Abstract
OBJECTIVE
The fetal inflammatory response syndrome (FIRS) is present in a fraction of fetuses exposed to intra-amniotic infection and is associated with the impending onset of labor and multisystem organ involvement. Neonates born with funisitis, the histologic counterpart of fetal systemic inflammation, are at increased risk for cerebral palsy and bronchopulmonary dysplasia. The aim of this study was to determine whether fetal and maternal granulocytes and monocytes have the phenotypic and metabolic characteristics of activation in cases with FIRS.
STUDY DESIGN
A case-control study was conducted with umbilical cord and maternal blood samples obtained from patients who delivered preterm with (n=30) and without funisitis (n=15). The phenotypic characteristics of granulocytes and monocytes were examined using flow cytometry and monoclonal antibodies including CD11b, CD14, CD15, CD16, CD18, CD49d, CD62L, CD64, CD66b, and HLA-DR. Intracellular reactive oxygen species (iROS) were measured at the basal state and after stimulation (oxidative burst). A p-value <0.01 was considered statistically significant.
RESULTS
1) Funisitis was associated with a significant increase in the median mean channel brightness (MCB) of CD14, CD64, and CD66b on granulocytes and the MCB of CD64 on monocytes collected from umbilical cord blood. 2) The basal iROS production and oxidative burst were higher in the umbilical cord monocytes of neonates with funisitis than in those without funisitis. 3) There were no differences in the immunophenotype, basal iROS production, and oxidative burst in maternal granulocytes or monocytes between the study groups.
CONCLUSION
Fetal systemic inflammation is associated with phenotypic and metabolic changes consistent with activation in fetal immune cells but not in maternal blood.
Keywords: preterm delivery, funisitis, leukocyte phenotype, fetal monocyte-granulocyte activation, flow cytometry, chorioamnionitis, prematurity, fetal inflammation, fetal inflammatory response syndrome
INTRODUCTION
The fetal inflammatory response syndrome (FIRS) is present in fetuses exposed to intra-amniotic infection and is characterized by the systemic activation of the fetal innate immune system [18]. This syndrome was originally described in fetuses with spontaneous preterm labor and preterm prelabor rupture of the membranes (preterm PROM), and was operationally defined by an elevated fetal plasma interleukin-6 (IL-6) [> 11 pg/mL] [18]. Prenatal exposure of the fetus to inflammation is associated with the impending onset of labor [42] and fetal multi-systemic organ involvement [43]. Funisitis, the histologic counterpart of FIRS, involves the sequential migration of inflammatory cells from the lumen to the muscular layers of the umbilical vessels, and into Wharton’s jelly [25]. The presence of funisitis is regarded as evidence of a fetal plasma cytokine response [37]. Activation of umbilical cord endothelial cells can be detected in preterm infants with chorioamnionitis and funisitis [10]. Moreover, neonates born with funisitis are at increased risk for neonatal sepsis [61], cerebral palsy [62], and bronchopulmonary dysplasia [59].
A solid body of evidence indicates that extensive infiltration of monocytes and granulocytes is essential to the execution of an acute inflammatory response [9]. Indeed, fetuses destined for premature delivery have a higher percentage of CD11c, CD13, CD15, and CD67 in fetal blood than those destined to deliver at term [7]. Furthermore, flow cytometry studies in maternal blood reveal that preterm parturition with intact or ruptured membranes is associated with phenotypic and metabolic changes in monocytes and granulocytes [16, 17]. Although it is well established that a FIRS is associated with the infiltration of inflammatory cells in the fetal compartment, it has not been addressed whether funisitis is associated with changes in the maternal compartment with flow cytometry. Analysis of the immunophenotype and metabolic activity of immune cells by flow cytometry is a useful tool which provides sensitive information about the detection of inflammation in vivo [5]. The present study was designed to investigate the changes in the phenotypic and metabolic characteristics of fetal and maternal immune cells associated with funisitis.
PATIENTS AND METHODS
Study design and population
A case-control study was conducted with umbilical cord and maternal blood samples obtained from patients with spontaneous preterm labor with intact membranes or preterm PROM who delivered before 35 completed weeks of gestation. The case group consisted of patients with funisitis (n=30) and the control group consisted of those without funisitis (n=15) which were matched for gestational age at delivery within two weeks. Patients with multiple pregnancies, preeclampsia, maternal medical disease, fetal death, and fetal congenital or chromosomal abnormalities were excluded. All patients provided written informed consent prior to the collection of samples. The collection and utilization of umbilical cord and maternal blood samples for research purposes were approved by the Institutional Review Boards of both Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Biological materials and some flow cytometric analyses of patients who were enrolled in this study have been used for studies of inflammation in pregnancy complications [16, 17, 36].
Clinical definitions
Spontaneous preterm labor was defined as the presence of regular uterine contractions occurring at a frequency of at least two every 10 minutes associated with cervical changes that required hospitalization before 37 completed weeks of gestation. The diagnosis of preterm PROM was confirmed by the pooling of amniotic fluid in the vagina in association with positive nitrazine and ferning tests or by positive amniocentesis-dye test before 37 completed weeks of gestation. Amniocentesis was performed at the discretion of the treating physician in a subset of patients; and amniotic fluid was cultured for the presence of microorganisms including aerobic and anaerobic bacteria as well as genital Mycoplasmas. Women received corticosteroids for fetal lung maturity and antibiotic therapy as clinically indicated. Clinical chorioamnionitis was diagnosed in the presence of fever (≥37.8°C) and two or more of the following criteria: uterine tenderness, malodorous vaginal discharge, maternal tachycardia (≥100 beats/minute), maternal leukocytosis (≥15,000 cells/mm3), and fetal tachycardia (≥160 beats/minute). A composite neonatal morbidity was defined as the presence of any of the following conditions: neonatal sepsis, respiratory distress syndrome, congenital pneumonia, bronchopulmonary dysplasia, intraventricular hemorrhage (grade III or IV), and necrotizing enterocolitis.
Umbilical cord and maternal blood samples collections
Maternal blood was obtained from patients upon admission to the labor and delivery unit, and umbilical cord blood was obtained from the umbilical vein at the time of delivery. The time interval between maternal blood sampling and delivery was less than 48 hours. The 48 hours interval was chosen to maintain the meaningful relationship of the phenotypic and metabolic characteristics of immune cells between umbilical cord and maternal blood. Blood samples were obtained using a syringe, added to an anticoagulant solution (20 μg/ml of the protease inhibitor, leupeptin), placed on ice, and transported to the laboratory. Blood samples were processed and analyzed within 60 minutes of sampling. The following were available for analysis: for the funisitis group, 30 samples of umbilical blood and 25 pairs of maternal blood; for the control group, 15 samples of umbilical cord blood and 12 pairs of maternal blood.
Flow cytometry
Evaluation of the granulocyte and monocyte surface markers was conducted according to the methods described previously [35]. To isolate leukocytes from red blood cells, LDS-751 (Molecular Probes, Eugene, OR, USA), a vital nucleic acid dye, was immediately added to the specimen (final concentration 0.0001%) upon arrival of the samples at the laboratory. Cell-surface antigens were phenotyped using monoclonal antibodies including CD11b, CD14, CD15, CD16, CD18, CD49d, CD62L, CD64, CD66b, and HLA-DR (Immunotech, Miami, FL, USA), which had been directly conjugated to fluorescein isothiocyanate (FITC). The isotype antibodies (IgG1, IgG2a, and IgM), matched by concentration, were used as the negative control. All antibodies were used in the concentrations recommended by the manufacturer. Flow cytometric analysis of umbilical cord and maternal blood was performed by a Coulter XL-MCL (Coulter Corp, Hialeah, FL, USA) equipped with a single 488-nm laser. FITC was detected at 525 nm, and LDS-751 was detected at 620 nm. Red blood cells, not labeled with LDS-751, were excluded from analysis. Granulocytes and monocytes were gated according to their forward and side scatter characteristics. The results were expressed as the mean fluorescence intensity (mean channel brightness [MCB]) after subtracting the fluorescence intensity of the isotype control antibody. The surface markers that were studied are described in Table 1.
Table 1.
Leukocyte surface antigens analyzed with specific monoclonal antibody and the staining of the leukocyte subpopulation
| Surface marker | Known or proposed functions |
|---|---|
| CD11b | Neutrophil and monocyte adhesion to endothelium and extracellular matrix proteins |
| CD14 | Receptor for lipopolysaccharide and its binding protein |
| CD15 | Leukocyte adhesion to endothelial cells; ligand for selectins |
| CD16 | Low affinity receptor for aggregated IgG |
| CD18 | Integrin β2 subunit mediated firm adhesion of leukocyte to endothelium |
| CD49d | Leukocyte adhesion to extracellular matrix; binds to VCAM-1 and fibronectin |
| CD62L | L-selectin mediates tethering and rolling of leukocytes |
| CD64 | High affinity receptor for IgG, mediates release of IL-1, IL-6, and TNF-α |
| CD66b | Role in cell-cell interaction |
| HLA-DR | Class II major histocompatibility antigen |
CD, cluster differentiation; HLA-DR, human leukocyte antigen-DR; IgG, immunoglobulin G; VCAM-1, vascular cell adhesion molecule-1; IL-1/6, interleukin-1/6; TNF-α, tumor necrosis factor-α
The presence of intracellular reactive oxygen species (iROS) within granulocytes and monocytes, including the determination of basal content and production in response to a stimulant (i.e., oxidative burst), were evaluated using the methods described by Himmelfarb et al [20]. Briefly, 2′7′ dichlorofluorescein diacetate (DCFH-DA) was added to blood samples and incubated for 15 minutes at 37°C. DCFH-DA reacts with the intracellular products of oxygen metabolism (primarily hydrogen peroxide) to form 2′7′-dichlorofluorescein (DCF), which is highly fluorescent and detected by flow cytometry. In addition to basal measurements, the oxidative burst was studied by adding 10 μL of N-formyl-methionylleucyl-phenylalanine (FMLP; Sigma, St Louis, MO, USA) to a tube containing 50 μL of blood sample and DCFH-DA. After incubation for 30 minutes at 37°C, 5 μL of LDS-751 in methanol (final concentration 0.0001%) was added for 1 minute at room temperature. The samples were then analyzed immediately by flow cytometry. The results were expressed as the MCB of basal iROS and oxidative burst.
Histopathologic examination of the umbilical cord and placenta
In all 45 patients, tissue samples taken for histopathologic examination included umbilical cord, chorionic plate and extraplacental membranes (amnion and choriodecidua). These samples were fixed in 10% neutral-buffered formalin and embedded in paraffin. Paraffin sections were stained with hematoxylin and eosin. Histopathologic examinations were performed systematically for inflammation based on the diagnostic criteria previously described [41]. Acute histologic chorioamnionitis was diagnosed if acute inflammatory changes were present in chorionic plate, amnion or choriodecidua [41]. Funisitis was defined as the presence of neutrophil infiltration into the wall of the umbilical vessels and/or into Wharton’s jelly [37]. Funisitis was classified as follows by inflammation in the umbilical cord: 1) umbilical phlebitis/chorionic vasculitis (stage 1 early); 2) umbilical arteritis (stage 2 intermediate); 3) necrotizing funisitis (stage 3 late); and 4) intense chorionic vasculitis with recent nonocclusive chorionic vessel thrombi (severe) [41]. Pathologists were blinded to the clinical information and flow cytometry analyses.
Statistical analysis
Shapiro-Wilk test was used to assess whether the data was normally distributed. Differences in the continuous variables between the two groups were estimated using nonparametric Mann-Whitney U test or parametric Student t-test based on the distribution of the data. Comparisons of proportions were performed by Chi-square and Fisher’s exact tests. All statistical analyses were performed using SPSS version 12.0 (SPSS Inc, Chicago, IL, USA). A p-value of < 0.01 was considered statistically significant. This threshold was chosen to have stringent criteria for significance, given the multiple comparisons performed in this study.
RESULTS
Demographic and clinical characteristics of the study population
The demographic and clinical characteristics of the study population are displayed in Table 2. The group with preterm delivery and funisitis had a higher rate of positive amniotic fluid culture and composite neonatal morbidity than those of the control group (p=0.007 and p=0.004, respectively). There were 12 cases of positive amniotic fluid culture. Gram negative microorganisms were detected in only two cases, while others were Candida albicans (n=1), genital Mycoplasmas (n=5) and Gram positive bacteria (n=4). The small number of patients in each group was insufficient to conduct an analysis. The majority of women in the case and control groups received steroids for fetal lung maturity (cases, 90.0%; controls, 86.7%; p=0.55) and antibiotics (cases, 96.7%; controls, 93.3%; p=0.56) before umbilical cord blood sampling. Overall, the median maternal blood sampling-to-delivery interval was seven hours, with an interquartile range of 2–16 hours.
Table 2.
Demographic and clinical characteristics of the study population
| Characteristic | Delivery before 35 completed weeks of gestation
|
p-value | |
|---|---|---|---|
| No funisitis (n=15) | Funisitis (n=30) | ||
| Maternal age (y)* | 26 (17–42) | 24 (17–35) | 0.32 |
| Race (African-American) | 14 (93.3%) | 22 (73.3%) | 0.23 |
| Nulliparity | 3 (20.0%) | 10 (33.3%) | 0.49 |
| Labor type | |||
| Spontaneous | 7 (46.7%) | 15 (50.0%) | 0.83 |
| Induced | 4 (26.7%) | 12 (40.0%) | 0.51 |
| No labor | 4 (26.7%) | 3 (10.0%) | 0.20 |
| Cesarean delivery | 4 (26.7%) | 4 (13.3%) | 0.41 |
| Clinical chorioamnionitis | 0 (0%) | 5 (16.7%) | 0.15 |
| Positive amniotic fluid culture† | 0 (0%) | 12 (54.5%) | 0.007§ |
| Prenatal steroid treatment | 13 (86.7%) | 27 (90.0%) | 1.00 |
| Prenatal antibiotic treatment | 14 (93.3%) | 29 (96.7%) | 1.00 |
| Causes of preterm delivery | |||
| PTL and intact membranes | 6 (40.0%) | 13 (43.3%) | 0.83 |
| Preterm PROM | 9 (60.0%) | 17 (56.7%) | 0.83 |
| Gestational age at delivery (wk)* | 29.6 (22.4–34.0) | 29.1 (23.0–34.0) | 0.41 |
| Birth weight (g)* | 1270 (400–2590) | 1115 (440–2300) | 0.45 |
| Neonatal Gender (male) | 9 (60.0%) | 15 (50.0%) | 0.53 |
| 5 -minute Apgar score <7 | 3 (20.0%) | 8 (26.7%) | 0.73 |
| Neonatal sepsis†† | 0 (0%) | 3 (12.0%) | 0.54 |
| Composite neonatal morbidity | 7 (46.7%) | 26 (86.7%) | 0.004§ |
PTL, preterm labor; preterm PROM, preterm prelabor rupture of membranes
Composite neonatal morbidity was defined as neonatal sepsis, respiratory distress syndrome, pneumonia, bronchopulmonary dysplasia, intraventricular hemorrhage (grade III or IV), or necrotizing enterocolitis.
Amniocenteses were performed on 8 women in the no funisitis group and 22 women in the funisitis group.
Neonatal blood cultures were performed on 11neonatesin the no funisitis group and 25 neonates in the funisitis group.
Values are given as median (range)
Statistically significant, p<0.01
Funisitis is associated with the changes in the phenotypic characteristics of granulocytes and monocytes in umbilical cord blood but not in maternal blood
Umbilical cord granulocytes of the funisitis group showed a significantly higher median MCB of CD14, CD64, and CD66b than those of the control group (p<0.01 for all comparisons, Table 3); the p-values for CD15 and CD16 on umbilical cord granulocytes were borderline (p=0.01 and p=0.02, respectively). The umbilical cord monocytes of the funisitis group had a significantly higher median MCB of CD64 than those of the control group (Table 3). Most of the funisitis cases were of stage 1 or 2 and these did not affect the results (data not shown).
Table 3.
Mean channel brightness of surface markers on umbilical cord granulocytes and monocytes in patients with preterm delivery with and without funisitis.
| Umbilical cord blood | Delivery before 35 completed weeks of gestation
|
p-value | |
|---|---|---|---|
| No funisitis (n=15) | Funisitis (n=30) | ||
| Granulocytes | |||
| CD11b | 4.64 (2.6–8.7) | 5.55 (2.3–16.5) | 0.40 |
| CD14 | 1.04 (0.8–1.4) | 1.34 (0.8–3.2) | 0.001† |
| CD15 | 124.00 (76.4–155.3) | 96.70 (58.1–126.4) | 0.01 |
| CD16 | 30.20 (0.9–64.9) | 13.75 (2.8–49.3) | 0.02 |
| CD18 | 8.93 (7.8–12.3) | 8.23 (6.2–20.4) | 0.10 |
| CD49d | 1.34 (0.9–2.7) | 1.54 (0.9–6.5) | 0.14 |
| CD62L | 11.80 (4.5–18.7) | 9.42 (6.2–16.2) | 0.13 |
| CD64 | 2.37 (1.5–3.9) | 8.75 (2.3–23.6) | <0.001† |
| CD66b | 5.45 (3.3–8.2) | 7.13 (2.8–28.1) | 0.003† |
| HLA-DR | 0.51 (0.4–1.0) | 0.57 (0.4–1.2) | 0.20 |
| Monocytes | |||
| CD11b | 4.75 (2.7–7.6) | 4.93 (1.9–15.0) | 0.59 |
| CD14 | 25.30 (17.7–36.4) | 24.30 (9.2–35.6) | 0.35 |
| CD15 | 4.88 (1.2–13.6) | 5.55 (1.5–35.3) | 0.61 |
| CD16 | 0.93 (0.7–2.4) | 0.84 (0.5–2.1) | 0.42 |
| CD18 | 14.00 (9.0–17.8) | 11.80 (6.2–20.4) | 0.37 |
| CD49d | 2.45 (1.6–3.8) | 2.70 (1.6–3.9) | 0.32 |
| CD62L | 14.90 (8.3–20.3) | 13.25 (8.5–21.3) | 0.95 |
| CD64 | 7.20 (4.6–13.5) | 15.30 (7.4–27.3) | <0.001† |
| CD66b | 0.80 (0.4–1.1) | 0.77 (0.6–1.3) | 0.66 |
| HLA-DR | 10.70 (4.8–22.2) | 6.99 (2.2–49.8) | 0.17 |
Values are given as median (range)
Statistically significant, p<0.01
In contrast, there was no difference in phenotypic characteristics in maternal granulocytes or monocytes between the study groups (Table 4); the p-values for CD49d, CD66b, and HLA-DR on maternal granulocytes were 0.03, 0.02, and 0.02, respectively. Only five cases with the diagnosis of clinical chorioamnionitis were included in this study. The small number of patients with clinical chorioamnionitis did not allow us to make a meaningful comparison in the phenotypic characteristics in maternal granulocytes or monocytes relative to the presence or absence of clinical chorioamnionitis.
Table 4.
Mean channel brightness of surface markers on maternal granulocytes and monocytes in patients with preterm delivery with and without funisitis.
| Maternal blood | Delivery before 35 completed weeks of gestation
|
p-value | |
|---|---|---|---|
| No funisitis (n=12) | Funisitis (n=25) | ||
| Granulocytes | |||
| CD11b | 6.14 (4.1–10.0) | 6.80 (3.0–15.8) | 0.42 |
| CD14 | 1.20 (0.9–1.7) | 1.27 (0.8–2.5) | 0.66 |
| CD15 | 96.20 (83.5–131.0) | 91.40 (70.2–119.3) | 0.47 |
| CD16 | 32.50 (9.1–71.9) | 28.20 (12.0–64.5) | 0.63 |
| CD18 | 9.33 (6.9–12.6) | 8.66 (4.8–13.9) | 0.31 |
| CD49d | 1.08 (0.9–1.4) | 0.93 (0.8–1.5) | 0.03 |
| CD62L | 6.23 (3.6–9.3) | 4.78 (2.2–11.1) | 0.15 |
| CD64 | 2.18 (1.4–3.1) | 2.35 (1.5–4.7) | 0.23 |
| CD66b | 4.65 (3.2–5.9) | 5.74 (3.3–10.9) | 0.02 |
| HLA-DR | 0.46 (0.4–0.7) | 0.41 (0.4–1.0) | 0.02 |
| Monocytes | |||
| CD11b | 8.73 (4.5–11.8) | 7.69 (3.5–21.0) | 0.77 |
| CD14 | 44.10 (32.9–54.5) | 39.90 (22.5–53.0) | 0.80 |
| CD15 | 2.92 (1.4–4.0) | 2.47 (1.3–9.4) | 0.39 |
| CD16 | 0.76 (0.5–1.1) | 0.73 (0.5–1.3) | 0.99 |
| CD18 | 16.45 (13.0–26.6) | 17.50 (9.2–24.3) | 0.81 |
| CD49d | 3.38 (2.2–4.1) | 3.15 (1.7–4.6) | 0.64 |
| CD62L | 7.12 (5.9–9.4) | 6.39 (3.8–14.0) | 0.16 |
| CD64 | 11.95 (8.6–19.3) | 12.60 (7.3–18.4) | 0.54 |
| CD66b | 0.83 (0.6–1.0) | 0.76 (0.6–1.3) | 0.23 |
| HLA-DR | 6.47 (3.3–12.7) | 7.28 (2.7–24.7) | 0.33 |
Values are given as median (range)
Funisitis is associated with the changes in metabolic activity of monocytes in umbilical cord blood but not in maternal blood
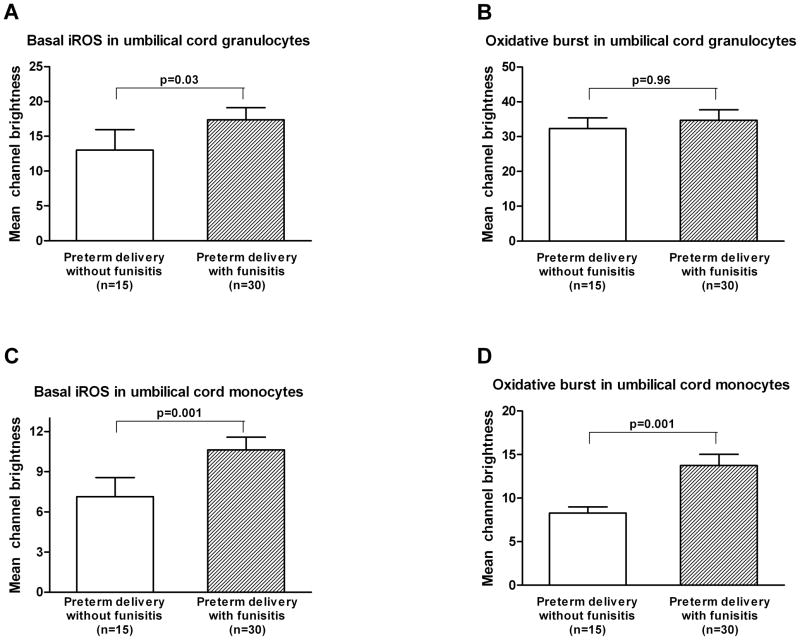
The basal iROS production and oxidative burst were significantly higher in umbilical cord monocytes of the funisitis group compared to those of the control group (the control group vs. the funisitis group; mean ± SEM: 7.1 ± 1.4 vs. 10.6 ± 1.0; p=0.001, and 8.3 ± 0.7 vs. 13.8 ± 1.3; p=0.001, respectively; Figures 1C and 1D). The basal iROS production was higher in umbilical cord granulocytes of the funisitis group than that of the control group, but the difference did not reach statistical significance (the control group vs. the funisitis group; mean ± SEM: 13.0 ± 2.9 vs. 17.4 ± 1.7; p=0.03, Figure 1A). There was no difference in oxidative burst in the umbilical cord granulocytes between the control and funisitis groups (the control group vs. the funisitis group; mean ± SEM: 32.3 ± 3.1 vs. 34.7 ± 3.0; p=0.96, Figure 1B).
Figure 1. Umbilical cord blood: mean channel brightness (mean ± SEM) of basal dichlofluorescein (DCF) activated intracellular reactive oxygen species (iROS) and DCF after stimulation (oxidative burst) in granulocytes and monocytes.
A and B) There was no difference in basal iROS production and oxidative burst in the umbilical cord granulocytes between the control and funisitis groups (mean ± SEM: 13.0 ± 2.9 vs. 17.4 ± 1.7; p=0.03, and 32.3 ± 3.1 vs. 34.7 ± 3.0; p=0.96, respectively). C and D) The basal iROS production and oxidative burst were significantly increased in the umbilical cord monocytes of the funisitis group compared to those of the control group (the control group vs. the funisitis group; mean ± SEM: 7.1 ± 1.4 vs. 10.6 ± 1.0; p=0.001, and 8.3 ± 0.7 vs. 13.8 ± 1.3; p=0.001, respectively).
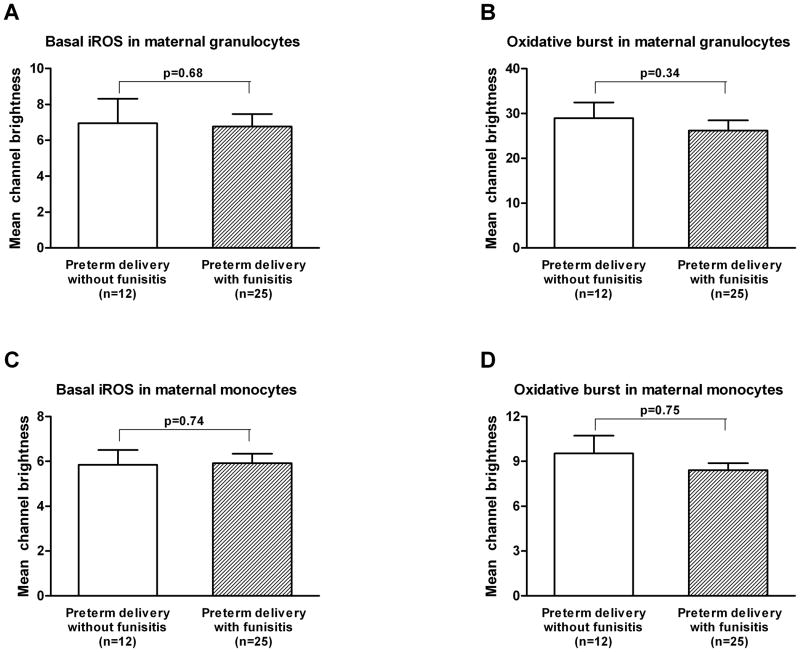
In contrast, there was no difference in basal iROS production and oxidative burst in the maternal granulocytes (mean ± SEM: 6.9 ± 1.4 vs. 6.8 ± 0.7; p=0.68, and 29.0 ± 3.5 vs. 26.2 ± 2.3; p=0.34, respectively; Figures 2A and 2B) or monocytes between the control and funisitis groups (mean ± SEM: 5.8 ± 0.7 vs. 5.9 ± 0.4; p=0.74, and 9.5 ± 1.2 vs. 8.4 ± 0.5; p=0.75, respectively; Figures 2C and 2D).
Figure 2. Maternal blood: mean channel brightness (mean ± SEM) of basal dichlofluorescein (DCF) activated intracellular reactive oxygen species (iROS) and DCF after stimulation (oxidative burst) in granulocytes and monocytes.
A and B) There was no difference in basal iROS production and oxidative burst in the maternal granulocytes between the control and funisitis groups (mean ± SEM: 6.9 ± 1.4 vs. 6.8 ± 0.7; p=0.68, and 29.0 ± 3.5 vs. 26.2 ± 2.3; p=0.34, respectively). C and D) There was no difference in basal iROS production and oxidative burst in the maternal monocytes between the control and funisitis groups (mean ± SEM: 5.8 ± 0.7 vs. 5.9 ± 0.4; p=0.74, and 9.5 ± 1.2 vs. 8.4 ± 0.5; p=0.75, respectively).
DISCUSSION
Principal findings of this study
1) Funisitis was associated with phenotypic changes in umbilical cord granulocytes and monocytes consistent with activation; 2) the basal iROS production and oxidative burst were higher in umbilical cord monocytes from neonates with funisitis than in those without this lesion; and 3) there were no significant differences in the immunophenotype, basal iROS production, and oxidative burst in maternal granulocytes or monocytes of mothers with or without funisitis.
Fetal systemic inflammation is associated with intravascular inflammation and the production of intracellular reactive oxygen radicals in the preterm neonate
We report herein changes in the immunophenotype and iROS production in neonates with evidence of in-utero systemic inflammation (funisitis is the histologic counterpart of FIRS). These observations have been made despite the traditional view that the preterm fetus and neonate have an immature and hyporesponsive immune system in comparison to the adult [32, 56]. Another important observation is that the maternal and fetal immune responses in the context of funisitis were dissociated from each other.
Previous studies of granulocytes and monocytes in preterm neonates
The changes in oxidative burst of granulocytes in the preterm neonate have been controversial. Indeed, some investigators have reported that the oxidative burst after stimulation with neonatal granulocytes is increased [44, 51], decreased [6], or unchanged [15, 50] compared to that of adults. Moreover, preterm newborns have been reported to have a lower [26] or higher [29] oxidative burst of granulocytes after stimulation than those of term newborns and adults. Our study fills a major gap in the literature, which is to determine the in vivo immunophenotypic and metabolic state of granulocytes and monocytes of fetuses with and without systemic inflammation at the time of birth.
The rationale for using funisitis to identify the fetal inflammatory response system
Funisitis, inflammation of the umbilical cord, is a fetal host response, and is the hallmark of the fetal inflammatory response syndrome. Previous studies have established the strengths of this association [10, 37]. Indeed, neonates with funisitis are at an increased risk for neonatal morbidity and long-term sequelae, such as cerebral palsy and bronchopulmonary dysplasia [59, 60]. The findings of this study are novel and present for the first time the evidence that funisitis is associated with the changes in the phenotypic and metabolic characteristics of umbilical cord granulocytes and monocytes.
Increased expression of CD14 by granulocytes of newborns with funisitis
CD14 has been implicated in the clearance of Gram-negative microorganisms by the enhancement of lipopolysaccharide (LPS) signaling pathway [58]. The cellular response involves the LPS-binding protein/CD14/Toll-like receptor 4 complex [2]. In addition, the CD14 receptor on immune cells, interacts with several other bacterial components such as the streptococcal cell-wall polysaccharides [46], mycobacterial lipoarabinomannan [39], and peptidoglycans [52]. Soluble CD14 (sCD14) is present in plasma, and the changes in CD14 gene expression and sCD14 concentration are associated with several inflammatory conditions [11, 19, 28]. It is interesting that the amniotic fluid concentration of sCD14 is higher in the context of intra-amniotic infection/inflammation [12]. Our results are consistent with previous studies reporting that neonates born to mothers with preterm labor with intact membranes and intra-amniotic infection/inflammation had a higher median concentration of sCD14 in umbilical cord plasma than those without intra-amniotic infection/inflammation [12].
Increased expression of CD64 by granulocytes and monocytes of newborns with funisitis
CD64 is a type of Fc receptor expressed on monocytes, macrophages and activated neutrophils, which promotes the phagocytosis of opsonized particles and mediate the release IL-1, IL-6 and TNFα [21]. Previous studies have shown that granulocytes that bind to endothelial monolayers express CD64 [13], which is considered to be an activation marker of granulocytes [1]. Our finding that the median MCB of CD64 on umbilical cord granulocytes in neonates born with funisitis is higher than that of neonates born without funisitis is consistent with previous studies, which found that the CD64 expression on granulocytes has been increased in newborn infants [14] and adult patients [40] with bacterial infections.
Increased expression of CD66b by granulocytes of newborns with funisitis
CD66b, expressed mainly on granulocytes, is a member of the carcino-embryonic antigen family [24] and participates in neutrophil function, including the regulation of endothelial adhesion [30] and activation of reactive oxygen species (ROS) production [49]. The enhanced expression of CD66b is also associated with systemic inflammatory response syndrome (SIRS) in adults [34]. In neonates, CD66b expression on peripheral blood granulocytes was significantly elevated in preterm neonates with sepsis [53]. We found an increased expression of CD66b in umbilical cord granulocytes from neonates with funisitis compared to that of neonates without funisitis. This is consistent with the known biology of CD66b.
Evidence of increased metabolic activity of fetal leukocytes in fetal systemic inflammation
Successful phagocytosis leads to the destruction of the microorganism through the generation of toxic ROS such as superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (-OH), during the oxidative burst [22]. Quantification of ROS is an index of the metabolic activity of phagocytes [38]. In this study, ROS was measured by the fluorescence intensity of DCF generated by the reaction between DCFH-DA and hydrogen peroxide inside the cell at the basal state and after stimulation by FMLP (oxidative burst). We demonstrated herein that basal iROS production and oxidative burst were increased in the umbilical cord monocytes of neonates born with funisitis compared to those of neonates born without funisitis. The increased basal iROS production suggests that umbilical cord monocytes had an enhanced intracellular metabolic activity, whereas the increased oxidative burst indicates that there was priming of monocytes in umbilical cord blood.
The excessive production of ROS and their release into the extracellular compartment can damage tissues [55] and may also be involved in the generation of neonatal morbidity associated with FIRS. Of note is the close relationship between oxidative stress and inflammation [31, 47].
Confounding factors influencing the immunophenotype and metabolic activity of fetal leukocytes
It is generally agreed that preterm newborns, when compared to term newborns, are more vulnerable to severe and life-threatening infections [33, 57]. This susceptibility has been largely attributed to the immaturity of leukocytes in preterm neonates [3, 45]. The expression of CD16 on neutrophils is lower in preterm than in term newborns [8]. The percentage of MHC Class II-positive monocytes increases over gestation, whereas the expression of CD11b and CD35 on monocytes fluctuates over the course of gestation [23]. Thus, gestational age at delivery may have an impact on the different results of the phenotypic characteristics of leukocytes in umbilical cord blood. However, in the present study, there was no difference in gestational age at delivery between the two study groups. Moreover, it has been proposed that spontaneous labor is associated with a fetal immune response [48, 54]. In this study, no differences in distribution of labor type or delivery mode were observed among study groups.
Antenatal corticosteroid administration can lead to changes in the number and response of fetal inflammatory cells [4, 27]. Although in the present study the effect of antenatal steroid administration in the response of fetal immune cells could not to be determined, it is unlikely that this is responsible for the differences in the immunophenotype and metabolic changes of leukocytes between the study groups as most cases included in this study received antenatal steroids.
Limitation of the study
Simultaneous collection of maternal and neonatal blood would have been the ideal condition for analysis. In this study, maternal blood samples were restricted to those obtained within 48 hours from delivery. Collection of maternal blood several hours before delivery may be too early to allow detection of a maternal systemic inflammatory response, which might be more evident if the maternal blood was obtained at the time of cord blood sampling. Although this can be considered as a limitation of the study, the median maternal blood sampling-to-delivery interval was less than eight hours, and only 13% (6/45) of cases had a maternal blood sampling-to-delivery interval between 24–48 hours.
In conclusion, this study provides evidence that fetal systemic inflammation is associated with phenotypic and metabolic changes consistent with activation in fetal immune cells but not in maternal blood. While most studies have focused on the developmental immaturity of the fetal innate immune system, the novelty of this study is the assessment of the differences in the immunophenotype and metabolic activity of umbilical cord granulocytes and monocytes between preterm neonates born with and without funisitis. Further studies are warranted to elucidate the comprehensive mechanisms involved in this differential immunological response.
Acknowledgments
This research was supported in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Akerley WL, III, Guyre PM, Davis BH. Neutrophil activation through high-affinity Fc gamma receptor using a monomeric antibody with unique properties. Blood. 1991;77:607–615. [PubMed] [Google Scholar]
- 2.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 3.Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res. 1986;20:899–904. doi: 10.1203/00006450-198609000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Barak M, Cohen A, Herschkowitz S. Total leukocyte and neutrophil count changes associated with antenatal betamethasone administration in premature infants. Acta Paediatr. 1992;81:760–763. doi: 10.1111/j.1651-2227.1992.tb12098.x. [DOI] [PubMed] [Google Scholar]
- 5.Barclay AN, Brown MH, Law SKA, McKnight AJ, Tomlinson MG, van der Merwe PA. The Leukocyte antigen facts book. 2. 1997. [Google Scholar]
- 6.Bektas S, Goetze B, Speer CP. Decreased adherence, chemotaxis and phagocytic activities of neutrophils from preterm neonates. Acta Paediatr Scand. 1990;79:1031–1038. doi: 10.1111/j.1651-2227.1990.tb11379.x. [DOI] [PubMed] [Google Scholar]
- 7.Berry SM, Romero R, Gomez R, Puder KS, Ghezzi F, Cotton DB, et al. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol. 1995;173:1315–1320. doi: 10.1016/0002-9378(95)91378-5. [DOI] [PubMed] [Google Scholar]
- 8.Carr R, Davies JM. Abnormal FcRIII expression by neutrophils from very preterm neonates. Blood. 1990;76:607–611. [PubMed] [Google Scholar]
- 9.Christensen RD, Harper TE, Rothstein G. Granulocyte-macrophage progenitor cells in term and preterm neonates. J Pediatr. 1986;109:1047–1051. doi: 10.1016/s0022-3476(86)80297-9. [DOI] [PubMed] [Google Scholar]
- 10.D’Alquen D, Kramer BW, Seidenspinner S, Marx A, Berg D, Groneck P, et al. Activation of umbilical cord endothelial cells and fetal inflammatory response in preterm infants with chorioamnionitis and funisitis. Pediatr Res. 2005;57:263–269. doi: 10.1203/01.PDR.0000148713.48218.86. [DOI] [PubMed] [Google Scholar]
- 11.Egerer K, Feist E, Rohr U, Pruss A, Burmester GR, Dorner T. Increased serum soluble CD14, ICAM-1 and E-selectin correlate with disease activity and prognosis in systemic lupus erythematosus. Lupus. 2000;9:614–621. doi: 10.1191/096120300678828749. [DOI] [PubMed] [Google Scholar]
- 12.Espinoza J, Chaiworapongsa T, Romero R, Gomez R, Kim JC, Yoshimatsu J, et al. Evidence of participation of soluble CD14 in the host response to microbial invasion of the amniotic cavity and intra-amniotic inflammation in term and preterm gestations. J Matern Fetal Neonatal Med. 2002;12:304–312. doi: 10.1080/jmf.12.5.304.312. [DOI] [PubMed] [Google Scholar]
- 13.Fadlon E, Vordermeier S, Pearson TC, Mire-Sluis AR, Dumonde DC, Phillips J, et al. Blood polymorphonuclear leukocytes from the majority of sickle cell patients in the crisis phase of the disease show enhanced adhesion to vascular endothelium and increased expression of CD64. Blood. 1998;91:266–274. [PubMed] [Google Scholar]
- 14.Fjaertoft G, Hakansson L, Foucard T, Ewald U, Venge P. CD64 (Fcgamma receptor I) cell surface expression on maturing neutrophils from preterm and term newborn infants. Acta Paediatr. 2005;94:295–302. doi: 10.1111/j.1651-2227.2005.tb03072.x. [DOI] [PubMed] [Google Scholar]
- 15.Frazier JP, Cleary TG, Pickering LK, Kohl S, Ross PJ. Leukocyte function in healthy neonates following vaginal and cesarean section deliveries. J Pediatr. 1982;101:269–272. doi: 10.1016/s0022-3476(82)80138-8. [DOI] [PubMed] [Google Scholar]
- 16.Gervasi MT, Chaiworapongsa T, Naccasha N, Blackwell S, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1124–1129. doi: 10.1067/mob.2001.117681. [DOI] [PubMed] [Google Scholar]
- 17.Gervasi MT, Chaiworapongsa T, Naccasha N, Pacora P, Berman S, Maymon E, et al. Maternal intravascular inflammation in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11:171–175. doi: 10.1080/jmf.11.3.171.175. [DOI] [PubMed] [Google Scholar]
- 18.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann W, Ecker D, Quast S, Klieden M, Rose S, Marzi I. Comparison of procalcitonin, sCD14 and interleukin-6 values in septic patients. Clin Chem Lab Med. 2000;38:41–46. doi: 10.1515/CCLM.2000.007. [DOI] [PubMed] [Google Scholar]
- 20.Himmelfarb J, Hakim RM, Holbrook DG, Leeber DA, Ault KA. Detection of granulocyte reactive oxygen species formation in whole blood using flow cytometry. Cytometry. 1992;13:83–89. doi: 10.1002/cyto.990130113. [DOI] [PubMed] [Google Scholar]
- 21.Hulett MD, Hogarth PM. The second and third extracellular domains of FcgammaRI (CD64) confer the unique high affinity binding of IgG2a. Mol Immunol. 1998;35:989–996. doi: 10.1016/s0161-5890(98)00069-8. [DOI] [PubMed] [Google Scholar]
- 22.Johnston RB., Jr Oxygen metabolism and the microbicidal activity of macrophages. Fed Proc. 1978;37:2759–2764. [PubMed] [Google Scholar]
- 23.Jones CA, Holloway JA, Warner JO. Phenotype of fetal monocytes and B lymphocytes during the third trimester of pregnancy. J Reprod Immunol. 2002;56:45–60. doi: 10.1016/s0165-0378(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 24.Khan WN, Frangsmyr L, Teglund S, Israelsson A, Bremer K, Hammarstrom S. Identification of three new genes and estimation of the size of the carcinoembryonic antigen family. Genomics. 1992;14:384–390. doi: 10.1016/s0888-7543(05)80230-7. [DOI] [PubMed] [Google Scholar]
- 25.Kim CJ, Yoon BH, Romero R, Moon JB, Kim M, Park SS, et al. Umbilical arteritis and phlebitis mark different stages of the fetal inflammatory response. Am J Obstet Gynecol. 2001;185:496–500. doi: 10.1067/mob.2001.116689. [DOI] [PubMed] [Google Scholar]
- 26.Komatsu H, Tsukimori K, Hata K, Satoh S, Nakano H. The characterization of superoxide production of human neonatal neutrophil. Early Hum Dev. 2001;65:11–19. doi: 10.1016/s0378-3782(01)00188-8. [DOI] [PubMed] [Google Scholar]
- 27.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Antenatal betamethasone changes cord blood monocyte responses to endotoxin in preterm lambs. Pediatr Res. 2004;55:764–768. doi: 10.1203/01.PDR.0000120678.72485.19. [DOI] [PubMed] [Google Scholar]
- 28.Kruger C, Schutt C, Obertacke U, Joka T, Muller FE, Knoller J, et al. Serum CD14 levels in polytraumatized and severely burned patients. Clin Exp Immunol. 1991;85:297–301. doi: 10.1111/j.1365-2249.1991.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kugo M, Sano K, Uetani Y, Nakamura H. Superoxide dismutase in polymorphonuclear leukocytes of term newborn infants and very low birth weight infants. Pediatr Res. 1989;26:227–231. doi: 10.1203/00006450-198909000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Kuijpers TW, van der Schoot CE, Hoogerwerf M, Roos D. Cross-linking of the carcinoembryonic antigen-like glycoproteins CD66 and CD67 induces neutrophil aggregation. J Immunol. 1993;151:4934–4940. [PubMed] [Google Scholar]
- 31.Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall-Clarke S, Reen D, Tasker L, Hassan J. Neonatal immunity: how well has it grown up? Immunol. Today. 2000;21:35–41. doi: 10.1016/s0167-5699(99)01548-0. [DOI] [PubMed] [Google Scholar]
- 33.Martinot A, Leclerc F, Cremer R, Leteurtre S, Fourier C, Hue V. Sepsis in neonates and children: definitions, epidemiology, and outcome. Pediatr Emerg Care. 1997;13:277–281. doi: 10.1097/00006565-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Martins PS, Brunialti MK, Martos LS, Machado FR, Assuncao MS, Blecher S, et al. Expression of cell surface receptors and oxidative metabolism modulation in the clinical continuum of sepsis. Crit Care. 2008;12:R25. doi: 10.1186/cc6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy DA, Macey MG. Novel anticoagulants for flow cytometric analysis of live leucocytes in whole blood. Cytometry. 1996;23:196–204. doi: 10.1002/(SICI)1097-0320(19960301)23:3<196::AID-CYTO3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 36.Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001;185:1118–1123. doi: 10.1067/mob.2001.117682. [DOI] [PubMed] [Google Scholar]
- 37.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 38.Perticarari S, Presani G, Mangiarotti MA, Banfi E. Simultaneous flow cytometric method to measure phagocytosis and oxidative products by neutrophils. Cytometry. 1991;12:687–693. doi: 10.1002/cyto.990120713. [DOI] [PubMed] [Google Scholar]
- 39.Pugin J, Heumann ID, Tomasz A, Kravchenko VV, Akamatsu Y, Nishijima M, et al. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 40.Qureshi SS, Lewis SM, Gant VA, Treacher D, Davis BH, Brown KA. Increased distribution and expression of CD64 on blood polymorphonuclear cells from patients with the systemic inflammatory response syndrome (SIRS) Clin Exp Immunol. 2001;125:258–265. doi: 10.1046/j.1365-2249.2001.01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation--a workshop report. Placenta. 2005;26 (Suppl A):S114–S117. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 43.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 44.Shigeoka AO, Charette RP, Wyman ML, Hill HR. Defective oxidative metabolic responses of neutrophils from stressed neonates. J Pediatr. 1981;98:392–398. doi: 10.1016/s0022-3476(81)80701-9. [DOI] [PubMed] [Google Scholar]
- 45.Smith JB, Campbell DE, Ludomirsky A, Polin RA, Douglas SD, Garty BZ, et al. Expression of the complement receptors CR1 and CR3 and the type III Fc gamma receptor on neutrophils from newborn infants and from fetuses with Rh disease. Pediatr Res. 1990;28:120–126. doi: 10.1203/00006450-199008000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Soell M, Lett E, Holveck F, Scholler M, Wachsmann D, Klein JP. Activation of human monocytes by streptococcal rhamnose glucose polymers is mediated by CD14 antigen, and mannan binding protein inhibits TNF-alpha release. J Immunol. 1995;154:851–860. [PubMed] [Google Scholar]
- 47.Speer CP. Inflammation and bronchopulmonary dysplasia. Semin Neonatol. 2003;8:29–38. doi: 10.1016/s1084-2756(02)00190-2. [DOI] [PubMed] [Google Scholar]
- 48.Steinborn A, Sohn C, Sayehli C, Baudendistel A, Huwelmeier D, Solbach C, et al. Spontaneous labour at term is associated with fetal monocyte activation. Clin Exp Immunol. 1999;117:147–152. doi: 10.1046/j.1365-2249.1999.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stocks SC, Kerr MA, Haslett C, Dransfield I. CD66-dependent neutrophil activation: a possible mechanism for vascular selectin-mediated regulation of neutrophil adhesion. J Leukoc Biol. 1995;58:40–48. doi: 10.1002/jlb.58.1.40. [DOI] [PubMed] [Google Scholar]
- 50.Strauss RG, Seifert MJ. Oxidative metabolism in cord-blood polymorphonuclear leucocytes. Arch Dis Child. 1978;53:78–80. doi: 10.1136/adc.53.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strauss RG, Snyder EL. Activation and activity of the superoxide-generating system of neutrophils from human infants. Pediatr Res. 1983;17:662–664. doi: 10.1203/00006450-198308000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Weidemann B, Schletter J, Dziarski R, Kusumoto S, Stelter F, Rietschel ET, et al. Specific binding of soluble peptidoglycan and muramyldipeptide to CD14 on human monocytes. Infect Immun. 1997;65:858–864. doi: 10.1128/iai.65.3.858-864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinschenk NP, Farina A, Bianchi DW. Premature infants respond to early-onset and late-onset sepsis with leukocyte activation. J Pediatr. 2000;137:345–350. doi: 10.1067/mpd.2000.107846. [DOI] [PubMed] [Google Scholar]
- 54.Weinschenk NP, Farina A, Bianchi DW. Neonatal neutrophil activation is a function of labor length in preterm infants. Pediatr Res. 1998;44:942–945. doi: 10.1203/00006450-199812000-00020. [DOI] [PubMed] [Google Scholar]
- 55.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 56.Wilson CB. Immunologic basis for increased susceptibility of the neonate to infection. J Pediatr. 1986;108:1–12. doi: 10.1016/s0022-3476(86)80761-2. [DOI] [PubMed] [Google Scholar]
- 57.Wolach B. Neonatal sepsis: pathogenesis and supportive therapy. Semin Perinatol. 1997;21:28–38. doi: 10.1016/s0146-0005(97)80017-1. [DOI] [PubMed] [Google Scholar]
- 58.Wright SD, Ramos RA, Hermanowski-Vosatka A, Rockwell P, Detmers PA. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991;173:1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181:773–779. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 60.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 61.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–1129. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 62.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–1440. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]