Abstract
BACKGROUND & AIMS
Colorectal cancer (CRC) screening with colonoscopy often requires expensive copayments from patients. The 2010 Patient Protection and Affordable Care Act mandated elimination of copayments for CRC screening, including colonoscopy, but little is known about the effects of copayment elimination on use. The University of Texas employee, retiree, and dependent health plan instituted and promoted a waiver of copayments for screening colonoscopies in fiscal year (FY) 2009; we examined the effects of removing cost sharing on colonoscopy use.
METHODS
We conducted a retrospective cohort study of 59,855 beneficiaries of the University of Texas employee, retiree, and dependent health plan, associated with 16 University of Texas health and nonhealth campuses, ages 50 – 64 years at any point in FYs 2002–2009 (267,191 person-years of follow-up evaluation). The primary outcome was colonoscopy incidence among individuals with no prior colonoscopy. We compared the age- and sex-standardized incidence ratios for colonoscopy in FY 2009 (after the copayment waiver) with the expected incidence for FY 2009, based on secular trends from years before the waiver.
RESULTS
The annual incidence of colonoscopy increased to 9.5% after the copayment was waived, compared with an expected incidence of 8.0% (standardized incidence ratio, 1.18; 95% confidence interval, 1.14 –1.23; P < .001). After adjusting for age, sex, and beneficiary status, the copayment waiver remained significantly associated with greater use of colonoscopy, with an adjusted hazard ratio of 1.19 (95% confidence interval, 1.12–1.26).
CONCLUSIONS
Waiving copayments for colonoscopy screening results in a statistically significant, but modest (1.5%), increase in use. Additional strategies beyond removing financial disincentives are needed to increase use of CRC screening.
Keywords: Colorectal Neoplasm, Cost Sharing, Early Detection, Colon Cancer
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States, resulting in treatment costs of more than $6.5 billion annually.1,2 Much of the morbidity, mortality, and costs associated with CRC may be preventable with effective screening, but screening participation remains suboptimal at 63% nationally.2 Barriers to screening may include limited access to care and failure of a physician to recommend screening.3
Financial costs to patients may be another major barrier to screening. Colonoscopy, the most commonly recommended test, usually involves heavy cost sharing,4 in which the copay can be $750 or more. This substantial financial disincentive could dampen patients willingness to undergo colonoscopy. Indeed, prior work has shown that cost sharing may reduce preventive health care use, including use of screening tests such as mammography, Papanicolaou (PAP) smear, and colonoscopy.5–7
To address financial barriers and boost CRC screening, the recently enacted Patient Protection and Affordable Care Act (ACA) mandates that insurers “not impose any cost-sharing requirements for [preventive] services that have in effect a rating of ‘A’ or ‘B’ in the current recommendations of the United States Preventive Services Task Force.”8 This mandate has been in effect since September 23, 2010, for all new private group and individual insurance plans, since January 1, 2011, for all Medicare beneficiaries, and will take effect in 2013 for Medicaid programs.9 For CRC screening, the United States Preventive Services Task Force recommends colonoscopy every 10 years for individuals age 50–75 at average risk for CRC.10 Thus, the ACA legislation has mandated waiver of copays for screening colonoscopies.
Although prior studies have shown that requiring copays reduces use of preventive health care, to date, there are limited empiric data on the extent to which removing copays will increase rates of colonoscopy when a copay previously was required.5–7,11,12 For patients who have had access to colonoscopy screening through insurance but with a cost-sharing requirement, it is unknown whether the primary reason for nonadherence to colonoscopy among persons not up to date with screening is cost sharing, or other factors such as time away from work and test invasiveness.13 Specifically, it is possible that implementing a cost-sharing waiver will simply provide savings to patients motivated to participate in screening who would have had colonoscopy with or without financial incentives to do so. Further, because of the high cost of removing copays, policies that waive them could result in substantial costs to insurers and society.
We sought to assess the early expected benefits of waiving the copay on colonoscopy use by taking advantage of a natural experiment in which the large health plan serving all University of Texas System employees, retirees, and dependents removed cost sharing for screening colonoscopy. We hypothesized that offering colonoscopy at no cost to beneficiaries would increase the rate of colonoscopy completion.
Methods
Study Setting and Data Source
We conducted a retrospective cohort study of individuals enrolled in the University of Texas employee, retiree, and dependent health plan (UT SELECT), a large health insurance plan that provides coverage for all employees of the University of Texas System and their beneficiaries. The University of Texas System includes 16 institutions of higher education across Texas, including 5 medical schools. A medical claims database maintained by UT SELECT for purposes of care delivery and billing was the primary data source for this study.
Colonoscopy Copay Waiver
To boost use of screening colonoscopy, beginning fiscal year (FY) 2009 (September 1, 2008 onward), the UT Office of Employee Benefits instructed the UT SELECT health plan to waive the copay for all screening colonoscopies. The copay waiver benefit was promoted in multiple ways, including through the following: (1) monthly e-mail newsletters from July 2008 to January 2009, (2) annual benefit enrollment campaign brochures distributed to all employees, (3) annual employee benefit fairs held at all UT campuses, and (4) a front-page story in the 2009 employee benefits mailer. The copay for colonoscopy before FY 2009 was $750 or higher.
Study Subjects
We extracted de-identified health claims data for beneficiaries ages 50 – 64 covered by UT SELECT at any point during fiscal years 2002–2009, defined as September 1, 2001, to August 31, 2009. Thus, data on colonoscopy use were available for a 7-year period before institution of the colonoscopy copay waiver, as well as for a 1-year period after the waiver. Individuals younger than age 50 years were excluded because colonoscopy is not recommended routinely for screening this group.10 We excluded individuals older than age 64 because they usually are covered by Medicare insurance.
Measurements
We extracted data on sex, age, UT employment campus (health vs nonhealth campus), status as either a primary beneficiary (employee) or secondary beneficiary (dependent such as husband, wife, or child), and colonoscopy use. Colonoscopy use was determined by the presence of a Current Procedural Terminology (CPT) code for colonoscopy. To facilitate sensitivity analyses excluding patients who may have had diagnostic colonoscopy for work-up of signs or symptoms of CRC, rather than screening colonoscopy, we queried for the presence of International Classification of Diseases 9th edition coding consistent with any of the following symptoms and diagnoses: blood in stool, iron-deficiency anemia, weight loss, change in bowel habits, abdominal pain, colorectal cancer, colon polyp, ulcerative colitis, Crohn’s disease, diverticulitis, and diverticulosis. To compare colonoscopy use trends with secular trends for other common cancer screening tests, as well as with diagnostic tests that should not have been affected by the colonoscopy copay waiver, we also queried for the presence of CPT coding consistent with any of the following medical tests: mammography, PAP smear, prostate-specific antigen (PSA) testing, echocardiography, liver biopsy, and upper endoscopy (see Supplementary Table 1 for CPT/International Classification of Diseases 9th edition codes used).
Statistical Analyses
The primary outcome was colonoscopy incidence among individuals with no prior record of colonoscopy. For our primary analysis, we computed the standardized incidence ratio (SIR) of observed colonoscopy incidence in fiscal year 2009 with copay waiver, vs expected colonoscopy incidence for 2009 estimated by the secular trend in colonoscopy incidence for fiscal years 2004 –2008. We chose to compare observed vs expected colonoscopy incidence, recognizing that there has been a consistent secular trend toward increasing annual colonoscopy incidence over time.1,2 Data from 2004 to 2008, rather than the entire period of 2002–2008, were used to compute the expected incidence because we noted the linear trend for the 2004 –2008 period to be distinctly different from that before 2004 (Figure 1). The SIR was age- and sex-standardized using direct standardization to the population distribution of individuals insured by UT SELECT at cohort inception. Specifically, we divided men and women separately into 3 age strata as follows: 50 –54, 55–59, and 60 – 64 years. Within each stratum, a linear trend in incidence rate was assumed and an expected incidence rate was calculated for year 2009. The final expected colonoscopy incidence for 2009 was obtained from combining the subgroup expected standardized incidences.
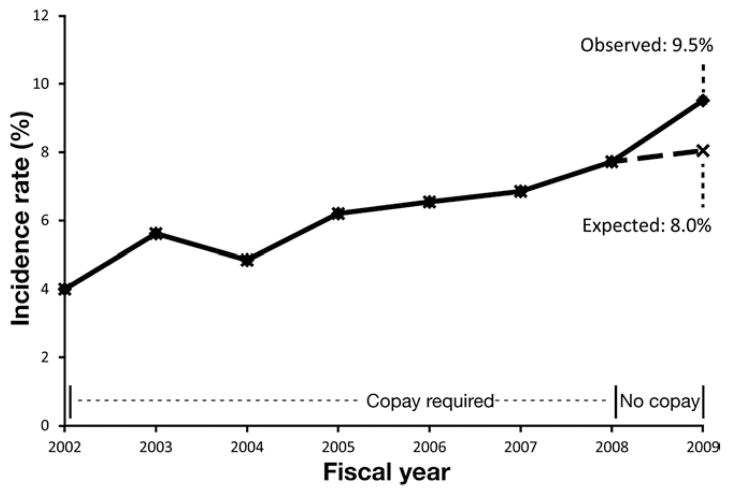
Figure 1.
Colonoscopy incidence before and after the colonoscopy copay waiver. Before institution of the colonoscopy copay waiver in FY 2002–2008, a linear trend in colonoscopy incidence was observed. After the copay waiver in FY 2009, the observed colonoscopy incidence was higher than expected based on prior trends (SIR, 1.18; 95% CI, 1.14 –1.23; P < .001).
To explore the impact of potential confounders of the association between copay waiver and colonoscopy use, we constructed a Cox proportional hazards model, with time to colonoscopy as the dependent variable, and copay waiver in 2009 (coded as a time-varying indicator variable) as the primary exposure variable. Individuals were followed up until incident colonoscopy, or censored at the end of last year of participation in UT SELECT or at the end of FY 2009. Age, sex, beneficiary status (primary vs secondary), and employment at a health campus were included as candidate covariates in the model. We further defined a time-varying variable to adjust for the linear time trend in colonoscopy incidence for FY 2002–2008. Forward selection and backward elimination procedures were used, in which predictor variables with an association of a P value of less than .2 were considered for inclusion, and those with a P value of less than .05 on multivariate analysis were retained in the final model.
In exploratory analyses, we computed the SIR limited to individuals without any of the previously mentioned symptoms or diagnoses that may have prompted a diagnostic, rather than a screening, colonoscopy. Further, an SIR was computed for individuals newly aged 50 in FY 2009 compared with those newly aged 50 in prior years to explore the impact of copay waiver on individuals eligible for CRC screening for the first time.10,14 In addition, we computed the SIR restricting the analysis to individuals continuously enrolled in the insurance program throughout the study follow-up period. Finally, secular trends in colonoscopy incidence were contrasted with secular trends in other common cancer screening tests (mammography, PAP smear, and PSA testing) and diagnostic tests (echocardiography, liver biopsy, and upper endoscopy), which should not have been affected by the colonoscopy copay waiver. For mammography, PAP smear, and PSA testing, no cost sharing has been required for plan members since 2001. Ninety-five percent confidence intervals (CIs) were computed for all rates and ratios, and P values less than .05 were considered statistically significant for all comparisons. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). The study was approved by the UT Southwestern Institutional Review Board.
Results
There were 59,855 beneficiaries with 267,191 person-years of follow-up evaluation in our analytical cohort. Most beneficiaries were younger than age 60 (85.7%), and more than half were women (Table 1). Sixty-six percent of the cohort was insured by UT SELECT for 5 or more years, and half (49.8%) was insured by UT SELECT for the entire follow-up period. Primary beneficiaries accounted for 72.5% of individuals, and 61% of individuals were insured through employment at a campus affiliated with a medical school.
Table 1.
Demographic Characteristics of Study Cohort (N = 59,855)
| % | n | |
|---|---|---|
| Age, y | ||
| 50–54 | 62.1 | 37,173 |
| 55–59 | 23.6 | 14,136 |
| 60–64 | 14.3 | 8546 |
| Sex | ||
| Men | 43.2 | 25,876 |
| Women | 56.8 | 33,979 |
| Beneficiary status | ||
| Primary beneficiary | 72.5 | 43,408 |
| Secondary beneficiary | 27.5 | 16,447 |
| Campusa | ||
| Health campus | 61.0 | 35,501 |
| Nonhealth campus | 39.0 | 22,730 |
NOTE. Based on characteristics for each individual at initiation of the follow-up period.
There were 1624 missing campus data.
Figure 1 depicts the age- and sex-standardized incidence of colonoscopy over time. Before institution of the copay waiver for FY 2002–2008, there was a linear increase in colonoscopy incidence over time of 3.7%. After institution of the copay waiver in FY 2009, the observed colonoscopy incidence was 9.5%, compared with an expected incidence based on the continuation of secular trends of 8.0%, an absolute increase in observed vs expected colonoscopy incidence of 1.49% (95% CI, 1.02–1.95), with an SIR of 1.18 (95% CI, 1.14 –1.23; P < .001; Figure 1). This increase in incidence translates into an estimated absolute increase of 232 incident colonoscopies attributable to the copay waiver of a total 2590 incident colonoscopies in FY 2009.
The copay waiver in FY 2009 similarly was associated with increases in colonoscopy in the Cox proportional hazards models even after adjustment for age, sex, and beneficiary status (adjusted hazard ratio, 1.19; 95% CI, 1.12–1.26; P < .0001; Table 2). In exploratory analyses, the results were not changed after excluding individuals with suspected signs or symptoms that may have triggered a diagnostic colonoscopy (data not shown). Analyses limited to individuals aged 50 who were newly eligible for screening did not show a significant increase in colonoscopy incidence (SIR, 1.07; 95% CI, 0.95–1.20; P >.05). Analysis of individuals aged 50 – 64 continuously enrolled throughout the study follow-up period showed similar results to our primary SIR estimate of colonoscopy use (SIR, 1.16; 95% CI, 1.10 –1.21; n = 32,339).
Table 2.
Factors Independently Associated With Incident Colonoscopy
| Predictor | Adjusted hazard ratioa | 95% CI |
|---|---|---|
| Colonoscopy copay waiver exposure, FY 2009 | 1.19b | 1.16–1.26 |
| Women | 1.16b | 1.12–1.20 |
| Primary beneficiary | 1.05c | 1.01–1.09 |
| Age (in 1-year increments) | 1.02b | 1.02–1.03 |
Estimated hazard ratio from a multivariate model with covariates: exposure to colonoscopy copay waiver, sex, primary beneficiary status, age, and secular trend in colonoscopy incidence.
P < .001.
P = .007.
In contrast to the statistically significant increase in colonoscopy incidence after the copay waiver in FY 2009, there were no significant increases in rates of other types of cancer prevention screening (mammography, PAP smear, and PSA). There were no increases in other common, non–CRC-related, diagnostic tests (echocardiography, liver biopsy, and upper endoscopy) in FY 2009 beyond secular trends (Figures 2 and 3).
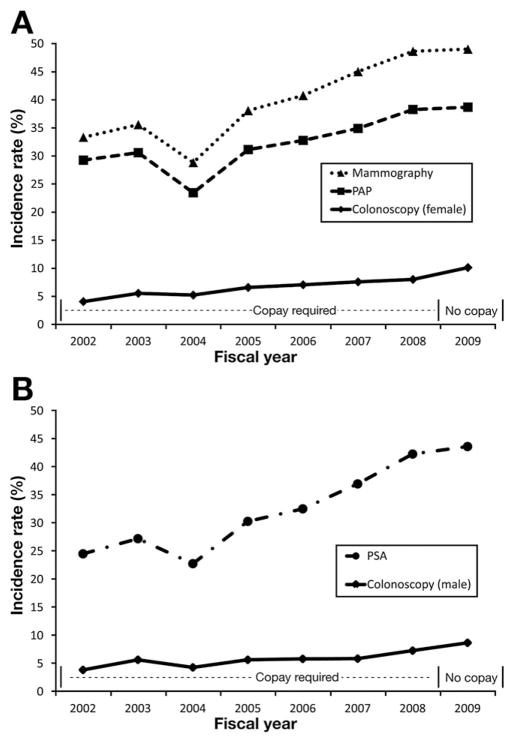
Figure 2.
(A) Colonoscopy, mammography, and PAP smear incidence before and after the colonoscopy copay waiver in FY 2009 among women. A linear increasing trend in test use was observed for all tests. After the colonoscopy copay waiver, the observed vs expected test incidence was higher for colonoscopy (SIR, 1.20; 95% CI, 1.14–1.25), but not mammography (SIR, 0.94; 95% CI, 0.92–0.96) or PAP smear (SIR, 0.96; 95% CI, 0.94 – 0.98). (B) Colonoscopy and PSA incidence before and after the colonoscopy copay waiver in FY 2009 among men. A linear increasing trend in test use was observed for all tests. After the colonoscopy copay waiver, the observed vs expected test incidence was higher for colonoscopy (SIR, 1.17; 95% CI, 1.10–1.24), but not PSA (SIR, 0.95; 95% CI, 0.93–0.98).
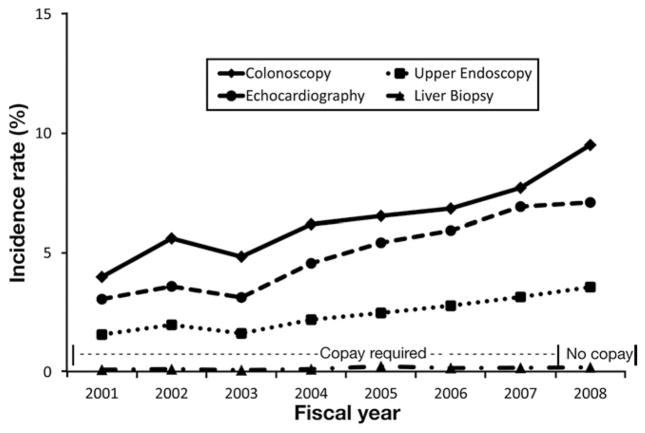
Figure 3.
Colonoscopy, echocardiography, liver biopsy, and upper endoscopy incidence before and after the colonoscopy copay waiver in FY 2009 among all beneficiaries. After the colonoscopy copay waiver, the observed vs expected test incidence was higher for colonoscopy (SIR, 1.18; 95% CI, 1.14 –1.23), but not echocardiography (SIR, 0.94; 95% CI, 0.91– 0.97), liver biopsy (SIR, 0.94; 95% CI, 0.74 –1.18), or upper endoscopy (SIR, 1.04; 95% CI, 0.99 –1.10).
Discussion
We took advantage of a natural experiment in which a large, statewide, employer-sponsored health plan waived copays for screening colonoscopy to understand the impact of such a policy on colonoscopy use. We found that early after the copay waiver, the observed incidence of colonoscopy increased from an expected incidence of 8.0% to 9.5%, an absolute increase of 1.5%, and an 18% relative increase. This statistically significant, but modest, increase in uptake appeared attributable to the copay waiver, not any larger trends in screening for cancer in general or non-CRC test use.
While the impact of imposing cost sharing on use of cancer screening has undergone substantial investigation, we report in detail on impact of cost-sharing removal on participation among patients previously required to cost share for CRC screening with colonoscopy.5–7,11,12,14,15 Somewhat similar to our analysis, Busch et al5 examined changes in use of a basket of preventives, including CRC screening, before and after removal of cost sharing among beneficiaries served by a large employer-sponsored plan. Busch et al5 did not find any increase in CRC screening after waiver of cost sharing. Their negative findings may have been because their study was conducted from 2003 to 2004, a period during which awareness and enthusiasm for CRC screening was lower. In addition, the removal of cost sharing in the Busch et al5 study was accompanied by the countervailing imposition of higher copays for nonpreventive health care visits, during which screening often is discussed. Our study provides evidence that among individuals previously subject to expensive cost sharing for colonoscopy, waiving that financial disincentive to screening can significantly, but modestly, increase uptake.
Our findings may have national public health and financial implications. We estimated that 232 of 2590 incident colonoscopies in FY 2009 were attributable to the copay waiver. Prior studies have suggested that the number needed to screen with endoscopy to detect one patient with advanced neoplasia (colorectal cancer, large polyp, or polyp containing villous histology and/or high grade dysplasia) ranges from 18 to 31 patients,16 and that the numbers needed to prevent a CRC diagnosis and a CRC death are 191 and 489, respectively.17 Thus, we would expect that in just 1 year, at least 18 additional patients would have had advanced colorectal neoplasia detected and at least 1 patient would have had a CRC diagnosis prevented as a result of the copay waiver.
Because waiving expensive cost sharing is itself costly to insurers, the benefits need to be put in context of its costs. From the insurer perspective, the total incremental costs of the copay waiver were substantial, including the following: (1) the copay cost previously incurred by the 2358 patients who would have been screened had the copay remained in effect; and (2) the additional full cost of screening of the incremental 232 patients who responded to the incentive provided by the copay waiver. Assuming that the full cost of a colonoscopy screening is approximately $1750, the marginal cost to the insurer for each screen without the copay waiver would have been $1000, because the patient’s $750 copay would have covered the rest. The total outlay by the insurer for the 2358 patients who would have been expected to undergo a colonoscopy without the copay waiver previously would have been $2,358,000. With the copay waiver, the insurer picked up the full cost for 2590 patients, of which 232 patients made up the increment of new screens induced by the copay waiver. The new total cost to the insurer after the copay waiver was $4,532,500, an additional $2,174,500 to detect an estimated 18 patients with advanced neoplasia ($120,806 per neoplasia detected) and prevent 1 patient from developing cancer ($2,174,500 per cancer prevented). Although these numbers represent only rough estimates, waiving copays, although moderately effective in increasing colonoscopy uptake, appears expensive.
Our results suggest that additional strategies, beyond removing financial disincentives, will be required to optimize CRC screening. Addressing nonfinancial barriers such as patient knowledge of screening through outreach invitations to complete CRC screening, and frequency of physician recommendation of screening, may be synergistic to boosting screening uptake with copay waivers.3 Small positive financial incentives, in addition to removing financial disincentives such as copays, also could improve screening use, and merit study.
Several limitations are worth noting. Our data were from one employer-based health plan in a single state serving academic institutions among individuals aged 50 – 64 years old, which may not be generalizable to other settings and populations. However, the sample was large, and included many diverse regions in the second largest state in the United States. In addition, the UT SELECT plan is similar to most employer-sponsored group health plans. The impact of financial incentives or disincentives also may be greater in older persons more likely to have fixed and lower incomes. We only had data for 1 year after the 2009 copay waiver. Because colonoscopy is recommended only every 10 years for average-risk individuals, a longer time period to assess the impact of an ongoing copay waiver program would be ideal, and ought to be the focus of additional research. In our sensitivity analysis seeking to examine the impact on colonoscopies likely performed for screening, rather than diagnostic, purposes, we did not use formal validated algorithms to separate screening from diagnostic examinations.18 –20 Thus, it is possible that misclassification of screening and diagnostic examinations may have occurred. However, because no published algorithms have greater than 80% sensitivity and specificity for identifying screening colonoscopy, even if we had used one of these algorithms, there would still be residual uncertainty regarding whether examinations were performed for screening, rather than diagnostic purposes.19 Finally, the extrapolations we made regarding the costs and possible cost effectiveness of the copay waiver policy should be seen as rough estimates. More formal cost-effectiveness analyses, particularly taking into account costs of cancer care avoided, are required to understand potential costs and benefits of waiving copays for cancer screening tests such as colonoscopy.
In conclusion, we found that waiving the copay for colonoscopy resulted in a statistically significant increase in use. However, the increase was modest, suggesting that copay waivers mandated by employer groups locally, and now by the ACA nationally, will need to be accompanied by other co-interventions to optimize CRC screening participation. Future research will be needed to rigorously examine both the effectiveness and costs of different strategies for improving CRC screening.
Acknowledgments
The authors would like to thank Lance T. Rowell for providing assistance with data extraction, as well as the UT Central Benefits Office for providing access to study data.
Funding
Supported by the Cancer Prevention and Research Institute of Texas grant PP100039 (S.G., PI); National Institutes of Health grant 1 KL2 RR024983-01 (Milton Packer, MD, PI; S.G., KL2 Scholar), from the National Center for Research Resources, a component of the National Institutes of Health and National Institutes of Health Roadmap for Medical Research; and the National Institutes of Health/National Cancer Institute grant 1U54CA163308-01 (Celette Sugg Skinner, PI; S.G., Co-Investigator).
Abbreviations used in this paper
- ACA
Affordable Care Act
- CI
confidence interval
- CPT
Current Procedural Terminology
- CRC
colo-rectal cancer
- FY
fiscal year
- PAP
Papanicolaou
- PSA
prostate-spe-cific antigen
- SIR
standardized incidence ratio
Footnotes
Conflicts of interest
The authors disclose no conflicts.
The contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or the National Institutes of Health.
Information on the National Center for Research Resources is available at http://www.ncrr.nih.gov/; and information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://doi:10.1016/j.cgh.2012.02.027.
References
- 1.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vital signs: colorectal cancer screening among adults aged 50 –75 years—United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59:808–812. [PubMed] [Google Scholar]
- 3.Holden DJ, Jonas DE, Porterfield DS, et al. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668–676. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 4.Klabunde CN, Riley GF, Mandelson MT, et al. Health plan policies and programs for colorectal cancer screening: a national profile. Am J Manag Care. 2004;10:273–279. [PubMed] [Google Scholar]
- 5.Busch SH, Barry CL, Vegso SJ, et al. Effects of a cost-sharing exemption on use of preventive services at one large employer. Health Aff (Millwood) 2006;25:1529–1536. doi: 10.1377/hlthaff.25.6.1529. [DOI] [PubMed] [Google Scholar]
- 6.Solanki G, Schauffler HH. Cost-sharing and the utilization of clinical preventive services. Am J Prev Med. 1999;17:127–133. doi: 10.1016/s0749-3797(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi AN, Rakowski W, Ayanian JZ. Effect of cost sharing on screening mammography in Medicare health plans. N Engl J Med. 2008;358:375–383. doi: 10.1056/NEJMsa070929. [DOI] [PubMed] [Google Scholar]
- 8.H.R. 3590 -111th Congress: Patient Protection and Affordable Care Act §2713; 2009.
- 9.Cassidy A. Health policy brief: preventive services without cost sharing. Health Aff (Millwood) 2010 Available at: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=37.
- 10.U S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 11.Newhouse JP. Free for all? Lessons from the RAND health insurance experiment. Boston, MA: Harvard University Press; 1993. [Google Scholar]
- 12.Brook RH, Ware JE, Jr, Rogers WH, et al. Does free care improve adults’ health? Results from a randomized controlled trial. N Engl J Med. 1983;309:1426–1434. doi: 10.1056/NEJM198312083092305. [DOI] [PubMed] [Google Scholar]
- 13.Jonas DE, Russell LB, Sandler RS, et al. Patient time requirements for screening colonoscopy. Am J Gastroenterol. 2007;102:2401–2410. doi: 10.1111/j.1572-0241.2007.01387.x. [DOI] [PubMed] [Google Scholar]
- 14.Wharam JF, Galbraith AA, Kleinman KP, et al. Cancer screening before and after switching to a high-deductible health plan. Ann Intern Med. 2008;148:647–655. doi: 10.7326/0003-4819-148-9-200805060-00004. [DOI] [PubMed] [Google Scholar]
- 15.Gruber J. The role of consumer copayments for health care: lessons from the RAND health insurance experiment and beyond; 2010. Menlo Park, CA: The Henry J. Kaiser Family Foundation; 2006. [Google Scholar]
- 16.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 17.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 18.El-Serag HB, Petersen L, Hampel H, et al. The use of screening colonoscopy for patients cared for by the Department of Veterans Affairs. Arch Intern Med. 2006;166:2202–2208. doi: 10.1001/archinte.166.20.2202. [DOI] [PubMed] [Google Scholar]
- 19.Fisher DA, Grubber JM, Castor JM, et al. Ascertainment of colonoscopy indication using administrative data. Dig Dis Sci. 2010;55:1721–1725. doi: 10.1007/s10620-010-1200-y. [DOI] [PubMed] [Google Scholar]
- 20.Haque R, Chiu V, Mehta KR, et al. An automated data algorithm to distinguish screening and diagnostic colorectal cancer endoscopy exams. J Natl Cancer Inst Monogr. 2005;35:116–118. doi: 10.1093/jncimonographs/lgi049. [DOI] [PubMed] [Google Scholar]