Abstract
Background & Aims
Colonoscopy is consistently associated with reduced left-sided, but not right-sided, colorectal cancer (CRC) incidence and mortality. This might be because polyps with advanced pathology are smaller and more easily missed in the right vs left colon. We explored this postulate by evaluating the relationship among size, location, and histology of polyps from a large nationwide sample.
Methods
We conducted a cross-sectional study of 233,414 polyps from 142,686 patients (47% women; mean age, 60 years), which were reviewed by Miraca Life Sciences in 2009. We assessed polyp histology, location, and size of largest fragment submitted. We compared size distribution of right vs left polyps with high-grade dysplasia (HGD) or adenocarcinoma as well as any advanced neoplasia.
Results
The average size of right-sided polyps was smaller than that of left-sided polyps with HGD or adenocarcinoma (8.2 vs 12.4 mm, respectively); the same was true for polyps with advanced neoplasia (7.6 vs 11.1 mm, respectively) (P < .001). Most right-sided polyps with HGD, adenocarcinoma, or any advanced neoplasia were ≤9 mm, whereas most left-sided polyps with these findings were >9 mm. Polyps with advanced pathology were 5-fold more likely to be <6 mm in the right vs left colon: odds ratio, 5.27; 95% confidence interval, 4.06–6.82 for HGD or adenocarcinoma; odds ratio, 4.89; 95% confidence interval, 4.34–5.51 for advanced neoplasia.
Conclusions
Polyps with features of HGD, adenocarcinoma, or advanced neoplasia were significantly smaller in the right vs left colon. Strategies to prevent right-sided CRC require more accurate detection of small, advanced polyps.
Keywords: Colorectal Neoplasms, Epidemiology, Colon Cancer Screening, Early Detection
Colorectal cancer (CRC) is currently the second leading cause of cancer death in the United States and accounts for 608,000 deaths worldwide annually.1,2 Increasing rates of CRC screening have contributed to reduced CRC mortality.3 Decreased CRC mortality appears to be attributable in part to detection and removal of adenomatous polyps, which are believed to be precursors of most CRCs.3,4
Despite the overall success of screening for reducing CRC mortality, it is unclear whether screening can prevent right-sided CRC incidence and mortality. Indeed, findings from multiple observational studies suggest that colonoscopy consistently protects against left-sided CRC but may not consistently protect against right-sided CRC.5–10
Quality factors as well as biological factors may contribute to inconsistent protection against right-sided CRC.11 For example, suboptimal bowel preparation and colonoscopy technique may lead to missed right-sided lesions. Alternatively, from a biological perspective, right-sided cancers may have unique molecular features such as a tendency toward microsatellite instability, which may be associated with rapid cancer development.11,12 Furthermore, some studies suggest that small polyps with advanced histology may be more common in the right than in the left colon.13 This observation represents a plausible unifying biological and quality explanation for persistently high right-sided CRC incidence and mortality despite screening. Prevention of right-sided CRC incidence and mortality may be inconsistent because right-sided polyps with advanced histology may tend to be small and therefore more likely to be missed and subsequently progress to cancer.13 Although it is established that small polyps are more likely to be missed at colonos-copy,14–16 whether advanced polyps in the right colon are more likely to be small in size compared with the left colon has not been explored in detail. Therefore, our aim was to determine whether polyps with advanced pathology in the right colon tend to be significantly smaller in size compared with polyps with advanced pathology in the left colon by using a large national sample of polyps from patients across the United States.
Methods
Study Setting and Data Source
We conducted a cross-sectional analysis of biopsies reviewed by Miraca Life Sciences (Irving, TX) January 1, 2009, through December 31, 2009. Miraca provides gastrointestinal pathology diagnostic services for more than 1900 endoscopists from 43 states, Washington, DC, and Puerto Rico. As such, patients with biopsies reviewed by Miraca may be representative of patients undergoing routine colonoscopy in the United States. Miraca gastrointestinal pathologists review biopsies from more than 450,000 patients yearly. At time of submission, biopsies are routinely accompanied by descriptions of biopsy type, ie, polyp, mucosal biopsy, etc. Biopsies are submitted in formalin jars and processed systematically. Processing of each polyp includes size measurement of the largest polyp fragment submitted after removal from the formalin jar. Indications for colonoscopy procedures are recorded on the basis of information submitted by endoscopists. Standardized consensus-based criteria are used to assign pathologic diagnoses, and challenging cases are reviewed daily in conference with other gastrointestinal pathologists. Each pathologic diagnosis has a highly specific, internally created code assigned at time of diagnosis that is systematically entered into a clinical database. Patient characteristics (including age and gender), polyp characteristics (including size of largest fragment, location, and pathologic findings), and procedure indications are also recorded, among other items. This information is abstracted from pathology requisitions and procedure reports submitted with specimens. The Miraca clinical pathology database was the study data source.
Study Sample
Of all biopsies submitted to Miraca Life Sciences for review January 1, 2009, through December 31, 2009 (n = 452,225), we first identified all colorectal polyps from men and women aged 40 – 85 years for this study (n = 261,473). Next, we excluded polyp specimens with multiple histologies (n = 5419) and those for which multiple locations were listed (n = 22,640). The final sample thus included 233,414 polyps.
Colon site was defined as right colon for all polyps proximal to the descending colon and left colon for all polyps including or distal to the descending colon. A polyp with high-grade dysplasia/adenocarcinoma (HGD/AdenoCa) was defined by any tubular adenoma, tubulovillous adenoma, villous adenoma, sessile serrated adenoma, or traditional serrated adenoma associated with HGD or any polyp containing AdenoCa. A polyp with advanced neoplasia (AdNeo) was defined by a polyp with HGD/AdenoCa and/or a polyp with >20% villous architecture. Criteria used to diagnose HGD, AdenoCa, and villous component are described in Table 1.
Table 1. Diagnostic Criteria for Polyps With HGD, AdenoCa, and Villous Features.
| Diagnosis | Criteria |
|---|---|
| HGD | Both criteria required (or one only if extensivea):
|
| AdenoCa | Evidence of submucosal invasion by identifying either malignant glands deep to the muscularis mucosae or desmoplastic stromal reaction |
| Villous component | At least 20% villous architecture |
Extensive is defined by having >50% of lesion meeting criteria.
Analytical Approach
The primary comparison was differences in size of polyps with HGD/AdenoCa in the right and left colon. To assess these differences, (1) mean and median size of polyps with HGD/AdenoCa and (2) size distribution of polyps with HGD/AdenoCa for 3 polyp size categories, <6 mm, 6–9 mm, and >9 mm, were computed for the right and left colon. All right vs left comparisons were also made for polyps with AdNeo. In addition, per-patient analyses, in which patients were characterized as having isolated right or left colonic polyps and then stratified by size of largest polyp, were conducted for comparisons of size distribution of polyps with HGD/AdenoCa as well as AdNeo. For our primary comparisons, we include single polyps submitted as single fragments, single polyps submitted as multiple fragments (with size determined by size of largest fragment), and multiple polyps submitted as multiple fragments as an aggregate specimen (with size determined by size of largest fragment) following the patterns with which polyps are submitted in usual practice.
Because rates of advanced pathology may differ by indication for colonoscopy (ie, screening vs diagnostic), we conducted sensitivity analyses restricted to colonoscopies likely to have been done for screening purposes. For this assessment, we selected patients whose indication for colonoscopy was consistent with screening, such as “average risk for colorectal cancer” and “colorectal cancer screening” (Supplementary Table 1 shows all indications used and frequency of each indication).
Polyp size was based on size of largest polyp fragment submitted for pathology analysis. Thus, we recognized potential for underestimation of true polyp size when multiple polyp fragments were submitted for analysis. To address this possibility, we conducted 3 secondary analyses. First, we identified all polyps where only a single fragment was submitted for analysis and compared size distribution of polyps with HGD/AdenoCa and AdNeo for the right vs left colon. Second, we repeated right vs left comparisons after creating 3 larger polyp categories, <9 mm, 9–12 mm, and >12 mm. Third, we compared the size distribution of all colon polyps, regardless of histology, to see whether there were systematic differences in the overall size of polyps in the right vs left colon.
We used descriptive statistics (mean, median, standard deviation, and frequencies) to characterize the patient and polyp samples. We then used t tests to compare mean polyp size and χ2 tests to compare polyp size distribution between right and left colon. We used logistic regression models to examine the association between polyps with advanced histology, polyp size, and polyp location. The dependent variable for this model was polyp with advanced histology (yes/no). The independent variables modeled as a logit function of the dependent variable were polyp size (<6 mm, 6–9 mm, >9 mm), polyp location (right or left), an interaction term polyp size × polyp location, patient age (40–49, 50–59, 60–69, 70–79, 80+ years), and patient gender (male or female). A key assumption for logistic regression models is that observations on the dependent variable are independent. In this study sample, multiple polyps may arise from a single patient, thus potentially leading to correlation between observations (ie, polyps). To explore the effect of this correlation between observations, we compared models (estimates, standard errors, and model fit) with and without including an indicator variable for patient. We did not find any substantial differences between the 2 models. We present the results obtained from the model that included an indicator variable for patient. For all comparisons, two-sided P values <.05 were considered statistically significant for all comparisons. Analyses were performed with SAS version 9.2 (SAS Institute Inc, Cary, NC) and Stata MP 11.0 (Stata Corporation, College Station, TX). The study was approved by the University of Texas Southwestern Medical Center and Miraca Life Sciences Institutional Review Boards.
Results
We included 233,414 polyps from 142,686 patients for analysis. Polyps were submitted by 1779 endoscopists from 43 states. Median patient age was 60 years; 47% were women; demographic characteristics of all patients as well as those with isolated right or isolated left colonic polyps are summarized in Table 2. Table 3 summarizes polyp characteristics, overall and stratified by colon site. Forty-nine percent of polyps were right-sided (n = 114,354). Tubular adenomas (45.3%) and hyperplastic polyps (35.1%) accounted for 80% of polyps identified.
Table 2. Demographic Characteristics of Included Patients Overall and for Patients With Isolated Left or Right Colonic Polyps.
| Over all (n = 140, 622) | Isolated right colon polyps (n = 49,566) | Isolated left, colon polyps (n = 62,075) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| n | % | n | % | n | % | |
| Female a,b | 65,512 | 46.6 | 23,328 | 47.1 | 30,623 | 49.3 |
| Age distribution, ya,b | ||||||
| 40–49 | 13,566 | 9.6 | 3860 | 7.8 | 7658 | 12.3 |
| 50–59 | 52,269 | 37.2 | 16,694 | 33.7 | 25,421 | 41.0 |
| 60–69 | 43,435 | 30.9 | 15,943 | 32.2 | 17,876 | 28.8 |
| 70–79 | 25,204 | 17.9 | 10,325 | 20.8 | 9067 | 14.6 |
| 80+ | 6148 | 4.4 | 2744 | 5.5 | 2053 | 3.3 |
May not add up to total because of missing values.
P value comparison of patients with isolated right vs left polyps <.0001.
Table 3. Polyp Characteristics, Overall and by Colon Site.
| Overall (n = 233,414) | Right colon (n = 114,354) | Left colon (n =119,060) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| n | % | n | % | n | % | |
| Polyp size, any histology, mean (SD), mm | 4.3 (2.9) | 4.3 (2.6) | 4.3 (3.1) | |||
| Size distribution, any histology (mm) | ||||||
| <6 | 180,479 | 77.32 | 87,428 | 76.45 | 93,051 | 78.15 |
| 6–9 | 42,277 | 18.11 | 22,572 | 19.74 | 19,705 | 16.55 |
| >9 | 10,658 | 4.57 | 4354 | 3.81 | 6304 | 5.29 |
| Polyp histology | ||||||
| Normal | 14,627 | 6.30 | 8056 | 7.04 | 6571 | 5.52 |
| Hyperplastic | 81,848 | 35.10 | 22,438 | 19.62 | 59,410 | 49.90 |
| Tubular adenoma | 105,719 | 45.30 | 66,632 | 58.27 | 39,087 | 32.83 |
| Tubulovillous/villous adenoma | 6021 | 2.60 | 2776 | 2.43 | 3245 | 2.72 |
| Sessile serrated adenoma | 8961 | 3.80 | 8126 | 7.11 | 835 | 0.70 |
| Traditional serrated adenoma | 826 | 0.40 | 172 | 0.15 | 654 | 0.55 |
| Mixed hyperplastic-adenomatous polyp | 110 | 0.00 | 33 | 0.03 | 77 | 0.06 |
| HGD/AdenoCa | 1689 | 0.70 | 585 | 0.51 | 1104 | 0.93 |
| Other | 11,202 | 4.80 | 4623 | 4.04 | 6579 | 5.53 |
| Unclassified | 2411 | 1.00 | 913 | 0.80 | 1498 | 1.26 |
| Total | 233,414 | 100.00 | 114,354 | 100.00 | 119,060 | 100.00 |
SD, standard deviation.
Mean and Median Size of Polyps With High-grade Dysplasia/Adenocarcinoma or Advanced Neoplasia in the Right vs Left Colon
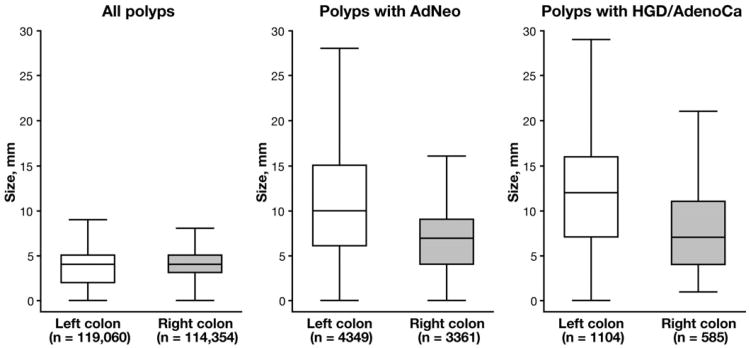
In the right colon, polyps with HGD/AdenoCa had a mean polyp size of 8.2 mm and a median polyp size of 7.0 mm, whereas in the left colon, polyps with HGD/AdenoCa had a mean polyp size of 12.4 mm and a median polyp size of 12.0 mm, P < .001. In the right colon, polyps with AdNeo had a mean polyp size of 7.6 mm and a median polyp size of 7.0 mm, whereas in the left colon, polyps with AdNeo had a mean size of 11.1 mm and a median size of 10.0 mm, P < .001. Figure 1 shows size distribution of all polyps, regardless of histology, as well as polyps with HGD/AdenoCa and AdNeo, stratified by colon location.
Figure 1.
Size distribution of all polyps, regardless of histology, as well as polyps with AdNeo and HGD/AdenoCa in right vs left colon. Distribution of all polyps, regardless of histology in right and left colon, were similar. However, polyps in right colon with AdNeo or HGD/AdenoCa were smaller than polyps with advanced histology in left colon, P < .001. For each box, the middle line represents median, the upper box boundary represents 75th percentile, and the lower boundary represents 25th percentile. For each plot, upper whisker represents distance to upper adjacent value within 1.5 interquartile range, and lower whisker represents distance to lower adjacent value within 1.5 interquartile range; outliers beyond the upper and lower adjacent values were suppressed to enhance plot clarity.
Size Distribution of High-grade Dysplasia/Adenocarcinoma in the Right vs Left Colon
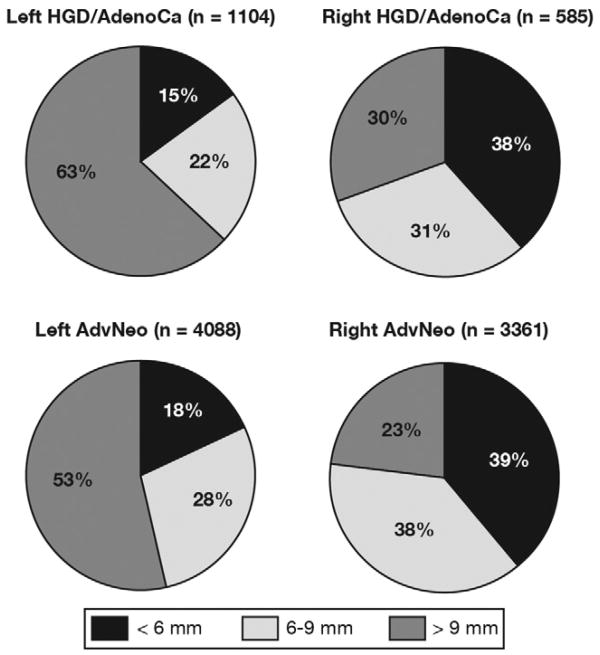
In the right colon, the majority of polyps with HGD/AdenoCa were <9 mm in size (69.7%, n = 408/585), whereas in the left colon, the majority of polyps with HGD/AdenoCa were >9 mm in size (62.7%, n = 692/1104; Figure 2, Table 4), P < .001 for all comparisons. Right colonic polyps with HGD/AdenoCa were 5-fold more likely to be <6 mm and 3-fold more likely to be 6 –9 mm than left colonic polyps with HGD/AdenoCa: odds ratio (OR), 5.27; 95% confidence interval (CI), 4.06–6.82 for <6 mm; OR, 2.92; 95% CI, 2.27–3.75 for 6–9 mm; Table 4. Analyses limited to polyps with AdenoCa showed even more pronounced differences, because right colonic polyps with AdenoCa were nearly 6-fold more likely to be <6 mm and nearly 4-fold more likely to be 6–9 mm than left colonic polyps with AdenoCa (Table 4). After adjusting for patient age and sex, estimates were slightly attenuated but remained statistically significant for right vs left comparisons of size distribution of polyps with HGD/AdenoCA as well as AdenoCA (Table 4).
Figure 2.
Distribution of polyps with HGD/AdenoCa and AdNeo in right and left colon for 3 clinically relevant size categories. In the left colon, the majority of polyps with advanced histology were >9 mm in size. However, in the right colon, the majority of polyps containing AdenoCa/HGD, AdNeo, and AdenoCa were ≤9 mm in size.
Table 4. Odds of Advanced Pathology in Right vs Left Colon, Stratified by Polyp Size.
| Right colon | Left colon | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| n | % | n | % | OR (95% CI) | Adjusted OR (95% CI)a | |
| All polypsb | ||||||
| HGD/AdenoCa (mm) | ||||||
| <6 | 225 | 38.5 | 167 | 15.1 | 5.27 (4.06–6.82) | 4.10 (3.15–5.34) |
| 6–9 | 183 | 31.3 | 245 | 22.2 | 2.92 (2.27–3.75) | 1.98 (1.45–2.42) |
| >9 | 177 | 30.3 | 692 | 62.7 | Reference | Reference |
| AdNeo (mm) | ||||||
| <6 | 1315 | 39.1 | 794 | 18.3 | 4.89 (4.34–5.51) | 4.39 (3.85–5.01) |
| 6–9 | 1262 | 37.6 | 1238 | 28.5 | 3.01 (2.69–3.36) | 2.19 (1.93–2.48) |
| >9 | 784 | 23.3 | 2317 | 53.3 | Reference | Reference |
| AdenoCa (mm) | ||||||
| <6 | 43 | 43.4 | 44 | 16.9 | 5.80 (3.27–10.24) | 4.28 (2.42–7.61) |
| 6–9 | 26 | 26.3 | 39 | 15.0 | 3.95 (2.10–7.41) | 2.42 (1.29–4.55) |
| >9 | 30 | 30.3 | 178 | 68.2 | Reference | Reference |
| All patients with isolated right or left colonic polyps (per-patient analyses) (mm)c | ||||||
| HGD/AdenoCa (mm) | ||||||
| <6 | 90 | 31.5 | 87 | 15.5 | 3.47 (2.4–5.0) | – |
| 6–9 | 93 | 32.5 | 130 | 23.1 | 2.40 (1.70–3.4) | – |
| >9 | 103 | 36.0 | 346 | 61.5 | Reference | – |
| AdNeo (mm) | ||||||
| <6 | 675 | 37.1 | 430 | 17.9 | 4.51 (3.83–5.30) | – |
| 6–9 | 698 | 38.4 | 697 | 29.0 | 2.87 (2.47–3.34) | – |
| >9 | 444 | 24.4 | 1276 | 53.1 | Reference | – |
| AdenoCa (mm) | ||||||
| <6 | 24 | 48.0 | 29 | 21.8 | 5.09 (2.29–11.3) | – |
| 6–9 | 13 | 26.0 | 24 | 18.0 | 3.33 (1.36–8.14) | – |
| >9 | 13 | 26.0 | 80 | 60.2 | Reference | – |
Adjusted for patient age and sex.
n = 114,354 right colonic polyps, n = 119,060 left colonic polyps.
n = 49,566 patients with isolated right colonic polyps, n = 62,075 patients with isolated left colonic polyps.
Size Distribution of Advanced Neoplasia in the Right vs Left Colon
In the right colon, the majority of polyps with AdNeo were <9 mm (76.7%, n = 2577/3361), whereas in the left colon, the majority of polyps with AdNeo were >9 mm (53.3%, n = 2317/4349; Figure 2, Table 4). Right colonic polyps with AdNeo were nearly 5-fold more likely to be <6 mm and 3-fold more likely to be 6–9 mm than left colonic polyps with AdNeo: OR, 4.89; 95% CI, 4.34–5.51 for <6 mm; OR, 3.01; 95% CI, 2.69– 3.36 for 6–9 mm; Table 4. After adjusting for patient age and sex, ORs were slightly attenuated but remained statistically significant (Table 4).
Per-Patient Analyses
Results of per-patient analyses limited to patients with isolated right or left colonic polyps were qualitatively similar to those of the overall study group with respect to size distribution of HGD/AdenoCA as well as AdNeo in the right vs left colon (Table 4).
Per-Polyp Secondary Analyses
A “screening indication” for colonoscopy accompanied 60,100 polyps. Right vs left differences in size distribution of polyps with HGD/AdenoCa and AdNeo were similar to those observed for polyps with any indication (Supplementary Table 2).
Separate analyses of polyps with HGD/AdenoCa and AdNeo submitted as a single fragment for pathology review (n = 100,447) showed similar differences in mean and median size between the right and left colon, although the magnitude of difference was smaller than observed for our overall study group. Similarly, statistically significant differences in size distribution (<6, 6–9, and >9 mm) of polyps with HGD/AdenoCa and AdNeo were observed between the right and left colon, but these differences were not as striking as for our primary analyses (P < .01 for all comparisons; Supplementary Figure 1, Supplementary Table 3).
By using upstaged size categories (<9, 9–12, and >12 mm), the size distribution of polyps with HGD/AdenoCa and AdNeo continued to show marked differences between the left and right colon (P < .001 for all comparisons).
Finally, evaluation of all colonic polyps, regardless of pathology, showed no relationship between size and location in the colon, suggesting that right vs left differences were limited to polyps with advanced pathology (Figure 1, Supplementary Table 4).
Discussion
By using a large national sample of polyps from patients undergoing routine colonoscopy, we found that the average size of polyps with advanced pathology was smaller in the right colon as compared with the left colon. Moreover, we found that the majority of right colonic polyps with advanced pathology were ≤9 mm, whereas the majority of left colonic polyps with advanced pathology were >9 mm. Polyps with advanced pathology were 5-fold more likely to be <6 mm in the right than in the left colon. The tendency for advanced polyps in the right colon to be small, coupled with the propensity for small polyps to be missed at time of colonoscopic screening, may explain why multiple observational studies have found little or no association between colonoscopy and protection from right-sided CRC incidence and mortality.5–10
Although many prior studies have examined the overall size distribution of advanced polyps, few studies have examined size distribution of advanced polyps stratified by colon site. By using a sample of 3720 patients with 2106 adenomas, Rondagh et al13 reported that 45% of right and 18.9% of left colonic advanced adenomas were <6 mm, similar to our findings. Furthermore, Rondagh et al also reported that advanced adenomas were more likely to be nonpolypoid in the right colon. They postulated that because right-sided advanced neoplasms are more likely to be subtle in appearance because of small size and morphology, these lesions might be more likely to be missed at colonoscopy and progress to CRC. Our findings support this postulate, although our data set did not contain endoscopic information regarding morphology such as sessile vs polypoid configuration. Taken together, the single-center findings reported by Rondagh et al as well as findings from our large national sample of more than 233,414 colorectal polyps from 142,686 patients submitted from usual clinical practice are consistent in identifying higher risk for advanced pathology in small polyps from the left vs right colon.
The strengths of our study include the very large sample size, inclusion of patients from diverse geographic locations from across the United States, sampling from “usual practice” settings, and pathology assessment conducted by a large group of gastrointestinal specialized pathologists with a consensus approach to diagnosis. However, we recognize potential limitations of our study. First, polyp size was measured on the basis of size of largest polyp fragment submitted. This approach theoretically could have led to systematic underestimation of polyp size, including size of polyps with advanced pathology. For example, it is possible that a 10-mm adenoma containing HGD resected in 2 pieces that measured 8 and 2 mm at time of pathology processing could have been counted as an 8-mm polyp with HGD rather than a 10-mm polyp with HGD. If this was a frequent scenario, it could have led to overestimation of advanced pathology prevalence among smaller polyps. Also, polyp size in our database was based on postfixation measurement. Some studies have suggested that formalin fixation leads to “shrinkage” and underestimation of polyp size,17–19 whereas others have reported that pathology measurements are the most accurate measure of polyp size.20 If underestimation occurred, it may have led to undersize classification of some advanced polyps. Nonetheless, any bias in polyp size measurement, whether because of overestimation or underestimation of polyp size, would be expected to be nondifferential across the colon and would not be expected to account for the right vs left differences in polyp epidemiology we observed.
We explored the potential impact of our polyp size measurement approach on our results in 3 separate ways. First, we conducted a secondary analysis restricted to polyps submitted as a single fragment. These analyses similarly showed marked differences between the right and left colon regarding average polyp size as well as proportion of small polyps with advanced pathology in the right colon. Second, we evaluated whether size distribution of polyps with advanced pathology continued to be different between the right and left colon when upstaged size categories (<9 mm, 9–12 mm, and >12 mm) were used for analysis. Again, we found similar differences between right and left colonic polyps. Finally, in a third auxiliary analysis, we evaluated size distribution of all polyps, regardless of histology, and found no differences between the right and left colon, suggesting that right vs left differences were limited to advanced polyps. Overall, our sensitivity analyses suggest that the size distribution differences we noted between the right and left colon for advanced polyps are unlikely to have been biased substantially by the method used for polyp size estimation, particularly because any bias would have been expected to affect both right-sided and left-sided polyps rather than right-sided polyps alone.
We have also considered the possibility that nonpolyp tissue (such as a mass) could have been misclassified as a polyp. Classification of a specimen as a polyp was based on the submitting endoscopist's labeling of biopsy specimens. If some endoscopists were systematically imprecise in labeling biopsy specimens, some masses may have been classified as polyps, perhaps leading to higher than true estimates of advanced pathology. We attempted to explore potential for this limitation by conducting a sensitivity analysis restricted to procedures associated with a screening rather than a diagnostic indication for colonoscopy. No qualitative differences in outcomes were observed. Again, even if misclassification of some mass lesions as polyps occurred, such misclassification would be expected to have been nondifferential and cannot account for the right vs left differences observed. We do not anticipate that endoscopists would be systematically more likely to misclassify right than left colonic mass lesions as polyps.
We have also taken into consideration that in usual practice, multiple polyps are sometimes placed in one specimen jar. In our primary analysis, we counted multiple polyps submitted together as a single polyp. It is possible such pooling could have resulted in overestimates of size-specific rates of AdNeo. However, the aforementioned single-fragment analysis found qualitatively similar results to our primary analysis. Moreover, even if pooling of multiple fragments resulted in overestimates of size-specific rates of advanced pathology, there is no reason to suspect that this bias would be more likely in the right than left colon.
Because our data set did not contain detailed information on polyp morphology (ie, flat, sessile, or pedunculated), we were unable to determine how our observations would be affected by this factor. Flat polyps may be more common in the right colon and may be more likely to contain advanced pathology, even if small in size, according to some prior reports.13,21 Thus, further evaluation of morphology and polyp size distribution could add a richer picture of the epidemiology of advanced polyps in the right vs left colon and should be the subject of future research.
A final theoretical limitation of our study was related to how endoscopists might approach removal of polyps with advanced pathology in different parts of the colon. It is conceivable that endoscopists systematically tend to more often biopsy, rather than remove, suspected advanced lesions in the right colon. Because biopsy fragments would generally be expected to be smaller than fragments submitted from a complete or piecemeal polypectomy, such a tendency could explain the finding of smaller polyp samples from advanced polyps in the right colon. However, to substantially affect our results, this theoretical tendency to biopsy, rather than resect, suspected advanced polyps of the right colon would have to be a common nationwide practice among endoscopists. To our knowledge, there are no data to support the presence of such a practice pattern, and we have not observed this tendency in our practice. Therefore, although we recognize this potential bias, we do not believe it to be a major study limitation.
Our observation that size distribution of polyps with advanced histology is markedly smaller in the right colon may have several implications. First, because smaller polyps are more likely to be missed at colonoscopy and because small polyps in the right colon may be more likely to contain advanced pathology, the clinical significance of small missed right colonic polyps is heightened. These findings may justify efforts to optimize colonoscopy technique, such as ensuring adequate withdrawal time and careful inspection of all folds. Furthermore, implementation of new innovations in colonoscopy may be required. Routine retroflexion in the cecum to facilitate examination of the right colon may improve detection of right-sided polyps and could be taught to all endoscopists.15 Technology such as the Third Eye Retroscope (Avantis Medical Systems, Sunnyvale, CA) may also improve detection of right-sided polyps.22,23 Beyond optimizing colonoscopy technique, endoscopists may benefit from identifying risk factors for small advanced right-sided lesions that could raise suspicion for finding these lesions at time of colonoscopy. Epidemiologic studies with sufficient sample sizes to identify clinical factors associated with small advanced right-sided lesions are needed. Furthermore, biomarkers in the blood or stool could be developed and calibrated to enhance identification of patients likely to have small advanced right-sided lesions.
On the basis of our analysis of a large national sample of polyps from patients undergoing colonoscopy in usual practice, we conclude that polyps with advanced pathology were significantly smaller in the right than left colon. This observation supports the hypothesis that colonoscopy inconsistently protects against right-sided CRC because right-sided lesions with advanced histology are more likely to be small and therefore more likely to be missed at time of colonoscopy and progress to cancer. These data may have important implications for developing strategies to reduce right-sided CRC incidence and mortality. Specifically, improving right-sided CRC detection and mortality may require strategies to optimize identification and removal of small right-sided polyps with advanced pathology.
Supplementary Material
Supplementary Figure 1. Distribution of polyps submitted as single fragments with HGD/AdenoCa and AdNeo in right and left colon for 3 clinically relevant size categories.
Supplementary Table 1. Screening Indications Used to Designate Biopsies Associated With CRC Screening
Supplementary Table 2. Distribution of Polyps With HGD and AdNeo Associated With a Screening Indicationa.
aP < .0001 for right vs left comparisons.
bPercentages may not add to 100% because of rounding.
Supplementary Table 3. Distribution of Polyps With HGD, AdenoCa, and AdNeo Submitted as Single Fragmentsa
aP < .001 for right vs left comparisons.
bPercentages may not add to 100% because of rounding.
Supplementary Table 4. Size Distribution of All Colorectal Polyps, Regardless of Histology (n = 233,414)
Acknowledgments
Funding: Supported by Cancer Prevention and Research Institute of Texas grant PP100039 (Samir Gupta, PI); National Institutes of Health/National Cancer Institute grant number 1U54CA163308-01 titled, “Parkland-UT Southwestern PROSPR Center: Colon cancer screening in a safety net” (Celette Sugg Skinner, PI). Also supported by National Institutes of Health grant number 1 KL2 RR024983-01, titled, “North and Central Texas Clinical and Translational Science Initiative” (Milton Packer, MD, PI) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. None of the study sponsors had a role in study design, data collection, analysis, interpretation, or decision to publish.
Abbreviations in this paper
- AdenoCa
adenocarcinoma
- AdNeo
advanced neoplasia
- CI
confidence interval
- CRC
colorectal cancer
- HGD
high-grade dysplasia
- OR
odds ratio
Footnotes
Conflicts of interest: The authors disclose no conflicts.
Supplementary Material: Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/doi:10.1016/j.cgh.2012.07.004.
References
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 19752006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 6.Brenner H, Chang-Claude J, Seiler CM, et al. Long-term risk of colorectal cancer after negative colonoscopy. J Clin Oncol. 2011;29:3761–3767. doi: 10.1200/JCO.2011.35.9307. [DOI] [PubMed] [Google Scholar]
- 7.Brenner H, Hoffmeister M, Arndt V, et al. Protection from right-and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 8.Mulder SA, van Soest EM, Dieleman JP, et al. Exposure to colorectal examinations before a colorectal cancer diagnosis: a casecontrol study. Eur J Gastroenterol Hepatol. 2010;22:437–443. doi: 10.1097/MEG.0b013e328333fc6a. [DOI] [PubMed] [Google Scholar]
- 9.Singh H, Nugent Z, Demers AA, et al. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128–1137. doi: 10.1053/j.gastro.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 10.Kahi CJ, Imperiale TF, Juliar BE, et al. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009;7:770–775. doi: 10.1016/j.cgh.2008.12.030. quiz 711. [DOI] [PubMed] [Google Scholar]
- 11.Carethers JM. One colon lumen but two organs. Gastroenterology. 2011;141:411–412. doi: 10.1053/j.gastro.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology. 2006;131:1700–1705. doi: 10.1053/j.gastro.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Rondagh EJ, Bouwens MW, Riedl RG, et al. Endoscopic appearance of proximal colorectal neoplasms and potential implications for colonoscopy in cancer prevention. Gastrointest Endosc. 2012;75:1218–1225. doi: 10.1016/j.gie.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Kaltenbach T, Friedland S, Soetikno R. A randomised tandem colonoscopy trial of narrow band imaging versus white light examination to compare neoplasia miss rates. Gut. 2008;57:1406–1412. doi: 10.1136/gut.2007.137984. [DOI] [PubMed] [Google Scholar]
- 15.Hewett DG, Rex DK. Miss rate of right-sided colon examination during colonoscopy defined by retroflexion: an observational study. Gastrointest Endosc. 2011;74:246–252. doi: 10.1016/j.gie.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 17.Morales TG, Sampliner RE, Garewal HS, et al. The difference in colon polyp size before and after removal. Gastrointest Endosc. 1996;43:25–28. doi: 10.1016/s0016-5107(96)70255-9. [DOI] [PubMed] [Google Scholar]
- 18.Gopalswamy N, Shenoy VN, Choudhry U, et al. Is in vivo measurement of size of polyps during colonoscopy accurate? Gastrointest Endosc. 1997;46:497–502. doi: 10.1016/s0016-5107(97)70003-8. [DOI] [PubMed] [Google Scholar]
- 19.Barancin C, Pickhardt PJ, Kim DH, et al. Prospective blinded comparison of polyp size on computed tomography colonography and endoscopic colonoscopy. Clin Gastroenterol Hepatol. 2011;9:443–445. doi: 10.1016/j.cgh.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Schoen RE, Gerber LD, Margulies C. The pathologic measurement of polyp size is preferable to the endoscopic estimate. Gastrointest Endosc. 1997;46:492–496. doi: 10.1016/s0016-5107(97)70002-6. [DOI] [PubMed] [Google Scholar]
- 21.Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–1035. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 22.DeMarco DC, Odstrcil E, Lara LF, et al. Impact of experience with a retrograde-viewing device on adenoma detection rates and withdrawal times during colonoscopy: the Third Eye Retroscope study group. Gastrointest Endosc. 2010;71:542–550. doi: 10.1016/j.gie.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Leufkens AM, DeMarco DC, Rastogi A, et al. Effect of a retrograde-viewing device on adenoma detection rate during colonoscopy: the TERRACE study. Gastrointest Endosc. 2011;73:480–489. doi: 10.1016/j.gie.2010.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Distribution of polyps submitted as single fragments with HGD/AdenoCa and AdNeo in right and left colon for 3 clinically relevant size categories.
Supplementary Table 1. Screening Indications Used to Designate Biopsies Associated With CRC Screening
Supplementary Table 2. Distribution of Polyps With HGD and AdNeo Associated With a Screening Indicationa.
aP < .0001 for right vs left comparisons.
bPercentages may not add to 100% because of rounding.
Supplementary Table 3. Distribution of Polyps With HGD, AdenoCa, and AdNeo Submitted as Single Fragmentsa
aP < .001 for right vs left comparisons.
bPercentages may not add to 100% because of rounding.
Supplementary Table 4. Size Distribution of All Colorectal Polyps, Regardless of Histology (n = 233,414)