidate the impact on neurobehavioral functioning for those who have comorbid SDB and depression.
It is important to note that the self-reported data did not yield the same rates of difficulties as the parent-reported ratings. The self-reported rates of difficulties were lower overall, suggesting that youth did not perceive themselves to have as many difficulties as their parents or they were underreporting their symptom-atology. However, for the most part, the pattern remained the same as found with parent-reported behaviors, in that, youth with persistent SDB had equal or higher rates of behaviors that were in the at-risk or clinical range than those who never had SDB. Youth-reported social stress was one self-reported difficulty worth noting as those who had SDB starting early in development, regardless of whether it was remitted, were more likely to meet the criteria on cutoff scores on the BASC-SRP Social Stress subscale compared to the other two groups. Since the odds lowered in the group with persistent SDB once BMI was entered into the model, it may be that those with SDB and who gain weight over time are not only vulnerable to worsening SDB16 but also to experiencing stress in social situations.
Adaptive Functioning
To our knowledge, this is the first sleep-related study to use a standardized questionnaire to assess adaptive functioning in typically developing youth with and without SDB. The data indicate that youth who evidenced SDB during earlier elementary school years and presumably continue to have it during the pre-adolescent and adolescent years are at-risk for problems in their ability to make decisions and work independently (BASC-2 PRF Leadership Skills subscale), effectively communicate (ABAS-2 Communication subscale), take care of themselves (ABAS-2 Self-Care subscale), and engage in social interactions and activities (ABAS-2 Social Domain). Further, youth who acquired SDB (incident SDB group), despite not having SDB earlier in their development, experienced compromised general adaptive behaviors as evidenced by greater impairment on the BASC-2 Adaptive Behavior Composite and the ABAS-2 General Adaptive Composite. The exact mechanism as to why those with concurrent SDB may have difficulties with adaptive skills is unknown. However, it is possible that fragmented sleep and/or hypoxemia associated with SDB affects the prefrontal cortex, an area of the brain that is associated with the ability to plan and organize23,24; these skills underlie successful acquisition of life-care skills. Schools and clinics that identify problems in these areas should consider the potential contribution of SDB to these issues. Conversely, when SDB is suspected or diagnosed, clinicians should determine if adaptive functioning is compromised. Although we used a cut-off of at least one SD below the mean, clinicians may want to closely monitor youth who have scores on the ABAS-II that are more than two-thirds of one SD below the mean.11 Along these same lines, future research on the long-term effects of SDB or the benefits of its treatment should consider incorporating measures of life-care skills into the protocol.
Impact on School Performance
As a secondary aim, we examined if youth with a history of SDB had more learning problems and lower grades. Our findings that there is an increased risk of learning problems among youth with SDB corroborates previous cross-sectional research1,9 and extends our previous reports of that learning problems were associated with concurrent SDB at time1 and time 2.8,10 Corresponding to the behavioral data, youth with persistent SDB had increased risk of having grades of C or lower. Beebe et al.1 documented similar findings using parent and self-reported grades among youth with SDB relative to those without SDB. School-based interventions to target academic difficulties or behavioral challenges may not have optimal effectiveness if youth have undiagnosed or untreated SDB. Nonetheless, only parent reports of learning problems and grades were available. Future research with objective indicators of SDB should obtain actual grades, attendance records, and state standardized test scores.25,26 For instance, one study25 that used sleep-associated gas exchange abnormalities as a marker of SDB found that young elementary school students with evidence SDB had lower grade point averages. Direct classroom observations or teacher ratings would also contribute to our understanding of the impact of SDB on classroom learning.
Limitations and Future Directions
Several limitations apply to this study. First, we did not consider the severity of SDB across time. As we have previously reported, the prevalence of SDB in this cohort decreased with age.16 Thus, the fewer number of youth with SDB at time 2 precluded meaningful longitudinal analyses of additional categories according to SDB severity. However, several studies have concluded that SDB of any severity places the child at risk for behavioral problems.1,4,5,9,27,28 Second, also consistent with our previous observations, incident SDB occurred more in boys. However, our analyses controlled for sex when it was related to the outcome, thus it is unlikely that gender differences contributed to our findings. Third, we chose to exclude parent-reported snoring a priori because of previous research suggesting behavioral problems associated with primary snoring.4,7 There are also limited data on the reliability of parental reporting for snoring in older youth, particularly given adolescents are rarely observed during sleep. Although the significance of the odds ratios did not change substantially when snorers were included, our data also suggest a lack of correspondence between parent-reported snoring and SDB history. Future research should examine the long-term effects of snoring in children. Lastly, the study also did not include the BASC-2 or ABAS-2 during the first examination. Thus, we are unable to determine prior psychosocial and adaptive functioning with these instruments.
Overall, the current study underscored the associated risks of SDB being left untreated. Other studies have demonstrated the benefits of adenotonsillectomy in younger cohorts in improving both SDB and behavioral problems.25,27–30 Clinicians need to work closely with school professionals to inform them of the potential for SDB to contribute to problematic behaviors, particularly, ADHD-like symptoms, and difficulties with life-care skills that are important to succeed in school. Even though SDB appears to decline into adolescence, taking a wait and see approach is risky and families and clinicians alike should identify potential treatments. Longitudinal research is needed to examine the persistence of SDB into adulthood and determine the impact on behavioral indicators that are relevant to home, school, and the workforce.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENT
TuCASA was supported by HL62373.
SUPPLEMENTAL MATERIAL
Means scores and standard deviations of the means scores on the Behavior Assessment Scale for Children (BASC)-Parent Report Form Based on SDB history
Means and standard deviations of the scores on the Adaptive Behavior Assessment System-2nd Edition (ABAS-2) based on SDB history
Means and standard deviations of the scores on the Behavior Assessment Scale for Children-Self Report-2nd Edition (BASC-2) based on SDB history
Odds of impairment on the Behavior Assessment Scale for Children Self-Report Form-2nd Edition (BASC-2 SRF) Relative to never having SDB
REFERENCES
- 1.Beebe DW, Ris MD, Kramer ME, Long E, Amin R. The association between sleep disordered breathing, academic grades, and cognitive and behavioral functioning among overweight subjects during middle to late childhood. Sleep. 2010;33:1447–56. doi: 10.1093/sleep/33.11.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourke RS, Anderson V, Yang JSC, et al. Neurobehavioral function is impaired in children with all severities of sleep disordered breathing. Sleep Med. 2011;12:222–9. doi: 10.1016/j.sleep.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Mulvaney SA, Goodwin JL, Morgan WJ, et al. Behavior problems associated with sleep disordered breathing in school-aged children – the Tucson Children's Assessment of Sleep Apnea Study. J Pediatr Psychol. 2005;31:322–30. doi: 10.1093/jpepsy/jsj035. [DOI] [PubMed] [Google Scholar]
- 4.Rosen CL, Storfer-Isser A, Taylor HG, Kirchner HL, Emancipator JL, Redline S. Increased behavioral morbidity in school-aged children with sleep-disordered breathing. Pediatrics. 2004;114:1640–8. doi: 10.1542/peds.2004-0103. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Q, Sherrill DL, Goodwin JL, Quan SF. Association between sleep disordered breathing and behavior in school-age children: The Tucson Children's Assessment of Sleep Apnea study. Open Epidemiol J. 2008;1:1–9. doi: 10.2174/1874297100801010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giordani B, Hodges EK, Guire KE, et al. Changes in neuropsychological and behavioral functioning with and without obstructive sleep apnea following Tonsillectomy. J Int Neuropsychol Soc. 2008;14:571–81. doi: 10.1017/S1355617708080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blunden S, Lushington K, Kennedy D, et al. Behavior and neurocognitive performance in children aged 5-10 years who snore compared to controls. J Clin Exp Neuropsychol. 2000;22:554–68. doi: 10.1076/1380-3395(200010)22:5;1-9;FT554. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin JL, Kaemingk KL, Mulvaney SA, Morgan WJ, Quan SF. Clinical screening of school children for polysomnography to detect sleep-disordered breathing–the Tucson Children's Assessment of Sleep Apnea Study (TuCASA) J Clin Sleep Med. 2005;1:247–54. [PMC free article] [PubMed] [Google Scholar]
- 9.Owens JA, Mehlenbeck R, Lee J, King MM. Effect of weight, sleep duration, and comorbid sleep disorders on behavioral outcomes in children with sleep-disordered breathing. Arch Pediatr Adolesc Med. 2008;162:313–21. doi: 10.1001/archpedi.162.4.313. [DOI] [PubMed] [Google Scholar]
- 10.Silva GE, Goodwin JL, Parthasarathy S, et al. Longitudinal association between short sleep, body weight, and emotional and learning problems in Hispanic and Caucasian children. Sleep. 2011;3:1197–205. doi: 10.5665/SLEEP.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ditterline J, Banner D, Oakland T, Becton D. Adaptive behavior profiles of students with disabilities. J Applied Sch Psychol. 2008;24:191–208. [Google Scholar]
- 12.Bonuck K, Grant R. Sleep problems and early developmental delay: implications for early intervention programs. Am J Intellect Dev Disabil. 2012;50:41–52. doi: 10.1352/1934-9556-50.1.41. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann LK. Chronotype and transition to college life. Chronobiol Int. 2011;28:904–10. doi: 10.3109/07420528.2011.618959. [DOI] [PubMed] [Google Scholar]
- 14.Vaughn BE, El-Sheikh M, Shin N, et al. Attachment representations, sleep quality, and adaptation in the preschool peer group. Attach Hum Dev. 2011;13:525–40. doi: 10.1080/14616734.2011.608984. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds CR, Kamphaus RW. Behavior Assessment System for Children. Second Edition manual. Circle Pines, MN: American Guidance Service Publishing; 2004. [Google Scholar]
- 16.Goodwin JL, Vasquez MM, Silva GE, Quan SF. Incidence and remission of sleep-disordered breathing and related symptoms in 6- to 17- year old children – The Tucson Children's Assessment of Sleep Apnea Study. J Pediatr. 2010;157:57–61. doi: 10.1016/j.jpeds.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin J, Enright P, Kaemingk K, et al. Feasibility of using unattended polysomnography in children for research: Report of the Tucson Children's Assessment of Sleep Apnea study (TuCASA) Sleep. 2001;24:937–44. doi: 10.1093/sleep/24.8.937. [DOI] [PubMed] [Google Scholar]
- 18.Rechtschaffen A, Kales AA. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. Manual of standardized terminology: techniques and scoring systems for sleep stages of human subjects. [Google Scholar]
- 19.Goodwin J, Kaemingk K, Fregosi M, et al. Clinical outcomes associated with sleep disordered breathing in Caucasian and Hispanic children–the Tucson Children's Assessment of Sleep Apnea Study (TuCASA) Sleep. 2003;26:587–91. doi: 10.1093/sleep/26.5.587. [DOI] [PubMed] [Google Scholar]
- 20.Harrison PL, Oakland T. Adaptive Behavior Assessment System. Second Edition. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 21.Centers for Disease Control. Body Mass Index. [Last accessed June 29, 2012]. http://www.cdc.gov/healthyweight/assessing/bmi/
- 22.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–87. [PubMed] [Google Scholar]
- 23.Macey PM, Kumar R, Woo MA, et al. Brain structural changes in obstructive sleep apnea et al. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–77. [PMC free article] [PubMed] [Google Scholar]
- 24.Joo EY, Tae WS, Lee MJ, et al. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010;33:235–41. doi: 10.1093/sleep/33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102:616–20. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 26.Perfect MM., Patel PG, Scott RE, et al. Sleep, glucose, and daytime functioning in youth with type 1 diabetes. Sleep. 2012;35:81–8. doi: 10.5665/sleep.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y-S, Guilleminault C, Li H-Y, Yang C-M, Wu YY, Chen N-H. Attention-deficit/hyperactivity disorder with obstructive sleep apnea: a treatment outcome study. Sleep Med. 2007;8:18–30. doi: 10.1016/j.sleep.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell RB. Adenotonsillectomy for obstructive sleep apnea in children: outcome evaluated by pre- and postoperative polysomnography. Laryngo-scope. 2007;117:1844–54. doi: 10.1097/MLG.0b013e318123ee56. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell RB, Kelly J. Behavioral changes in children with mild sleep-disordered breathing or obstructive sleep apnea after adenotonsillectomy. Laryngoscope. 2007;117:1685–8. doi: 10.1097/MLG.0b013e318093edd7. [DOI] [PubMed] [Google Scholar]
- 30.Dillon JE, Blunden S, Ruzicka DL, et al. DSM-IV diagnoses and obstructive sleep apnea in children before and 1 year after adenotonsillectomy. J Am Acad Child Adolesc Psychiatry. 2007;46:1425–36. doi: 10.1097/chi.0b013e31814b8eb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Means scores and standard deviations of the means scores on the Behavior Assessment Scale for Children (BASC)-Parent Report Form Based on SDB history
Means and standard deviations of the scores on the Adaptive Behavior Assessment System-2nd Edition (ABAS-2) based on SDB history
Means and standard deviations of the scores on the Behavior Assessment Scale for Children-Self Report-2nd Edition (BASC-2) based on SDB history
Odds of impairment on the Behavior Assessment Scale for Children Self-Report Form-2nd Edition (BASC-2 SRF) Relative to never having SDB


