Abstract
Rationale
Cardiac myosin binding protein C (cMyBP-C) regulates cross-bridge cycling kinetics and thereby fine-tunes the rate of cardiac muscle contraction and relaxation. Its effects on cardiac kinetics are modified by phosphorylation. Three phosphorylation sites (Ser275, Ser284, Ser304) have been identified in vivo, all located in the cardiac-specific M-domain of cMyBP-C. However recent work has shown that up to four phosphate groups are present in human cMyBP-C.
Objective
To identify and characterize additional phosphorylation sites in human cMyBP-C.
Methods and results
Cardiac MyBP-C was semi-purified from human heart tissue. Tandem mass-spectrometry analysis identified a novel phosphorylation site on serine 133 in the proline-alanine (Pro-Ala) rich linker sequence between the C0 and C1 domains of cMyBP-C. Unlike the known sites, Ser133 was not a target of protein kinase A. In silico kinase prediction revealed glycogen synthase kinase 3β (GSK3β) as the most likely kinase to phosphorylate Ser133. In vitro incubation of the C0C2 fragment of cMyBP-C with GSK3β showed phosphorylation on Ser133. In addition, GSK3β phosphorylated Ser304, although the degree of phosphorylation was less compared to PKA-induced phosphorylation at Ser304. GSK3β treatment of single membrane-permeabilized human cardiomyocytes significantly enhanced the maximal rate of tension redevelopment.
Conclusion
GSK3β phosphorylates cMyBP-C on a novel site, which is positioned in the Pro-Ala rich region and increases kinetics of force development, suggesting a non-canonical role for GSK3β at the sarcomere level. Phosphorylation of Ser133 in the linker domain of cMyBP-C may be a novel mechanism to regulate sarcomere kinetics.
Keywords: Cardiac myosin binding protein-C, phosphorylation, GSK3β, heart failure, contractile proteins, hypertrophy/remodeling, myocardial contractility
INTRODUCTION
Cardiac myosin binding protein C (cMyBP-C) is increasingly being recognized as an important regulator of sarcomere function with consequences for in vivo cardiac performance. The main regulatory role of cMyBP-C seems to be its effect on cross-bridge cycling kinetics of sarcomere contraction.1, 2 Cardiac MyBP-C itself is regulated by phosphorylation.3 It has been proposed that cMyBP-C acts as a structural constraint limiting cross-bridge formation and that phosphorylation of cMyBP-C accelerates cross-bridge kinetics, which is required for enhanced rates of relaxation and force development in diastole and systole, respectively.2
Classically, protein kinase A (PKA), which is activated upon β-adrenergic receptor stimulation, was described as the main kinase responsible for cMyBP-C phosphorylation.4 At least three sites on cMyBP-C can be phosphorylated in vivo by PKA,4, 5 i.e. Ser275, Ser284, Ser304 (numbering based on human sequence), while Ser311 phosphorylation was shown to be phosphorylated by PKA in vitro.6 All these phosphorylation sites are located in the cardiac isoform specific M-domain (Figure 1A). More recent work has shown that the phosphorylation sites in the M-domain can be targeted by many other kinases (reviewed by Bardswell7). Moreover, additional sites have been described on the basis of animal experiments8 and studies on human cardiac tissue revealed that at least one additional phosphorylation site should exist in humans, as up to four phosphate groups are present in isolated human cMyBP-C.5
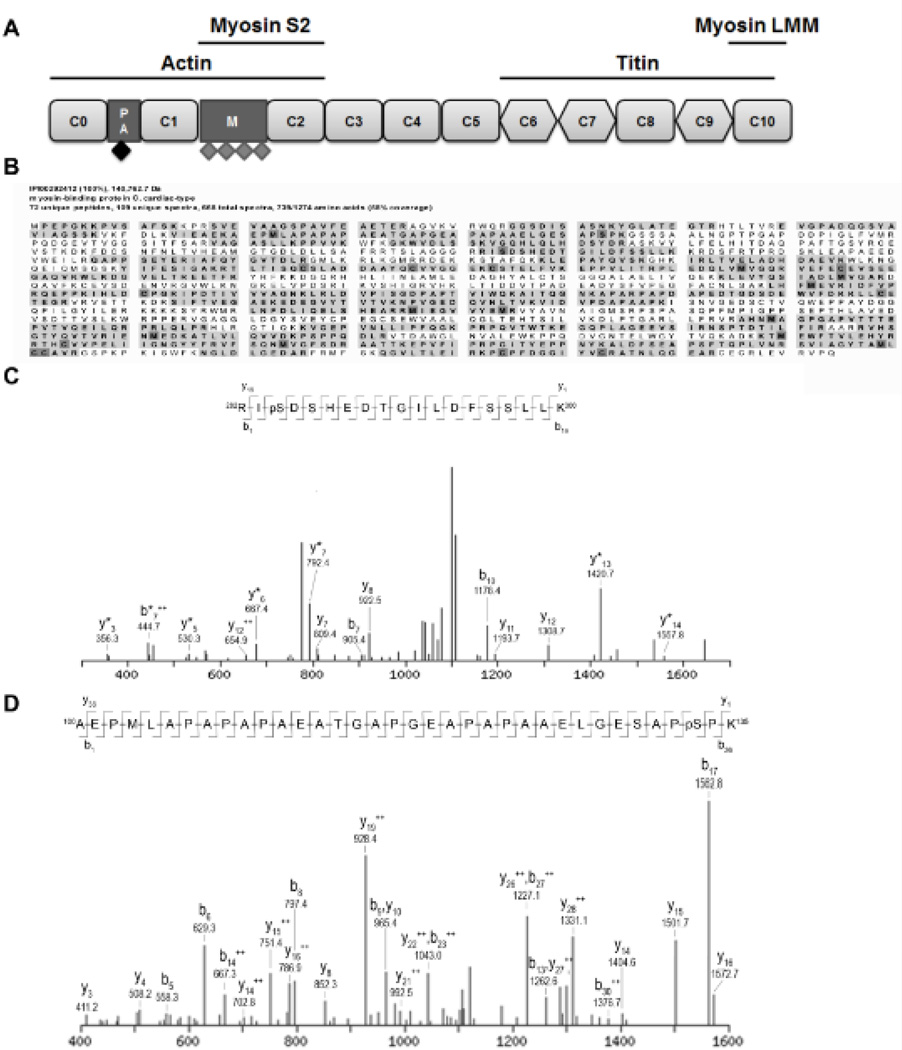
Figure 1. Identified phosphorylated peptides from cMyBP-C.
A. Schematic domain structure of cMyBP-C. Cardiac MyBP-C consists of 8 Ig (rounded rectangle) and 3 fibronectin (diamond) domains labeled C0 (N-terminus) through C10 (C-terminus). Two additional domains are present in the N-terminal part of the protein, the Proline-Alanine rich region (PA) and the M-domain (M). Four previously described phosphorylation sites, Ser275, Ser284, Ser304 and Ser311 (indicated by grey diamonds), are located in the M-domain. Cardiac MyBP-C was semi-purified from donor and failing human myocardium. After trypsin digestion, peptides were scanned by tandem mass spectrometry for phosphorylated peptides. B. Peptide coverage (bold with gray background) of cMyBP-C semi-purified from donor myocardium. 58% of the total amino acid sequence was covered by the peptides identified by the MS/MS analysis. Modified amino acids are indicated in dark gray. C. Fragmentation spectrum with b and y ions indicated of peptide spanning from amino acid 282–300 showed a phosphorylated serine at position 284 in cMyBP-C from donor myocardium. D. Fragmentation spectrum of peptide spanning from amino acids 100–135 revealed a novel phosphorylation site on serine 133 in cMyBP-C semi-purified from donor heart tissue. Serine 133 is located in the PA region (indicated by black diamond in Figure 1A).
To identify phosphorylation sites on cMyBP-C, we performed tandem mass-spectrometry on semi-purified cMyBP-C from human heart tissue. A novel phosphorylation site (Ser133) was identified in the linker domain of cMyBP-C, which was not a target of PKA, but was phosphorylated by the “hypertrophy” kinase, glycogen synthase kinase 3β (GSK3β). Interestingly, GSK3β treatment of single membrane-permeabilized human cardiomyocytes significantly enhanced kinetics of force redevelopment, suggesting a novel functional role for GSK3β at the sarcomere level.
METHODS
An expanded Methods section is available in the Online Data Supplement.
In vitro kinase assays
Recombinant C0C2 fragment was incubated for 2 h at 37°C in relax solution (pH 7.0; in mmol/L: free Mg2+ 1, KCl 145, EGTA 2, ATP 4, imidazole 10) with 750U PKA (Calbiochem) or 10 µl GSK3β (Sigma Aldrich). This reaction was stopped using 2D clean-up kit (GE Healthcare).
RESULTS
Novel phosphorylation site in human cMyBP-C
Previous data showed that at least one additional phosphorylation site is present in human cMyBP-C.5 To investigate the presence of novel phosphorylation sites, cMyBP-C was semi-purified from donor and failing (idiopathic dilated cardiomyopathy, IDCM) heart tissue and was analyzed for phosphorylated peptides by tandem mass spectrometry. Peptide coverage was 58% of the total amino acid sequence (Figure 1B). In donor cMyBP-C two phosphorylation sites were identified. One corresponded to the known Ser284 site4 (Figure 1C), while a novel phosphorylation site was identified at serine 133 (Figure 1D). Analysis of failing cMyBP-C did not reveal any phosphorylated peptides (data not shown).
Levels of phosphorylated Ser133 in human cardiac tissue
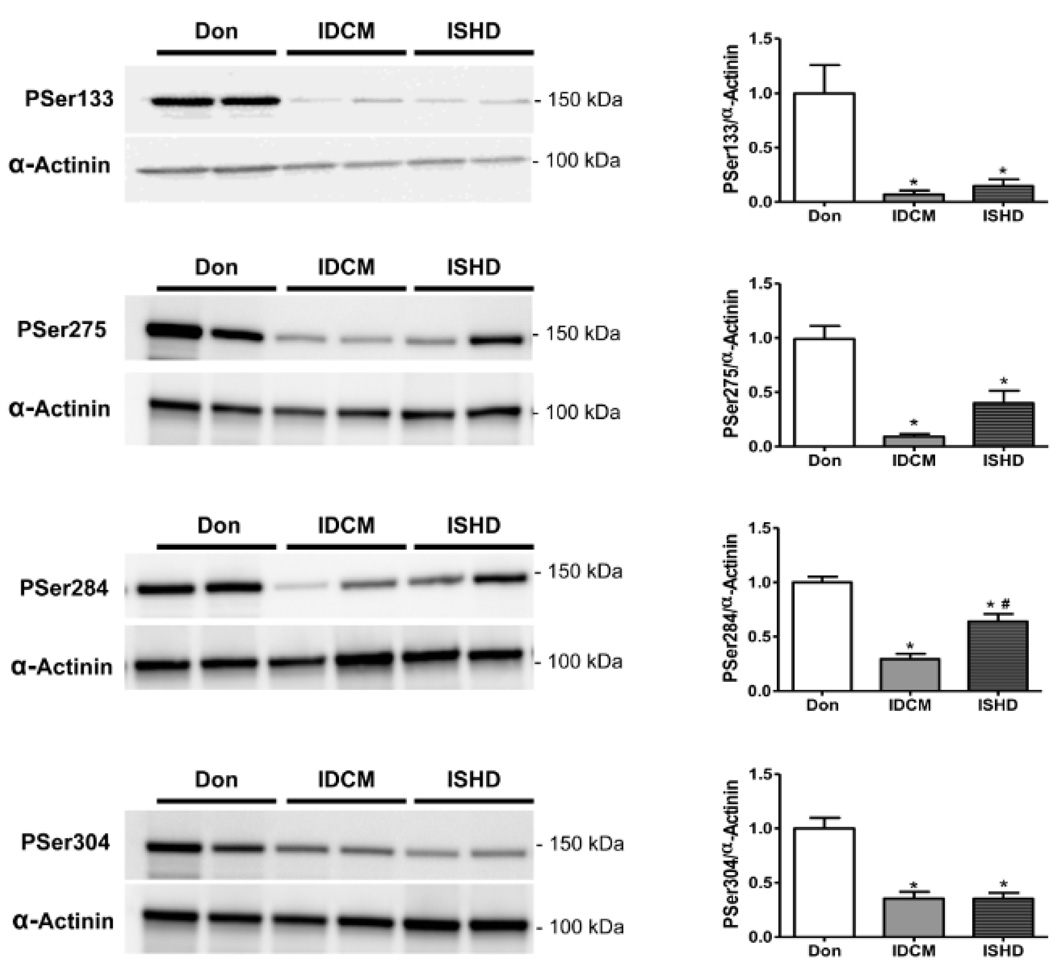
To assess Ser133 phosphorylation levels in non-failing and failing human hearts, a phospho-specific antibody was generated. Phosphorylation levels in failing heart samples from dilated as well as ischemic heart disease patients were significantly lower than in donors (Figure 2), which was in agreement with the MS/MS data. Phosphorylation at the in vivo established sites on cMyBP-C (Ser275, Ser284 and Ser304) was significantly lower in IDCM and ISHD compared to donor hearts (Figure 2).
Figure 2. Site specific phosphorylation of cMyBP-C in donor and end-stage failing hearts.
Immunoblot analysis of tissue homogenates from donor (n=5), and end-stage failing hearts from patients with idiopathic (IDCM; n=6) or ischemic (ISHD; n=6) cardiomyopathy revealed lower phosphorylation of Ser133 in failing compared to donor hearts. Phosphorylation of previously identified sites, Ser275, Ser284 and Ser304, was also lower. *P<0.01 IDCM and ISHD versus donor in 1-way ANOVA followed by Bonferroni post-hoc test; #P<0.05 ISHD versus IDCM.
Ser133 is a target of GSK3β
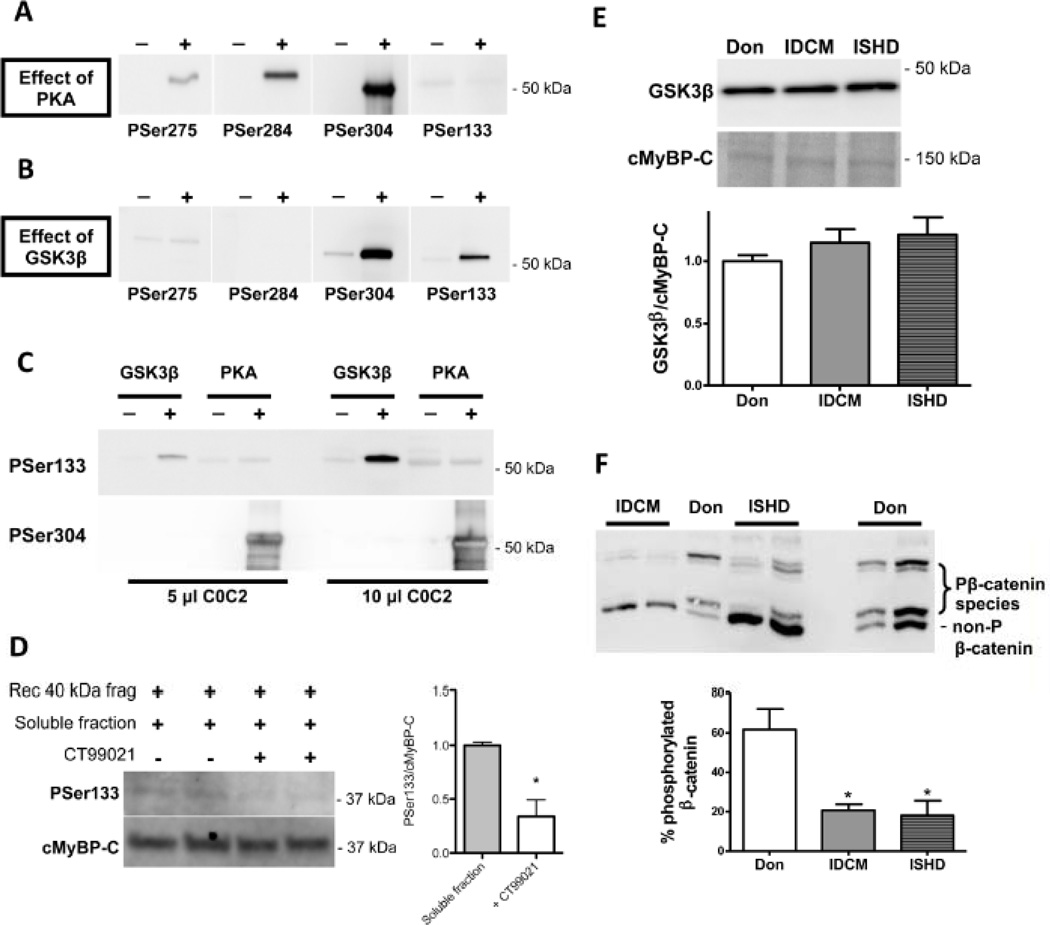
PKA is the archetypical kinase that phosphorylates cMyBP-C at all previously identified sites.4, 6, 9 To study if PKA can also phosphorylate Ser133, the N-terminal human recombinant peptide spanning the C0C2 domain (amino acids 1–451) of cMyBP-C was incubated with PKA. Robust phosphorylation of Ser275, Ser284 and Ser304 sites was detected, whereas Ser133 was not phosphorylated by PKA (Figure 3A). To identify the kinase responsible for Ser133 phosphorylation, in silico kinase prediction was performed. This yielded GSK3β as the most likely candidate (score 0.52). In vitro incubation of the C0C2 peptide with GSK3β revealed marked phosphorylation at Ser133 and Ser304, whereas the other sites were not phosphorylated (Figure 3B). Analysis of C0C2 treated with GSK3β or PKA loaded on the same immunoblot and stained with the antibodies against phosphorylated Ser133 and Ser304 (Figure 3C) confirmed that Ser133 was phosphorylated by GSK3β, but not by PKA. Interestingly, no phosphorylation signal was obtained at Ser304 for GSK3β-treated C0C2, while phosphorylation signals for the PKA-treated C0C2 were extremely intense even though PKA activity was lower compared to GSK3β activity (respectively, 10 versus 168 pmol/min/µg). Overall, this suggests that Ser133 may be the preferred target of GSK3β on cMyBP-C.
Figure 3. Ser133 is phosphorylated by GSK3β.
Human recombinant C0C2 fragment was incubated with either PKA or GSK3β for 2 hours at 37°C. Phosphorylation at serines 275, 284, 304 and 133 was determined with phospho-specific antibodies. A. PKA phosphorylated Ser275, 284 and 304, but not Ser133. B. GSK3β phosphorylated Ser304 and 133. C. To directly compare the relative capability of PKA and GSK3β to phosphorylate Ser133 and Ser304, human recombinant C0C2 was incubated without kinase, with PKA (10 pmol/min/µg) or with GSK3β (168 pmol/min/µg) and loaded onto the same gel in two different amounts followed by immunoblotting with site-specific antibodies. Ser133 was only phosphorylated by GSK3β while Ser304 was predominately phosphorylated by PKA. D. Recombinant 40kDa cMyBP-C (amino acids 1–271 from the human sequence) was incubated with a rough cytosolic fraction isolated from donor heart tissue with (n=3) or without (n=3) 2µM CT99021 (GSK3β antagonist) to determine whether endogenous GSK3β is responsible for Ser133 phosphorylation. PSer133 signal was normalized to total cMyBP-C (with antibody against C0 domain) and 40kDa + soluble fraction was set at 1. CT99021 significantly decreased PSer133 phosphorylation. *P<0.05, with versus without CT99021 in unpaired Student’s t-test. E. GSK3β protein levels were not changed in the end-stage failing hearts compared with donor hearts. F. Phosphorylation of another GSK3β target protein β-catenin was studied by Phos-tag analysis. Reversible interaction between phosphorylated proteins and Phos-tag reagent slow down migration of phosphorylated proteins through the gel. End-stage failing hearts showed a greater proportion of unphosphorylated β-catenin compared with donor hearts suggesting a reduced GSK3β activity. *P<0.01 IDCM and ISHD versus donor in 1-way ANOVA followed by Bonferroni post-hoc test.
To show that endogenous GSK3β targets Ser133, the recombinant human 40kDa fragment (amino acids 1–271, also known as the 29kDa fragment100) was incubated with a rough cytosolic fraction from donor heart tissue with and without 2 µM GSK3β antagonist CT99021. At this dose, CT99021 almost completely prevented the ability of exogenous GSK3β to phosphorylate Ser133 (Online Figure I). Phosphorylation of Ser133 was significantly lower in the presence of CT99021 (Figure 3D), suggesting that GSK3β present in the cytosolic fraction is able to phosphorylate cMyBP-C at Ser133.
GSK3β protein levels were similar in donor and failing samples (Figure 3E), while phosphorylation of β-catenin (determined by Phos-tag analysis11), another cellular substrate of GSK3β, was lower in IDCM and ISHD compared to donor (Figure 3F) consistent with low Ser133 cMyBP-C phosphorylation in failing myocardium (Figure 2).
Effect of GSK3β on sarcomere function
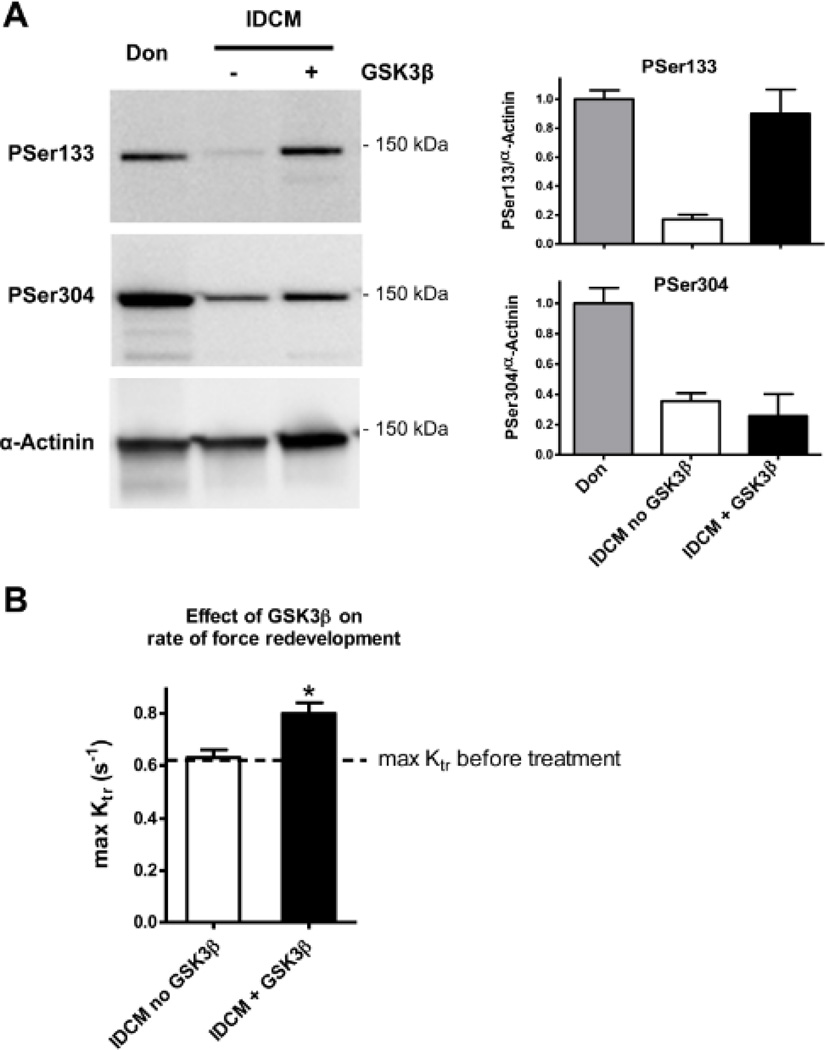
To study the effects of GSK3β on sarcomere function, force measurements in membrane-permeabilized cardiomyocytes were performed. Cardiomyocytes from failing human tissue (low basal Ser133 phosphorylation, Figure 2) were used and sarcomere function was measured before and after incubation with GSK3β. Incubations in kinase buffer without enzyme served as control. IDCM cardiac tissue incubated with GSK3β showed increased phosphorylation at Ser133, while no increase was found at Ser304 (Figure 4A). GSK3β incubation had no effect on maximal and passive force (data not shown). However, GSK3β significantly increased the maximal rate of tension redevelopment, while no effect was seen in the absence of the kinase (Figure 4B).
Figure 4. Effect of GSK3β on the rate of force redevelopment.
A. Incubation of skinned human left ventricular tissue from end-stage failing hearts with GSK3β (n=3) led to an increase of Ser133 phosphorylation while Ser304 phosphorylation was unchanged. B The rate of force redevelopment was determined at maximal calcium activation (max ktr) in Triton-permeabilized single cardiomyocytes isolated from left ventricular myocardium from end-stage heart failure patients (4 hearts; 11 cells). GSK3β significantly increased max Ktr, while upon control treatments (in the absence of GSK3β) no effect was found. *P<0.05, before versus after GSK3β in paired Student’s t-test.
DISCUSSION
Tandem MS identified a novel phosphorylation site at Ser133 in human cMyBP-C. We also revealed that GSK3β, but not PKA, could phosphorylate Ser133. Functional studies in cardiomyocytes from failing hearts showed that GSK3β also has a modulating effect on sarcomere function by increasing the rate of tension redevelopment.
To date several phosphorylation sites have been identified in the M-domain of human cMyBP-C (Figure 1A),4, 6, 9 and our recent study suggested that at least one more site exists.5 Starting from this, we performed tandem MS experiments to identify the “missing” site in cMyBP-C semi-purified from donor myocardium. This resulted in the identification of one of the known sites (Ser284) and a novel site Ser133. The other PKA sites (Ser275, 304 and Ser311) were not found in our analysis, because they were not present in the identified peptides (Figure 1B). Complete sequence coverage of a protein is typically not achieved in MS/MS experiments, due to a variety of reasons, such as the generation of peptides that are either too large or too small to be adequately detected.12
To our knowledge, we are the first to link phosphorylation of cMyBP-C to GSK3β. GSK3β is a key signaling molecule in the Insulin/PI3K/Akt signaling cascade13 and is active under resting conditions, and inactivated by phosphorylation.14 GSK3β is considered to be anti-hypertrophic,15 through the inhibition of pro-hypertrophic transcription factors.13, 15 Reduced GSK3β activity has been reported in human heart failure, while protein levels were similar (Haq et al.16 and Figure 3E). In line with reduced GSK3β activity, the level of phosphorylated Ser133 was significantly lower in end-stage failing than in donor myocardium (Figure 2). Ser133 is positioned within the Pro-Ala rich linker domain between C0 and C1 (Figure 1A), which was thought to interact with the thin filament actin,17 although recently it has been shown that N-terminal fragments of cMyBP-C lacking the M-domain bind actin only weakly in vitro.18 Other proposed interaction partners for the N-terminal part of cMyBP-C are myosin S2 domain and possibly the regulatory light chain (RLC).19 The true worth of actin, myosin S2 and RLC interactions with cMyBP-C on sarcomere function are yet to be established. Phosphorylation of Ser133 likely affects these protein interactions, and might explain the observed change in cross-bridge kinetics. To directly link cMyBP-C Ser133 phosphorylation to the observed increase in cross-bridge kinetics additional experiments using transgenic mice or exchange with recombinant protein are needed. Previous studies showed that PKA-mediated cMyBP-C phosphorylation is centrally involved in enhancement of cardiac kinetics during β-adrenergic receptor stimulation. Likewise, we showed that GSK3β increases the rate of force redevelopment in human cardiomyocytes and may represent another kinase, which is able to modify myocardial kinetics, possibly via phosphorylation of a newly identified site in the linker domain of cMyBP-C.
Supplementary Material
Novelty and Significance.
What Is Known?
Cardiac myosin binding protein C (cMyBP-C, or MYBPC3), a thick filament associated protein, is an important regulator of cardiac contractility.
Phosphorylation cMyBP-C regulates cardiac function.
Phosphorylation of cMyBP-C is reduced in end-stage heart failure (HF).
What New Information Does This Paper Contribute?
A new phosphorylation site (Ser133) was identified in the proline-alanine rich linker sequence between the C0 and C1 domains of cMyBP-C.
Ser133 is phosphorylated by glycogen synthase kinase 3β (GSK3β). Level of Ser133 phosphorylated cMyBP-C protein is lower in HF.
Incubation of the skinned ventricular myocytes isolated from failing human hearts with GSK3β leads to an increase in the kinetics of cardiac contraction.
Phosphorylation of cMyBP-C is required for normal cardiac function. We have identified a novel phosphorylation site on cMyBP-C, i.e. Ser133 in the proline-alanine rich linker sequence between C0 and C1 domains. Hearts from end-stage HF patients have reduced level of Ser133 phosphorylated cMyBP-C compared with donor hearts. Ser133 is phosphorylated by the anti-hypertrophy kinase GSK3β. Incubation of skinned ventricular myocytes isolated from failing human hearts with GSK3β increased kinetics of contraction. These findings implicate GSK3β is implicated in direct regulation of cardiac contractility through phosphorylation of a myofilament protein. Regulating GSK3β activity may represent a new mechanism for regulating the kinetics of cardiac contraction in human myocardium.
Acknowledgments
SOURCES OF FUNDING
We acknowledge support from the 7th Framework Program of the European Union (“BIG-HEART,” grant agreement 241577), the Netherlands organization for scientific research (NWO;VIDI grant) and National Institutes of Health grants R01HL105826 and K02HL114749.
Non-standard Abbreviations
- cMyBP-C
Cardiac myosin binding protein C
- PKA
Protein Kinase A
- GSK3β
Glycogen synthase kinase 3β
- Ser
Serine
- IDCM
Idiopathic dilated cardiomyopathy
- ISHD
Ischemic heart disease
- ESI
Electron spray ionization
- CID
Collision induced dissociation
- ETD
Electron-transfer dissociation
- EGTA
Ethylene glycol tetraacetic acid
- PMSF
Phenylmethylsulfonyl fluoride
- DTT
Dithiothreitol
- MS
Mass spectrometry
- Ktr
Rate of tension redevelopment
- cTnT
Cardiac troponin T
- Pro
Proline
- Ala
Alanine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Stelzer JE, Dunning SB, Moss RL. Ablation of cardiac myosin-binding protein-C accelerates stretch activation in murine skinned myocardium. Circ Res. 2006;98:1212–1218. doi: 10.1161/01.RES.0000219863.94390.ce. [DOI] [PubMed] [Google Scholar]
- 2.Stelzer JE, Patel JR, Moss RL. Protein kinase A-mediated acceleration of the stretch activation response in murine skinned myocardium is eliminated by ablation of cMyBP-C. Circ Res. 2006;99:884–890. doi: 10.1161/01.RES.0000245191.34690.66. [DOI] [PubMed] [Google Scholar]
- 3.Kuster DW, Bawazeer AC, Zaremba R, Goebel M, Boontje NM, van der Velden J. Cardiac myosin binding protein C phosphorylation in cardiac disease. J Muscle Res Cell Motil. 2011 doi: 10.1007/s10974-011-9280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautel M, Zuffardi O, Freiburg A, Labeit S. Phosphorylation switches specific for the cardiac isoform of myosin binding protein-C: a modulator of cardiac contraction? Embo J. 1995;14:1952–1960. doi: 10.1002/j.1460-2075.1995.tb07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copeland O, Sadayappan S, Messer AE, Steinen GJ, van der Velden J, Marston SB. Analysis of cardiac myosin binding protein-C phosphorylation in human heart muscle. J Mol Cell Cardiol. 2010;49:1003–1011. doi: 10.1016/j.yjmcc.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Jia W, Shaffer JF, Harris SP, Leary JA. Identification of novel protein kinase A phosphorylation sites in the M-domain of human and murine cardiac myosin binding protein-C using mass spectrometry analysis. J Proteome Res. 2010;9:1843–1853. doi: 10.1021/pr901006h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardswell SC, Cuello F, Kentish JC, Avkiran M. cMyBP-C as a promiscuous substrate: phosphorylation by non-PKA kinases and its potential significance. J Muscle Res Cell Motil. 2011 doi: 10.1007/s10974-011-9276-3. [DOI] [PubMed] [Google Scholar]
- 8.Yuan C, Guo Y, Ravi R, Przyklenk K, Shilkofski N, Diez R, Cole RN, Murphy AM. Myosin binding protein C is differentially phosphorylated upon myocardial stunning in canine and rat hearts-- evidence for novel phosphorylation sites. Proteomics. 2006;6:4176–4186. doi: 10.1002/pmic.200500894. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed AS, Dignam JD, Schlender KK. Cardiac myosin-binding protein C (MyBP-C): identification of protein kinase A and protein kinase C phosphorylation sites. Arch Biochem Biophys. 1998;358:313–319. doi: 10.1006/abbi.1998.0857. [DOI] [PubMed] [Google Scholar]
- 10.Previs MJ, Beck Previs S, Gulick J, Robbins J, Warshaw DM. Molecular mechanics of cardiac myosin-binding protein C in native thick filaments. Science. 2012;337:1215–1218. doi: 10.1126/science.1223602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamdani N, Borbely A, Veenstra SP, Kooij V, Vrydag W, Zaremba R, Dos Remedios C, Niessen HW, Michel MC, Paulus WJ, Stienen GJ, van der Velden J. More severe cellular phenotype in human idiopathic dilated cardiomyopathy compared to ischemic heart disease. J Muscle Res Cell Motil. 2010;31:289–301. doi: 10.1007/s10974-010-9231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn K, Goshe MB. Challenges and strategies for targeted phosphorylation site identification and quantification using mass spectrometry analysis. Brief Funct Genomic Proteomic. 2009;8:90–103. doi: 10.1093/bfgp/eln051. [DOI] [PubMed] [Google Scholar]
- 13.Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- 14.Sugden PH, Fuller SJ, Weiss SC, Clerk A. Glycogen synthase kinase 3 (GSK3) in the heart: a point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. Br J Pharmacol. 2008;153(Suppl 1):S137–S153. doi: 10.1038/sj.bjp.0707659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welsh GI, Proud CG. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J. 1993;294(Pt3):625–629. doi: 10.1042/bj2940625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin JD. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 17.Squire JM, Luther PK, Knupp C. Structural evidence for the interaction of C-protein (MyBP-C) with actin and sequence identification of a possible actin-binding domain. J Mol Biol. 2003;331:713–724. doi: 10.1016/s0022-2836(03)00781-2. [DOI] [PubMed] [Google Scholar]
- 18.Govindan S, Sarkey J, Ji X, Sundaresan NR, Gupta MP, de Tombe PP, Sadayappan S. Pathogenic properties of the N-terminal region of cardiac myosin binding protein-C in vitro. J Muscle Res Cell Motil. 2012;33:17–30. doi: 10.1007/s10974-012-9292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfuhl M, Gautel M. Structure, interactions and function of the N-terminus of cardiac myosin binding protein C (MyBP-C): who does what, with what, and to whom? J Muscle Res Cell Motil. 2012;33:83–94. doi: 10.1007/s10974-012-9291-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.