Abstract
Since the effect of alcohol and its environmental cues on brain dopamine have been implicated in the maintenance of heavy drinking, drugs that modify dopamine might be useful in reducing drinking or promoting abstinence. The goal of the current study was to utilize an established brain imaging paradigm to explore the effect of aripiprazole (final dose 15mg over a 14 day period), a dopamine stabilizer medication, on alcohol cue-induced brain activation and drinking in alcoholics. Non-treatment seeking alcoholics were randomly assigned aripiprazole (n= 14) or identical placebo (n=16) and reported their alcohol use while taking study medication for 14 days prior to an alcohol cue induced brain fMRI imaging study. In a Philips 3.0 Tesla MRI scanner, subjects were given a sip of alcohol before viewing a randomized presentation alcohol and non alcohol beverage pictures while subjects rated their urge to drink. During picture presentation, changes in regional brain activity were measured and differences between viewing alcoholic beverage and non-alcoholic beverages were compared within and between groups. Brain activity analysis revealed increased activation for placebo-treated subjects in the right ventral striatum (p<.005, threshold 15 voxels) while there was a blunting of activation in this area in the aripiprazole-treated subjects. Aripiprazole-treated subjects, compared to placebo-treated subjects, also had significantly less heavy drinking during the 14-day medication period. The study provides both novel and valuable information regarding the effect of aripiprazole on cue-induced brain activation and voluntary drinking during treatment.
Keywords: Neuroimaging, Alcoholism, Craving, Aripiprazole
INTRODUCTION
Although there have been numerous advances in the understanding of the mechanisms underlying alcoholism, relapse rates remain high. Unfortunately, disulfiram, naltrexone, and acamprosate, three FDA-approved medications for the treatment of alcoholism, have not been found to be powerfully or universally effective in altering relapse to alcohol use.1–5 As such, there has been considerable interest in identifying more efficacious medications for the treatment of alcoholism.
Aripiprazole is considered a partial dopaminergic agonist, acting on both postsynaptic D2 receptors and presynaptic autoreceptors.6, 7 Additionally, aripiprazole displays partial agonism at serotonin 1A (5-HT 1A) receptors and antagonism at 5-HT 2A receptors.8, 9 Aripiprazole is a well-tolerated drug and overdose has been shown to be very unlikely.10, 11 During aripiprazole therapy, there are no clinically significant changes in hematological or liver function tests even with the dose of 180 mg.11 This decreases concern about potential hepatotoxicity in an already alcohol-compromised liver or with an interaction with alcohol and other drugs on the liver. In addition, unlike other antipsychotic medications, aripiprazole has considerably less undesirable qualities such as extrapyramidal symptoms, especially at lower dosages. 12
Aripiprazole’s partial dopamine agonist activity has stimulated interest in its potential utility as an agent to treat addictive disorders. Aripiprazole has been found efficacious in an open-label trial in cocaine dependence13, decreasing the effects of amphetamine in a challenge paradigm14, and reducing the use and craving for alcohol.15, 16 A multi-site trial of aripiprazole (up to 30mg per day – mean dose 23mg) in the treatment of alcoholism was conducted in the United States. The side effect profile of the medication above 15 mg may have limited the effectiveness of the medication in maintaining abstinence, but it appeared to be effective in reducing drinking if a person did initiate alcohol use.17 In addition, compared to placebo-treated subjects, aripiprazole-treated subjects reported significantly fewer drinks per drinking day (p=0.001), had significantly lower end-of-study Alcohol Dependence Scale scores (p=0.004), had a greater reduction in %CDT (p=0.02), a marker of heavy alcohol consumption, and endorsed more positive effects on craving reduction and drinking modification. Lastly, a recent double-blind comparison with naltrexone supports the utility of aripiprazole in alcohol dependence.18
Human alcohol-cue based laboratory paradigms have been used to identify potential alcohol treatment medications.19–21 Aripiprazole has been evaluated in two clinical lab studies. Kranzler and colleagues found that aripiprazole dose-dependently reduce the euphoric effects of alcohol22 when given as a single dose prior to alcohol exposure. Our group evaluated the safety, tolerance, and ability of aripiprazole to reduce drinking compared to placebo in a subacute dosing (8 days up to 15mg) natural drinking and bar-lab study in non-treatment seeking alcoholics (n=30). Aripiprazole was well tolerated, and reduced drinking and alcohol-induced stimulation compared to placebo.23
Brain activation can be produced and measured in imaging paradigms involving alcohol cues.24–29 An area of cue-stimulated activation noted by our group has been the ventral striatum.27, 30 The mesolimbic dopamine pathway that projects from the ventral tegmental area (VTA) to a structure within the ventral striatum, the nucleus accumbens (Nac), has been implicated as a major site for the reinforcing actions of many addictive drugs including ethanol.31–35 Recently, our group reported that naltrexone, and to a lesser degree, ondansentron, or the combination of these medications could attenuate cue-induced activation of the ventral striatum.30 This is noteworthy since both medications have been reported to be effective in reducing drinking and/or relapse.
The exact mechanism by which aripiprazole may influence alcohol use behaviors is not known. Therefore, the goal of the current study was to explore the effect of aripiprazole on ventral striatum activation as a proposed site of action. We reasoned that if aripiprazole stabilized, or blocked, alcohol cue-induced dopamine release then this might be reflected in reduced ventral striatal activity to alcohol cues during our fMRI paradigm. Secondarily, we wished to explore in whether aripiprazole would be well tolerated at a dose of 15mg per day and reduce drinking when taken over a longer period (14 days vs. 7 days previously) in non-treatment seeking alcoholics. A priori hypotheses were that participants treated with aripiprazole would have lower ventral striatum activation to alcohol cues compared to placebo-treated participants and that the attenuation in cue-induced brain activation caused by medication treatment would correlate with decreased alcohol use as compared to placebo-treated participants. An active comparator drug was not used in this study.
MATERIALS AND METHODS
Participants
Non-treatment seeking individuals (n=30) meeting criteria for alcohol dependence were screened for participation. After baseline evaluation, participants were URN randomized, based on gender, smoking history and family history of alcohol dependence, by the investigational pharmacist to receive either aripiprazole or placebo in a double-blind manner. Participants received study drugs for 14 days. On day 14, after a minimum of 24 hours of abstinence, participants underwent a functional MRI brain scan with cue stimulation.
Potential participants were told that the study was investigating effects of a medication that may have beneficial effects for alcoholics in treatment. All participants consumed at least 20 drinks/week and met Diagnostic and Statistical Manual IV (DSM-IV) criteria for alcohol dependence, including loss of control drinking or an inability to cut down or quit, but they denied any active involvement in, or desire for, alcohol treatment. Exclusion criteria for all participants were as follows: current DSM-IV criteria for other drug abuse or dependence by verbal report and urine drug screens, other major DSM-IV Axis I disorders, psychoactive medication or substance use (except marijuana) in the past 30 days or a positive urine drug screen, current suicidal or homicidal ideation, past history of alcohol-related medical illness, liver enzymes ≥ 2.5 times above normal, or significant health problems. All participants were screened for DSM-IV criteria using the entire Structured Clinical Interview for all the DSM-IV Axis 1 Disorders (SCID).36
Procedures
Upon arrival for the baseline visit, the study was described in detail to the participant and Informed Consent was obtained using a form and procedures approved by the Investigational Review Board at our institution. Each participant was then evaluated with a number of standard interview, questionnaire, and medical diagnostic procedures similar to those in other studies reported by our group.19, 21 Interview procedures included a demographic form, the alcohol and drug section of the SCID administered by a trained physician, and a timeline follow-back interview to quantify drinking during the preceding 90 days.37 The Obsessive-Compulsive Drinking Scale (OCDS)38, the Self-Administered Alcohol Screening Test (SAAST)39, the Alcohol Dependence Scale (ADS)40, Clinical Institute Withdrawal Assessment for Alcohol - Revised (CIWA-Ar)41 and a symptom checklist were administered. Finally, a breath alcohol level was obtained, a urine specimen was collected to screen for abused drugs, and a blood sample collected for liver function and general health screening. In addition, a physical exam was conducted by a physician assistant and reviewed by a physician.
On study Day 1, participants who passed all screening and eligibility criteria were randomly assigned to receive aripiprazole (n=14) in 5mg or identical placebo capsules (n=16) for fourteen days. The following dose titration for aripiprazole or matching placebo was employed: 5 mg/day on Days 1–3, 10mg/day on Days 4–7, and 15 mg/day for Days 8 through 14. Participants were given no explicit instructions regarding use of alcohol or modification of their drinking behavior for Days 1 – 12. However, they were required to abstain completely from drinking for the 24-hour period prior to the imaging session on Day 14.
On Day 7, several assessments were completed. A 6-Day version of the timeline follow-back interview (in which they reported their alcohol consumption since the outset of the medication period) and breath alcohol level were completed. The symptom checklist, the CIWA-Ar, and the OCDS were repeated. Participants were instructed to return on Day 14 for the imaging session.
The cue-induced MRI scanning procedures are similar to those used in prior work by our group.27, 30 Briefly, on the day of the imaging session, participants completed assessment questionnaires (TLFB, OCDS, CIWA-Ar), were breathalyzed, and a rapid urine drug screen obtained. No participant had evidence of alcohol use or a positive urine drug screen prior to the imaging session. After positioning in the scanner, participants were checked to ensure that they could view the cues comfortably on a video screen mounted to the headcoil. During initial scanner tuning and structural scanning (T1 weighted 3-D volume and T1-weighted structural scan in the functional scan plane), participants were shown relaxation pictures. They were given a sip of preferred spirits in non-carbonated juice through a straw placed in their mouth, and then shown the 12 minutes of alternating stimuli with BOLD image acquisition. Subjects self-rated their craving in real-time (after each picture block) using a hand-pad (from 0–4 with 0 being “none” and 4 being “extreme”) during the picture viewing and brain image acquisition. After the imaging session, they were escorted out of the scanner room, rinsed their mouths with water, and given a breathalyzer test (the sip of alcohol does not produce measurable breath alcohol readings).
Alcohol Cues
The alcohol cues used during the MRI scanning procedures are similar to those used in our prior work.27, 30 Briefly, alcohol and non-alcohol beverage picture cues were selected primarily from the Normative Appetitive Picture System (NAPS n=38) but were supplemented with 22 additional cues selected from advertisements to avoid repeating the same stimuli during the scanning sequence. Visual control pictures match the alcohol cues in color and hue but lack any object recognition. A sequence for stimulus presentation has been created consisting of six, 120-second, epochs. Each epoch contains three 24-second blocks (1 block each of alcohol, non-alcohol beverage and visual control pictures) and one 24-second rest (cross-hair). Each 24-second block is made up of 5 individual pictures, each displayed for approximately 4.8 seconds. The alcohol blocks are specific to a beverage type (beer, wine or liquor), with two blocks per type. In order to control for time and order effects across subjects, the order of the individual pictures, the blocks within the epoch, and the epochs are all randomly presented. The pictorial scripts were created and displayed by E-prime on an Integrated Functional Imaging System (IFIS) (Invivo, Orlando, Fl.). After each 24 second block, participants rated their “urge to consume alcohol” on a hand-pad that was connected to IFIS with a real-time ability to measure changes in craving. Prior to entering the scanner the participants were trained to rate their “urge to drink alcohol” by using the hand-pad.
MRI Image Acquisition
Participants wore earplugs and head movement was restricted using cushions surrounding the head. MRI scans were performed in a Philips 3.0 T MR scanner (Intera, Philips Medical System, The Netherlands) with an eight-channel SENSE head coil. Following a manual tuning for echoplanar imaging, the cue-induction paradigm was performed while also acquiring Blood Oxygen Level Dependent (BOLD) weighted transverse scans using a gradient echo, echo-planar (EPI) fMRI sequence (Flip Angle=90°, Echo Time (TE) =30.0 ms, Repetition Time (TR)=1867ms, Field of view (FOV)=208 mm, matrix 64×64, SENSE factor 2, 36 slices, 3 mm thick with 0 mm gap, giving a voxel size of 3.25 ×3.25 × 3.00 mm. A high-resolution 160 slice 1 mm thick sagittal T1 weighted scan was obtained for later volumetric and co-registration analysis and to ensure there was no significant anatomical brain pathology.
Statistical Analysis
Baseline Characteristics
Analyses of baseline drinking and demographics were performed with either ANOVA (continuous variables) or Chi-square (categorical variables).
fMRI Data Analyses
Images were converted from the Philips format into the ANALYZE format with MRIcron (http://www.sph.sc.edu/comd/rorden/mricron/). The post-acquisition preprocessing and statistical analysis was performed using Statistical Parametric Mapping software 5 (SPM5, The Wellcome Department of Cognitive Neurology, University College London, http://www.fil.ion.ucl.ac.uk/spm), run under the Matlab 7.6 (The Mathworks, Inc., Natick, MA) environment. Default settings were used unless indicated otherwise. For each subject, all volumes were realigned to the first volume. After realignment (including the adjustment for sampling errors), movement across the entire scan was less than 1 mm in 3 axes and less than 1 degree in 3 orientations for all subjects. The images were stereotactically normalized into a standard space with a resolution of 3×3×3 mm voxels using the averaged functional EPI image -the Montreal Neurological Institute (MNI) EPI template in SPM5. Subsequently, the data were smoothed with an anisotropic 8×8×8 mm Gaussian kernel and high-pass filtered (cut-off period=240s). This first level of statistical analysis used a boxcar function convolved with the modeled hemodynamic response function as the basic function for the general linear model. Contrast-maps were obtained of the difference between alcohol minus beverage and visual control minus rest for each patient individually with the six head movement parameters included as covariates. The subject-specific contrasts maps were then entered into a second-level analysis-full factorial model, to obtain random effects of activation in each group as well as main effects of treatment. The group t maps were thresholded using p<.005 uncorrected and cluster statistical weight (spatial extent threshold) of 15 voxels and were analyzed without knowledge of specific medication group assignment.
In order to identify activity in the ventral striatum among all subjects, a location in the center of the nucleus accumbens (MNI coordinates 6, 21, -3) was used to define as a center for extracting time courses. A small volume of 6 mm radius spherical volume of interest (VOI) was calculated as the first eigenvariate of a singular value decomposition across voxels. With this mask, averaged time courses of these voxels were generated from each individual’s data.
In order to explore the relationship between imaging and drinking, a covariate-only model was estimated with percent heavy drinking days as the covariate. The covariate t maps were thresholded using p<.005 uncorrected and cluster statistical weight (spatial extent threshold) of 15 voxels and were analyzed without knowledge of specific medication group assignment. Interestingly, a clear area in the ventral striatum (MNI coordinates 3, 9, -6), but distinct from the anatomically defined region above was detected as being highly related to drinking, across both medication groups. Data from this area were further analyzed in a hierarchical linear model with medication group, percent heavy drinking days and their interaction as level 2 predictors.
Specific Effects of Medication on Alcohol Cue Induced Ventral Striatum Activation
Analysis of the ventral striatum data was performed as a three-level hierarchical linear regression (HLM 6.04) with fMRI paradigm run-time (level 1) nested within condition (level 2) nested within subject (level 3). Level 2 predictors, the dummy-coded variables representing the contrasts of neutral-beverage relative to each of the other conditions (rest, visual control, alcohol), were analyzed as random variables. Level three predictors were those distinguishing between subjects, i.e. drug group and any constant, subject-level covariate, e.g. age. This a priori defined analysis focused on the differential effects of each of the between-subject variables (different medication conditions) on the difference in ventral striatum activation generated during neutral-beverage versus alcohol visual stimuli (the dependent variable of interest). The medication conditions were represented with an indicator coded dummy variable using the placebo group as a reference.
Craving Analysis
Analysis of craving scores was performed in a hierarchical linear regression similar to that used for ventral striatum activation, but was only a two level model, craving after each stimulus condition nested within subject. The primary analysis was directed at estimating the difference in craving during the neutral-beverage versus alcohol conditions. Estimates of the alcohol minus beverage contrast for ventral striatum activation were calculated from the Bayesian residuals of the overall hierarchical model and were compared (using standard regression techniques) with the overall craving experienced by the participants while in the scanner.
Drinking Analysis
Drinking data (percent heavy drinking days) were analyzed only over the first 12 days of the treatment period to avoid contamination by scan preparation and study mandated abstinence. Data were subjected to Analysis of Covariance (ANCOVA) controlling for baseline drinking and education (which was found a priori to be associated with heavy drinking days). Secondary drinking variables (percent days abstinent, drinks per day and drinks per drinking day) were analyzed similarly.
Both the anatomically- and correlation- defined regions were analyzed similarly with respect to the relationship between percent heavy drinking days by including the percent heavy drinking days reported during treatment, and its interaction with drug (aripiprazole or placebo) group, in evaluating activation (alcohol minus neutral beverage cues) in the ROI analysis.
RESULTS
Demographics and Subjective Ratings
As can be seen in Table 1, there were no significant baseline differences in demographics or alcohol use parameters between the medication groups. Subjects were drinking on more that 70% of the days and 50% of the days were heavy drinking days with an average of about 8 drinks per drinking day with moderate to high OCDS scores.
Table 1.
Demographics and Drinking History
| Aripiprazole (n=14) | Placebo (n=16) | Statistics | |
|---|---|---|---|
| Age | 26.8 ±6.5 | 30.9 ±7.5 | Not Significant |
| Education | 15.0 ±1.6 | 15.6 ±2.6 | Not Significant |
| Gender (% Male) | 64% | 81% | Not Significant |
| Race (%Caucasian/AA/Other) | 100 / 0 / 0 | 81 / 6 / 13 | Not Significant |
| Baseline Drinks/day | 5.4 ±1.7 | 5.5 ±3.1 | Not Significant |
| Baseline % days abstinent | 28.1 ±15 | 27.3 ±17 | Not Significant |
| Baseline % heavy drinking days | 53.3 ±18 | 52.1 ±21 | Not Significant |
| Baseline Drinks/drinking day | 7.74 ±2.7 | 7.81 ±4.4 | Not Significant |
| Alcohol Dependence Scale | 10.6 ±5.0 | 12.9 ±5.0 | Not Significant |
| OCDS Total Score | 15.1 ±7.2 | 16.8 ±6.7 | Not Significant |
Craving
There were no significant differences between the drug groups with regards to the alcohol craving ratings during visual presentation within the scanner.
Alcohol Withdrawal
As expected no subject experienced significant alcohol withdrawal symptoms during mandated pre-scan abstinence with the average CIWA-Ar score below 1 in both medication groups.
Comparison of Alcohol Cues with Beverage Cues
The brain areas that significantly activated within each group during the comparison of alcohol cues and beverage cues by SPM5 analysis are summarized in Table 2 and depicted in Figure 1. Consistent with our previous studies27, 30, the placebo-treated non-treatment seeking alcoholics had activation in limbic regions including ventral striatum. Alcoholics treated with aripiprazole did not experience the ventral striatum activation observed in the placebo-treated alcoholics.
Table 2.
Brain Areas Activated in the Alcohol Minus Beverage Comparison
| Group | Region | X | Y | Z | T value | Cluster Size |
|---|---|---|---|---|---|---|
|
| ||||||
| Placebo | Right inferior occipital | 33 | −90 | −6 | 8.41 | 472 |
| Left superior/medial frontal | −21 | 33 | 57 | 5.46 | 142 | |
| Left inferior occipital | −39 | −69 | −9 | 4.68 | 68 | |
| Left VTA | −3 | 3 | −18 | 4.68 | 21 | |
| Left middle occipital | −30 | −96 | −3 | 4.29 | 119 | |
| Inferior temporal | −48 | 6 | −36 | 3.82 | 17 | |
| Left parahippocapus | −21 | −18 | −24 | 3.77 | 21 | |
| Right ventral striatum | 6 | 21 | −3 | 3.75 | 16 | |
|
| ||||||
| Aripiprazole | Right lingual | 24 | −60 | 0 | 8.08 | 264 |
| Middle cingulate | −3 | 9 | 30 | 5.78 | 50 | |
| Left prefrontal | −27 | 0 | 48 | 4.7 | 57 | |
| Left superior occipital | −6 | 99 | 21 | 4.36 | 50 | |
| Right occipital | 30 | −69 | 18 | 4.28 | 40 | |
| Left occipital | −42 | −90 | 12 | 4.08 | 105 | |
| Inferior frontal | 39 | 9 | 30 | 3.96 | 17 | |
Figure 1.
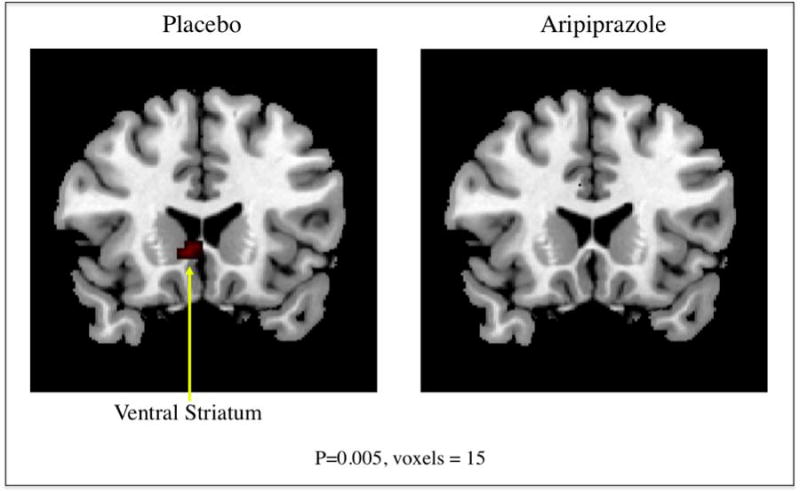
Brain regions with significantly increased activation during viewing alcohol beverages compared to viewing non-alcohol beverages are depicted in color on coronal structural magnetic resonance imaging scans (p ≤.005, spatial extent threshold 15 voxels).
Ventral Striatum Activation
Figure 2 shows the estimated Alcohol minus Beverage contrast for placebo versus aripiprazole. The cross level interaction indicating the difference in cue-induced activation between the two groups (Drug Group by Alcohol/Beverage contrast) was highly significant (B=1.04 + .42, t(27)=2.37, p=.025). Aripiprazole-treated subjects showed less activation than placebo-treated subjects, though in both groups the Alcohol/Beverage contrast was greater than zero (p<.01).
Figure 2.
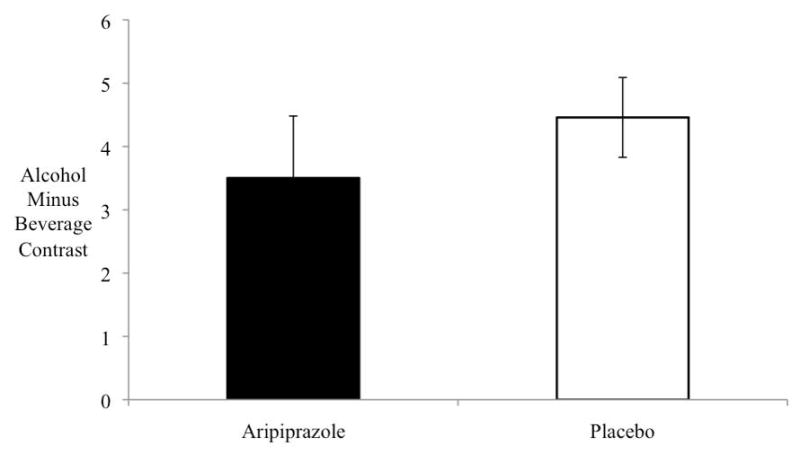
Ventral striatum activation (contrast of alcohol cue activation minus beverage cue activation) was significantly decreased in the aripiprazole group as compared to the placebo group (p=.025). Bars indicate mean values; error bars, standard error of the mean.
Drinking Outcomes
During the 12-day natural observation period, aripiprazole decreased percentage of heavy drinking days (33.1± 5.2) as compared to placebo-treated participants (49.7 ± 4.8), f(1,26)=5.48, p=.027) adjusted for baseline drinking and education. While secondary drinking variables all favored a reduction in the aripiprazole group compared to the placebo group, none reached statistical significance. No subject remained completely abstinent during the study period in either medication group.
Relationship Between Ventral Striatum Activation and Drinking
Overall, pooled across drug groups, we were unable to detect a relationship between activation of the anatomically defined ventral striatum and percent heavy drinking (B=.005 + .009, t(27)=.54, p=.59). Further, there was no interaction between drinking and drug group (B=.009+ .019, p=.62) nor between drinking and activation within either drug group (both p’s >.5). However, in the separate ventral striatal region defined by maximum correlation with drinking, a very strong positive relationship was detected in the aripiprazole-treated subjects (B=.059 + .018, t(26)=3.37, p=.003) and a substantial interaction of drinking and drug group was present (B=.085+.177, t(26)=−3.84, p=.001) such that subjects in the placebo group who had more heavy drinking days exhibited less ventral striatal activation and those in the aripiprazole group who had more heavy drinking days showed more ventral striatal activation. These data are shown in figure 3. The strong relationship between heavy drinking days and activation is clear in the aripiprazole group (B=.059 + .018, t(26)=3.37, p=.003) while the relationship is actually slightly negative in the placebo group (B=-.02 + .014, t(26)=−1.89, p=.07). We would expect a positive relationship between drinking and activation since the region was defined by the pooled subjects’ drinking/activation correlation statistical map, however, the difference between the medication groups is of interest because it is less dependent on how the region was defined. It should be noted that there are more aripiprazole subjects with both lower ventral striatal activation and lower heavy drinking days.
Figure 3.
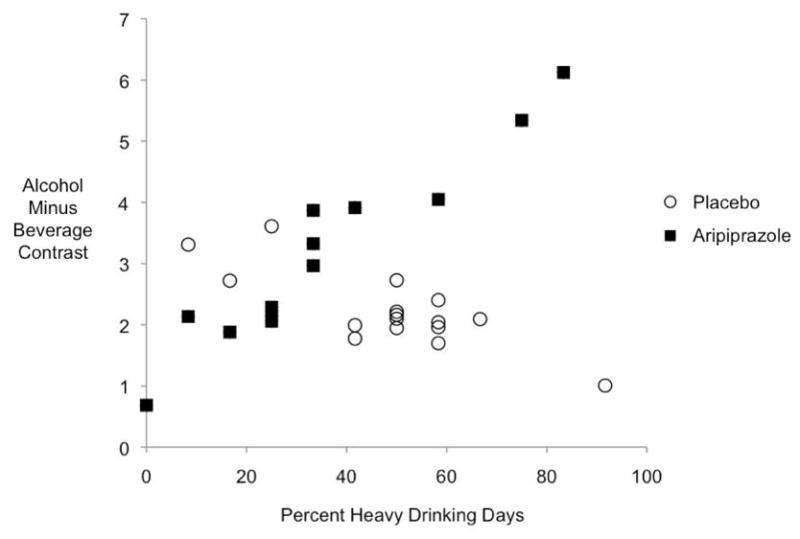
Relationship between ventral striatum activation and drinking
DISCUSSION
The results of the study indicate that aripiprazole can attenuate alcohol cue-induced activation of the brain structures that are thought to underlie craving for addictive substances. Brain activity analysis revealed increased alcohol cue-induced activation for placebo-treated participants in the right ventral striatum and the left ventral tegmental area (p<.005, threshold 15 voxels). In aripiprazole-treated participants there was a complete blockade of the alcohol cue-induced activation in these areas (p<.005, threshold 15 voxels). These results are consistent with prior work by our group in which the opiate antagonist naltrexone and, to a lesser extent, the serotonin receptor antagonist ondansetron attenuated alcohol cue-induced activation of the ventral striatum.30 In addition, aripiprazole-treated participants had a decrease in percent heavy drinking days as compared to placebo-treated participants.
The ventral striatum, particularly the nucleus accumbens (Nac), has long been recognized as an important component of the brain’s reward pathway and has extensive cortical and subcortical connections.42–44 Dopamine concentration in the Nac is increased by systemic and oral ethanol administration.45–50 In animal models, dopaminergic projections from the ventral tegmental area (VTA) to the Nac have been found to fire in response to presentation of reward cues and reward anticipation34, 51–53 and alcohol-associated cues (light or environmental) have been found to increase dopamine output from the Nac prior to actual alcohol consumption.32, 54
In human imaging studies, PET studies have implicated striatal dopamine systems in alcohol effects55–57 and fMRI studies have found anticipation of increasing monetary rewards yield increasing Nac activation58 and that memory of rewarding stimuli was preceded by differential activation of the VTA and Nac.59 Imaging studies utilizing alcohol administration paradigms have also supported the importance of the ventral striatum in alcoholism. PET studies have found increased activation in the ventral striatum during alcohol administration.60, 61
Recent fMRI work has correlated ventral striatal activation with ratings of intoxication during intravenous alcohol administration.62 This suggests that an individual’s subjective feeling of intoxication is modulated by increased neuronal activity in the ventral striatum. Our findings suggest that aripiprazole can attenuate cue-induced activation in the ventral striatum. Given that the US multi-site aripiprazole study found an effect on drinking in those individuals who had initiated alcohol use, aripiprazole could potentially be decreasing the salience of the alcohol cue and decreasing alcohol use. This effect would be similar to that of naltrexone which has been found to block ventral striatal dopamine release in anticipation of alcohol consumption in animal models.54, 63
Several dopamine agonist and antagonist (particularly D2 receptor agents) have been studied in the treatment of alcoholism but have not found clinical utility and have troublesome side effects.64–66 As a partial agonist at the D2 receptor, aripiprazole acts as a functional antagonist in hyperdopamine states and as a functional agonist at hypodopamine states.67 As such, aripiprazole’s partial dopaminergic agonist activity could potentially block the rewarding aspects of alcohol use thought to be mediated by increased dopamine in the ventral striatum. Therefore, aripiprazole has the potential of reducing acute alcohol consumption reinforcement and may normalize these crucial brain dopamine systems during initial attempts at abstinence, thereby, hypothetically, reducing the urge to drink and preventing relapse. The current study directly tested this hypothesis and found that aripiprazole was able to attenuate cue-induced brain activity in the ventral striatum. This would suggest that aripiprazole might be capable of disrupting “alcohol-induced reward memory” in alcoholics leading to reduced cue-responsiveness, craving and relapse.
Aripiprazole’s effect on reduction of heavier alcohol consumption is consistent with our previous work23 and that in several clinical trials.15–18 Another mechanism of action, suggested by our previous work, is the possibility that aripiprazole might work by breaking the relationship between trait-impulsivity and drinking since it seemed to work best in individuals with higher trait-impulsivity.23 This information was not available at the start of this study so we did not randomize based on trait-impulsivity, but future work should take this into consideration. It is of interest in this regard that several brain areas showing a placebo-aripiprazole difference, including the ventral striatum have been shown to play a role in the choice of immediate versus delayed reinforcers.
The cue-induced activation in the placebo group is consistent with our previous work27, 30 and other published cue-induced imaging studies involving substances of abuse. Regions activated include both limbic and cortical areas. These include various portions of the prefrontal cortex 68–70 and the ventral striatum.25, 26, 29, 71, 72
Of interest was the interaction of heavy drinking days and ventral striatum area activation by drug group. Given the correlative nature of the data it is impossible to determine if the ventral striatal activation is a cause or effect of the heavy drinking. However, it would appear, at least for a few aripiprazole-treated subjects that if ventral striatal activation is strongly present so is heavy drinking days, while for the majority of aripiprazole subjects both ventral striatal activation and heavy drinking were low. Whether this suggests individual differences (genetic or phenotypic) in response to aripiprazole, or just a chance finding, is hard to know at this time. Also, for the majority of the aripiprazole subjects it would appear that cue responsivity did not translate into more heavy drinking days while this was not as observable in placebo treated-subjects. Although more data is needed, it is possible that, at least for some individuals, aripiprazole might break a link between cue reactivity and heavy drinking, while for others it might exacerbate it. This idea might be consistent with our previous finding of aripiprazole being effective at reducing drinking only in individuals with higher trait-impulsivity and with it breaking the relationship between alcohol-induced stimulation and heavier drinking.23
In summary, the current study provides evidence of the potential utility of aripiprazole in the treatment of alcoholism. Consistent with animal data suggesting that alcohol and alcohol cues can stimulate dopamine output in the ventral striatum, the current study found alcohol cue-induced activation in the ventral striatum in the placebo group that was attenuated by aripiprazole. The modulating effect on dopamine systems by aripiprazole may have directly effected the cue-induced activation. It should be noted that the subjects in this study had no motivation to stop or reduce drinking and that aripiprazole was given over a period when alcohol was being consumed under natural conditions. There is no guarantee that with a single dose of aripiprazole, or if imaging had been done without prior alcohol experience while taking the medication, that the same effect on ventral striatal imaging would be observed. The relationship between this deactivation, craving, alcohol consumption and relapse drinking during treatment all require further exploration.
Acknowledgments
This work was funded by the Charleston Alcohol Research Center (ARC) (NIAAA P50 AA10761). Dr. Myrick is also funded through NIAAA K23 AA00314 and the VA Research and Development Service, Ralph H. Johnson Department of Veterans Affairs Medical Center.
Footnotes
Presented in part as an oral presentation at the 2006 American College of Neuropsychopharmacology meeting and a poster presentation at the 2009 Research Society on Alcoholism meeting.
FINANCIAL DISCLOSURES
Drs. Anton and Myrick have received grant support from Bristol-Myers Squib. Dr. Myrick is a member of the Bristol Myers Squib speakers bureau and Dr. Anton has been a consultant for Bristol Myers Squib. Drs. Voronin, Li, Randall and Mr. Henderson reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism. A Veterans Administration cooperative study. JAMA. 1986;256:1449–1455. [PubMed] [Google Scholar]
- 2.Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1335–1341. [PubMed] [Google Scholar]
- 3.Krystal JH, Cramer JA, Krol WF, et al. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- 4.Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta-analysis of randomized controlled trials. Alcohol Alcohol. 2001;36:544–552. doi: 10.1093/alcalc/36.6.544. [DOI] [PubMed] [Google Scholar]
- 5.Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 6.Aihara K, Shimada J, Miwa T, et al. The novel antipsychotic aripiprazole is a partial agonist at short and long isoforms of D2 receptors linked to the regulation of adenylyl cyclase activity and prolactin release. Brain Res. 2004;1003:9–17. doi: 10.1016/j.brainres.2003.09.082. [DOI] [PubMed] [Google Scholar]
- 7.Yokoi F, Grunder G, Biziere K, et al. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27:248–259. doi: 10.1016/S0893-133X(02)00304-4. [DOI] [PubMed] [Google Scholar]
- 8.Burris KD, Molski TF, Xu C, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302:381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- 9.Taylor DM. Aripiprazole: a review of its pharmacology and clinical use. Int J Clin Pract. 2003;57:49–54. [PubMed] [Google Scholar]
- 10.Argo TR, Carnahan RM, Perry PJ. Aripiprazole, a novel atypical antipsychotic drug. Pharmacotherapy. 2004;24:212–228. doi: 10.1592/phco.24.2.212.33145. [DOI] [PubMed] [Google Scholar]
- 11.DeLeon A, Patel NC, Crismon ML. Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clin Ther. 2004;26:649–666. doi: 10.1016/s0149-2918(04)90066-5. [DOI] [PubMed] [Google Scholar]
- 12.Lindsey RL, Kaplan D, Koliatsos V, et al. Aripiprazole and extrapyramidal symptoms. J Am Acad Child Adolesc Psychiatry. 2003;42:1268–1269. doi: 10.1097/01.chi.0000095540.18151.b0. [DOI] [PubMed] [Google Scholar]
- 13.Beresford TP, Clapp L, Martin B, et al. Aripiprazole in schizophrenia with cocaine dependence: a pilot study. J Clin Psychopharmacol. 2005;25:363–366. doi: 10.1097/01.jcp.0000169419.38899.5b. [DOI] [PubMed] [Google Scholar]
- 14.Lile JA, Stoops WW, Vansickel AR, et al. Aripiprazole attenuates the discriminative- stimulus and subject-rated effects of D-amphetamine in humans. Neuropsychopharmacology. 2005;30:2103–2114. doi: 10.1038/sj.npp.1300803. [DOI] [PubMed] [Google Scholar]
- 15.Janiri L, Martinotti G, Di Nicola M. Aripiprazole for relapse prevention and craving in alcohol-dependent subjects: results from a pilot study. J Clin Psychopharmacol. 2007;27:519–520. doi: 10.1097/JCP.0b013e318150c841. [DOI] [PubMed] [Google Scholar]
- 16.Warsi M, Sattar SP, Bhatia SC, et al. Aripiprazole reduces alcohol use. Can J Psychiatry. 2005;50:244. doi: 10.1177/070674370505000415. [DOI] [PubMed] [Google Scholar]
- 17.Anton RF, Kranzler H, Breder C, et al. A randomized, multicenter, double-blind, placebo-controlled study of the efficacy and safety of aripiprazole for the treatment of alcohol dependence. J Clin Psychopharmacol. 2008;28:5–12. doi: 10.1097/jcp.0b013e3181602fd4. [DOI] [PubMed] [Google Scholar]
- 18.Martinotti G, Di Nicola M, Di Giannantonio M, et al. Aripiprazole in the treatment of patients with alcohol dependence: a double-blind, comparison trial vs. naltrexone. J Psychopharmacol. 2009;23:123–129. doi: 10.1177/0269881108089596. [DOI] [PubMed] [Google Scholar]
- 19.Drobes DJ, Anton RF, Thomas SE, et al. A clinical laboratory paradigm for evaluating medication effects on alcohol consumption: naltrexone and nalmefene. Neuropsychopharmacology. 2003;28:755–764. doi: 10.1038/sj.npp.1300101. [DOI] [PubMed] [Google Scholar]
- 20.Drobes DJ, Thomas SE. Assessing craving for alcohol. Alcohol Res Health. 1999;23:179–186. [PMC free article] [PubMed] [Google Scholar]
- 21.O’Malley SS, Krishnan-Sarin S, Farren C, et al. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- 22.Kranzler HR, Covault J, Pierucci-Lagha A, et al. Effects of aripiprazole on subjective and physiological responses to alcohol. Alcohol Clin Exp Res. 2008;32:573–579. doi: 10.1111/j.1530-0277.2007.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voronin K, Randall P, Myrick H, et al. Aripiprazole effects on alcohol consumption and subjective reports in a clinical laboratory paradigm--possible influence of self-control. Alcohol Clin Exp Res. 2008;32:1954–1961. doi: 10.1111/j.1530-0277.2008.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braus DF, Wrase J, Grusser S, et al. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm. 2001;108:887–894. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- 25.George MS, Anton RF, Bloomer C, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- 26.Kareken DA, Sabri M, Radnovich AJ, et al. Olfactory system activation from sniffing: effects in piriform and orbitofrontal cortex. Neuroimage. 2004;22:456–465. doi: 10.1016/j.neuroimage.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Myrick H, Anton RF, Li X, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 28.Schneider F, Habel U, Wagner M, et al. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- 29.Wrase J, Grusser SM, Klein S, et al. Development of alcohol-associated cues and cue- induced brain activation in alcoholics. Eur Psychiatry. 2002;17:287–291. doi: 10.1016/s0924-9338(02)00676-4. [DOI] [PubMed] [Google Scholar]
- 30.Myrick H, Anton RF, Li X, et al. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- 32.Katner SN, Weiss F. Neurochemical characteristics associated with ethanol preference in selected alcohol-preferring and -nonpreferring rats: a quantitative microdialysis study. Alcohol Clin Exp Res. 2001;25:198–205. [PubMed] [Google Scholar]
- 33.Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- 34.Melendez RI, Rodd-Henricks ZA, Engleman EA, et al. Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res. 2002;26:318–325. [PubMed] [Google Scholar]
- 35.Wise RA. Opiate reward: sites and substrates. Neurosci Biobehav Rev. 1989;13:129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-1 Clinical Version) Washington, DC: American Psychiatric Publishing, Inc; 1997. [Google Scholar]
- 37.Sobell LC, Sobell MB, Leo GI, et al. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 38.Anton RF, Moak DH, Latham PK. The obsessive compulsive drinking scale: A new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996;53:225–231. doi: 10.1001/archpsyc.1996.01830030047008. [DOI] [PubMed] [Google Scholar]
- 39.Davis LJ, Jr, Hurt RD, Morse RM, et al. Discriminant analysis of the Self-Administered Alcoholism Screening Test. Alcohol Clin Exp Res. 1987;11:269–273. doi: 10.1111/j.1530-0277.1987.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 40.Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 42.Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- 43.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 44.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blomqvist O, Engel JA, Nissbrandt H, et al. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- 46.Mocsary Z, Bradberry CW. Effect of ethanol on extracellular dopamine in nucleus accumbens: comparison between Lewis and Fischer 344 rat strains. Brain Res. 1996;706:194–198. doi: 10.1016/0006-8993(95)01200-1. [DOI] [PubMed] [Google Scholar]
- 47.Weiss F, Lorang MT, Bloom FE, et al. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- 48.Weiss F, Mitchiner M, Bloom FE, et al. Free-choice responding for ethanol versus water in alcohol preferring (P) and unselected Wistar rats is differentially modified by naloxone, bromocriptine, and methysergide. Psychopharmacology (Berl) 1990;101:178–186. doi: 10.1007/BF02244123. [DOI] [PubMed] [Google Scholar]
- 49.Yim HJ, Schallert T, Randall PK, et al. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res. 1998;22:367–374. [PubMed] [Google Scholar]
- 50.Yoshimoto K, McBride WJ, Lumeng L, et al. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- 51.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 52.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 53.Schultz W, Apicella P, Scarnati E, et al. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Middaugh LD, Szumlinski KK, Van Patten Y, et al. Chronic ethanol consumption by C57BL/6 mice promotes tolerance to its interoceptive cues and increases extracellular dopamine, an effect blocked by naltrexone. Alcohol Clin Exp Res. 2003;27:1892–1900. doi: 10.1097/01.ALC.0000099264.36220.48. [DOI] [PubMed] [Google Scholar]
- 55.Heinz A, Siessmeier T, Wrase J, et al. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162:1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- 56.Martinez D, Gil R, Slifstein M, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 57.Yoder KK, Kareken DA, Seyoum RA, et al. Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Res. 2005;29:965–970. doi: 10.1097/01.alc.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]
- 58.Knutson B, Adams CM, Fong GW, et al. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adcock RA, Thangavel A, Whitfield-Gabrieli S, et al. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 60.Schreckenberger M, Amberg R, Scheurich A, et al. Acute alcohol effects on neuronal and attentional processing: striatal reward system and inhibitory sensory interactions under acute ethanol challenge. Neuropsychopharmacology. 2004;29:1527–1537. doi: 10.1038/sj.npp.1300453. [DOI] [PubMed] [Google Scholar]
- 61.Wang GJ, Volkow ND, Franceschi D, et al. Regional brain metabolism during alcohol intoxication. Alcohol Clin Exp Res. 2000;24:822–829. [PubMed] [Google Scholar]
- 62.Gilman JM, Ramchandani VA, Davis MB, et al. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carlsson C, Gullberg B. A double blind study with melperone and placebo in the treatment of chronic alcoholics. Int J Clin Pharmacol Biopharm. 1978;16:331–332. [PubMed] [Google Scholar]
- 65.Naranjo CA, Dongier M, Bremner KE. Long-acting injectable bromocriptine does not reduce relapse in alcoholics. Addiction. 1997;92:969–978. [PubMed] [Google Scholar]
- 66.Walter H, Ramskogler K, Semler B, et al. Dopamine and alcohol relapse: D1 and D2 antagonists increase relapse rates in animal studies and in clinical trials. J Biomed Sci. 2001;8:83–88. doi: 10.1007/BF02255975. [DOI] [PubMed] [Google Scholar]
- 67.Kikuchi T, Tottori K, Uwahodo Y, et al. 7-(4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butyloxy)-3,4-dihydro-2(1H)-qui nolinone (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J Pharmacol Exp Ther. 1995;274:329–336. [PubMed] [Google Scholar]
- 68.Grant S, London ED, Newlin DB, et al. Activation of memory circuits during cue- elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maas LC, Lukas SE, Kaufman MJ, et al. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- 70.Wang GJ, Volkow ND, Fowler JS, et al. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- 71.Breiter HC, Gollub RL, Weisskoff RM, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 72.Kilts CD, Schweitzer JB, Quinn CK, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]


