Abstract
Multiple signaling pathways participate in the regulation of bone remodeling, and pathological negative balance in the regulation results in osteoporosis. However, interactions of signaling pathways that act comprehensively in concert to maintain bone mass are not fully understood. We investigated roles of parathyroid hormone receptor (PTH/PTHrP receptor) signaling in osteoblasts in unloading-induced bone loss using transgenic mice. Hind limb unloading by tail suspension reduced bone mass in wild-type mice. In contrast, signaling by constitutively active PTH/PTHrP receptor (caPPR), whose expression was regulated by the osteoblast-specific Col1a1 promoter (Col1a1-caPPR), suppressed unloading-induced reduction in bone mass in these transgenic mice. In Col1a1-caPPR transgenic (Tg) mice, hind limb unloading suppressed bone formation parameters in vivo and mineralized nodule formation in vitro similarly to those observed in wild-type mice. In addition, serum osteocalcin levels and mRNA expression levels of type I collagen, Runx2 and Osterix in bone were suppressed by unloading in both wild-type mice and Tg mice. However, in contrast to unloading-induced enhancement of bone resorption parameters in wild-type mice, Col1a1-caPPR signaling suppressed, rather than enhanced, osteoclast number and osteoclast surface as well as urinary deoxypyridinoline excretion upon unloading. Col1a1-caPPR signaling also suppressed mRNA expression levels of RANK and c-fms in bone upon unloading. Although the M-CSF and monocyte chemoattractant protein 1 (MCP-1) mRNA levels were enhanced in control Tg mice, these levels were suppressed in unloaded Tg mice. These results indicated that constitutive activation of PTH/PTHrP receptor signaling in osteoblastic cells suppresses unloading-induced bone loss specifically through the regulation of osteoclastic activity.
Bone mass is strictly maintained through dynamic equilibrium of bone formation and bone resorption under the control of various signals including hormones, cytokines, and mechanical stimuli (1). Combined actions of these multiple signals regulate osteoblasts and osteoclasts responsible for bone formation and bone resorption, respectively. Dysregulation of the balance of bone formation and bone resorption leads to pathological bone loss and results in osteoporosis (2).
Mechanical loading exerts anabolic action on bone and is essential for the integrity of the bone architecture. Loss of mechanical loading (unloading) results in rapid bone loss caused by enhanced bone resorption and simultaneous suppression of bone formation as seen in disuse osteoporosis (3–8). Mechanisms underlying this catabolic balance of bone metabolism are largely unknown. Although inhibitors for bone resorption have been used clinically, they still fail to fully restore bone mass in patients with severe osteoporosis. Hind limb unloading, in which mice are subjected to tail suspension, results in bone loss and is widely used as a model of disuse osteoporosis. In these animal models, inhibitors for bone resorption such as bisphosphonates are able to partially suppress bone loss caused by unloading-induced bone resorption, but these agents do not inhibit unloading-induced suppression of bone formation (9).
One of the possible measures to treat patients suffering from severe disuse osteoporosis could be the use of anabolic agents such as PTH3 to activate bone formation. Intermittent systemic administration of PTH enhances bone formation and exerts anabolic effects on bone, whereas continuous systemic administration of PTH causes bone loss due to enhanced bone resorption (10). PTH/PTHrP receptor signaling is responsible for these diverse effects (11). Jansen-type mutation (H223R) renders constitutive activation of the PTH/PTHrP receptor (12). Cells in the osteoblast lineage express PTH/PTHrP receptor during embryonic and postnatal development. Overexpression of the mutant receptor (H223R) under the control of 2.3-kb mouse Col1a1 promoter, whose activity is directed in mature bone-forming osteoblasts and late stage precursors (pre-osteoblast), reveals anabolic effects in trabecular bone mass and bone formation and bone resorption are both activated in these transgenic mice (13). Forced expression of the constitutively active mutant PTH/PTHrP receptor also activates niche activity for hematopoietic stem cells (14). Thus, constitutively active PTH/PTHrP receptor signaling in osteoblastic cells alters the bone marrow environment (15).
Mechanical stimuli affect cells in the bone microenvironment and appear to be involved in PTH actions. Hind limb unloading alleviates anabolic effects of intermittent systemic PTH administration on bone (16–18). PTH/PTHrP receptor is expressed by relatively mature bone-forming osteoblasts and their precursors. Thus, PTH would exert its effects on bone through the regulation of these cells in osteoblastic lineage. As these cells have been suggested to be targets of mechanical signaling in bone (1, 4, 5), modulation of PTH/PTHrP receptor signaling in these cells would alter the unloading-induced bone phenotypes. Therefore, we examined effects of osteoblast-specific transgenic expression of constitutively active PTH/PTHrP receptor on bone metabolism in mice subjected to tail suspension.
EXPERIMENTAL PROCEDURES
Animals
Col1a1-caPPR transgenic mice in a FVB/N background were previously reported (13). Briefly, a mouse 2.3-kb fragment of Col1a1 promoter was ligated upstream to the entire coding region of the human mutated Jansen-type PTH/PTHrP receptor (HKrk-H223R), along with the cloning vector pcDNA I sequence that contains a poly(A) signal. Transgenic male mice were crossed with female FVB/N mice to generate littermates. 8-Week-old female transgenic mice and their wild-type littermates were used for experiments. Genomic DNA from the tail was used for PCR genotyping, using a forward 5′-GAGTCTACATGTCTAGGGTCTA-3′, and a reverse 5′-TAGTTGGCCCACGTCCTGT-3′ primer under the following conditions: 94 °C for 1 min, 58 °C for 45 s, and 72 °C for 1 min for 35 cycles. All experiments were performed according to institutionally approved guidelines for animal welfare.
Tail Suspension Model
Tail suspension was conducted as described previously (6). A metal paper clip in S-shape was made and one of the two ends was fixed to the tail by adhesive tape. The other end was hung from an overhead cage wire and adjusted to maintain the mice at an ~30 degree head down tilt. Hind limbs were elevated above the floor, whereas forelimbs maintained contact with the cage floor. As the S-shaped paperclip slid freely on the wire, suspended mice were able to move around using their forelimbs. Half of the littermates were subjected to tail suspension for 14 days. Three to four tail-suspended mice were housed per cage. The other half was used as control and housed under similar conditions, but without tail suspension for the same duration. Animals were housed in a temperature controlled room on a 12-h light-dark cycle and allowed to access to food and water ad libitum.
Measurement of Bone Mineral Density
Bone mineral density of the whole femora was measured by dual x-ray absorptiometry using a PIXImus system (GE Lunar, Madison, WI).
Two-dimensional Micro-CT Analysis of Bone
Imaging of distal metaphyses of the femora was performed using a micro-CT apparatus (Musashi, Nittetsu-ELEX Co., Japan). Two-dimensional micro-CT images were analyzed and quantified using an automated image analyzer (Luzex-F, Nireco, Japan). Bone volume/tissue volume (BV/TV) of the secondary trabeculae was measured in an area of 0.49 mm2 with its closest and furthest edges at 0.24 and 0.94 mm, respectively, from the growth plate in the distal ends of the femora. The threshold level for the measurements was set at 110 for analysis.
Histomorphometric Analysis of Bone
Calcein (1.6 mg/kg body weight) was injected intraperitoneally twice, 4 and 2 days before sacrifice. Femora were embedded in methyl methacrylate and 3-μm thick sagittal sections of distal metaphyses and horizontal sections of midshaft regions were prepared. Calcein labeling was visualized using confocal laser microscopy (LSM510, Carl Zeiss, Germany) with an excitation wavelength of 488 nm and a 550-nm band-pass filter. Tibiae were decalcified in 10% EDTA, embedded in paraffin, and 7-μm thick saggital serial sections of proximal metaphyses were made. Tartrate-resistant acid phosphatase (TRAP) staining was used to quantify osteoclast number and osteoclast surface.
Bone Marrow Cell Cultures
Bone marrow cells were flushed out from control and unloaded tibiae and cultured in α-minimal essential medium supplemented with 10% fetal bovine serum. For mineralized nodule formation, bone marrow cells were plated in 12-well plate (Costar) at 3.0 × 106 cells per well and cultured for 21 days in the presence of 50 μg/ml ascorbic acid and 10 mm sodium β-glycerophosphate. The culture medium was changed every 3 days. Alizarin red staining was performed to assess mineral deposition. For TRAP-positive multinucleated cell development, bone marrow cells were plated in a 24-well plate (Costar) at 1.5 × 106 cells per well and cultured for 11 days in the presence of 10 nm 1,25-(OH)2 vitamin D3 and 100 nm dexamethasone. Some of the cultures were carried out in the presence of recombinant mouse M-CSF (30 ng/ml) and monocyte chemoattractant protein 1 (MCP-1) (100 ng/ml) (R&D Systems) during vitamin D3 and dexamethasone-induced osteoclastic development. The number of TRAP-positive multinucleated cells with three nuclei or more was counted. For osteogenic cell cultures, the cells were incubated for 6 days.
Urinary Deoxypyridinoline
Urine samples were individually collected in metabolic cages (Natsume, Japan) during the last 24 h before sacrifice. Urinary deoxypyridinoline levels were measured by enzyme-linked immunosorbent assay (DPD EIA kit, Metra Biosystems).
Serum Osteocalcin
Blood samples were collected immediately after killing the mice and the osteocalcin levels determined in the serum using an IRMA kit (Immutopics).
Quantitative Real-time PCR Analysis
Control and unloaded femora were snap-frozen in liquid nitrogen immediately after excision, crushed, and homogenized in TRIzol reagent (Invitrogen) using a rotary homogenizer (Polytron 3100, Kinematica, Germany). One μg of total RNA was treated with DNase I (Invitrogen) prior to reverse transcription. First-strand cDNA was synthesized using SuperScript II transcriptase and oligo(dT)12–18 primers (Invitrogen). Quantitative real-time PCR analysis was carried out using iCycler (Bio-Rad) and iQ5 data analyzing software. The reaction was performed in a 25-μl reaction mixture containing 2 μl of cDNA samples, 1 μl of sense and antisense primer mixture (5 μM), and 12.5 μl of iQ SYBR Green Supermix. The primer sequences were designed based on the Beacon Designer (Bio-Rad) program, and are listed in Table 1. The PCR conditions were 95 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s for 40–50 cycles.
TABLE 1.
Primer sequences for quantitative real-time PCR
| Gene | Forward | Reverse | Product size |
|---|---|---|---|
| bp | |||
| Col1a1 | 5′-ctgactggaagagcggagag-3′ | 5′-gcacagacggctgagtagg-3′ | 123 |
| Runx2 | 5′-tggcttgggtttcaggttaggg-3′ | 5′-tcggtttcttagggtcttggagtg-3′ | 106 |
| Osterix | 5′-gcaactggctaggtggtggtc-3′ | 5′-gcaaagtcagatgggtaagtaggc-3′ | 115 |
| RANK | 5′-agaagcacaccaggggacaac-3′ | 5′-acagagatgaagaggagcagaacg-3′ | 126 |
| c-fms | 5′-gcctgcctgtaaagtggatgg-3′ | 5′-ccagaggaggatgccgtagg-3′ | 92 |
| RANKL | 5′-gaaactcacagccctctctcttg-3′ | 5′-gcatcggaatacctctcccaatc-3′ | 150 |
| OPG | 5′-tacctggagatcgaattctgctt-3′ | 5′-ccatctggacattttttgcaaa-3′ | 110 |
| M-CSF | 5′-gcttctggagaggctagtgagg-3′ | 5′-agggaatggagatgctgagagg-3′ | 126 |
| MCP-1 | 5′-agagccagacgggaggaagg-3′ | 5′-atgagtagcagcaggtgagtgg-3′ | 193 |
Statistical Analysis
The results were expressed as mean ± S.D. Statistical evaluation was conducted based on analysis of variance followed by Fisher’s protected least significant difference test after the Bartlett test. A p value of <0.05 was considered significant.
RESULTS
Col1a1-caPPR Transgenic Mice Were Resistant to Unloading-induced Bone Loss
As reported previously, Tg mice showed a significant increase in trabeculation and porosity in cortical bone in the femora (Fig. 1a). Such phenotypes were at least in part similar to those in hyperthyroidism. Col1a1-caPPR transgenic mice (Tg mice) and their wild-type littermates were subjected to tail suspension for 2 weeks. A two-week period has been used to observe the effects of tail suspension on bone. Micro-CT analysis revealed that trabecular BV/TV of Tg mice was about 2.5-fold higher than that of wild-type mice in this protocol as reported previously (Fig. 1b) (13). Unloading reduced BV/TV of wild-type mice by more than 50%. In contrast, unloading did not reduce BV/TV of Tg mice (Fig. 1b). Effects of tail suspension were also examined in the weight bearing bones other than the hind limbs. Trabecular BV/TV in the 4th lumbar vertebrae of wild-type was reduced after tail suspension for 2 weeks and this reduction was not observed in caPPR Tg mice (Fig. 1, c and d). In contrast, non-weight bearing bone such as calvariae did not show any alteration by tail suspension. These data indicated that constitutively active PTH/PTHrP signaling in the Col1-expressing cell population in osteoblastic lineage in vivo suppressed unloading-induced bone loss.
FIGURE 1. Col1a1-caPPR Tg mice were resistant to unloading-induced bone loss.
Two-dimensional micro-CT images and BV/TV in the secondary trabeculae of the distal metaphyses of the femora (a and b) and the 4th lumbar vertebrae (c and d). Unloading-induced bone loss was observed in wild-type (Wt), but not in Tg mice after 14 days of tail suspension (Susp.) (*, p < 0.05, **, p < 0.01, n = 9 per group for the femora, n = 5 per group for the vertebra).
Constitutively Active PTH/PTHrP Receptor Signaling in Osteoblastic Cells Did Not Prevent Unloading-induced Suppression of Bone Formation
As bone mass level is determined by the balance of two activities, i.e. bone formation and bone resorption, we first investigated whether unloading-induced suppression of bone formation activity was modulated in the presence of constitutively active PTH/PTHrP receptor signaling in osteoblastic cells. Unloading suppressed bone formation parameters in wild-type mice as reported previously (Fig. 2, a–d). Constitutively active PTH/PTHrP receptor signaling in osteoblastic cells did not prevent unloading-induced suppression on bone formation parameters including mineral apposition rate, mineralized surface relative to bone surface, and bone formation rate (Fig. 2, a–d). Moreover, the magnitudes of unloading-induced suppression in these bone formation parameters in Col1a1-caPPR Tg mice were similar to those in wild-type mice.
FIGURE 2. caPPR signaling in osteoblastic cells did not prevent unloading-induced suppression of trabecular bone formation parameters.

Quantification of dynamic bone formation parameters in the trabeculae of the distal metaphyses of the femora (b–d). Calcein was injected at 2-day intervals, and undecalcified sections were scanned with confocal laser microscopy (a). Mineral apposition rate (MAR) (b), mineralized surface per bone surface (MS/BS) (c), and bone formation rate (BFR) (d) are shown (*, p < 0.05; **, p < 0.01, n = 4 per group). caPPR signaling in osteoblastic cells did not prevent unloading-induced suppression of trabecular bone formation parameters. Susp, suspension; Wt, wild type.
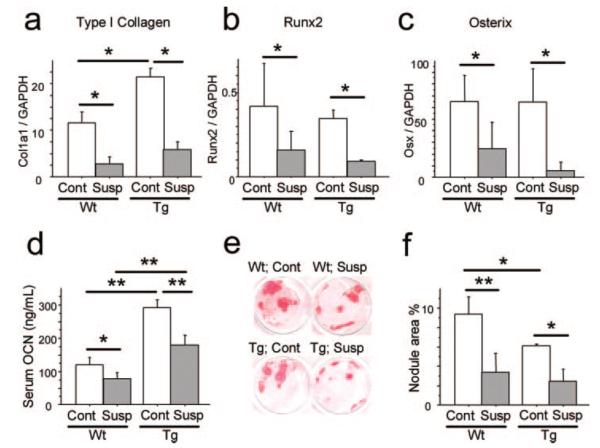
In addition to the histomorphometric observations on bone formation parameters in long bones, the molecular basis for these phenomena were examined by quantitative real-time PCR analysis of mRNA of the whole bone. Unloading reduced mRNA expression levels of genes characteristically expressed by osteoblasts such as type I collagen, Runx2, and Osterix in wild-type mice as well as in caPPR Tg mice (Fig. 3, a–c), coinciding with the observations on the bone formation rate and mineral apposition rate. Unloading reduced serum OCN levels in wild-type mice (Fig. 3d). Control Tg mice exhibited about 2-fold higher levels of serum osteocalcin compared with wild-type controls. However, unloading also reduced serum osteocalcin levels in Col1a1-caPPR Tg mice (Fig. 3d). To analyze the cellular effects of the constitutive expression of the PTH/PTHrP receptor, bone marrow cells were obtained from mice after unloading for 2 weeks and were cultured in the presence of ascorbic acid and β-glycerophosphate to induce mineralized nodule formation. Both wild-type and Tg mice showed unloading-induced reduction in the area of mineralized nodules (Fig. 3, e and f). Overall, these data revealed that constitutively active PTH/PTHrP signaling in the Col1-expressing cell population in the osteoblastic lineage did not alter unloading-induced suppression of bone formation activities.
FIGURE 3. caPPR signaling in osteoblastic cells did not alter unloading-induced suppression of bone formation activities in vivo.
mRNA expression levels of type 1 collagen (a), runx2 (b), and osterix (c) in hind limbs were determined by quantitative real-time PCR analysis (*, p < 0.05; **, p < 0.01, n = 4 per group except for runx2, n = 6 for wild-type (Wt)). Unloading universally suppressed genes characteristically expressed by osteogenic cells in both wild-type and Tg mice. Serum osteocalcin (OCN) level after 14 days of tail suspension is shown in d (n = 4 per group). Unloading suppressed serum OCN levels similarly in both wild-type and Tg mice. Mineralized nodule formation of bone marrow cells from control and unloaded tibiae of wild-type and Tg mice are shown in e and f. Alizarin red staining was done after 21 days of culture in the presence of ascorbic acid and β-glycero- phosphate (n = 3 per group). Bone marrow cell culture of unloaded tibiae yielded a less mineralized area both in wild-type and Tg mice. Susp, suspension; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Constitutively Active PTH/PTHrP Receptor Signaling in Osteoblastic Cells Suppressed Bone Resorption upon Unloading in Vivo
Unloading enhanced the levels of osteoclast number and osteoclast surface per bone surface (N.Oc/BS and OcS/BS, respectively) in wild-type mice by about 2-fold as shown previously (Fig. 4, a–c). Steady state bone resorption parameters in control Tg mice were enhanced by about 2-fold over the wild-type control as previously reported. In contrast to wild-type mice, constitutively active PTH/PTHrP receptor signaling in the transgenic mice suppressed, rather than enhanced, osteoclast parameters (Oc. number and Oc. surface) upon unloading of the hind limbs in Tg mice (Fig. 4, a–c). Unloading also increased the levels of urinary deoxypyridinoline excretion into urine in wild-type mice. The steady state level of deoxypyridinoline in urine was 3-fold higher in control Tg mice than in the wild-type control. Again, consistent with the histomorphological observation in bone, constitutively active PTH/PTHrP signaling in osteoblastic cells suppressed deoxypyridinoline excretion into urine by about 40% upon unloading (Fig. 4d). Thus, based on these analyses of both local and systemic parameters, constitutively active PTH/PTHrP signaling in the Col1-expressing cell population in osteoblastic lineage suppressed bone resorption upon unloading.
FIGURE 4. caPPR signaling in osteoblastic cells suppressed bone resorption activities upon unloading in vivo.
TRAP staining of the decalcified sections of the mesial metaphyses of the tibiae (a). Osteoclast number per bone surface (N.Oc/BS) (b) and osteoclast surface per bone surface (OcS/BS) (*, p < 0.05; **, p < 0.01, n = 5 per group) (c) are shown. Urinary deoxypyridinoline (Dpyd) level after 14 days of tail suspension (n = 12 per group) is shown in d. caPPR signaling in osteoblastic cells suppressed bone resorption activities upon unloading in vivo. Susp, suspension; Wt, wild type.
Constitutively Active PTH/PTHrP Receptor Signaling in Osteoblastic Cells Suppressed Osteoclast Differentiation in Vitro after Unloading in Vivo
To examine cellular mechanisms underlying caPPR suppression of bone resorption upon unloading in vivo, osteoclast differentiation in culture was investigated using bone marrow cells obtained from the mice subjected to hind limb unloading. Bone marrow cells were cultured in the presence of vitamin D and dexamethasone to induce TRAP-positive osteoclastic cell formation. Unloading in wild-type mice in vivo did not alter the levels of TRAP-positive cell development in vitro significantly. However, in the cells obtained from Tg mice, unloading in vivo suppressed TRAP-positive cell development in vitro (Fig. 5, a and b). Thus, constitutively active PTH/PTHrP signaling in the Col1-expressing cell population in osteoblastic lineage suppressed osteoclastic differentiation activity of the bone marrow cells upon unloading.
FIGURE 5. caPPR signaling in osteoblastic cells suppressed osteoclast differentiation in vitro upon unloading in vivo.

TRAP-positive multinucleated cell development of bone marrow cells from control and unloaded tibiae of wild-type (Wt) and Tg mice (a and b). Bone marrow cells were cultured for 11 days in the presence of 1,25-(OH)2 vitamin D3 and dexamethasone (*, p < 0.05; **, p < 0.01, n = 9 per group). Suppression on osteoclast progenitors due to unloading was observed only in Tg mice. Expression levels of RANK (c) and c-fms (d) in hind limbs was quantified by real-time PCR analysis (n = 4 per group). All data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Unloading suppressed expression levels of osteoclast differentiation-related genes in hind limbs in both wild-type and Tg mice. Susp, suspension.
To elucidate the molecular basis of caPPR signaling upon unloading, expression levels of osteoclastogenesis-related genes in whole bone were examined by quantitative real-time PCR analysis. In wild-type mice, unloading stimulated the expression levels of RANK mRNA about 5-fold. The steady state levels of RANK mRNA expression in control Tg mice were higher than those in wild-type control. CaPPR signaling suppressed RANK mRNA levels upon unloading in Tg mice(Fig.5c).Different from RANK mRNA, c-fms mRNA levels in wild-type mice were not obviously altered by unloading. Steady state levels of c-fms mRNA in control Tg mice were up-regulated. CaPPR signaling again suppressed c-fms mRNA levels upon unloading in Tg mice (Fig. 5d). Thus, these results suggest that, on unloading, constitutively active PTH/PTHrP signaling in the Col1-expressing cell population in osteoblastic lineage suppresses the expression of mRNAs encoding receptors that are involved in osteoclast differentiation.
We examined whether the effects of constitutively active PTH/PTHrP receptor (caPPR) signaling on unloading-induced suppression on osteoclast differentiation in “bone marrow cell cultures” were a direct action of caPPR signaling on bone marrow macrophages (BMMs), and not requiring the osteoblastic populations, which were present in our bone marrow cultures shown in the experiments presented in Fig. 5b. To exclude the contribution of osteoblast/stromal cell populations as much as possible, we conducted BMM cultures using cells obtained from bone marrow of the mice after unloading (tail suspension) or control housing. Bone marrow cells of control or unloaded wild-type or caPPR Tg mice were initially plated for 24 h, and then non-adherent cells were recovered from the cultures. These cells were replated and cultured in the presence of M-CSF for 2 days to obtain BMMs. BMMs were further cultured for 4 days in the presence of M-CSF and RANKL to induce osteoclast development. In these experiments, levels of TRAP-positive multinucleated cell development were similar between wild-type and caPPR transgenic mice. Moreover, unloading (tail suspension) did not alter the levels of TRAP-positive multinucleated cell development in cultures of Tg-derived BMMs. Thus, unloading-induced suppression in osteoclast development in caPPR Tg mice would not be observed in the absence of osteoblastic or fibroblastic cells. These data suggest the requirement for the presence of these osteoblast/stromal cell population.
Constitutively Active PTH/PTHrP Receptor Signaling in Osteoblastic Cells Suppressed M-CSF and MCP-1 Expression Levels upon Unloading
In the bone marrow environment, osteoblasts play a central role in maintenance of bone turnover as they secrete local factors to regulate bone resorption under the control of systemic signals, such as PTH. RANKL and its decoy receptor, osteoprotegerin (OPG) are major factors secreted by osteoblasts. We therefore examined the expression levels of RANKL and OPG. Unloading tended to increase RANKL mRNA levels and decrease OPG mRNA levels in wild-type without any statistically significant difference when compared with the control group individually (Fig. 6, a and b). However, unloading increased significantly the ratio of RANKL/OPG in wild-type mice. In contrast, unloading did not alter the RANKL/OPG ratio in Col1a1-caPPR Tg mice (Fig. 6c).
FIGURE 6. caPPR signaling in osteoblasts suppressed M-CSF and MCP-1 expression levels upon unloading.
mRNA expression levels of RANKL (a), OPG (b), RANKL/OPG ratio (c), M-CSF (d), and MCP-1 (e) in hind limbs of wild-type (Wt) and Tg mice were quantified by real-time PCR analysis (*, p < 0.05; **, p < 0.01, n = 4 per group). Unloading suppressed M-CSF and MCP-1 expression levels specifically in Tg mice. Messenger RNA expression levels of caPPR, RANKL, OPG, M-CSF, and MCP-1 in bone marrow-derived cells cultured in osteogenic medium were also shown (f) (*, p < 0.05; **, p < 0.01, n = 3 per group). All data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Because these studies did not reveal target genes associated with the unloading-induced suppression of osteoclastic activity in the Col1a1-caPPR Tg mice, we examined other osteoblast-derived factors required for osteoclastic activities. M-CSF mRNA levels in bone were not altered by unloading in wild-type mice, whereas they were elevated in control Tg mice (Fig. 6d). In contrast to wild-type mice, caPPR signaling suppressed M-CSF levels upon unloading down to one-fifth of those in the control Tg mice (Fig. 6d). Thus, constitutively active PTH/PTHrP receptor signaling suppressed the expression of M-CSF under the unloaded conditions. These studies also identified MCP-1, a member of the chemokine superfamily that induces chemotaxis of monocytes especially in inflammation (19, 20), as a putative target gene for the observed suppression of osteoclast activity. Similar to M-CSF, the MCP-1 expression level was not affected by unloading in wild-type mice, whereas it was up-regulated in control Tg mice compared with the wild-type control. In contrast to wild-type mice, constitutively active PTH/PTHrP receptor signaling in osteoblastic cells suppressed MCP-1 mRNA expression levels upon unloading down to one-fifth of those in control Tg mice (Fig. 6e).
To determine whether the effects of the caPPR are on cytokines produced by the osteogenic cells, adherent bone marrow cells from wild-type and caPPR transgenic mice were cultured in osteogenic medium (supplemented with ascorbic acid and β-glycerophosphate). After 6 days caPPR (hPTHR1(H223R)) mRNA was expressed in the cultures of Tg-derived osteoblastic cells (Fig. 6f). At this time, mRNA expression levels of RANKL and MCP-1 were enhanced in Tg-derived osteoblastic cells compared with wild-type cells (Fig. 6f). Although the OPG and M-CSF levels were also higher, the increases were not statistically significant.
Supplementation of M-CSF and/or MCP-1 in Vitro Rescued the Unloading-induced Suppression of Osteoclast Development in the Culture of Cells from caPPR Tg Mice
We observed that unloading (tail suspension) suppressed the levels of osteoclast number per bone surface, osteoclast surface per bone surface, and deoxypyridinoline excretion in caPPR Tg mice. These were in parallel to unloading-induced suppression in the levels of osteoclast development in culture and unloading-induced suppression in M-CSF and MCP-1 mRNA levels in bone. Therefore, we examined whether supplementation of M-CSF and/or MCP-1 in vitro would rescue the unloading-induced suppression of osteoclast development in the culture of cells from caPPR Tg mice. To examine the direct effects of M-CSF and MCP-1 on osteoclast development, we carried out the following experiments. Bone marrow cells of control or unloaded wild-type or caPPR Tg mice were cultured in the osteoclastogenic medium (supplemented with vitamin D3 and dexamethasone) to induce TRAP-positive osteoclastic cell development in the presence or absence of M-CSF or MCP-1 or both (Fig. 7). First, in unloaded (tail-suspended) caPPR Tg mice, suppression in the levels of TRAP-positive multinucleated cell development (Fig. 7, lane 1 versus 5) was partially recovered by supplementation of either M-CSF (lane 5 versus 6) or MCP-1 (lane 5 versus 7) alone. Furthermore, supplementation of both M-CSF and MCP-1 almost fully recovered TRAP-positive multinucleated cell development (lane 5 versus 8) to the levels comparable with those in loaded (control) caPPR Tg mice or wild-type mice (lane 8 versus 1). Therefore, unloading-induced suppression in the levels of osteoclast development in the cultures of bone marrow cells prepared from caPPR Tg mice was at least in part due to the suppression of endogenous production of M-CSF and MCP-1. Second, as reference, supplementation of either M-CSF or MCP-1, or both did not affect TRAP-positive multinucleated cell development in the cultures of bone marrow cells prepared from control wild-type mice. Although the reason is not clear, supplementation of M-CSF mildly suppressed TRAP-positive multinucleated cell development in the cultures of bone marrow cells from control Tg mice.
FIGURE 7. Osteoclast development in the osteoclastogenic cultures of bone marrow cells in the presence or absence of M-CSF or MCP-1.

Bone marrow cells from control and unloaded tibiae of wild-type (Wt) and Tg mice were cultured in the presence of 1,25-(OH)2 vitamin D3 and dexamethasone supplemented with either M-CSF or MCP-1 or both (*, p < 0.05; **, p < 0.01, n = 5 per group).
DISCUSSION
In this report, we demonstrated that constitutively active PTH/PTHrP receptor (caPPR) signaling in the Col1-expressing cell population in osteoblastic lineage prevented unloading-induced bone loss in long bones of the hind limbs. Unloading also reduced the levels of bone volume in vertebral bodies in wild-type mice, and caPPR signaling prevented unloading-induced bone loss. These data suggest that the observed phenomena were not a local event limited to hind limb bones but also occurring in other bones such as vertebral bones. As PTH exerts its effects on bone through osteoblasts and these cells have been reported to be targets of mechanical signaling, one could speculate that caPPR signaling in osteoblastic cells may counteract unloading-induced suppression of osteoblastic activities. However, individual osteoblastic activities (mineral apposition rate) and bone formation rate were suppressed by unloading regardless of the presence or absence of constitutively active PTH receptor signaling in osteoblastic cells. Thus, bone formation activity would not be a major component to explain the prevention of unloading-induced bone loss in caPPR Tg mice.
We, therefore, analyzed the unloading effects on bone resorption parameters in vivo in caPPR Tg mice. As known before, caPPR Tg mice exhibited steady state elevation in the levels of histomorphometric bone resorption parameters. Strikingly, unloading suppressed, rather than increased, osteoclast number (Oc.N/BS) as well as the osteoclast surface (Oc.S/BS) in caPPR Tg mice. Similarly, in caPPR Tg mice, unloading suppressed the level of deoxypyridinoline (biochemical parameters) excretion into urine.
Subsequent analyses on the osteoblast-derived factors controlling osteoclast differentiation indicated that unloading increased in RANKL/OPG ratio in wild-type mice to levels similar to those of this ratio in control caPPR Tg mice, although interestingly, unloading did not alter the ratio of RANKL/OPG mRNA in Tg mice. Thus, neither expression levels of RANKL and OPG mRNA nor the ratios of RANKL over OPG alone could explain why unloading suppressed bone loss and how unloading suppressed bone resorption in caPPR Tg mice. Further search for the targets of caPPR signaling that inhibit unloading-induced osteoclast differentiation identified that caPPR signaling reduced mRNA expression levels of the genes encoding pro-osteoclastic cytokines including M-CSF and chemokine MCP-1 upon unloading.
Our data point to the fact that there are at least two groups of regulators that contribute to enhanced bone resorption upon unloading. One group of regulators would be involved in the signaling being governed by M-CSF and MCP-1. The other group of regulators for osteoclastic development and their activities includes RANKL and OPG. Constitutively active PTH/PTHrP receptor signaling in osteoblastic cells enhances the levels of mRNA of M-CSF, MCP-1, and the RANKL/OPG ratio under normal conditions. However, the two groups of regulators are different with respect to the regulation by unloading. CaPPR-induced elevation in the RANKL/OPG ratio was not further enhanced by unloading in Tg mice. In contrast to the RANKL and OPG group, whose ratio (RANKL/OPG) was increased by unloading in wild-type mice, mRNA expression levels of regulators belonging to the other group, i.e. M-CSF and MCP-1, were not increased by unloading in wild-type mice. However, these levels were decreased in caPPR Tg mice when the mice were subjected to the unloading condition. Our identification of M-CSF and MCP-1 mRNAs to be suppressed by unloading in caPPR Tg mice is compatible with the observation that unloading reversed the direction of its effects on bone resorption from activation to suppression in caPPR Tg mice when subjected to tail suspension. These observations indicated that unloading-induced bone loss, in which osteoclastic bone resorption is activated, did not affect all the cytokine levels related to bone resorption activities in bone in a similar fashion. Rather, caPPR signaling differentially affects at least two distinct groups of regulators that are involved in diverse points of regulation of osteoclastic bone resorption upon unloading.
There are few studies of systemic administration of PTH, either through intermittent or continuous regimens, on the expression of pro-osteoclastic cytokines by osteoblastic cells. Previous studies have shown that the intermittent administration of PTH in vivo did not alter the expression of RANKL mRNA in bone marrow cells immediately prepared from bone (18) but regulates the expression of MCP-1 in bone (27). The expression of M-CSF in animals subjected to PTH treatment has not been reported. However, in vitro, PTH has been reported to enhance mRNA expression levels of both RANKL, via the cAMP-protein kinase A (PKA)-cAMP-response element-binding protein (CREB) pathway (21–24) and M-CSF in stromal cells and an osteoblastic cell line (24, 25).
It has been considered that osteocytes may play a role in mechanosensing in bone. Osteocytes express sclerostin and hence regulate osteoblastogenesis and osteoclastogenesis in bone. Chronic elevation of PTH serum levels in mice reduces the expression of sclerostin by osteocytes (26). This reduction in sclerostin expression, in turn, results in a decreased repression of Wnt signaling in osteoblasts, thereby increasing osteoblast anabolic activities. The CaPR Tg mice that were cage active controls showed higher levels of BV/TV than WT cage active mice (Fig. 1). These CaPR TG mice may be manifesting decreased sclerostin expression by constitutively active PTR signaling (mimicking constitutively high levels of serum PTH levels). Although the activity of 2.3-kb Col1a1 promoter would not be at least high in osteocytes, we cannot exclude the possibilities that forced caPPR expression in osteoblastic cells may indirectly alter sensitivity or activity of osteocytes.
Overall effects of constitutively active PTH/PTHrP receptor signaling resemble those of PTH administration. For instance, simultaneous activation of bone formation and bone resorption with net gain in bone mass would be observed in both cases. It has been postulated that effects of constitutively active PTH/PTHrP receptor signaling would share aspects with those physiological effects of PTH (and possibly PTHrP) signaling in bone remodeling. How our observation on the interaction of mechanical signaling and constitutively active PTH/PTHrP receptor signaling would relate to the physiological condition still needs to be elucidated by further analysis. In conclusion, our data indicate that constitutively active PTH/PTH receptor signaling in osteoblastic cells suppresses osteoclastic activity upon unloading in association with specific suppression on pro-osteoclastic cytokines, M-CSF and MCP-1.
Footnotes
This work was supported by grants-in-aid from the Japanese Ministry of Education (21st Century Center of Excellence (COE) Program, Frontier Research for Molecular Destruction and Reconstitution of Tooth and Bone, 17012008, 18109011, 18659438, 18123456), grants from the Japan Space forum NASDA and ABJS (Advanced Bone and Joint Science) Strategic Research Networks/Projects, in Japan Society for Promotion of Science Core to Core Program, Research for the Future Program, Genome Science).
The abbreviations used are: PTH, parathyroid hormone; PTHrP, parathyroid hormone related peptide; caPPR, constitutively active PTH/PTHrP receptor; RANK, receptor activator of NF-κB; RANKL, RANK ligand; M-CSF, macrophage colony stimulating factor; MCP-1, macrophage chemoattractant protein 1; BV/TV, bone volume per tissue volume; TRAP, tartrate-resistant acid phosphatase; Tg, transgenic; BMM, bone marrow macrophage; OPG, osteoprotegerin.
REFERENCES
- 1.Harada S, Rodan GA. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 2.Raisz LG. J. Clin. Investig. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanyon LE, Skerry T. J. Bone Miner. Res. 2001;16:1937–1947. doi: 10.1359/jbmr.2001.16.11.1937. [DOI] [PubMed] [Google Scholar]
- 4.Ehrlich PJ, Lanyon LE. Osteoporosis Int. 2002;13:688–700. doi: 10.1007/s001980200095. [DOI] [PubMed] [Google Scholar]
- 5.Bikle DD, Halloran BP. J. Bone Miner. Metab. 1999;17:233–244. doi: 10.1007/s007740050090. [DOI] [PubMed] [Google Scholar]
- 6.Ishijima M, Rittling SR, Yamashita T, Tsuji K, Kurosawa H, Nifuji A, Denhardt DT, Noda M. J. Exp. Med. 2001;193:399–404. doi: 10.1084/jem.193.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin TJ, Sims NA. Trends Mol. Med. 2005;11:76–81. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Kondo H, Nifuji A, Takeda S, Ezura Y, Rittling SR, Denhardt DT, Nakashima K, Karsenty G, Noda M. J. Biol. Chem. 2005;280:30192–30200. doi: 10.1074/jbc.M504179200. [DOI] [PubMed] [Google Scholar]
- 9.Kodama Y, Nakayama K, Fuse H, Fukumoto S, Kawahara H, Takahashi H, Kurokawa T, Sekiguchi C, Nakamura T, Matsumoto T. J. Bone Miner. Res. 1997;12:1058–1067. doi: 10.1359/jbmr.1997.12.7.1058. [DOI] [PubMed] [Google Scholar]
- 10.Qin L, Raggatt LJ, Partridge NC. Trends Endocrinol. Metab. 2004;15:60–65. doi: 10.1016/j.tem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Murray TM, Rao LG, Divieti P, Bringhurst FR. Endocr. Rev. 2005;26:78–113. doi: 10.1210/er.2003-0024. [DOI] [PubMed] [Google Scholar]
- 12.Schipani E, Langman CB, Parfitt AM, Jensen GS, Kikuchi S, Kooh SW, Cole WG, Juppner H. N. Engl. J. Med. 1996;348:618–629. doi: 10.1056/NEJM199609053351004. [DOI] [PubMed] [Google Scholar]
- 13.Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. J. Clin. Investig. 2001;107:277–286. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 15.Kuznetsov SA, Riminucci M, Ziran N, Tsutsui TW, Corsi A, Calvi LM, Kronenberg HM, Schipani E, Robey PG, Bianco P. J. Cell Biol. 2004;167:1113–1122. doi: 10.1083/jcb.200408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halloran BP, Bikle DD, Harris J, Tanner S, Curren T, Morey-Holton ER. J. Bone Miner. Res. 1997;12:1068–1074. doi: 10.1359/jbmr.1997.12.7.1068. [DOI] [PubMed] [Google Scholar]
- 17.Kostenuik PJ, Harris J, Halloran BP, Turner RT, Morey-Holton ER, Bikle DD. J. Bone Miner. Res. 1999;14:21–31. doi: 10.1359/jbmr.1999.14.1.21. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka S, Sakai A, Tanaka M, Otomo H, Okimoto N, Sakata T, Nakamura T. J. Bone Miner. Res. 2004;19:1813–1820. doi: 10.1359/JBMR.040808. [DOI] [PubMed] [Google Scholar]
- 19.Zhu JF, Valente AJ, Lorenzo JA, Carnes D, Graves DT. J. Bone Miner. Res. 1994;9:1123–1130. doi: 10.1002/jbmr.5650090721. [DOI] [PubMed] [Google Scholar]
- 20.Graves DT, Jiang Y, Valente AJ. Histol. Histopathol. 1999;14:1347–1354. doi: 10.14670/HH-14.1347. [DOI] [PubMed] [Google Scholar]
- 21.Fu Q, Manolagas SC, O’Brien CA. Mol. Cell. Biol. 2006;26:6453–6468. doi: 10.1128/MCB.00356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dossing DA, Stern PH. J. Cell. Biochem. 2005;95:1029–1041. doi: 10.1002/jcb.20470. [DOI] [PubMed] [Google Scholar]
- 23.Fu Q, Jilka RL, Manolagas SC, O’Brien CA. J. Biol. Chem. 2002;277:48868–48875. doi: 10.1074/jbc.M208494200. [DOI] [PubMed] [Google Scholar]
- 24.Kondo H, Guo J, Bringhurst FR. J. Bone Miner. Res. 2002;17:1667–1679. doi: 10.1359/jbmr.2002.17.9.1667. [DOI] [PubMed] [Google Scholar]
- 25.Itoh K, Udagawa N, Matsuzaki K, Takami M, Amano H, Shinki T, Ueno Y, Takahashi N, Suda T. J. Bone Miner. Res. 2000;15:1766–1775. doi: 10.1359/jbmr.2000.15.9.1766. [DOI] [PubMed] [Google Scholar]
- 26.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL. Endocrinology. 2005;146:4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 27.Partridge NC, Li X, Quin L. Ann. N.Y. Acad. Sci. 2006;1068:187–193. doi: 10.1196/annals.1346.024. [DOI] [PubMed] [Google Scholar]