Abstract
Nucleocytosolic and secreted proteins are commonly glycosylated. However, reports of glycosylated mitochondrial proteins are rare. Using lectin chromatography on bovine heart, we detected low-abundance glycoforms of nuclear-encoded proteins with well-established mitochondrial function: pyruvate dehydrogenase E1α, NADH dehydrogenase [ubiquinone] iron-sulfur protein 3, ADP/ATP translocase, ATP synthase d and oligomycin sensitivity-conferring protein. Notably, the latter two have been previously detected at the plasma membrane. Our findings indicate that glycosylation of classic mitochondrial proteins may be more common than previously appreciated. We discuss the implication that glycosylation could represent an unexplored mechanism for regulating these proteins’ functions within mitochondria or at extra-mitochondrial locations.
Keywords: glycosylation, PDH E1α, ANT, NDUFS3, ATP synthase subunit d, OSCP
1. Introduction
Glycosylation is a common post-translational modification capable of altering a protein’s activity, stability, localization, and interactions (Marth and Grewal, 2008). Importantly, many essential cellular functions are mediated by glycoproteins, including molecular trafficking, cell signaling, development, and immune response (Ohtsubo and Marth, 2006).
Although the glycosylation of secreted, membrane, and nucleocytosolic proteins has been well-studied, knowledge regarding the glycosylation of mitochondrial proteins is limited. Other post-translational modifications of mitochondrial proteins have been investigated (Distler et al., 2007; Kerner et al., 2004) but until recently there had been only a few isolated reports of glycosylated mitochondrial proteins (Chandra et al., 1998; Levrat et al., 1989). Recent reports have suggested that several proteins with mitochondrial function or with mitochondrial-annotation, and thus putative mitochondrial function, may be glycosylated (Clark et al., 2008; Hu et al., 2009; Kung et al., 2009; Teo et al., 2010). With a few exceptions (Hu et al., 2009), most of these recent observations have come from high-throughput proteomics data, in which hundreds of candidate glycoproteins are reported without secondary verification. One caveat of this approach is that although each individual glycoprotein candidate may have a low probability of being a false-positive, the probability of at least one false-positive amongst the entire dataset is much higher. Despite this, reports of even unconventional glycoprotein candidates such as the classic mitochondrial protein ATP synthase α-subunit (Clark et al., 2008; Kung et al., 2009; Teo et al., 2010) have been verified by additional observations using independent and individualized experimental approaches (Schmidt et al., 2008) and our work (Burnham-Marusich, et al., in review). Intriguingly, glycosylated ATP synthase α-subunit subunit resides at the cell surface of rat neuroblastoma cells, where it is part of a cell-surface F1F0-ATP synthase complex whose ATPase activity is necessary for normal short- and long-term synaptic plasticity (Schmidt et al., 2008).
Identifying extra-mitochondrial localizations for classic mitochondrial proteins and understanding their functions in extra-mitochondrial locations is an emerging field. For example, fumarase participates in the tricarboxylic acid cycle when in the mitochondria, and in the DNA damage response when in the cytosol and nucleus (Yogev et al., 2010). Indeed, a recent report indicates that one-third of the mitochondrial proteome may be dual-localized (Ben-Menachem et al., 2011). Because ATP synthase α-subunit is the only mitochondrial protein for which the subcellular localization of its glycosylated isoform has been determined, the possibility that glycosylation of a subset of classic mitochondrial proteins may be associated with their extra-mitochondrial localization cannot be ruled out.
The increasing number of putative glycoproteins with mitochondrial-annotation (Clark et al., 2008; Hu et al., 2009; Kung et al., 2009; Teo et al., 2010) and our work (Burnham-Marusich, et al., in review) prompted us to investigate whether proteins with well-established mitochondrial function have verifiable glycosylated isoforms. Here we provide data indicating that multiple proteins with well-characterized and essential mitochondrial functions are glycosylated. Because these previously undetected glycosylated isoforms must reside either within the mitochondria or outside the mitochondria in an unconventional location, we discuss the implications that 1) glycosylation may be an unexplored regulatory mechanism within the mitochondria, or 2) these glycosylated isoforms may be the first indication of an extra-mitochondrial location and possible unconventional function for these proteins.
2. Materials and methods
2.1 Preparation of bovine heart mitochondria and lectin affinity chromatography
Mitochondria were enriched as in (Smith, 1967), with modifications described in the Supplementary Methods. Mitochondria were solubilized at 1.8mg/ml for 30min on ice in detergent buffer (3% w/v CHAPS, 7M urea, 2M thiourea, 9mM Tris-acetate, pH 7.0, 0.5mM PMSF), then centrifuged 30min at 16,000 × g. Supernatant was incubated 1hr with wheat germ agglutinin (WGA) agarose beads (Vector) at a ratio of 16mg solubilized protein per 1ml packed WGA beads. Beads were washed 6X in 6.5 volumes PBS, then proteins eluted 2X in 1 volume PBS with 0.5M GlcNAc.
2.2 Metabolic labeling, Click Chemistry detection, and enrichment of labeled proteins
COS-7 cells were metabolically labeled for 48hrs with 50μM tetraacetylated N-azidoacetylgalactosamine (azido-GalNAc) or GalNAc and then harvested by incubating 30min on ice in 1% SDS 50mM TrisHCl, pH 8 with 1X protease inhibitor cocktail (Calbiochem) and 300U/ml Benzonase (Novagen). In parallel, equal amounts of azido-GalNAc and GalNAc COS-7 lysates were reacted as follows: 200μg of cellular protein in 50μl of lysis buffer were reacted with final concentrations of 20μM biotin-alkyne (Invitrogen), 47.5% DMSO, 2mM fresh ascorbic acid, 200μM Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine, and 2mM CuSO4 in a 200μl reaction volume. Click Chemistry reactions were incubated for 1hr at room temperature (RT). Samples used for Western blot analysis were precipitated with methanol-chloroform, while samples used for avidin enrichment were subjected to repeated concentration and dilution with 10kDa filters (Amicon) and 0.2% SDS in PBS to remove Click reaction components to <0.1% of original concentration. Avidin enrichment samples were incubated with streptavidin beads (GE) for 1hr and washed as described (Nandi et al., 2006). Proteins were eluted in one bead volume Laemmli buffer with 6mM D-biotin.
2.3 Western blot analysis
Protein loading was verified with Sypro Ruby (Invitrogen). Blots were incubated with antibodies for 2hrs at RT and developed as per company recommendations: PDH E1α (1:1000), NDUFS3 (1:890), OSCP (1:2000), ATP synthase subunit d (1:1000), ANT (1:1000), Complex IV subunit 1 (1:1000), MCAD (1:8000) all from MitoSciences. Calnexin (Enzo, ADI-SPA-860) and integrin α5 (gift from Dr. Valencik, UNR) were diluted 1:1000 and 1:2000, respectively, in 5% milk/PBS and developed like the antibodies above. Goat anti-mouse and anti-rabbit IgG:HRP secondary antibodies (Jackson ImmunoResearch) were used at 1:10,000 and 1:20,000, respectively. To detect azido-proteins reacted with biotin-alkyne, blots were incubated 1hr with 0.01μg/ml avidin:HRP (Vector).
2.4 In silico protein sequence analysis for potential glycosylation sites
The following Uniprot sequences were analyzed: A7MB35 (PDH E1α), P13621 (OSCP), P13620 (ATP synthase subunit d), P23709 (NDUFS3), P02722 (ANT1). For O-GlcNAc glycosylation predictions, YinOYang v1.2 (Gupta and Brunak, 2002), and OGlcNAcScan (Wang et al., 2011) were used. Potential N-linked glycosylation sites were identified manually using the N-!P-[S/T] consensus motif. Signal peptide predictions were made using SignalP v3.0 (Bendtsen et al., 2004).
3. Results
3.1 Pyruvate dehydrogenase E1α (PDH E1α) has a glycosylated isoform
Mitochondria were first enriched from bovine heart tissue in order to provide a starting material with increased quantities of mitochondrial proteins. Since some of the mitochondrial proteins to be analyzed have been reported at the plasma membrane (ATP synthase subunit d and OSCP; (Yonally and Capaldi, 2006), mitochondria were not further purified. Western blotting for subcellular markers revealed that compared to the starting homogenate, the mitochondrial fraction contained increased quantities of Complex IV, subunit 1, which is a mitochondrially-encoded, transmembrane protein of the mitochondrial inner membrane, and decreased quantities of the ER marker, Calnexin, (Figure 1A). Comparable amounts of integrin α5, a plasma membrane marker, were found in both fractions (Figure 1A).
Fig. 1. An isoform of PDH E1α is glycosylated.

(A) Bovine heart homogenate (Hom.) and mitochondria enriched from it (Mito.) were separated by SDS-PAGE. Protein loading was verified by staining with Sypro. Membranes were probed with antibodies for ER (Calnexin), mitochondria (Complex IV, subunit 1), and plasma membrane (integrin α5) markers. (B) WGA-binding glycoproteins were purified from 5.1mg of detergent-solubilized protein from enriched mitochondria. Input and flowthrough (FT) lanes contain 0.03% of each fraction; wash and eluate lanes contain all protein that was released from the WGA-beads during the last PBS wash (wash lane) or during the subsequent elution with PBS + 0.5M GlcNAc (eluate lane). PDH E1α was detected by western blot. Images are representative of two independent experiments. (C) COS-7 cells were incubated with 50μM azido-GalNAc (+) or 50μM GalNAc (-). In parallel, 2.3mg each of azido-GalNAc and GalNAc lysates were reacted with biotin-alkyne, and biotinylated azido-labeled proteins were captured with streptavidin beads. Input and FT lanes contain 2% of each fraction; eluate lanes contain all protein released from the washed beads by boiling in Laemmli + 6mM biotin. Metabolically labeled proteins were detected by avidin-HRP. PDH E1α was detected by western blot.
The enriched mitochondria were then solubilized with a harsh detergent solution to dissociate protein complexes, and individual glycoproteins were affinity purified from the lysate using beads conjugated to the lectin wheat germ agglutinin (WGA). Western blotting revealed that a fraction of the total PDH E1α bound the beads (eluate vs. flowthrough lanes, Figure 1B). PDH E1α was not released from the WGA-beads during the last PBS wash (wash lane, Figure 1B), but was specifically released during the subsequent elution with PBS + 0.5M GlcNAc (eluate lane, Figure 1B).
In an independent approach, the glycosylation status of PDH E1α was assessed by azido-sugar metabolic labeling. Equal amounts of COS-7 lysates from cells incubated with GalNAc or peracetylated azido-GalNAc were reacted via Click Chemistry with biotin-alkyne. The biotinylated, azido-labeled proteins were then purified by avidin enrichment. A PDH E1α western blot showed signal only in the azido-GalNAc eluate, not the control GalNAc eluate (Figure 1C). Together, these data indicate that glycosylated PDH E1α is expressed in bovine heart tissue and a primate cell line.
3.2 Multiple proteins with essential mitochondrial functions have glycosylated isoforms
The observation of a glycosylated isoform of PDH E1α prompted us to assess the glycosylation status of other proteins with well-established mitochondrial function. Using the same lectin purification technique with detergent-solubilized proteins as above (Section 3.1), we determined that glycosylated isoforms of ADP/ATP translocase (ANT), NADH dehydrogenase [ubiquinone] iron-sulfur protein 3 (NDUFS3, a Complex I subunit), ATP synthase OSCP subunit, and ATP synthase d subunit also specifically bound the WGA-beads (Figure 2). The former three proteins were released from the WGA-beads only by PBS + 0.5M GlcNAc (eluate lane, Figure 2) and not by PBS alone (last wash lane, Figure 2). A small quantity of the fourth protein, ATP synthase d subunit, was detected in the final PBS wash (last wash lane, Figure 2), but a much greater amount was released by the subsequent incubation with PBS + 0.5M GlcNAc (eluate lane, Figure 2).
Fig. 2. Multiple proteins with mitochondrial function have glycosylated isoforms, including ANT, NDUFS3, and the ATP synthase d- and OSCP-subunits.
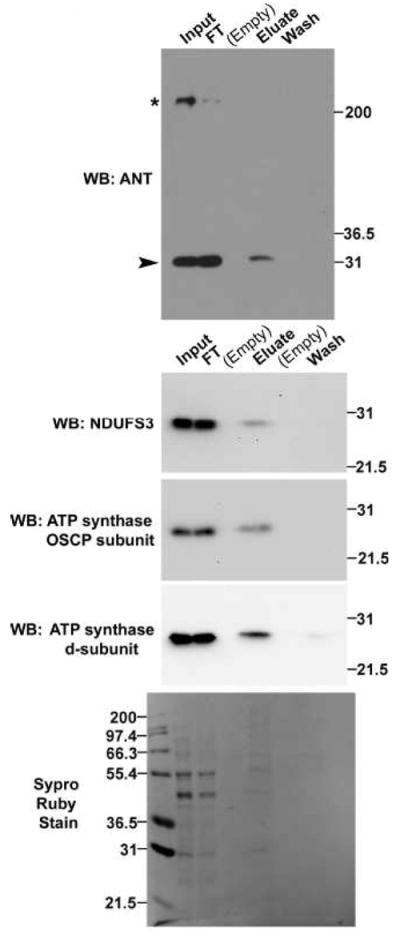
WGA-binding glycoproteins were purified from 5.1mg of detergent-solubilized protein from enriched mitochondria. Input and flowthrough (FT) lanes contain 0.03% of each fraction; wash and eluate lanes contain all protein released from the WGA-beads during the last PBS wash (wash lane) or during the subsequent elution with PBS + 0.5M GlcNAc (eluate lane). Proteins were detected by western blot. In the western blot for ANT, the arrowhead indicates a band of the expected molecular weight and the asterisk indicates a non-specific band. Images are representative of two independent experiments. All blots were stained with Sypro, and one representative image is shown.
The lack of WGA-binding for another abundant mitochondrial protein, medium-chain acyl-CoA dehydrogenase (MCAD), in an identical experiment (Supplementary Figure 1) further indicates that not every mitochondrial protein binds WGA and supports the specificity of the WGA beads. Moreover, lack of MCAD signal in the eluate lane of Supplementary Figure 1 is not due to insufficient protein in the lane, because signal was detected in this lane when the blot was probed with ANT antibody. Poor cross-reactivity of antibodies against ANT, NDUFS3 and OSCP in COS-7 extracts prevented the anaysis of azido-GalNAc labeled proteins, while analysis with ATP synthase subunit d antibody was inconclusive.
In an additional experiment, we used liquid chromatography with mass spectrometry (LC-MS/MS) to analyze the eluate and last wash fractions from a WGA enrichment experiment (Supplementary Methods). We observed multiple peptides that provided 44%, 19%, and 22% coverage for ANT-1, ATP synthase d subunit and OSCP, respectively, which enabled the high-confidence identifications (>99%) of these proteins exclusively within the eluate sample (Supplementary Table 1). Because only a fraction of the total eluate sample was analyzed by LC-MS/MS to avoid overloading the LC column, the glycoforms of PDH E1α and NDUFS3 may not be abundant enough to be detected by LC-MS/MS. Notably, although the ANT antibody recognizes all four similarly-sized ANT isoforms, the LC-MS/MS experiment identified peptides unique to ANT1, thereby identifying the glycosylated ANT isoform as ANT1 (Supplementary Table 2).
3.3 In silico analysis of predicted glycosylation sites in the identified glycoproteins
Two different O-GlcNAc site prediction algorithms both predict that all five of the identified glycoproteins are O-GlcNAc modified. Moreover, two of the five proteins (PDH E1α, NDUFS3) have potential O-GlcNAc glycosylation sites recognized by both algorithms. The remaining three proteins, OSCP, ANT1 and ATP synthase d, are predicted to be O-GlcNAc modified, but the algorithms do not agree on which amino acid would be modified.
Only two proteins (ATP synthase subunit d and ANT1) have a potential N-glycosylation site of the canonical form (N-!P-[S/T]). However, N-glycosylation classically occurs when proteins with signal sequences are imported into the ER (Marth and Grewal, 2008), and as expected of mitochondrial proteins, none of these five proteins received high scores when analyzed with the signal peptide prediction algorithm, SignalP.
4. Discussion
We have identified glycosylated isoforms of multiple proteins with established mitochondrial function. These glycoproteins are all nuclear-encoded and include: PDH E1α, ATP synthase d and OSCP subunit, NDUFS3, and ANT1. To our knowledge, the latter four glycoproteins represent novel isoforms that have not been previously reported. Moreover, although one other publication reports PDH E1α as a putative glycoprotein (Clark et al., 2008), the data came from a single high-throughput experiment without independent verification or commentary on the significance of finding that a protein with established function in the mitochondrial matrix has a glycosylated isoform.
Increasingly, high-throughput experiments are reporting proteins with mitochondrial annotation and thus likely mitochondrial function as putative glycoproteins (Clark et al., 2008; Kung et al., 2009; Teo et al., 2010). Our data indicates that glycosylated isoforms of proteins with established mitochondrial function exist and may be more common than previously appreciated. This poses a number of interesting questions regarding the nature of the glycosylation (glycan structure and attachment site), the subcellular compartment in which these proteins become glycosylated, the subcellular compartment in which the glycoforms reside, and the biological role of the modification in regulating these proteins’ functions either in the mitochondria or at an extra-mitochondrial location.
Since the lectin WGA binds multiple glycan residues (terminal GalNAc, GlcNAc, sialic acid, or polysialic acid) (Peters et al., 1979) and because both O-linked and N-linked glycoproteins can contain these glycan motifs, the WGA-binding data alone cannot distinguish to which glycoprotein class our five identified glycoproteins belong. However, an N-glycosylation model for these glycoproteins is unlikely for the following reasons. First, only two of the five proteins contain the N-glycosylation consensus motif. Second, unmodified N-glycosylation consensus sites are common. Although only a subset of yeast proteins are N-glycosylated, the canonical N-!P-[S/T] motif occurs 4.5 times per protein on average in the yeast proteome (Kung et al., 2009). Lastly, although there is one report of N-glycosylation occurring in the mitochondria (Levrat et al., 1989), it is commonly accepted that N-glycosylation occurs within the ER and Golgi via the sequential activities of multiple ER- and Golgi-localized glycosyltransferases. In support of this, there is one report of an N-linked glycoprotein synthesized in the ER migrating to the mitochondria (Chandra et al., 1998). But because PDH E1α, ANT, NDUFS3, ATP synthase subunit d and OSCP all lack signal sequences to target them to the ER, it is unlikely that the potential N-glycosylation sites within ATP synthase d and ANT1 are actually modified.
Conversely, O-GlcNAc glycosylation requires only a single glycosyltransferase (O-GlcNAc glycosyltransferase, OGT), of which two splice variants exist: a nucleocytosolic isoform with established catalytic activity, and a mitochondrially-targeted isoform (mOGT) that has shown catalytic activity in vitro (Love et al., 2003). Recently, the O-GlcNAc modification of a mitochondrially-encoded protein (Complex IV, subunit 1) was detected in cardiac myocytes, which suggests that O-GlcNAcylation may occur within the mitochondria (Hu et al., 2009). Thus although we cannot eliminate the possibility of an N-glycosylation, the body of O-GlcNAc literature and our site prediction analysis suggest that O-GlcNAc modification of these glycoproteins is likely.
An additional implication of our data is that it raises the question of where in the cell and, consequently, by which glycosyltransferases PDH E1α, ANT1, NDUFS3, ATP synthase subunit d and OSCP become glycosylated. Furthermore, until a survey of the glycan attachment sites of a large population of proteins with mitochondrial function is completed, it remains an open question as to whether the peptide sequences of the sites are actually conserved with those of classic nucleocytoplasmic (O-GlcNAc) or secreted (N-linked and other) glycoproteins.
Only a small fraction of the total pool of each of our identified glycoproteins bound the WGA beads during the affinity purification. It is unlikely that this was caused by limited WGA-bead binding capacity, because enough beads were used that even if one of every two milligrams of solubilized protein were N-acetylglucosaminyl glycoproteins, which is far in excess of what the total protein staining on the eluate lanes shows, then there would have been sufficient binding capacity in the resin to capture all the glycoproteins. Since the lectin purification was performed with enriched bovine heart mitochondria that contained residual ER and plasma membrane components, the glycoforms we detected must thus reside either within the mitochondria or in an unconventional location outside the mitochondria, such as the remaining ER or plasma membrane. If the glycoforms we detected reside within the mitochondria, then their glycosylation may be an unexplored mechanism for regulating their well-described metabolic functions. To this end, we note that dysfunction of N-glycosylation in yeast alters mitochondrial function (Mendelsohn et al., 2005) and increased O-GlcNAcylation of other subunits of Complex I, III, and IV is associated with impaired respiratory activity in rat cardiac myocytes (Hu et al., 2009), though the mechanisms underlying the glycosylation-related changes remain to be determined.
Alternatively, the glycosylated isoforms may be localized outside the mitochondria. Although an extra-mitochondrial localization for proteins with established mitochondrial function would be unconventional, it is not without precedent. Multiple subunits of the ATP synthase complex, including the OSCP and d subunits, have been observed at the plasma membrane (Moser et al., 1999; Vantourout et al., 2008; Yonally and Capaldi, 2006). Furthermore, the only mitochondrial protein for which the subcellular localization of its glycosylated isoform has been determined is ATP synthase α-subunit, and its glycoform is localized to the cell surface (Schmidt et al., 2008). Whether glycosylation of ATP synthase α, d, or OSCP subunits is important for their plasma membrane localization remains to be tested. Although ANT can associate with the ATP synthase complex (Chen et al., 2004) and thus could conceivably be at the cell surface in association with the ATP synthase complex, there are no reports of ER or plasma membrane localization for ANT, NDUFS3 or PDH E1α to our knowledge. In sum, if the glycosylated isoforms of ANT, NDUFS3 or PDH E1α do not reside within the mitochondria, then they must reside outside the mitochondria. Thus these unexpected glycosylated isoforms may be the first observation to raise the question of such an extra-mitochondrial location and unconventional function for ANT, NDUFS3 and PDH E1α.
In conclusion, we describe the novel glycosylation of several proteins with well-established mitochondrial localization and function. These glycosylated isoforms, which comprise only a fraction of the total expressed protein, could either reside within the mitochondria, where glycosylation may be an unexplored mechanism for regulating their well-described metabolic functions, or at an extra-mitochondrial location. The latter would represent an unconventional localization, which may provide the glycoforms an opportunity to participate in unconventional functions. Future research should focus on elucidating where these unusual glycoproteins reside, as well as on clarifying in which subcellular compartment they become glycosylated, which glycosyltransferases participate, and what the biological functions are of the attached glycans.
Supplementary Material
Highlights.
> Several nuclear-encoded mitochondrial proteins have glycosylated isoforms. > These include PDH E1α, NDUFS3, ANT, ATP synthase subunit d and OSCP. > Glycosylation of mitochondrial proteins may be more common than previously appreciated. > Glycosylation could represent a mechanism for regulating their functions.
Acknowledgments
We thank Rebekah Woolsey and Dr. David Quilici of the Nevada Proteomics Center (NPC) for mass spectrometry analysis, Dr. Michael Marusich for helpful comments on the manuscript, and Drs. Grant Mastick, Chris von Bartheld, Scott Clark and John McDonald for discussions. NPC instrument acquisition was funded by NIH grant #S10RR023587. This work was supported by NIH Grants P20 RR-016464 from the INBRE Program and P20 RR024210 from the COBRE Program of the National Center for Research Resources.
Abbreviations
- PDH E1α
pyruvate dehydrogenase E1 α-subunit
- ANT
ADP/ATP translocase
- NDUFS3
NADH dehydrogenase [ubiquinone] iron-sulfur protein 3
- OSCP
oligomycin sensitivity-conferring protein
- GalNAc
N-acetylgalactosamine
- GlcNAc
N-acetylglucosamine
- WGA
wheat germ agglutinin
- ER
endoplasmic reticulum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amanda R. Burnham-Marusich, Email: amanda.marusich@gmail.com.
Patricia M. Berninsone, Email: berninsone@unr.edu.
References
- Ben-Menachem R, Tal M, Shadur T, Pines O. A third of the yeast mitochondrial proteome is dual localized: A question of evolution. Proteomics. 2011;11:4468–76. doi: 10.1002/pmic.201100199. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–95. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Chandra NC, Spiro MJ, Spiro RG. Identification of a glycoprotein from rat liver mitochondrial inner membrane and demonstration of its origin in the endoplasmic reticulum. J Biol Chem. 1998;273:19715–21. doi: 10.1074/jbc.273.31.19715. [DOI] [PubMed] [Google Scholar]
- Chen C, Ko Y, Delannoy M, Ludtke SJ, Chiu W, Pedersen PL. Mitochondrial ATP synthasome: three-dimensional structure by electron microscopy of the ATP synthase in complex formation with carriers for Pi and ADP/ATP. J Biol Chem. 2004;279:31761–8. doi: 10.1074/jbc.M401353200. [DOI] [PubMed] [Google Scholar]
- Clark PM, Dweck JF, Mason DE, Hart CR, Buck SB, Peters EC, Agnew BJ, Hsieh-Wilson LC. Direct in-gel fluorescence detection and cellular imaging of O-GlcNAc-modified proteins. J Am Chem Soc. 2008;130:11576–7. doi: 10.1021/ja8030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler AM, Kerner J, Hoppel CL. Post-translational modifications of rat liver mitochondrial outer membrane proteins identified by mass spectrometry. Biochim Biophys Acta. 2007;1774:628–36. doi: 10.1016/j.bbapap.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Brunak S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac Symp Biocomput. 2002:310–22. [PubMed] [Google Scholar]
- Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, Trauger SA, Han W, Oyeleye MO, Dillmann WH. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J Biol Chem. 2009;284:547–55. doi: 10.1074/jbc.M808518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerner J, Distler AM, Minkler P, Parland W, Peterman SM, Hoppel CL. Phosphorylation of rat liver mitochondrial carnitine palmitoyltransferase-I: effect on the kinetic properties of the enzyme. J Biol Chem. 2004;279:41104–13. doi: 10.1074/jbc.M406570200. [DOI] [PubMed] [Google Scholar]
- Kung LA, Tao SC, Qian J, Smith MG, Snyder M, Zhu H. Global analysis of the glycoproteome in Saccharomyces cerevisiae reveals new roles for protein glycosylation in eukaryotes. Mol Syst Biol. 2009;5:308. doi: 10.1038/msb.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levrat C, Ardail D, Morelis R, Louisot P. Study of the N-glycoprotein biosynthesis through dolichol intermediates in the mitochondrial membranes. Int J Biochem. 1989;21:265–78. doi: 10.1016/0020-711x(89)90184-5. [DOI] [PubMed] [Google Scholar]
- Love DC, Kochan J, Cathey RL, Shin SH, Hanover JA. Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J Cell Sci. 2003;116:647–54. doi: 10.1242/jcs.00246. [DOI] [PubMed] [Google Scholar]
- Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol. 2008;8:874–87. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn RD, Helmerhorst EJ, Cipollo JF, Kukuruzinska MA. A hypomorphic allele of the first N-glycosylation gene, ALG7, causes mitochondrial defects in yeast. Biochim Biophys Acta. 2005;1723:33–44. doi: 10.1016/j.bbagen.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Moser TL, Stack MS, Asplin I, Enghild JJ, Hojrup P, Everitt L, Hubchak S, Schnaper HW, Pizzo SV. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci U S A. 1999;96:2811–6. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi A, Sprung R, Barma DK, Zhao Y, Kim SC, Falck JR, Zhao Y. Global identification of O-GlcNAc-modified proteins. Anal Chem. 2006;78:452–8. doi: 10.1021/ac051207j. [DOI] [PubMed] [Google Scholar]
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–67. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Peters BP, Ebisu S, Goldstein IJ, Flashner M. Interaction of wheat germ agglutinin with sialic acid. Biochemistry. 1979;18:5505–11. doi: 10.1021/bi00591a038. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Lepsverdize E, Chi SL, Das AM, Pizzo SV, Dityatev A, Schachner M. Amyloid precursor protein and amyloid beta-peptide bind to ATP synthase and regulate its activity at the surface of neural cells. Mol Psychiatry. 2008;13:953–69. doi: 10.1038/sj.mp.4002077. [DOI] [PubMed] [Google Scholar]
- Smith AL. [13] Preparation, properties, and conditions for assay of mitochondria: Slaughterhouse material, small-scale. Methods Enzymol. 1967;10:81–86. [Google Scholar]
- Teo CF, Ingale S, Wolfert MA, Elsayed GA, Nöt LG, Chatham JC, Wells L, Boons GJ. Glycopeptide specific monoclonal antibodies suggest new roles for O-GlcNAc. Nat Chem Biol. 2010;6:338–43. doi: 10.1038/nchembio.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantourout P, Martinez LO, Fabre A, Collet X, Champagne E. Ecto-F1-ATPase and MHC-class I close association on cell membranes. Mol Immunol. 2008;45:485–92. doi: 10.1016/j.molimm.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Wang J, Torii M, Liu H, Hart GW, Hu ZZ. dbOGAP - an integrated bioinformatics resource for protein O-GlcNAcylation. BMC Bioinformatics. 2011;12:91. doi: 10.1186/1471-2105-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev O, Singer E, Shaulian E, Goldberg M, Fox TD, Pines O. Fumarase: a mitochondrial metabolic enzyme and a cytosolic/nuclear component of the DNA damage response. PLoS Biol. 2010;8:e1000328. doi: 10.1371/journal.pbio.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonally SK, Capaldi RA. The F(1)F(0) ATP synthase and mitochondrial respiratory chain complexes are present on the plasma membrane of an osteosarcoma cell line: An immunocytochemical study. Mitochondrion. 2006;6:305–14. doi: 10.1016/j.mito.2006.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


