Abstract
Objectives
This study aimed to update and validate a prediction rule for respiratory syncytial virus (RSV) hospitalization in preterm infants 33–35 weeks gestational age (WGA).
Study Design
The RISK study consisted of 2 multicenter prospective birth cohorts in 41 hospitals. Risk factors were assessed at birth among healthy preterm infants 33–35 WGA. All hospitalizations for respiratory tract infection were screened for proven RSV infection by immunofluorescence or polymerase chain reaction. Multivariate logistic regression analysis was used to update an existing prediction model in the derivation cohort (n = 1,227). In the validation cohort (n = 1,194), predicted versus actual RSV hospitalization rates were compared to determine validity of the model.
Results
RSV hospitalization risk in both cohorts was comparable (5.7% versus 4.9%). In the derivation cohort, a prediction rule to determine probability of RSV hospitalization was developed using 4 predictors: family atopy (OR 1.9; 95%CI, 1.1–3.2), birth period (OR 2.6; 1.6–4.2), breastfeeding (OR 1.7; 1.0–2.7) and siblings or daycare attendance (OR 4.7; 1.7–13.1). The model showed good discrimination (c-statistic 0.703; 0.64–0.76, 0.702 after bootstrapping). External validation showed good discrimination and calibration (c-statistic 0.678; 0.61–0.74).
Conclusions
Our prospectively validated prediction rule identifies infants at increased RSV hospitalization risk, who may benefit from targeted preventive interventions. This prediction rule can facilitate country-specific, cost-effective use of RSV prophylaxis in late preterm infants.
Introduction
Respiratory syncytial virus (RSV) bronchiolitis is one of the most common causes of infant hospitalization during the winter season and is associated with a large burden of disease and high costs.[1]–[5] Hospitalization for RSV lower respiratory tract infection in Europe and the United States is estimated to be 1–3% of all infants aged less than 13 months. Important risk groups for RSV bronchiolitis are infants with prematurity with or without chronic lung disease, congenital heart disease, Down syndrome and immunodeficiencies.[6]–[9] Although risk groups for RSV bronchiolitis have been identified, the precise incidence of hospitalization for RSV bronchiolitis in these patient populations is generally not known. There is no effective therapy for RSV infection, so treatment is mainly symptomatic.[10] Due to the increased risk most high risk groups receive RSV immunoprophylaxis to prevent RSV infection. Palivizumab, a humanized immunoglobin monoclonal antibody, specific for RSV, has been proven effective and safe for preterm infants with gestational age ≤35 weeks, infants with bronchopulmonary dysplasia and infants with congenital heart disease.[11], [12] Efficacy of 55% of RSV prophylaxis has been demonstrated for late preterm infants 33–35 weeks gestational age (WGA). Subgroup analysis showed 80% efficacy of RSV prophylaxis in 32–35 WGA preterm infants.[12] In many countries RSV immunoprophylaxis is not used in late preterm infants 33–35 WGA because of high costs.[13] Within health care, limited budgets force the need to selectively apply high cost treatments to a proportion of infants identified as having increased risk for severe disease. Costs may be reduced by targeting RSV immunoprophylaxis to 33–35 WGA late preterm infants with additional risk factors.[14] Several environmental and clinical risk factors have been described which compound the risk for severe RSV disease. Presence of siblings, daycare attendance, month of birth and protective factors like breastfeeding have been described as independent risk factors for severe disease due to RSV infection.[15]–[21] In a recent paper it was emphasized that validated prediction rules are required to improve the care of our patients with infectious diseases.[22] Two prediction rules for late preterm infants 33–35 WGA have been published but these have not yet been validated prospectively.[23], [24] To develop a practical and accurate prediction model for the Netherlands the prediction rule previously developed by Simoes et al. may have inferior performance in countries, such as the Netherlands, in which most children visit day care facilities.[24] We therefore aimed to update and validate a RSV prediction rule for 33–35 WGA late preterm infants using 2 prospective birth cohorts.[24]
Methods
Study design
RISK is an ongoing study prospectively performed in late preterm infants born at 32 weeks and 1 day to 35 weeks and 6 days weeks gestational age (referred to as 33–35 WGA) in 41 hospitals of the RSV Neonatal Network in the Netherlands. Between June 2008 and January 2011 infants were included in hospitals located across the Netherlands. The study population consisted of newborn infants born at 33–35 WGA from 1 university hospital and 40 regional hospitals. Infants with gross abnormalities or Down syndrome, and those who received palivizumab for any reason were excluded. The study consists of 2 subsequent birth cohorts: a derivation cohort and a validation cohort.
Ethics statement
The study was reviewed and approved by the Institutional Review Board of the University Medical Center Utrecht and subsequently approved by Institutional Review Boards of all participating hospitals. All parents provided written informed consent for screening of hospital records. The study was conducted in compliance with the Declaration of Helsinki and the standards of Good Clinical Practice.
Data collection
At birth, a questionnaire containing questions on family history of wheeze, asthma and hay fever, smoking during pregnancy and in the household, the number of siblings and their age, parental education level, potential breastfeeding, potential day-care attendance, household pets and pregnancy details was filled out by parents. Clinical data on the mode of delivery, gestational age, respiratory support, birth weight, Apgar score and delivery details were derived from patient charts. The following 7 variables from the prediction rule previously developed by Simoes et al. were noted: ‘birth within 10 weeks of the start of the season,’ ‘birth weight,’ ‘breast-feeding ≤2 months,’ ‘number of siblings ≥2 years of age,’ ‘number of family members with atopy,’ ‘male sex,’ and ‘number of family members with wheeze’[24]. Breast-feeding was defined as either exclusive breastfeeding or mixed with formula feeding. Atopy was defined as the presence of asthma, eczema or hay fever. At one year of age, parents were contacted by telephone to determine whether hospitalization for respiratory disease had occurred. If any data were missing from questionnaires completed by the parents/legal guardians or from the clinical records, the respective physician was contacted for information, which ensured that all baseline data were assembled. If the parents could not be reached by telephone, the hospital and general practitioner were contacted for updated information. If no valid telephone number was available, an e-mail or letter was sent to the parents.
Outcome definition
When parents reported hospitalization for respiratory disease during the first year of life, we analysed the medical hospital record for RSV hospitalization, including routine virology results. The main study endpoint, hospitalization for RSV bronchiolitis was defined as hospitalization for lower respiratory tract infection with proven RSV infection determined by routine practice laboratory testing in the participating hospitals, i.e. either by rapid RSV immunofluorescence test or polymerase chain reaction.
Statistical analysis
Sample size calculation: According to a generally accepted rule of thumb that at least 10 cases are required per variable in the prediction rule. For a 7-variable model we calculated a priori, a sample size of 70 infants hospitalized for RSV bronchiolitis.[24] With an estimated incidence of 4%, the projected sample size of the derivation cohort was 1,750. To validate a 4-variable prediction rule, the estimated sample size of the validation cohort was 1,000.
Derivation and validation of the prediction rule
We assessed the test performance of the clinical prediction rule to identify infants at high risk for hospitalization with RSV bronchiolitis. To evaluate the models' calibration, the Hosmer-Lemeshow statistic was used in which observations are grouped based on deciles of predicted probability and compared with the observed risk of RSV bronchiolitis in the derivation and validation cohort. This was graphically assessed with a calibration plot and tested with the Hosmer-Lemeshow statistic, where a non-significant test indicated good model fit.[25], [26] Discrimination is the ability of the rule to distinguish between infants hospitalized from those not hospitalized for RSV bronchiolitis, and will be quantified with the Area Under the Receiver Operating Characteristic curve (AUROC). An AUROC area ranges from 0.5 (no discrimination.) to 1.0 (perfect discrimination).
We anticipated that the prediction rule previously developed by Simoes et al. may have inferior performance in countries, such as the Netherlands, in which most children visit day care facilities. Therefore we planned to update the model. Multivariable logistic regression was used to update the independent contribution of each of the variables to the discrimination of the model. The updated model was reduced by excluding variables from the model with univariate p-values >0.15, using the log likelihood ratio test. The AUROC was used to determine whether the variables provided added predictive value beyond the existent prediction rule.[27] Other, additional variables with a univariate p-value of <0.15 not included in the original prediction rule were added to increase the discrimination and reliability of the prediction rule. Subsequently, the model shrinkage was applied in the derivation dataset using bootstrapping, to adjust the model's estimated regression coefficients in order to reduce overfitting.[25], [28] We repeated the modelling process in 1,000 bootstrap samples. For each individual infant the risk score was calculated using the bootstrap-corrected coefficients of the updated prediction rule. The value of each risk factor was multiplied by its coefficient and the sum of all resulting values and the model intercept, i.e. the linear predictor, was calculated. The results of the validation were examined primarily by classification tables and by calculating the AUROC. To make the model easy to use in a clinical setting we calculated a point score.
The updated prediction rule was externally validated in a new cohort of infants. The two cohorts were derived by making a non-randomized split according to birth date.[29]
We defined our derivation cohort as all infants born between June 2008 and September 2009, and our validation cohort as all infants born between September 2009 and January 2011. We calculated performance of the rule as sensitivity, specificity, positive likelihood ratio and negative likelihood ratio. Statistical analysis was performed by using SPSS 15.0. (SPSS Inc, Chicago, Ill).
Results
Patient characteristics
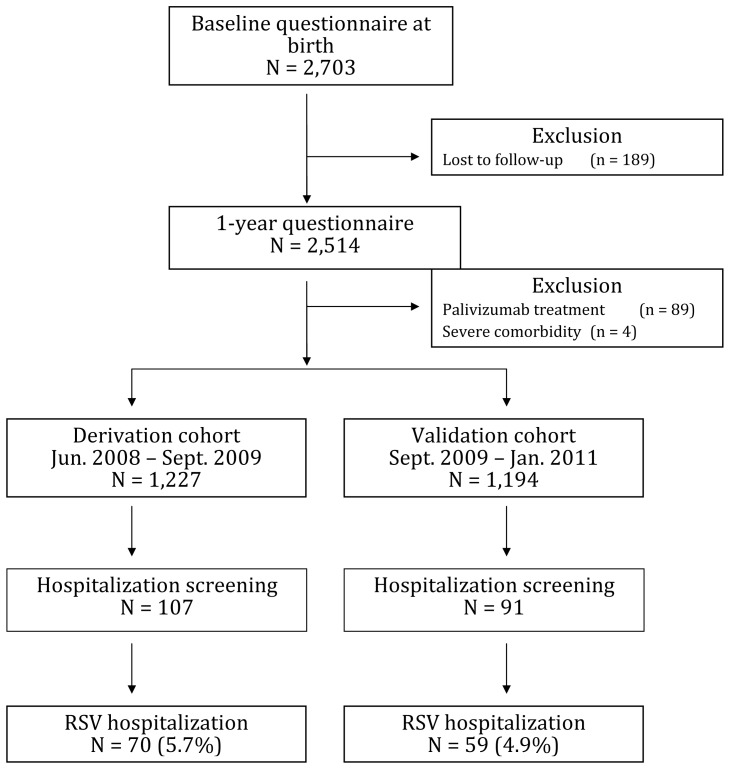
In total, 2,703 infants born in the 41 participating hospitals were included (figure 1, table 1); 186 infants (7%) were lost to follow-up after a year. Three infants died of RSV-unrelated causes. Of the 2,514 included infants, 198 parents reported hospitalization for respiratory tract symptoms during the first year of life and these were verified through hospital medical records. For these 198 hospitalizations, tests for RSV were positive in 129 instances (5.1%) and negative in another 41 (1.6%). Testing for RSV was not performed in 28 cases.
Figure 1. Patient flowchart derivation and validation cohort.
Table 1. Distribution of Baseline Patient Characteristics in the Derivation and Validation Cohort (Number(percentage)).
| Derivation cohort (n = 1,227) | Validation cohort (n = 1,194) | |
| Male gender | 676 (55.1%) | 659 (55.2%) |
| Gestational age (wk) | 34 | 34 |
| 32 | 115 (9.4%) | 124 (10.4%) |
| 33 | 296 (24.1%) | 240 (20.1%) |
| 34 | 371 (30.2%) | 429 (35.9%) |
| 35 | 445 (36.3%) | 401 (33.6%) |
| Birth Weight (g) (Mean(SD)) | 2214 (452) | 2225 (427) |
| Multiple pregnancy | 426 (34.7%) | 422 (35.3%) |
| Caesarean section | 409 (33.3%) | 436 (36.5%) |
| Continuous positive airway pressure | 166 (13.5%) | 217 (18.2%) |
| Mechanical ventilation* | 46 (3.7%) | 35 (2.9%) |
| Born Aug 14 th to Dec 1 st | 324 (26.4%) | 496 (41.5%) |
| Breastfeeding** less than 2months or not # | 416 (33.9%) | 376 (31.5%) |
| Presence of siblings | 504 (41.1%) | 463 (38.8%) |
| Atopy in 1st degree family member | 642 (52.3%) | 729 (61.1%) |
| Fur bearing pets | 571 (46.5%) | 548 (45.9%) |
| Maternal smoking during pregancy | 164 (13.4%) | 136 (11.4%) |
| Subject daycare attendance # | 730 (59.5%) | 714 (59.8%) |
| Number of house hold residents (Median (95%CI)) | 3 (2–4) | 3 (2–4) |
| Siblings or subject daycare attendance | 959 (78.2%) | 918 (76.9%) |
No infants developed BPD ** either exclusive breastfeeding or mixed with formula feeding # predicted by parents at birth.
Derivation of the prediction rule
Table 2 shows the distribution of potential predictors of RSV bronchiolitis. In the derivation cohort we updated a previously published prediction rule.[24] Of the seven predictors in this original model the following four variables ‘birth within 10 weeks of the start of the season,’ ‘breast-feeding ≤2 months’, ‘number of siblings ≥2 years of age’, ‘number of family members with atopy’, contributed significantly. Updating the model by adjusting the four original variables to increase discrimination and by stepwise backward selection in the derivation cohort resulted in the final 4-variable model including ‘born Aug 14 th to Dec 1 st’, ‘presence of siblings or day care attendance’, ‘atopy in a 1st degree family member’ and ‘breast-feeding ≤2 months’. The AUROC of this updated model was 0.703 (95% CI 0.64–0.76) before bootstrapping and 0.702 (0.64–0.76) afterwards (Table 3). We used point values generated from the five times multiplied and rounded regression coefficients to develop a score. We entered the scores of each patient in a logistic regression model to generate the individual predicted probability of RSV hospitalization. For scores ≥16 mean predicted probabilities were 10.0% (95% CI 7.0–14.2%) versus 3.5% in scores <16.
Table 2. Distribution of potential predictors across cases and non-cases in the derivation and validation cohort.
| Derivation cohort (n = 1,227) | Validation cohort (n = 1,194) | |||
| Characteristic (Number (%)) | RSV hospitalization (n = 70) | Controls (n = 1,157) | RSV hospitalization (n = 59) | Controls (n = 1,135) |
| Born Aug 14 th to Dec 1 st | 32 (45.7%) | 292 (25.2%) | 35 (59.3%) | 461 (40.6%) |
| Gestational age (weeks) (Median (95%CI)) | 34 (32−35) | 34 (32−35) | 34(32−35) | 34 (32−35) |
| Birth weight, gr (Mean (SD)) | 2216 (483) | 2214 (450) | 2215 (395) | 2200 (428) |
| Breast fed* ≤ 2 months or not# | 32 (45.7%) | 384 (33.2%) | 20 (33.9%) | 356 (31.4%) |
| Presence of siblings | 46 (65.7%) | 458 (39.6) | 33 (55.9%) | 430 (37.9%) |
| Atopy in 1st degree family member | 46 (65.7%) | 596 (51.5%) | 41 (69.5%) | 688 (60.6%) |
| Male gender | 39 (55.7%) | 637 (55.1%) | 29 (49.2%) | 630 (55.5%) |
| Fur bearing pets | 27 (38.6%) | 544 (47.0%) | 22 (37.3%) | 526 (46.3%) |
| Maternal smoking during pregancy | 11 (15.7%) | 153 (13.2%) | 9 (15.3%) | 127 (11.2%) |
| Subject daycare attendance# | 47 (67.1%) | 683 (59.0%) | 41 (70.7%) | 673 (59.4%) |
| Number of residents | 3.1 (0.84) | 2.8 (0.80) | 3.0 (0.80) | 3.0 (0.80) |
| Siblings or subject daycare attendance | 66 (94.3%) | 893 (77.2%) | 55 (93.2%) | 863 (76.0%) |
| Multiple birth | 25 (35.7%) | 401 (34.7%) | 14 (23.7%) | 408 (36.1%) |
either exclusive breastfeeding or mixed with formula feeding # predicted by parents at birth.
Table 3. Results of the multivariable logistic regression analyses in the derivation cohort (n = 1227) and the performance of the model in the validation cohort (n = 1194): predictors for RSV hospitalization after bootstrapping.
| Characteristics | RISK model | RISK point score | ||
| Regression coefficient | Odds ratio (95% CI) | p-value | ||
| Born Aug 14 th to Dec 1 st | 0.96 | 2.6 (1.6−4.2) | <0.001 | 5 |
| Presence of siblings or subject daycare attendance # | 1.65 | 4.7 (1.7−13.1) | 0.003 | 8 |
| Breast fed * 2months or not # | 0.51 | 1.7 (1.0−2.7) | 0.04 | 3 |
| Atopy in 1st degree family member | 0.67 | 1.9 (1.1−3.2) | 0.01 | 3 |
| Intercept | −4.20 | |||
| AUROC (95%CI) derivation cohort | 0.702 (0.64−0.76) | |||
| AUROC (95%CI) validation cohort | 0.678 (0.61−0.74) | |||
either exclusive breastfeeding or mixed with formula feeding # predicted by parents at birth.
Validation of the prediction rule
In our independent validation sample, the updated prediction rule demonstrated satisfactory discrimination (AUROC, 0.678; 95% CI 0.61–0.74) (Table 3). In the calibration plot, the intercept was 0.0, the slope was 1.0, indicating good calibration. The Hosmer-Lemeshow test resulted in a p-value of 0.26, and the average absolute difference in predicted and calibrated probabilities was 0.008. We calculated sensitivity, specificity and diagnostic likelihood ratios for each score defined as high-risk categories (Table 4). Using a threshold score ≥16 we observed that 27 infants (positive predictive value 10%) were hospitalized for RSV bronchiolitis in the validation cohort. We calculated the following other characteristics of the RISK prediction rule: negative predictive value of 96%, sensitivity of 46% (95% CI 34–58%), a specificity of 79% (95% CI 76–81%), a positive likelihood ratio of 2.1 (95% CI 1.6–2.9) and a negative likelihood ratio of 0.7 (95% CI 0.5–0.9).
Table 4. Operating Characteristics for Each Threshold of the RISK model in the validation cohort (n = 1194).
| RISK score | ||||
| ≥ 8 | ≥11 | ≥16 | ≥19 | |
| True positive | 56 (4.7%) | 53 (4.4%) | 27 (2.3%) | 8 (0.7%) |
| False positive | 957 (80%) | 745 (62%) | 243 (20%) | 62 (5.0%) |
| True negative | 178 (15%) | 390 (33%) | 892 (75%) | 1073 (90%) |
| False negative | 3 (0.2%) | 6 (0.5%) | 32 (2.6%) | 51 (5.0%) |
| Sensitivity | 0.95 | 0.90 | 0.46 | 0.14 |
| Specificity | 0.16 | 0.34 | 0.79 | 0.95 |
| Positive likelihood ratio | 1.1 | 1.4 | 2.1 | 2.5 |
| Negative likelihood ratio | 0.3 | 0.3 | 0.7 | 0.9 |
Discussion
We showed that the overall RSV hospitalization risk was 5.1% in this population of healthy late preterm infants 33–35 WGA. As far as we are aware, this is the first prospective validation study for RSV hospitalization in late preterm infants. The sample size was large enough for both updating and validating the updated prediction rule. The 4-variable prediction rule can be used to further target preventive interventions at those infants who have the highest risk for hospitalization caused by RSV infection.
Two previous studies described prediction rules for RSV hospitalization in late preterm infants.[23], [24] The group of Figueras-Aloy developed a 7-variable prediction rule for RSV hospitalization in a group of late preterms born between 33–35 weeks of gestation. This model was retrospectively validated in French, Italian and Danish cohort studies or case-control studies.[30]–[33] We updated the Spanish prediction rule aiming to produce a model which is both valid and practical in clinical use. The predictors in our prediction rule are also in agreement with a Canadian prediction model.[31] This model was retrospectively validated in the case-control study used to develop the Spanish prediction rule.[23] Although the Canadian study has not been prospectively validated, this study is used for targeted prophylaxis in Canada. The performance of the RISK prediction model is remarkably similar to the actual impact of the Canadian model as it targets 22% of the late preterm cohort which is comparable to the performance of the prediction rule used in Canada which targets 18% of late preterms of 33–35 WGA.[31]
The major strengths of our study include: that data from 2 large prospective cohorts were collected allowing further validation of an existing RSV prediction rule, the retrieval of complete baseline data, and palivizumab was used by less than 5% in our study population because it is not reimbursed. The majority of infants who received palivizumab in our study population had either a congenital anomaly or chronic lung disease. Some potential limitations included the following. First, an underestimation of RSV hospitalization may have occurred, because not all infants hospitalized for respiratory tract infections were routinely tested. Underestimation of the risk of RSV hospitalization is unlikely to have affected the AUROC of the prediction rule, but would result in an underestimation of the positive predictive value. Second, of all infants with a score <16, 3.5% will be hospitalized for RSV bronchiolitis while not classified as high risk. Third, 6.1% of parents could not be contacted after 1 year despite attempts to obtain contact details via the hospital, general practitioner or a web-based search and this could be a potential selection bias. Since the vast majority of parents were contacted we believe this does not significantly jeopardize the conclusions of this study. Fourth, this study does not answer the on-going question of cost-effectiveness of RSV immunoprophylaxis in late preterm infants.[13], [14], [34]–[39] Conflicting reports on this matter have recently been published.[36], [40]–[42] However, applying the RISK prediction rule will certainly improve cost-effectiveness of RSV prophylaxis. Five, because there is no gold standard for RSV prediction we were unable to assess the criterion validity of the RISK prediction model. Content, construct and face validity were accounted for because our analyses covered all relevant RSV risk factors and the outcome of our model is based on laboratory confirmed RSV hospitalizations. Since we externally validated the prediction model in a prospective and independent second cohort we believe the model was sufficiently validated.
The RISK prediction model incorporates four simple clinical variables which combined can be used for risk stratification in the birth period among late preterm infants. The RISK model provides an important foundation for targeted prevention for those infants most at risk for severe RSV disease. With the RISK prediction rule a high risk group can be identified with a hospitalization risk >10% which is comparable to the hospitalization risk in preterm infants <32 weeks gestational age and other high risk groups.[6], [7] If a risk score of 16 is applied, then infants with a risk score exceeding this threshold comprise 22% of all preterm infants 33–35 weeks gestational age. By targeting only 22% of this large birth cohort of late preterm infants for prophylaxis, the potential impact of our model is not dissimilar to the Canadian findings.[31] Future research should focus on the confirmation of the impact of the RISK prediction rule during implementation in clinical guidelines.
Conclusion
The risk of hospitalization for RSV bronchiolitis in late preterms is 5.1%. The RISK prediction rule is a simple clinical rule identifying a subgroup of 33–35 WGA late preterm infants with increased risk of hospitalization for RSV bronchiolitis. Implementation of the RISK prediction rule will further improve cost-effectiveness of RSV prophylaxis.
Acknowledgments
We thank Niek Achten, Koos Korsten, Katrien Castelijns and Annemiek Nooijer who helped in data collection. We also thank the infants and their families who graciously provided their time and effort to participate in this study.
Contributors
We thank all collaborating nurses, nurse practitioners, supporting staff and pediatricians in the hospitals of the Dutch RSV Neonatal Network who collaborated in the study: M.A. Breukels, Elkerliek hospital Helmond; M.M. Pestman-Harms, St. Anna hospital Geldrop; M. Westra, Zaans Medical Center Zaandam; A.O.A. Adeel, Kennemer Gasthuis Haarlem; J.T.C.M Verhallen, St. Franciscus Gasthuis Rotterdam; J.G.C.M. van Zoest, Diaconessenhuis Leiden; H. Rijk-van Gent, Antonius hospital Sneek; G.W. ten Tusscher, Westfries Gasthuis Hoorn; A.E. Hoffman-van der Meer, St. Franciscus hospital Roosendaal; C.C.J.M. Smeets, St. Elisabeth hospital Tilburg; E.H.G. van Leer, Groene Hart hospital Gouda; A.A.M.W. van Kempen, Onze Lieve Vrouwe Gasthuis Amsterdam; R. van Gent, Máxima Medical Center Veldhoven; C.J. Miedema, Catharina hospital Eindhoven; E.P. de Groot, Isala Klinieken Zwolle; A.H.P.M. Essink, Jeroen Bosch hospital ‘s Hertogenbosch; G.J. Blok, Medical Center Alkmaar;. J.C. Smal, Hospital Rijnstate Arnhem; M.J. Oele, Medical Center Haaglanden Westeinde Den Haag; L.H. van der Meer-Kappelle, Reinier de Graaf Gasthuis Delft; T. Hubregtse, Laurentius hospital Roermond; M.A.M. Jacobs, Slingeland hospital Doetinchem; T.E. Faber, Medical Center Leeuwarden; J.P. de Winter, Spaarne hospital Hoofddorp; A.C.M. van Kessel, Diaconessenhuis Meppel; P.P.R. Rosias, Orbis Medical Center Sittard; S.L. Schellekens, Bernhoven hospital Veghel; K.D. Liem, University Medical Center St. Radboud; A.J. Sprij, Juliana Children’s Hospital Den Haag; P.W.T. van Rijssel, Maashospital Pantein Boxmeer; I.A. von Rosenstiel, Slotervaart hospital Amsterdam; E.I.M. Blankman, Bovenij hospital Amsterdam; J.J.B. Rehbock, Lange Land hospital Zoetermeer; C.G. Massar, Waterland hospital Purmerend; C.L.M. Geesing, Bernhoven hospital Oss; W.F. Heikens, Hospital Tjongerschans Heerenveen; J.C. Bakker, Streekhospital Midden-Twente Hengelo; K.M. Dolman, St. Lucas Andreas hospital Amsterdam; J.H. Kreijen-Meinesz, Bronovo hospital Den Haag; E.A. Smit-Kleinlugtenbeld, Albert Schweitzer hospital Dordrecht
Funding Statement
This investigator driven study was funded by Abbott. This study is an investigator driven study, which means generation of the study hypothesis, study design, data collection, analysis and interpretation, decision to publish and the preparation of the manuscript were performed independently by the researchers. No honorarium, grant or other form of payment was given to anyone to produce the manuscript. Netherlands Organization for Health Research and Development (ZonMw), NWO-AGIKO grant 920-035-89 (M.O. Blanken). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bos JM, Rietveld E, Moll HA, Steyerberg EW, Luytjes W, et al. (2007) The use of health economics to guide drug development decisions: Determining optimal values for an RSV-vaccine in a model-based scenario-analytic approach. Vaccine 25: 6922–6929. [DOI] [PubMed] [Google Scholar]
- 2. Jansen AG, Sanders EA, Hoes AW, van Loon AM, Hak E (2007) Influenza- and respiratory syncytial virus-associated mortality and hospitalisations. Eur Respir J 30: 1158–1166. [DOI] [PubMed] [Google Scholar]
- 3. Rietveld E, De Jonge HC, Polder JJ, Vergouwe Y, Veeze HJ, et al. (2004) Anticipated costs of hospitalization for respiratory syncytial virus infection in young children at risk. PIDJ 23: 523–529. [DOI] [PubMed] [Google Scholar]
- 4. Smyth RL, Openshaw PJ (2006) Bronchiolitis. Lancet 368: 312–322. [DOI] [PubMed] [Google Scholar]
- 5. Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, et al. (2009) The burden of respiratory syncytial virus infection in young children. N Engl J Med 360: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bloemers BL, van Furth AM, Weijerman ME, Gemke RJ, Broers CJ, et al. (2007) Down syndrome: a novel risk factor for respiratory syncytial virus bronchiolitis--a prospective birth-cohort study. Pediatrics 120: e1076–e1081. [DOI] [PubMed] [Google Scholar]
- 7. Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR (2000) Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr 137: 865–870. [DOI] [PubMed] [Google Scholar]
- 8. Weisman LE (2003) Populations at risk for developing respiratory syncytial virus and risk factors for respiratory syncytial virus severity: infants with predisposing conditions. PIDJ 22: S33–S37. [DOI] [PubMed] [Google Scholar]
- 9. Welliver RC (2003) Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr 143: S112–S117. [DOI] [PubMed] [Google Scholar]
- 10. Lenney W, Boner AL, Bont L, Bush A, Carlsen KH, et al. (2009) Medicines used in respiratory diseases only seen in children. Eur Respir J 34: 531–551. [DOI] [PubMed] [Google Scholar]
- 11. Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, et al. (2003) Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 143: 532–540. [DOI] [PubMed] [Google Scholar]
- 12. The IMpact-RSV study group (1998) Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102: 531–537. [PubMed] [Google Scholar]
- 13. Wang D, Cummins C, Bayliss S, Sandercock J, Burls A (2008) Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: A systematic review and economic evaluation. Health Technol Assess 12. [DOI] [PubMed] [Google Scholar]
- 14. Wang D, Bayliss S, Meads C (2011) Palivizumab for immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high-risk infants and young children: a systematic review and additional economic modelling of subgroup analyses. Health Technol Assess 15: iii–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carbonell-Estrany X, Figueras-Aloy J, Law BJ (2004) Identifying risk factors for severe respiratory syncytial virus among infants born after 33 through 35 completed weeks of gestation: different methodologies yield consistent findings. PIDJ 23: S193–S201. [DOI] [PubMed] [Google Scholar]
- 16. Chantry CJ, Howard CR, Auinger P (2006) Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics 117: 425–432. [DOI] [PubMed] [Google Scholar]
- 17. Doering G, Gusenleitner W, Belohradsky BH, Burdach S, Resch B, et al. (2006) The risk of respiratory syncytial virus-related hospitalizations in preterm infants of 29 to 35 weeks' gestational age. PIDJ 25: 1188–1190. [DOI] [PubMed] [Google Scholar]
- 18. Houben ML, Bont L, Wilbrink B, Belderbos ME, Kimpen JL, et al. (2011) Clinical prediction rule for RSV bronchiolitis in healthy newborns: prognostic birth cohort study. Pediatrics 127: 35–41. [DOI] [PubMed] [Google Scholar]
- 19. Lanari M, Giovannini M, Giuffre L, Marini A, Rondini G, et al. (2002) Prevalence of respiratory syncytial virus infection in Italian infants hospitalized for acute lower respiratory tract infections, and association between respiratory syncytial virus infection risk factors and disease severity. Pediatr Pulmonol 33: 458–465. [DOI] [PubMed] [Google Scholar]
- 20. Law BJ, Langley JM, Allen U, Paes B, Lee DS, et al. (2004) The Pediatric Investigators Collaborative Network on Infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. PIDJ 23: 806–814. [DOI] [PubMed] [Google Scholar]
- 21. Sinha A, Madden J, Ross-Degnan D, Soumerai S, Platt R (2003) Reduced risk of neonatal respiratory infections among breastfed girls but not boys. Pediatrics 112: e303. [DOI] [PubMed] [Google Scholar]
- 22. Ferrero F, Nascimento-Carvalho CM (2012) Clinical prediction rules and pediatric infectious diseases. Pediatr Infect Dis J 31: 628–629. [DOI] [PubMed] [Google Scholar]
- 23. Sampalis JS, Langley J, Carbonell-Estrany X, Paes B, O'Brien K, et al. (2008) Development and validation of a risk scoring tool to predict respiratory syncytial virus hospitalization in premature infants born at 33 through 35 completed weeks of gestation. Med Decis Making 28: 471–480 0272989X08315238 [pii];10.1177/0272989X08315238 [doi]. [DOI] [PubMed] [Google Scholar]
- 24. Simoes EA, Carbonell-Estrany X, Fullarton JR, Liese JG, Figueras-Aloy J, et al. (2008) A predictive model for respiratory syncytial virus (RSV) hospitalisation of premature infants born at 33–35 weeks of gestational age, based on data from the Spanish FLIP Study. Respir Res 9: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 26.Hosmer DW, Lemeshow S (1989) Applied Logistic Regression. New York: John Wiley & Sons, Inc. [Google Scholar]
- 27. Janssen KJ, Vergouwe Y, Kalkman CJ, Grobbee DE, Moons KG (2009) A simple method to adjust clinical prediction models to local circumstances. Can J Anaesth 56: 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, et al. (2001) Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 54: 774–781. [DOI] [PubMed] [Google Scholar]
- 29. Steyerberg EW, Neville BA, Koppert LB, Lemmens VE, Tilanus HW, et al. (2006) Surgical mortality in patients with esophageal cancer: development and validation of a simple risk score. J Clin Oncol 24: 4277–4284. [DOI] [PubMed] [Google Scholar]
- 30. Stensballe LG, Fullarton JR, Carbonell-Estrany X, Simoes EA (2010) Population based external validation of a European predictive model for respiratory syncytial virus hospitalization of premature infants born 33 to 35 weeks of gestational age. Pediatr Infect Dis J 29: 374–376. [DOI] [PubMed] [Google Scholar]
- 31. Paes B, Steele S, Janes M, Pinelli J (2009) Risk-scoring tool for respiratory syncytial virus prophylaxis in premature infants born at 33–35 completed weeks' gestational age in Canada. Curr Med Res Opin 25: 1585–1591. [DOI] [PubMed] [Google Scholar]
- 32. Carbonell-Estrany X, Simoes EA, Fullarton JR, Ferdynus C, Gouyon JB (2010) Validation of a model to predict hospitalization due to RSV of infants born at 33–35 weeks' gestation. J Perinat Med 38: 411–417. [DOI] [PubMed] [Google Scholar]
- 33. Simoes EA, Carbonell-Estrany X, Fullarton JR, Rossi GA, Barberi I, et al. (2011) European risk factors' model to predict hospitalization of premature infants born 33–35 weeks' gestational age with respiratory syncytial virus: validation with Italian data. J Matern Fetal Neonatal Med 24: 152–157. [DOI] [PubMed] [Google Scholar]
- 34. Carbonell-Estrany X, de Mercado PL (2009) Health economics and RSV. Paediatr Respir Rev Jun; 10 Supll 1: 12–13. [DOI] [PubMed] [Google Scholar]
- 35. Resch B, Sommer C, Nuijten MJ, Seidinger S, Walter E, et al. (2012) Cost-effectiveness of palivizumab for respiratory syncytial virus infection in high-risk children, based on long-term epidemiologic data from Austria. Pediatr Infect Dis J 31: e1–e8. [DOI] [PubMed] [Google Scholar]
- 36. Lanctot KL, Masoud ST, Paes BA, Tarride JE, Chiu A, et al. (2008) The cost-effectiveness of palivizumab for respiratory syncytial virus prophylaxis in premature infants with a gestational age of 32–35 weeks: A Canadian-based analysis. Curr Med Res Opin 24: 3223–3237. [DOI] [PubMed] [Google Scholar]
- 37. Prescott WA Jr, Doloresco F, Brown J, Paladino JA (2010) Cost effectiveness of respiratory syncytial virus prophylaxis: a critical and systematic review. Pharmacoeconomics 28: 279–293. [DOI] [PubMed] [Google Scholar]
- 38. Krilov LR, Weiner LB, Yogev R, Fergie J, Katz BZ, et al. (2009) The 2009 COID recommendations for RSV prophylaxis: issues of efficacy, cost, and evidence-based medicine. Pediatrics 124: 1682–1684. [DOI] [PubMed] [Google Scholar]
- 39. Meissner HC, Bocchini JA Jr, Brady MT, Hall CB, Kimberlin DW, et al. (2009) The role of immunoprophylaxis in the reduction of disease attributable to respiratory syncytial virus. Pediatrics 124: 1676–1679. [DOI] [PubMed] [Google Scholar]
- 40. Elhassan NO, Sorbero MES, Hall CB, Stevens TP, Dick AW (2006) Cost-effectiveness analysis of palivizumab in premature infants without chronic lung disease. Arch Pediatr Adolesc Med 160: 1070–1076. [DOI] [PubMed] [Google Scholar]
- 41. Joffe S, Ray GT, Escobar GJ, Black SB, Lieu TA (1999) Cost-effectiveness of respiratory syncytial virus prophylaxis among preterm infants. Pediatrics 104: 419–427. [DOI] [PubMed] [Google Scholar]
- 42. Nuijten M, Lebmeier M, Wittenberg W (2009) Cost effectiveness of palivizumab for RSV prevention in high-risk children in the Netherlands. J Med Econ [DOI] [PubMed] [Google Scholar]