Abstract
Peripheral arterial disease (PAD) is characterized by reduced limb blood flow due to arterial obstruction. Current treatment includes surgical or endovascular procedures, the failure of which may result in amputation of the affected limb. An emerging therapeutic approach is cell therapy to enhance angiogenesis and tissue survival. Small clinical trials of adult progenitor cell therapies have generated promising results, although large randomized clinical trials using well-defined cells have not been performed. Intriguing pre-clinical studies have been performed using vascular cells derived from human embryonic stem cells (hESC) or human induced pluripotent stem cells (hiPSCs). In particular, hiPSC-derived vascular cells may be a superior approach for vascular regeneration. The regulatory roadmap to the clinic will be arduous, but achievable with further understanding of the reprogramming and differentiation processes; with meticulous attention to quality control; and perseverance.
Keywords: Angiogenesis, Peripheral arterial disease, Regenerative medicine, Regeneration, Nuclear reprogramming
1. Introduction
PAD is generally secondary to atherosclerotic lesions which may obstruct the ileofemoral, infrainguinal and/or infrapopliteal arteries, reducing pulsatile blood flow. Smoking, diabetes, hypertension, dyslipidemia and sedentary nature contribute to the development of PAD, superimposed upon a genetic predisposition (Leeper et al., 2010; Thorgeirsson et al., 2008; Wilson et al., 2011). Patients may have leg pain with walking, and in severe cases pain at rest or gangrene. Medical therapy is limited, with only modest benefit from cilostazol, although supervised exercise therapy may improve walking distance up to two-fold. Surgical bypass or endovascular interventions are reserved for patients with life-style limiting symptoms or to salvage tissue.
Cell therapy is a promising new therapeutic approach under investigation. Cell therapy approaches include the use of adult progenitor cells; the administration of therapeutic cells derived from embryonic stem cell (ESC); or the application of therapeutic cells derived from induced pluripotent stem cells (iPSCs). Each of these approaches has useful feature for vascular regenerative therapies, and potential deficits. We will review the evidence supporting these different approaches, and will explore the regulatory roadmap that these therapies must traverse on their way to the clinic for vascular regeneration.
2. What is vascular regeneration?
Broadly speaking, vascular regeneration encompasses the restoration of all vascular functions. These include the distribution of blood flow; the control of vascular resistance in response to hemodynamic, humoral, and local tissue factors; the regulation of immune response and the trafficking of circulating cells; the tissue trophic effects of paracrine factors elaborated by the vasculature; the modulation of blood fluidity and hemostasis; the permeation of nutrients and macromolecules through the systemic microvasculature, and the recirculation of plasma transudate by the lymphatics. For the purpose of this discussion, we are largely focused on the use of cell therapy to restore nutritive blood flow, so as to relieve symptoms and to reduce limb loss in patients with PAD. Furthermore, this review focuses on the potential of cell therapies derived from pluripotential stem cells. However, a brief review of adult stem cell therapy is useful, because pioneering trials using adult stem cells provide some illumination to visualize the regulatory roadmap for pluripotential stem cell therapies.
3. Adult stem and progenitor cells
Adult stem or progenitor cells are partially lineage-committed and thus give rise only to cells of one of the three germ layers, i.e. they are multipotent (as opposed to pluripotent cells that can give rise to ectodermal, mesodermal or endodermal lineages). These multipotent cells are found in the bone marrow, the circulation, or within specific tissues and share a number of traits that make them appealing as candidates for cell-based regeneration. The first is that these cells, if autologous, do not need to overcome an immunologic barrier. Also, this approach is not burdened by the ethical concerns that surround the use of human embryos.
Asahara's discovery of the “endothelial progenitor cell”(EPC) sub-population in 1997 marked the dawn of vascular regeneration as a therapeutic approach (Asahara and Kawamoto, 2004). Since then, a comprehensive body of pre-clinical studies have made a compelling case for pursuing cell therapy trials for cardiovascular regeneration. The use of such cells in patients with CAD or in PAD has been recently reviewed (Dimmeler et al., 2008; Leeper et al., 2010). Most cell therapy trials in PAD or CAD have utilized peripheral or bone-marrow derived mononuclear cells. In general, pre-clinical studies support the notion that a fraction of these cells support angiogenesis and vasculogenesis via paracrine effects. There also appear to be progenitor cells of endothelial lineage that can incorporate into the existing vasculature to increase capillary density (Aicher et al., 2007). In a parabiotic model, hindlimb ischemia led to the incorporation of circulating c-kit+CD45– progenitors, most of which were not derived from the bone marrow. The authors proposed that these cells were endothelial cells that were recruited into the blood, and circulated to the site of hindlimb ischemia, presumably under the influence of homing signals such as SDF-1.
The TACT study in 2002 was a pilot study of cell therapy in PAD (Tateishi-Yuyama et al., 2002). This unblinded study suggested that in patients with intermittent claudication, the IM injection of BMMNCs improved laser Doppler skin perfusion, transcutaneous oxygen measurements, BI and walking distance at 4 weeks, by comparison to PBMNC injection. Since this publication, there have been 30 reported therapeutic cell trials in patients with PAD. In reviewing this literature for a grant application, we found that about two-thirds of these studies have been in patients with critical limb ischemia (CLI) as defined by rest pain or tissue necrosis, and the other 1/3 in patients with symptomatic PAD, i.e., intermittent claudication. Most of the trials have utilized BMMNCs, with fewer using GCSF-mobilized PBMNCs [one recent analysis did not find any difference in outcome between CLI patients that received BMMNCs versus GCSF-mobilized PBMNCs] (Onodera et al., 2011). Most of the trials have utilized IM administration to the calf and thigh (40–80 sites), using a total of 1–10×107 CD34+ cells. Endpoints have included ankle-brachial pressure index, transcutaneous oxygen level, laser doppler perfusion, pain-free or maximal walking distance, relief of rest pain or healing of ischemic ulcers.
Using these endpoints, modest signals of efficacy have been reported in most trials, but the confidence intervals are large. Most trials enrolled relatively small numbers of patients, followed for short periods of time. Furthermore, in all trials, the characterization of the therapeutic cells has been very limited; typically only 1 or 2 surface markers are used to purify the cells, and a more comprehensive FACS analysis is not available. Often, there is no functional assessment of an aliquot of the cells. Furthermore, the persistence of these cells in the ischemic tissues is unknown; and the mechanism(s) of their therapeutic effect (if any) remains unknown. As a result, almost 10 years later we do not know for certain if cell therapy in PAD is useful, nor do we understand the mechanism of any clinical benefit. Furthermore, in the case of autologous adult stem cells, the conditions that give rise to PAD (e.g. diabetes mellitus and tobacco exposure) may also decrease the number and function of the therapeutic cells (Vasa et al., 2001). For this reason, allogeneic progenitor cells (such as mesenchymal stromal cells derived from adipose or placental tissue) may represent an alternative approach. In addition, these allogeneic cells are easier to procure and amenable to tissue banking. Nevertheless, the limitations of adult stem/progenitor cells provide a rationale for deriving therapeutic cells from other sources.
4. Embryonic stem cells as a source of therapeutic cells
The inner cell mass of the blastocyst is the source of embryonic stem cells (ESCs). ESCs are pluripotent, and can differentiate into any cell type of endodermal, mesodermal, or ectodermal lineage. Furthermore, they have almost unlimited replicative capacity. These two features favorably differentiate them from adult stem cells (Yu and Thomson, 2008). ESCs have been differentiated into vascular cells using 3D embryoid body (EB) or 2D growth factor-supplemented differentiation procedures (Kane et al., 2010; Sone et al., 2007). ESC-derived endothelial cells (ESC-ECs) are defined by their expression of surface markers (e.g. CD31) intracellular proteins (e.g. von Willebrand factor and endothelial nitric oxide synthase), and functions (e.g. uptake of acetylated-LDL and formation of endothelial tubes in Matrigel), that are associated with endothelial lineage (Huang et al., 2010a; Li et al., 2007). In evaluating the efficacy of the differentiation process, the most definitive marker for endothelial phenotype is inosculation, that is, the ability of a putative endothelial cell to integrate into a preexisting network of endothelial cells (Hirschi et al., 2008).
Animal studies have shown that ESC-EC incorporate into the vasculature of the ischemic limb or myocardium (Huang et al., 2010a; Li et al., 2007). We and others have shown that ESC-EC can enhance limb blood flow in murine models of PAD, via paracrine effects on angiogenesis and cell survival, as well as by direct incorporation into the existing vasculature (Cho et al., 2007; Huang et al., 2010a; Moon et al., 2011). Recently, we examined the effect of delivery modality on the survival, localization, and functional effects of syngenic ESC-EC in the murine model of PAD (Huang et al., 2010a). Murine ESC-ECs were stably transduced with a construct for bioluminescence imaging (BLI) and for immunofluorescent detection. The cells were delivered by intramuscular, intrafemoral artery, or intrafemoral vein injections in animals previously subjected to femoral artery ligation. At two weeks, BLI showed that ESC-ECs delivered by all 3 modalities localized to the ischemic limb. Intriguingly, the imaging studies indicated that ESC-EC administered intravenously initially lodged in the lung, but over a period of several days, eventually localized to the ischemic limb [the mechanisms responsible for the homing of intravenously administered ESC-EC to the ischemic limb are under investigation in our laboratory; stromal derived factor 1 i.e. SDF-1, is one likely homing signal produced by the ischemic tissue]. The engraftment of ESCECs into the limb vasculature was confirmed by immunohistochemistry. Notably, ESC-ECs administered by the intravenous or intra-arterial routes improved limb perfusion and neovascularization, whereas intramuscular administration was less effective. Administration of the parental ESCs or the vehicle had no beneficial effect. Similarly, administration of ECs derived from human ESCs also appears to have some benefit in this murine model of PAD (Cho et al., 2007; Moon et al., 2011).
Although ESCs have the advantages of greater proliferative capacity and pluripotentiality, these properties also raise a concern. Specifically, the accidental administration to a patient of a pluripotent ESC would risk teratoma formation. Accordingly, the clinical development of this cell therapy will require robust differentiation and purification protocols, supported by data showing the long term safety of these cells. The therapeutic use of these cells is further complicated because they are allogeneic and therapeutic engraftment may require immunosuppression, which carries additional risk. Finally, the clinical use of these cells may remain conflicted by the ethical debate surrounding the use of human embryos. Accordingly, there is great interest in a new form of pluripotential cell that can obviate some of these concerns.
5. Induced pluripotent stem cells
The generation of induced pluripotential stem cells (iPSC) by Yamanaka and colleagues, was a seminal moment in regenerative medicine (Takahashi and Yamanaka, 2006). By overexpressing four key transcriptional factors in somatic cells, pluripotentiality could be induced. The set of transcriptional factors used by Yamanaka and colleagues were octamer-binding transcription factor-3/4 (Oct 3/4), SRY-related high-mobility-group (HMG)-box protein-2 (Sox2), Kruppel-like factor 4 (Klf4), and c-Myc. In parallel studies, Thomson and colleagues achieved similar results using Oct 3/4 and Sox2, together with Nanog and Lin28 (Yu et al., 2007). These transcription factors are required only for the induction of, and not the maintenance of, pluripotency (Okita et al., 2007). Since this landmark work, iPSCs have been induced in a variety of somatic cells (some of which are easier to reprogram than fibroblasts); using subsets of these genes or other core pluripotency factors; with or without small molecules that enhance chromatin remodeling (Aasen et al., 2008; Desponts and Ding, 2010; Wernig et al., 2008).
Initial efforts to generate iPSCs used retroviruses or lentiviruses to overexpress the genes encoding the reprogramming factors in somatic cells. The use of these vectors is complicated by the integration of viral DNA into the genome, which raises the risk of silencing indispensable genes and/or inducing oncogenesis. These risks are reduced, but not abolished, with the use of adenoviruses, plasmid constructs, piggyBAC transposable elements, and the Cre/LoxP system (Kaji et al., 2009; Okita et al., 2008; Stadtfeld et al., 2008; Woltjen et al., 2009), but all of these approaches carry a risk of DNA integration. These concerns will be overcome using non-viral methodologies such as cell permeant reprogramming proteins, microRNA, and/or small molecules (Anokye-Danso et al., 2011; Desponts and Ding, 2010; Zhou et al., 2009). Small molecules that enhance reprogramming include those that act on epigenetic modifiers, such as inhibitors of histone deacetylase (HDAC). By inhibiting HDAC, agents such as valproic acid or trichostatin A promote a more open configuration of the chromatin, so as to facilitate the activation of genes encoding pluripotential factors. For a review of agents that facilitate reprogramming, see Desponts and Ding (2010).
6. Preclinical development of hiPSCs for vascular regeneration
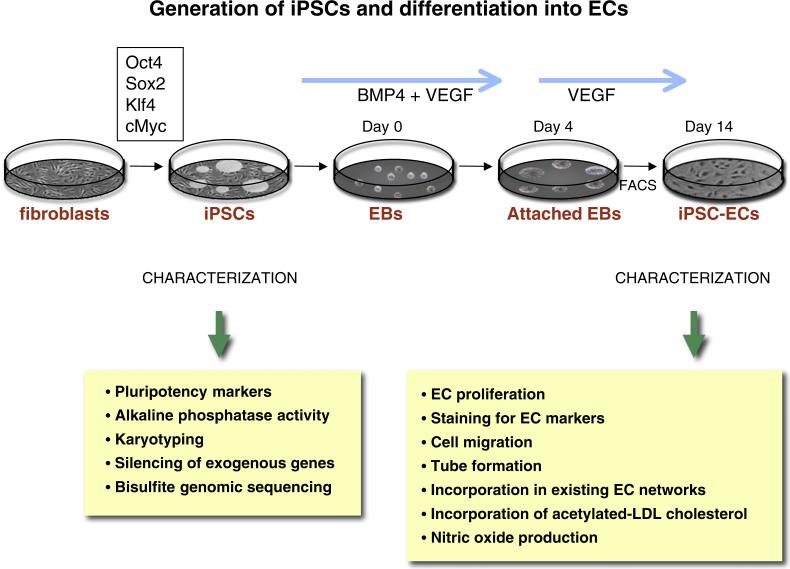
We have differentiated human iPSCs (hiPSCs) into endothelial cells (hiPSC-ECs) to assess their ability to improve perfusion in the murine model of peripheral arterial disease (Rufaihah et al., 2011). A schematic of the generation and testing of these human iPSC-ECs is shown in Fig. 1. In brief, endothelial differentiation was initiated by culturing hiPSCs for 14 days in differentiation media supplemented with bone morphogenetic protein 4 and vascular endothelial growth factor. We purified the heterogenous mixture of cells by FACS using an antibody directed against CD31. The purified hiPSCECs generated capillary-like structures when grown in matrigel and incorporated acetylated-LDL cholesterol. Endothelial markers such as KDR, CD31, CD144, and eNOS were expressed by these cells. When exposed to hypoxia the hiPSC-ECs produced angiogenic cytokines and growth factors. Subsequently, we transduced the cells with a double fusion construct comprising firefly luciferase for BLI and green fluorescence protein for histochemistry. The hiPSC-ECs were administered at day 0 and day 7 after femoral artery ligation into the ischemic hindlimb of immunodeficient mice. Over a two week period, BLI revealed attrition of cells, but some hiPSC-ECs survived in the ischemic limb for at least 2 weeks. At that time, and for up to 4 weeks, perfusion was improved by over 30% by comparison to the vehicle treated group, as assessed by laser Doppler imaging. This effect was associated with a 60% increase in the total number of capillaries in the ischemic limb of mice receiving hiPSC-EC injections by comparison to the vehicle-treated group. This pre-clinical work was proof-of-concept for the use of hiPSC-EC in PAD.
Fig. 1.
Generation of iPSCs and differentiation into ECs. IPSCs may be generated by overexpressing Oct4, Sox2, Klf 4 and cMyc (using retroviral, mmRNA, or cell-permeant peptide constructs). The iPSCs are characterized by staining for pluripotency markers and alkaline phosphatase activity. Other tests for quality of the reprogramming include spectral karyotyping, and bisulfite genomic sequencing to assess genomic and epigenomic quality. Endothelial differentiation is initiated by culturing hiPSCs for 14 days in differentiation media supplemented with 50 ng/ml bone morphogenetic protein 4 (BMP4) and 50 ng/ml vascular endothelial growth factor (VEGF). The heterogenous mixture of cells is purified by FACS using an antibody directed against CD31. The ECs can be characterized for functionality by looking at the ability of iPSC-ECs to proliferate (using BrdU), the ability to generate capillary-like structures (when grown in matrigel) and the ability to incorporate in a pre-existing EC network. Other functional tests include the ability of the IPSCs-ECs to incorporate acetylated-LDL cholesterol, to produce nitric oxide, and to express markers such as KDR, CD31, CD144, and eNOS.
This is a first step toward clinical development, and there remain many hurdles to overcome before iPSC-derived ECs are ready for clinical trials. Genetic or epigenetic errors may be introduced during nuclear reprogramming, and the generation of tissues from pluripotent stem cells employs laboratory methods for cell generation and expansion that increase the risk of genetic instability, epigenetic modification, and the generation of tumorigenic cells. These issues may be addressed in part by using non-viral reprogramming methods.
In addition, it is well known that cell culture can alter cell phenotype. For example, muscle stem cells lose their capacity for self-renewal in standard cell culture. Such alterations in cell phenotype might be attenuated or prevented by recapitulating the tissue niche during cell culture. With respect to the latter point, the Blau group recently showed that hydrogels that are substantially “softer” than plastic (i.e. elasticity values of 12 kPa vs 106kPa) are vastly superior in promoting muscle cell stem phenotype, enhancing cell proliferation and reducing cell death (Gilbert et al., 2010).
For generating therapeutic cells, it may be better to replace the current empirical approach to vascular differentiation with protocols of directed differentiation. Directed differentiation will require much more knowledge regarding the transcriptional factors and epigenetic modifiers that are involved in the differentiation of pluripotential stem cells to the somatic cell of choice. In unpublished work, we are attempting to gain this information using mixed species heterokaryons, together with RNA seq technology. Our general approach is to fuse human iPSCs with murine endothelial cells. The transcriptional factors, epigenetic modifiers, microRNA and other factors that maintain the endothelial phenotype in the murine endothelial cell are now free to act on the human iPSC nucleus. Over the course of hours, we observe a temporal sequence of gene expression in the human iPSC which appears to mirror ontogeny, i.e. we observe early expression of genes known to be expressed by mesodermal precursors, and later expression of genes that are associated with endothelial specification. In addition, we have observed the expression of novel genes that encode transcriptional factors and other relevant candidate genes not known to be involved in endothelial development. Gain- or loss-of-function studies will determine the importance of these novel genes in directed differentiation of iPSCs to endothelial lineage.
Furthermore, it will be necessary to avoid teratoma formation as an adverse event of using cells derived from pluripotential stem cells. Accordingly, we need to develop effective techniques of negative selection against pluripotential cells. In this regard, the recent discovery of a novel surface marker for pluripotential cells may be a useful target (Tang et al., 2011). These investigators have discovered a surface glycoprotein (termed SSEA 5) that appears to be specific to pluripotential stem cells. Antibodies directed against SSEA5 efficiently deplete pluripotential stem cells from a heterogenous cell mixture.
Another concern is the possibility that autologous iPSC-derived cells will harbor the genetic or acquired defects that predisposed a patient to a particular disease. For example, the iPSC-derived endothelial cells from patients with PAD may be more likely to manifest adhesion molecules, chemokines and pro-thrombotic factors that could lead to further vascular disease. Finally, as with any potential regenerative cell therapy, one must be aware of possible adverse events. In the case of iPSC-ECs for therapeutic angiogenesis, adverse events could result tumor angiogenesis, pathological retinopathy, or neovascularization of atheromatous plaque.
In addition to safety concerns, there are manufacturing hurdles to overcome for therapeutic application. Current reprogramming methods are inefficient, although reprogramming methods continue to improve. Initially, the induction of, iPSCs from human fibroblasts took 3–4 weeks (Nicholas and Kriegstein, 2010) and the yield was low (~0.01 to 0.1%) (Fu et al., 2010). However, the recent development of modified mRNA to deliver the reprogramming factors has halved the time to achieve pluripotentiality (Warren et al., 2010; Yakubov et al., 2010). The efficiency of nuclear reprogramming, and differentiation to the desired cell fate, will improve with the expansion of our knowledge regarding the genetic and epigenetic determinants of reprogramming and cell fate.
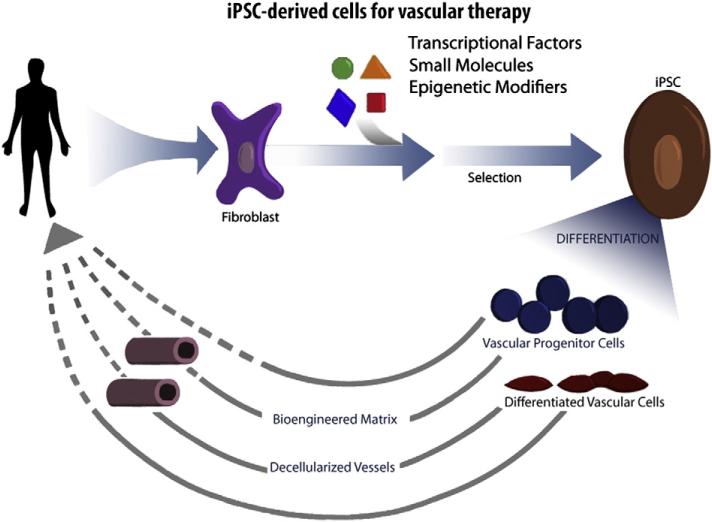
Finally, it is important to consider how the cells will be delivered. In the case of PAD, there are pre-clinical data, and small clinical trials with adult progenitor cells, indicating that some benefit may be achieved simply by injecting therapeutic cells into the ischemic muscle (Fig. 2). However, to restore maximal levels of perfusion, it is likely that large diameter, low resistance conduits will be necessary. In this case, it seems likely that iPSC-derived vascular cells will need to be provided as a biological conduit, incorporated into cylindrical bioengineered matrix or decellularized cadaveric vessels (Fig. 2). These bioengineered conduits would serve to replace autologous saphenous vein in patients that have insufficient or diseased veins.
Fig. 2.
Development of therapeutic cells from human induced pluripotential cells. Readily accessible somatic cells (e.g. skin fibroblasts) are harvested from a patient, and expanded in culture. Cells are exposed to reprogramming transcriptional factors, in the form of cell permeant peptides or modified mRNA. The resulting iPSC colonies are differentiated into vascular progenitor cells, that are administered directly to patients with vascular disease, or which are incorporated into matrices as a biological conduit for surgical implantation.
7. Translation from bench to bedside
7.1. General considerations
Clinical translation of a stem cell therapy product embodies all stages from derivation of the cell, preclinical characterization, development of regulatory-compliant processes, to planning and initiation of the clinical trials, towards regulatory approval. Translating a cell therapy to the clinic requires special considerations when compared to small molecule drug development. The complex nature of stem cells and their derivatives presents several challenges. The stem cell is a complex living entity which cannot be defined by a single parameter, such as a chemical structure would characterize a small molecule drug. A stem cell cannot be synthetically made (with current technology) — it must be derived from embryonic, fetal or adult tissues. Stem cell populations derived from different sources or donors may not have the same safety and activity profiles, despite the use of the same processing methods on each population. Moreover, alterations to cell processing methods may lead to unintended changes in the safety and activity profile. Stem cells can also undergo genetic or epigenetic changes during cell culture, during isolation, expansion or in cold storage. These factors are some of the liabilities when attempting to generate a stable, renewable, and consistent cell product.
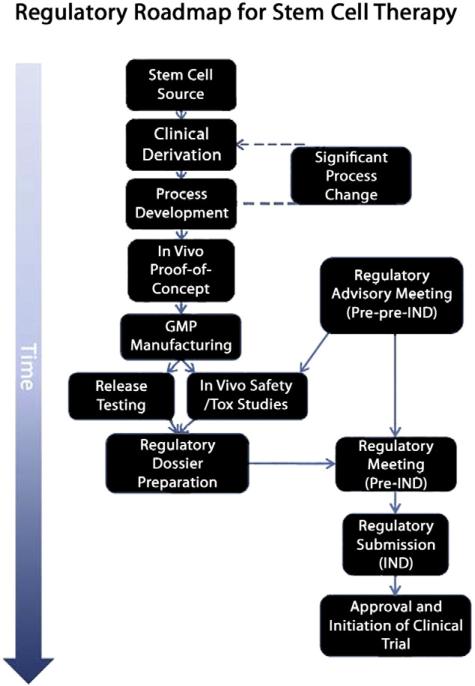
One challenge in moving a stem cell therapy through to the clinical stage is that processes developed in research laboratories may not recreate, for logistic and practical reasons, a process possible in human therapeutic regimens. The research laboratory provides a controlled and idealized environment; researchers are able to harvest stem cells from tissue culture and directly administer them to animal models. In clinical trials, such immediate administration is not always feasible. Accordingly, meticulous planning is important in translating a cell therapy to clinical trials. The translational roadmap from bench to clinical trial is shown (Fig. 3). From a practical perspective, it is difficult to justify the derivation of a clinical grade cell line before proof-of-concept studies have been conducted. Typically, research prototype cell line(s) are derived and tested in proof-of-concept animal model studies. Clinical grade prototype(s) must then be derived and validated using the same protocols in order to demonstrate comparability between the research and therapeutic approaches. The development of clinical grade cell derivation and regulatory-compliant procedures (process development) requires a significant investment of time and money. Additionally, the more that is changed from the research prototype, the more uncertainty that is introduced into the clinical grade cell line.
Fig. 3.
Roadmap to the clinic. Clinical derivation of the cell line of interest represents the initial stage of translating a cell therapy to the clinic. The ability to manufacture the stem cell product to current clinical expectations is addressed in process development before the clinical prototype cell is shown to be efficacious in animal models of disease. Note that significant late-stage changes to processes may result in rederivation and repeating key experiments. Based on advisory meetings with regulatory bodies (in this case the US FDA), release testing and safety/toxicology (tox) programs are initiated with the GMP manufactured cell product or equivalent. The regulatory dossier is compiled and further advice sought from the regulatory agency before submitting the Investigational New Drug (IND) application for a phase 1 clinical trial.
7.2. Special considerations related to choice of cell type
The focus of this review is on the regulatory roadmap for cell therapies derived from pluripotential stem cells. Although there are many similarities in the regulatory roadmaps for adult stem cell, embryonic stem cell and induced pluripotential stem cells (as discussed below) there are key differences. The regulatory roadmap for adult stem cells is somewhat simpler, particularly if autologous cells are used, and even more so if the generation of the product does not require passaging in vitro. Nevertheless, there is evidence that adult stem cells harvested from patients with cardiovascular disease are dysfunctional, and fewer in number, by comparison to healthy subjects. (Vasa et al., 2001). Furthermore, the cell products used to date in clinical trials are heterogenous in nature, and differ from one patient to the next. Accordingly, regulatory authorities require assessment some measure of cell efficacy, number and characterization for each lot.
For embryonic and induced pluripotential stem cells, the regulatory bar is higher. As discussed below, the generation and passaging of these cells in vitro may lead to genomic alterations that could potentially silence indispensable genes or increase oncogenicity. Furthermore, differentiation of the cells must be well-controlled, as a pluripotential cell could give rise to teratoma. Methods to preserve, and to document, the fidelity of reprogramming and differentiation, will be critical for regulatory approval. Finally, in the case of embryonic stem cells (and in the case of allogeneic iPSCs), the immunological barrier is an important issue. Allogeneic cell therapies may lead to inflammation or rejection in the case of inadequate immunosuppressive therapy. The immunosuppressants themselves carry risks, including predisposition to infection, to metabolic perturbations (e.g. Insulin resistance), and hypertension. It has been proposed that these issues might be minimized by creating banks of allogenic ESCs or iPSCs that represent the combinations of most prevalent human leukocyte antigens (HLA). In this way, cell therapies could be provided using allogeneic cells that are matched on the major HLA antigens (similar to the process observed in cord blood stem cell banks).
8. Derivation, differentiation and purification of therapeutic cells
Derivation of clinical grade cell lines is more involved and rigorously regulated than derivation of cells destined for research applications. Applicable laws and regulations regarding the derivation of ES cells are governed by Federal, State and local authorities in the United States. Within the United Kingdom, a license is required from the Human Fertilization and Embryo Agency (HFEA) in order to derive human embryonic stem (ES) cell lines. In addition, compliance with Good Tissue Practice (GTP) in the United States and/or approval from the Human Tissue Authority (HTA) within the United Kingdom, is required to process, test, store and distribute human tissue, and cells derived thereof, for human application. The GTP and HTA regulations ensure the donor is suitable, the tissue/cells are obtained ethically, the quality of the tissue/cells are safeguarded during processing and storage and distribution is regulated. Before clinical cell line derivation can begin, facilities must be in place, equipment calibrated, procedures documented (for example, Standard Operating Procedures, SOPs) and a quality system to document, monitor and maintain standards.
Manipulations and processing of the stem cells during derivation can impact both their activity and lessen the chance of clinical application. All information from the derivation of the cells is submitted to the regulatory agency for review; therefore, the providence and specifications of all reagents in contact with the cells during derivation and processing must be documented. Operators trained in relevant procedures work with quality and regulatory personnel to derive and collate the SOPs. This phase represents a significant challenge in translating a cell therapy to the clinic because here the research stops and development begins. The scientists on the product development team must agree upon and adhere to standardized procedures for derivation, processing, storing, transporting and delivering the cells. The amount of basic research conducted before attempts at clinical derivation can reduce the risk of problems in development, which inevitably lead to changes and delays (Fig. 3).
Ultimately, the goal is to achieve derivation, differentiation and purification procedures that are robust, reproducible and compliant with therapeutic applications. Roslin Cells (Edinburgh, United Kingdom) commercially produce clinical grade ESC lines that meet current expectations for therapeutic applications; however, the development of iPSCs to clinical grade is less defined. In particular, the generation of iPSCs requires the introduction of reprogramming factors to induce pluripotency (Takahashi et al., 2007; Takahashi and Yamanaka, 2006). Two findings have advanced the potential of iPSC based therapies towards the clinic: 1) the proto-oncogene c-Myc was not an absolute requirement of the four key genes originally described (Nakagawa et al., 2008); and 2) Oct4, Sox2, KLF4 and C-Myc transgene expressions are silenced in the resulting iPSC clones (Park et al., 2008; Takahashi et al., 2007). This led to the finding that non-integrating and transient expression of reprogramming factors is sufficient to induce pluripotency (Stadtfeld et al., 2008). These two findings may provide for approaches that reduce the tumorigenicity risk of iPSCs (Yu et al., 2009). Recently, Wang et al. showed co-expressing Rarg (RAR-γ) and Lrh-1 (liver receptor homologue 1; Nr5a2) with the four factors greatly accelerated reprogramming (Wang et al., 2011). Reprogramming somatic cells with non-integrating genetic constructs (Si-Tayeb et al., 2010; Warren et al., 2010; Zhou and Freed, 2009), cell permeant proteins (Kim et al., 2009; Yang et al., 2009; Zhou et al., 2009) and/or small molecules (Desponts and Ding, 2010; Li et al., 2009; Lin et al., 2009; Shi et al., 2008; Xu et al., 2008) may improve iPSC lines to clinical grade. Despite the advances made and numerous reports, Yamanaka's original approach remains the gold standard for comparison — 5 years after the original description.
Differentiation of iPSC lines to the desired derivative is another hurdle for this regenerative therapy. Current differentiation protocols are somewhat empirical, although administration of growth factors, combined with improved matrices that may mimic the stem cell niche (Dawson et al., 2008; Fu et al., 2010; Huang et al., 2010b; Lutolf et al., 2009), has improved the yields of desired cells. Functional endothelial differentiation of human iPSCs has been successfully carried out (Rufaihah et al., 2011; Taura et al., 2009). The entire field of regenerative medicine would be benefitted by more detailed understanding of the genetic determinants of differentiation, and their temporal sequence of activation or repression (Hanna et al., 2010). Other determinants of differentiation, including epigenetic modifications, miRNA modulation, matrix interactions, are not fully understood. Another concern is the efficiency in which pluripotency and differentiation is induced by different technologies using different donors (Masip et al., 2010; McDevitt and Palecek, 2008). This is less of a concern in an allogenic paradigm, where derivation and differentiation procedures can be optimized for a specific cell line, than in an autologous approach, where the age, health status of the patient donor and differences between individual cell lines may significantly compromise the efficiency of the processes. Regardless, no process can guarantee complete efficiency of differentiation to the desired cell type(s); thus, purification is an important aspect that ensures homogeneity and identity of the cell product. Antibodies used in the purification procedure should be produced to clinical standards for positive and negative selection approaches.
9. Good manufacturing practice for regenerative cell therapies
Good manufacturing practice (GMP) ensures that the quality and consistency of the stem cell product is suitable for human administration. Quality of the stem cell product is embodied by the facilities, instruments, reagents and consumables used in processing the stem cells. Standard operating procedures (SOPs) and training of the operators capture these quality parameters which define the operations within a certified GMP manufacturing facility. Early process development strategies should be designed to be compatible with GMP manufacturing constraints. Synthetic reagents containing no animal products are preferred; however, animal derived products can often be sourced to an acceptable quality. In the latter case, the reagents used in the processing of the stem cells must have a low transmissible spongiform encephalopathies (TSE) risk assessment and be of acceptable purity as documented by a Certificate of Origin (CoO) and a Certificate of Analysis (CoA). Ideally, all reagents themselves would be manufactured in GMP facilities and even approved for human administration. There is a logistical conflict where the use of clinical grade reagents is used in the preparation of stem cell products: clinical drugs are regulated for the use in humans, and are not readily supplied to researchers or manufacturing facilities where they are simply used as processing reagents. This is particularly relevant for drugs that are not generic. For example, trade name drugs are specially formulated for a particular use in humans, and the regulatory approval is for the drug in a specific formulation. This formulation may contain excipients that do not accommodate its use in cell culture. Furthermore (particularly if the formulation is altered), the regulatory authority will likely require substantial additional testing of the drug for the new “indication” in manufacturing stem cells.
The compatibility of the pluripotent stem cell processing strategies with GMP manufacture remains a significant challenge. Although embryonic stem cells have by definition the unlimited ability to renew, current ESC tissue culture expansion strategies may be limited because they are performed manually in T-Flasks. Important advances have been made in developing culture medium additives (McDevitt and Palecek, 2008) and synthetic surface coatings that support pluripotent cell growth, eliminating the need to use a fibroblast feeder layer (Brafman et al., 2010; Derda et al., 2010; Klim et al., 2010). Where an allogenic treatment is concerned, the manual T-Flask expansion of pluripotent stem cells could be scaled-up to a point or even automated with the robotic instrumentation (Thomas et al., 2009); however, the number of patients that could be treated would inevitably be limited. Scale-up manufacture approaches, such as culture in bioreactors (Serra et al., 2010), are needed for the anticipated future widespread demand of the stem cell drug product. Zwi-Dantsis et al. (2010) demonstrated a method for the scalable production of functional cardiomyocytes from iPSCs without the use of c-Myc. Large scale expansion of the parental cells (e.g. fibroblast line) followed by a one step induction of pluripotency and differentiation would be a cost-effective approach to manufacture iPSCs for clinical application (Nicholas and Kriegstein, 2010). Direct conversion of fibroblasts to multilineage blood progenitors (Szabo et al., 2010), or to functional neurons (Vierbuchen et al., 2010), has been successful, and transdifferentiation of fibroblasts to vascular cells is under investigation (Xie et al., 2010).
10. Banking and distribution of therapeutic cells
The following remarks are most relevant for the banking and distribution of allogenic cells. In this case, GMP manufacturing process is conducted in multiple stages. The processes are initially setup and evaluated within the GMP facility through a technical transfer. The technical transfer will produce a batch of cells using the GMP procedures and reagents but is carried out solely for training and evaluation purposes. Pending successful completion of the technical transfer, a GMP bank of cells would then be manufactured. [For an autologous cell therapy, the cell bank need only be large enough to conduct release testing and to treat the patient.] A multi-tier approach is generally applied for an allogeneic cell therapy. The first GMP bank produced is referred to as the Master Cell Bank (MCB). From the MCB one vial of cells is removed and a second bank produced, referred to as the Working Cell Bank (WCB). Each vial from the working cell bank would be expanded and processed to produce the Drug Product (DP) bank. The DP is designated to be administered to the patient. When the WCB is depleted another vial from the MCB is revived to generate a new WCB. Using this multi-tiered banking approach the number of doses available for wide-spread patient use can be maximized. For example, if each bank contains one hundred vials, assuming one vial per dose, than potentially (depending upon replicative capacity of the parental stem cell) as many as one million doses might be available.
Beyond the manufacturing process itself, the characteristics of the end-product generated impacts the clinical program. Depending on the formulation and packaging, the DP may or may not require further processing prior to patient administration. A formulation that can be simply defrosted and directly administered to the patient alleviates many logistical issues with distribution. Transportation of cryopreserved cell preparations is carried out in a liquid nitrogen cooled dry shipper that has been certified and fitted with a temperature tracker. At a minimum, the GMP banks would be shipped from the manufacturer to GMP storage and testing facilities, a clinical supply organization and to the clinical trial sites. Minimal processing may be acceptable at the clinical site, such as the washing and final formulation of the cell preparation to provide sterility and quality. However, if the cells require further manipulations (such as tissue culture expansion or differentiation prior to patient administration) then these processes would have to be conducted in a GMP facility within, or in close proximity to, the clinical trial site. Such manipulations may make full release testing on the product necessary prior to patient administration.
11. Characterization, release and stability testing for therapeutic cells
There are specific guidelines for the testing of the GMP manufactured cell product. The quality and release testing requirements are defined in guidance notes for the United States (Center for Biologics Evaluation and Research, 1998) and Europe (Committee for Medicinal Products for Human Use, 2010) for biological medicinal products. Relevant sections of the current Code of Federal Regulations (21 CFR 210, 211, 312 and 600) and International Conference on Harmonization guidelines (ICH Q5) should be consulted for reference. Cell banks and the product are characterized and released with reference to its sterility, identity, purity, constituent materials, potency and stability. Sterility tests are conducted by a suitably certified facility using validated testing methods and include USP sterility, endotoxin, mycoplasma and viral tests (for example, for opportunistic viruses such as HIV1&2, Hepatitis B&C, HTLV-1&2, EBV, CMV, HHV, Parvovirus B19 and WNV). Production specific tests may be warranted if a risk is introduced by the donor, reagent or process (for example, bovine virus tests are required if any processing and/or constituent materials contain, or have been in contact with, bovine products or derivatives thereof). Currently, there are no approved testing methods for TSE; therefore, any TSE exposure risk will be deem the cells unacceptable for use in human applications. In addition, certain reagents used in the production of the cell product may invalidate a particular release test. For example, the presence of antibiotics in the cell culture medium will compromise the USP sterility test. Identity (determined by a cell surface marker or genetic test) is a unique way to discriminate the product from others. Purity of the cell product includes viability but may also address heterogeneity of different cell types within the population. Constituent materials determine the actual ingredients of the product (for example diluents, excipients and/or carry-over materials from the manufacturing process). Potency tests measure directly or indirectly the biological function of the product. Currently, there are no accepted standardized potency tests to measure cell based products. Ideally the potency test would be a relatively brief in vitro assay, validated with positive and negative controls, with a quantitative readout that unequivocally defines a pass or fail of the product. Several angiogenesis assays might be used as potency assays where a cell therapy has angiogenic activity (Bishop et al., 1999; Donovan et al., 2001). For example, Rufaihah et al. demonstrated a positive functional correlation between the activity of human iPSC-ECs to form vessels in matrigel, and their therapeutic effect in vivo in a murine model of peripheral arterial disease (Rufaihah et al., 2011). Stability testing includes karyotype analysis and a stability-testing program if the product is intended to be stored. A stability-testing program involves carrying out relevant release tests on the stored product at time intervals over which the product is expected to be used and beyond (for example 3, 6, 12 and 18 months for an anticipated 1 year shelf-life). In most cases, full release testing and reports are completed before administration to patient.
The number of cells and vials required for release and stability testing is an important consideration when planning the scale in which the cell therapy should be manufactured. Sterility testing may require up to 10% of the vials in a bank, for example. In addition, the release and stability testing can introduce significant costs to the cell product. Batch release of large banks of cell product in an allogenic therapy is the most economical approach in this respect; whereas, an autologous therapy would require release testing for each individual patient.
12. Key regulatory issues in cell therapy
The primary concern of the regulatory bodies in an early phase 1 trial is safety. Many of the straight-forward regulatory issues and requirements described throughout the preceding translational sections can all be readily addressed with current technologies and adequate planning. However, a significant challenge is the assessment of the tumorigenicity risk of a cell preparation purified from differentiated pluripotent stem cells. Furthermore, although not an absolute requirement for phase 1 trials, a good understanding of the mode-of-action of a cell therapy makes it easier for regulatory officials to adequately assess toxicology.
Traditionally, the tumorigenicity test involves the subcutaneous implantation of 10×106 cells in nude (athymic) immunocompromised mice followed by observations over a couple of weeks to months. A positive control tumorigenic cell line, such as Hela or HT1080, is typically used to validate the model. However, the design and duration of the tumorigenicity test applied to a pluripotent stem cell derived product are more complex and dictated by the cell type, mode and site of delivery and patient population to be treated. The main concern is the possibility that undifferentiated pluripotent cell(s) within the product may represent a tumorigenicity risk (Blum and Benvenisty, 2008, 2009); moreover, it is believed that the tumorigenicity risk is not presented over a finite period but may exist over a life-time.
To recognize and define the risk presented, the undifferentiated parental pluripotent cells (from which the cell product is derived) may be used as a positive control in tumorigenicity studies. Advances in imaging and reporter gene cell labeling techniques provide the ability to accurately define the number of pluripotent cells that give rise to tumor formation (Kooreman and Wu, 2010). Using bioluminescence imaging in combination with a reporter gene, it was shown that a maximal dose of approximately 100 undifferentiated ESCs injected in the skeletal muscle or cardiac muscle, respectively, in immunodeficient mice did not form teratomas; furthermore, the absence of teratoma formation was demonstrated over a 365 day observation period (Lee et al., 2009). These findings show that tumor formation is dependent on the number of pluripotent cells administered and injection site, or niche. Moreover, all animals receiving more than the threshold doses of cells developed tumors within 2 weeks, without exception; indicating tumorigenicity risk may not endure over a long-term. Significantly, the minimum number of undifferentiated pluripotent cells required to form tumors exceeds the sensitivity and detection levels for current labeling and tracking technologies. This is supported by findings reported by Geron that showed no teratomas formed in over 400 mice and rats treated with differentiated embryonic stem cells; however, if mixed in a ratio of 10:90 (undifferentiated: differentiated) teratomas formed in 4 out of 31 animals. Furthermore, no teratomas formed in mix ratios 5:95 and 1:99 (Machida et al., 2009).
The use of cell labeling and tracking, in combination with biodistribution studies, can be applied in pre-clinical studies to address the tumorigenicity risk presented by a pluripotent cell derived product [of course, the genetic modifications used in cell labeling could also increase tumorigenicity risk]. The susceptibility of the mouse strain to form tumors is also an important factor in the design of the tumorigenesis assay. The NOD/Shi-scid IL2Rg (null) (NOG) mouse strain is becoming increasingly popular for tumorigenicity studies because it is deficient in T, B and NK cells and has been shown to have a higher sensitivity to form tumors derived from human injected cells when compared to SCID (T and B-cell deficient) or Nude mice (T-cell deficient). A further consideration is the pathological disease state of the host. Yamashita et al. (2010) demonstrated an increased propensity for undifferentiated iPSCs to form tumors in ischemic brain compared to normal brain. A large body of evidence will be needed to determine the most practical and reliable tumorigenicity test for pluripotent cells.
It is reasonable to conclude that current understanding of how cell therapies give rise to their regenerative effects is poorly understood in animal models and even less so in patients. Regulatory bodies welcome and encourage the ability to articulate, measure and monitor the biological activity and/or potency of the cellular product. In vitro biopotency or biomarker assays that can be used to assess the consistency and activity of the manufactured cell product are advantageous in the assessment of the cell therapy going to clinic by the regulatory bodies. As the stem cell field evolves, both scientists and regulatory reviewers will gain knowledge from both basic research findings and the clinical experience of adult stem cell trials, thus paving the way for cell therapies to be translated to the clinic.
References
- Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilic J, Pekarik V, Tiscornia G, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- Aicher A, Rentsch M, Sasaki K-I, Ellwart JW, Fändrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ. Res. 2007;100:581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, Zhang Y, Yang W, Gruber PJ, Epstein JA, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am. J. Physiol. Cell Physiol. 2004;287:C572–C579. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- Bishop ET, Bell GT, Bloor S, Broom IJ, Hendry NF, Wheatley DN. An in vitro model of angiogenesis: basic features. Angiogenesis. 1999;3:335–344. doi: 10.1023/a:1026546219962. [DOI] [PubMed] [Google Scholar]
- Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv. Cancer Res. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- Blum B, Benvenisty N. The tumorigenicity of diploid and aneuploid human pluripotent stem cells. Cell Cycle. 2009;8:3822–3830. doi: 10.4161/cc.8.23.10067. [DOI] [PubMed] [Google Scholar]
- Brafman DA, Chang CW, Fernandez A, Willert K, Varghese S, Chien S. Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials. 2010;31:9135–9144. doi: 10.1016/j.biomaterials.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SW, Moon SH, Lee SH, Kang SW, Kim J, Lim JM, Kim HS, Kim BS, Chung HM. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation. 2007;116:2409–2419. doi: 10.1161/CIRCULATIONAHA.106.687038. [DOI] [PubMed] [Google Scholar]
- Dawson E, Mapili G, Erickson K, Taqvi S, Roy K. Biomaterials for stem cell differentiation. Adv. Drug Deliv. Rev. 2008;60:215–228. doi: 10.1016/j.addr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- Derda R, Musah S, Orner BP, Klim JR, Li L, Kiessling LL. High-throughput discovery of synthetic surfaces that support proliferation of pluripotent cells. J. Am. Chem. Soc. 2010;132:1289–1295. doi: 10.1021/ja906089g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desponts C, Ding S. Using small molecules to improve generation of induced pluripotent stem cells from somatic cells. Methods Mol. Biol. 2010;636:207–218. doi: 10.1007/978-1-60761-691-7_13. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2008;28:208–216. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- Donovan D, Brown NJ, Bishop ET, Lewis CE. Comparison of three in vitro human ‘angiogenesis’ assays with capillaries formed in vivo. Angiogenesis. 2001;4:113–121. doi: 10.1023/a:1012218401036. [DOI] [PubMed] [Google Scholar]
- Fu RH, Wang YC, Liu SP, Huang CM, Kang YH, Tsai CH, Shyu WC, Lin SZ. Differentiation of stem cells: strategies for modifying surface biomaterials. Cell Transplant. 2010;20:37–47. doi: 10.3727/096368910X532756. [DOI] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143:508–525. doi: 10.1016/j.cell.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang NF, Niiyama H, Peter C, De A, Natkunam Y, Fleissner F, Li Z, Rollins MD, Wu JC, Gambhir SS, et al. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arterioscler. Thromb. Vasc. Biol. 2010a;30:984–991. doi: 10.1161/ATVBAHA.110.202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang NF, Patlolla B, Abilez O, Sharma H, Rajadas J, Beygui RE, Zarins CK, Cooke JP. A matrix micropatterning platform for cell localization and stem cell fate determination. Acta Biomater. 2010b;6:4614–4621. doi: 10.1016/j.actbio.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane NM, Meloni M, Spencer HL, Craig MA, Strehl R, Milligan G, Houslay MD, Mountford JC, Emanueli C, Baker AH. Derivation of endothelial cells from human embryonic stem cells by directed differentiation: analysis of microRNA and angiogenesis in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2010;30:1389–1397. doi: 10.1161/ATVBAHA.110.204800. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klim JR, Li L, Wrighton PJ, Piekarczyk MS, Kiessling LL. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat. Methods. 2010;7:989–994. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooreman NG, Wu JC. Tumorigenicity of pluripotent stem cells: biological insights from molecular imaging. J. R. Soc. Interface. 2010;7(Suppl. 6):S753–S763. doi: 10.1098/rsif.2010.0353.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS, Tang C, Cao F, Xie X, van der Bogt K, Hwang A, Connolly AJ, Robbins RC, Wu JC. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle. 2009;8:2608–2612. doi: 10.4161/cc.8.16.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation. 2010;122:517–526. doi: 10.1161/CIRCULATIONAHA.109.881441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116:I46–I54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, Lin X, Hahm HS, Hao E, Hayek A, et al. A chemical platform for improved induction of human iPSCs. Nat. Methods. 2009;6:805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida K, Suemizu H, Kawai K, Ishikawa T, Sawada R, Ohnishi Y, Tsuchiya T. Higher susceptibility of NOG mice to xenotransplanted tumors. J. Toxicol. Sci. 2009;34:123–127. doi: 10.2131/jts.34.123. [DOI] [PubMed] [Google Scholar]
- Masip M, Veiga A, Izpisua Belmonte JC, Simon C. Reprogramming with defined factors: from induced pluripotency to induced transdifferentiation. Mol. Hum. Reprod. 2010;16:856–868. doi: 10.1093/molehr/gaq059. [DOI] [PubMed] [Google Scholar]
- McDevitt TC, Palecek SP. Innovation in the culture and derivation of pluripotent human stem cells. Curr. Opin. Biotechnol. 2008;19:527–533. doi: 10.1016/j.copbio.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SH, Kim JS, Park SJ, Lee HJ, Do JT, Chung HM. A system for treating ischemic disease using human embryonic stem cell-derived endothelial cells without direct incorporation. Biomaterials. 2011;32:6445–6455. doi: 10.1016/j.biomaterials.2011.05.026. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Nicholas CR, Kriegstein AR. Regenerative medicine: cell reprogramming gets direct. Nature. 2010;463:1031–1032. doi: 10.1038/4631031a. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Onodera R, Teramukai S, Tanaka S, Kojima S, Horie T, Matoba S, Murohara T, Matsubara H, Fukushima M. Bone marrow mononuclear cells versus GCSF-mobilized peripheral blood mononuclear cells for treatment of lower limb ASO: pooled analysis for long-term prognosis. Bone Marrow Transplant. 2011;46:278–284. doi: 10.1038/bmt.2010.110. [DOI] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Rufaihah AJ, Huang NF, Jame S, Lee JC, Nguyen HN, Byers B, De A, Okogbaa J, Rollins M, Reijo-Pera R, et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler. Thromb. Vasc. Biol. 2011;31:e72–e79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Brito C, Sousa MF, Jensen J, Tostoes R, Clemente J, Strehl R, Hyllner J, Carrondo MJ, Alves PM. Improving expansion of pluripotent human embryonic stem cells in perfused bioreactors through oxygen control. J. Biotechnol. 2010;148:208–215. doi: 10.1016/j.jbiotec.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Shi Y, Desponts C, Do JT, Hahm HS, Scholer HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Si-Tayeb K, Noto FK, Sepac A, Sedlic F, Bosnjak ZJ, Lough JW, Duncan SA. Generation of human induced pluripotent stem cells by simple transient transfection of plasmid DNA encoding reprogramming factors. BMC Dev. Biol. 2010;10:81. doi: 10.1186/1471-213X-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone M, Itoh H, Yamahara K, Yamashita JK, Yurugi-Kobayashi T, Nonoguchi A, Suzuki Y, Chao TH, Sawada N, Fukunaga Y, et al. Pathway for differentiation of human embryonic stem cells to vascular cell components and their potential for vascular regeneration. Arterioscler. Thromb. Vasc. Biol. 2007;27:2127–2134. doi: 10.1161/ATVBAHA.107.143149. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tang C, Lee AS, Volkmer JP, Sahoo D, Nag D, Mosley AR, Inlay MA, Ardehali R, Chavez SL, Pera RR, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat. Biotechnol. 2011;29:829–834. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- Taura D, Sone M, Homma K, Oyamada N, Takahashi K, Tamura N, Yamanaka S, Nakao K. Induction and isolation of vascular cells from human induced pluripotent stem cells—brief report. Arterioscler. Thromb. Vasc. Biol. 2009;29:1100–1103. doi: 10.1161/ATVBAHA.108.182162. [DOI] [PubMed] [Google Scholar]
- Thomas RJ, Anderson D, Chandra A, Smith NM, Young LE, Williams D, Denning C. Automated, scalable culture of human embryonic stem cells in feeder-free conditions. Biotechnol. Bioeng. 2009;102:1636–1644. doi: 10.1002/bit.22187. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yang J, Liu H, Lu D, Chen X, Zenonos Z, Campos LS, Rad R, Guo G, Zhang S, et al. Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1. Proc. Natl. Acad. Sci. U. S. A. 2011;108:18283–18288. doi: 10.1073/pnas.1100893108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Cassady JP, Jaenisch R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008;2:10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Wilson AM, Sadrzadeh-Rafie AH, Myers J, Assimes T, Nead KT, Higgins M, Gabriel A, Olin J, Cooke JP. Low lifetime recreational activity is a risk factor for peripheral arterial disease. J. Vasc. Surg. 2011;54:427–432. 432, e421–424. doi: 10.1016/j.jvs.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hamalainen R, Cowling R, Wang W, Liu P, Gertsenstein M, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Sun A, Huang Z, Zhu W, Wang S, Zou Y, Ge J. Another possible cell source for cardiac regenerative medicine: reprogramming adult fibroblasts to cardiomyocytes and endothelial progenitor cells. Med. Hypotheses. 2010;76:365–367. doi: 10.1016/j.mehy.2010.10.041. [DOI] [PubMed] [Google Scholar]
- Xu Y, Shi Y, Ding S. A chemical approach to stem-cell biology and regenerative medicine. Nature. 2008;453:338–344. doi: 10.1038/nature07042. [DOI] [PubMed] [Google Scholar]
- Yakubov E, Rechavi G, Rozenblatt S, Givol D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem. Biophys. Res. Commun. 2010;394:189–193. doi: 10.1016/j.bbrc.2010.02.150. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Kawai H, Tian F, Ohta Y, Abe K. Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transplant. 2010;20:883–891. doi: 10.3727/096368910X539092. [DOI] [PubMed] [Google Scholar]
- Yang WC, Patel KG, Lee J, Ghebremariam YT, Wong HE, Cooke JP, Swartz JR. Cell-free production of transducible transcription factors for nuclear reprogramming. Biotechnol. Bioeng. 2009;104:1047–1058. doi: 10.1002/bit.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22:1987–1997. doi: 10.1101/gad.1689808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:2667–2674. doi: 10.1002/stem.201. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwi-Dantsis L, Mizrahi I, Arbel G, Gepstein A, Gepstein L. Scalable production of cardiomyocytes derived from c-Myc free induced pluripotent stem cells. Tissue Eng. Part A. 2010;17:1027–1037. doi: 10.1089/ten.TEA.2010.0235. [DOI] [PubMed] [Google Scholar]