Abstract
Estrogens are the primary female sex hormones and play important roles in both reproductive and non-reproductive systems. Estrogens can be synthesized in non-reproductive tissue as liver, heart, muscle, bone and brain. The tissue-specific estrogen synthesis is consistent with a diversity of estrogen actions. Here, we will focus on tissue and cell-specific estrogen synthesis and estrogen receptor signaling. This review will include three parts: (I) tissue and cell-specific estrogen synthesis and metabolism, (II) tissue and cell-specific distribution of estrogen receptors and signaling and (III) tissue-specific estrogen function and related disorders, including cardiovascular diseases, osteoporosis, Alzheimer's disease and Parkinson disease. This comprehensive review provides new insights into estrogens by giving a better understanding of the tissue-specific estrogen effects and their roles in various diseases.
Keywords: estrogen synthesis, estrogen metabolism, estrogen receptor signaling, aging, degenerative diseases
Increasing evidence that estrogen synthesis and signaling can be both tissue- and cell-specific suggests that estrogens are not simply female sex hormones for gonadal organ development and function. Estrogens have critical functions in extragonadal tissues including liver, heart, muscle, bone, and brain, and estrogen treatments are currently under evaluation in clinical trials for several aging-related diseases. But due to great discrepancies in the clinical and basic research studies, there is debate as to whether estrogen is beneficial or harmful. Bearing this in mind, it is important to recognize that estrogen may play different roles in different tissues, further complicated by crosstalk between circulating and tissue-specific estrogens. Here, we review tissue and cell-specific aspects of estrogen from several perspectives: (I) estrogen synthesis, (II) estrogen receptors (ERs) and signaling pathways, and (III) the impact of estrogen in age-associated diseases. We believe that a better understanding of tissue and cell-specific estrogen and ERs are not only necessary for the study of physiological and pathological changes during aging, but are important for developing new therapeutics to prevent and treat osteoporosis, heart disease, Parkinson's Disease (PD), and Alzheimer's disease (AD).
Estrogens are important for early development, not only for primary and secondary sexual characteristics but also embryonal and fetal development of the brain networks [1]. Estrogens act through 2 types of their receptors: classical nuclear receptors (ERα and ERβ) and novel cell surface membrane receptors (GPR30 and ER-X). Both types of estrogen receptors are expressed in periphery and brain with cell- and tissue-specific distributions. For example, estrogens from ovary play major roles in regulation of the reproductive system, such as pubertal onset, fertility, and estrous cycle mainly through interacting with nuclear estrogen receptors, while recent studies showed that brain estrogens protect against insults-induced neuronal damages via both nuclear and cell surface membrane receptors [2, 3]. The cell- and tissue-specific action of estrogens and their receptors contribute healthy ageing as well as divergent outcomes from estrogen therapy in age-related diseases.
Estrogen systhesis
In premenopausal women, estrogens are produced primarily in the ovaries, corpus luteum, and placenta, although a small but significant amount of estrogens can also be produced by nongonad organs, such as the liver, heart, skin, and brain. There are three major forms of physiological estrogens in females: estrone (E1), estradiol (E2, or 17β-estradiol), and estriol (E3). Each form of the estrogens presents different product delivered from cholesterol by series reactions throughout estrogen biosynthesis. E2 is the major product from the whole biosynthesis process and is the most potent estrogen during the premenopausal period in a woman's life, whereas E1 plays a larger role after menopause, when it is synthesized in adipose tissue from adrenal dehydroepiandrosterone. E3 is the least potent estrogen and is formed from E1 through 16α-hydroxylation, plays a larger role during pregnancy when it is produced in large quantities by the placenta. Estrogen deactivation such as level of E2, can be regulated by estrogens metabolisms, including conversion from E2 to a less-active form, such as E1 or E3 [4], and formation of E2 sulfation by estrogen sulfotransferase to form 17beta-estra-1,3,5-trien-3,17-diol 3-sulfate, which is no longer interacting with estrogen receptors [5]. Furthermore, studies showed that deficiency of lipocalin 2, a novel adipose-derived cytokine, can also prohibit E2 synthesis by down regulation of aromatase in adipose tissue of female mice [6]. Therefore, the ratio of circulating estrogens might serve as an indicative of a dynamic metabolism: the balance between estrogen synthesis and deactivation. One of the most widely used mechanisms for controlling estrogen synthesis in the body is regulation of the enzyme aromatase.
Aromatase: a key estrogen synthase
The enzyme aromatase is responsible for the last step in synthesis of E2, as shown in figure 1. Aromatase is a member of the cytochrome P450 superfamily and is widely expressed in many sites, including brain, gonads, blood vessels, liver, bone, skin, adipose tissue, and endometrium [7]. Tissue-specific expression of aromatase depends on three major factors: alternative splicing mechanisms, tissue-specific promoters, and different transcription factors. As shown in figure 2, the human aromatase gene CYP19 comprises a 93 kb 5'-regulatory region and a 30 kb 3'-coding region. The regulatory region contains 10 tissue-specific promoters for local estrogen biosynthesis under normal physiological or pathological conditions such as breast cancer and endometriosis [8]. Activation of each promoter gives rise to alternatively spliced forms of mature mRNA with the first exon a tissue-specific, untranslated region (5'-UTR) upstream of the coding region. The coding region spans nine exons (exon II-X), which are identical in all mRNA species and encode the same protein and 3'-UTR of the mRNA regardless of the tissue or the promoter used.
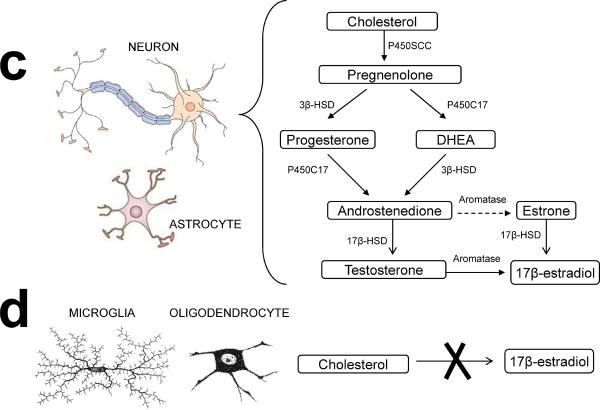
Figure 1. Estrogen synthesis in the ovary and brain.
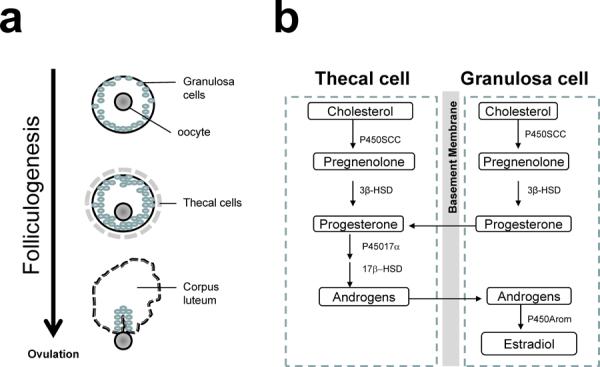
(a) Folliculogenesis. A primordial follicle consists of an oocyte and a layer of granulosa cells at the beginning of folliculogenesis. Thecal cells form a layer surrounding the granulosa cells when the follicle is activated. At end of folliculogenesis, thecal cells luteinize to form the corpus luteum after ovulation. (b) Cell-specific estrogen synthesis in the ovary. Production of estrogens starts with the synthesis of pregnenolone from cholesterol, catalyzed by the cytochrome P450 side chain cleavage enzyme (P450scc). Pregnenolone is then converted to progesterone by 3-beta-hydroxysteroid dehydrogenase (3β-HSD) in both thecal and granusola cells. Progesterone is converted to androgens via cytochrome P450 17α-hydroxylase (P45017α) and 17-beta-hydroxysteroid dehydrogenase (17β-HSD) in thecal cells during the follicular phase. The conversion of E2 is catalyzed by aromatase (P450Arom) in granulosa cells. (c) Cell-specific estrogen synthesis in the brain. Neurons and astrocytes express all enzymes required for estrogen synthesis to produce brain estrogen. (d) Microglial cells and oligodendrocytes are not able to produce estrogen. Dotted line indicates weak enzymatic activity converting testosterone to estrone in the brain.
Figure 2. Schematic representation of the human CYP19 gene reveals the alternative splicing and tissue specific promoters.
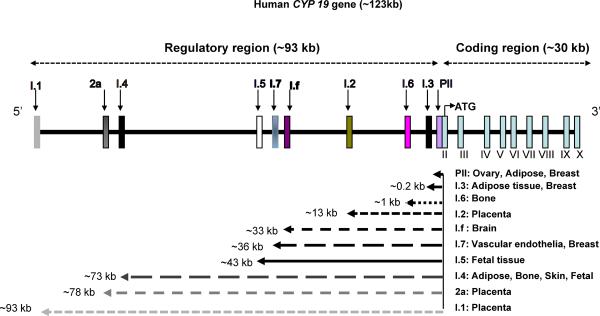
The regulatory region (~93 kb) contains 10 tissue-specific promoters and initial exons that differ in both location and size. These are alternatively spliced onto a common site just upstream of the ATG codon in exon II. The untranslated Exon I of mRNA species may be viewed as a signature of the tissue-specific promoter. Different promoters are named according to the 5'-UTR of their corresponding mature mRNA species. The coding region (~30 kb) spans exon II–X and is identical in all tissues and encodes the aromatase protein.
Distinct aromatase promoters are specifically regulated in different tissues by distinct sets of hormones or cytokines and second messenger signaling pathways, among other factors. For example, aromatase gene expression in the ovaries is regulated by follicle-stimulating hormone (FSH), which acts through cAMP via the proximal promoter II. In the placenta, aromatase gene expression is regulated via the distal promoter I.1, responsible for increasing circulating estrogen levels in pregnant women [9]. Aromatase gene expression in adipose and bone is driven by the distal promoter I.4 in response to glucocorticoids, class 1 cytokines, and tumor necrosis factor alpha (TNFα) [8], whereas in breast cancer patients, the increase in aromatase expression in breast adipose is partly due to a switch in promoter usage from the distal promoter I.4 to the cAMP responsive promoter II [10].
In addition to transcriptional regulation, aromatase activity can be changed by post-translational modifications such as phosphorylation [11]. These modifications can have an effect in much less time than is required to produced alternatively spliced transcripts, and may play an especially important role in the brain. Aromatase activity is potently inhibited by increased concentrations of ATP, Mg2+, or Ca2+, effects that are dependent on the activity of protein kinases. The presence of either genistein (a tyrosine kinases inhibitor) or staurosporine (a serine/threonine kinase inhibitor) can block the ATP, Mg2+ or Ca2+-induced inhibition completely [11, 12], supporting the idea that aromatase activity can be inhibited by phosphorylation.
Aromatase is expressed in the gonad of both sexes. In the ovary, restricted expression of aromatase is found only in granulosa and luteal cells. In the male gonads, this enzyme is widely expressed in the testis and accessory glands, maintaining the high levels of E2 needed for normal spermiogenesis, sperm maturation and sperm motility [13]. In humans, it has been clearly shown that the activity of aromatase in subcutaneous adipose stromal cells, as well as aromatase mRNA levels in the adipose tissue increase with advancing age [14].
Tissue- and cell-specific E2 synthesis
Estrogen synthesis differs between reproductive and non-reproductive women. In nonreproductive women, such as young females before puberty or women after menopause, extragonadal sites are the main sources of estrogens, including kidney, adipose tissue, skin, and brain. Unlike ovarian-synthesized estrogen, which is mainly released into the bloodstream, estrogen synthesized within these extragonadal compartments mostly acts locally at the site of synthesis and functions as a paracrine and/or intracrine factor to maintain important tissue specific functions [15]. Studies have shown that osteoclastic cell lines and cultured primary human fetal osteoblasts exhibit high levels of aromatase activity, indicating local synthesis of estrogen in bone [16]. Localized estrogen syntheses are more active in mesenchymal cells of adipose tissue and osteoblasts of bone in postmenopausal women than women at a reproductive age [17], suggesting that localized estrogen production plays significant physiological roles in tissue-specific functions. Notably, estrogen synthesis is also occurring in male testes and acts locally to regulate normal male gonadal development and spermatogenesis, in particular spermiogenesis [18]. However, although estrogen production in all tissues contributes to cellular health, the two most well-studied and influential target sites of E2 productions are the ovaries and brain.
E2 synthesis in ovaries
The ability to synthesize estrogens is an essential characteristic of healthy ovaries in reproductive females. Within the ovary, several cell types support normal ovulation during menses cycles, and as shown in figure 1, during the process of folliculogenesis both thecal and granulose cells are involved in cell-specific estrogen synthesis. Thecal cells can not produce estrogen, but do produce androgens; conversely, granulose cells cannot produce androgen from progesterone, but can convert androgen into E2. Therefore, in growing follicles androgen is released from thecal cells and transported into granulose cells where it is metabolized into estrogen by aromatase [19]. These ovary-derived estrogens are released into general circulation and target distal estrogen-responsive tissues including reproductive and non-reproductive organs. The level of estrogen synthesis is dependent upon the reproductive status of the individual and reaches highest during the reproductive years, and declines during transition and the postmenopausal period. During the menstrual cycle, E2 reaches its highest level immediately before ovulation; levels of circulating E2 during the follicular phase, pre-ovulatory phase, and luteal phase are 19–140 pg/ml, 110–410 pg/ml, and 19–160 pg/ml, respectively, whereas in postmenopausal women they are below 35 pg/ml [20]. During the menopause transition, serum E2 levels decrease by 85–90% and serum E1 levels decrease by 65–75% from mean pre-menopausal levels [21].
E2 synthesis in the brain
The brain is an additional extragondal site of estrogen synthesis, and it can synthesize estrogen from cholesterol [22], as evidenced by the presence of all enzymes required for E2 synthesis. P450scc, the single enzyme that mediates the conversion of cholesterol to pregnenolone, has been observed in the hippocampus, hypothalamus, amygdala, caudate nucleus, thalamus, cerebral cortex, cerebellum, and some circumscribed limbic areas. In addition, in the human brain, the constitutive expression and activity of aromatase, the final enzyme in the synthesis of E2, was first described in hypothalamic and limbic areas [23, 24], where estrogen plays important roles in sex differentiation and controlling sex behavior [25, 26]. Later, aromatase expression is also discovered in the “non-primary reproductive” brain areas including regions of basal forebrain, hippocampus, thalamus, cerebral cortex, cerebellum and brainstem where are involved in the regulation of neural development, synaptic plasticity and cell survival [27]. At the cellular level, aromatase is found in astrocytes and neurons, based on in situ hybridization, immunohistochemistry, and activity assays [28]. This cell-specific expression of aromatase in this brain region suggests that local E2 production may be important for specific brain functions. For instance, conditional aromatase-knockout mice are impaired in various behaviors, such as sexual, aggressive, and locomotive behaviors [29], whereas aromatase depletion in an animal model for AD led to earlier and more severe neuropathology than was observed in ovariectomized control mice [30].
As shown in figure 1, cell-specific E2 can be produced from circulating testosterone or cholesterol by neurons and astrocytes, but not by microglia or oligodendrocytes [31, 32]. Under normal physiological conditions, neurons are the major site for E2 synthesis in the brain, but elevated aromatase expression in astrocytes has been found following brain injury, suggesting that neuronal impairment can induce E2 production in astrocytes [31, 33]. By contrast, microglia and ologodendrocytes fail to produce brain E2 from cholesterol, although microglial cells do express 17β-HSD, which can convert E1 to E2 and, in theory, convert androstenedione to testosterone [32]. Brain-driven E2 is very stable and not converted to E1 in significant amounts, which differs from the situation in the ovary and other peripheral organs [34]. Together, the brain produces its own E2, in addition to utilizing circulating E2 and C19 steroid precursors (a substrate for estrogen synthesis) to provide essential substrates for estrogen synthesis in the brain [35]. It has been reported that E2 levels in the brain are 0.08–0.19 ng/g wet weight [36]; therefore, levels of brain estrogens might not be the same as those in the blood, which is often used as diagnostic marker in clinic. While age-related decline in serum E2 levels has been well characterized, brain levels of E2 can significantly differ from circulating levels, due to sequestration by sex hormone binding globulin, the presence of steroid converting enzymes in brain, as well as neurosteroidogenesis [36] and activity of local estrogen synthesis in the brain [30]. However, female brain E2 levels have been found to qualitatively mirror circulating E2 levels and show significant declines in brains of postmenopausal women compared to premenopausal women, but there is very little additional decrease with age after menopause in healthy individuals [36]
The level of E2 synthesis during ageing is critical biochemical change for females and sharp reduction of E2 during menopausal period is commonly associated with various diseases observed in post-menopausal women as well as decline of cognitive function [37].
Tissue-specific ERs and signaling
Estrogen signaling is primarily mediated through ERs. ERs include classic receptors as ERα and ERβ, members of the nuclear receptor superfamily acting as transcription factors and modulate the transcription of target genes, and membrane receptors such as GPR30 (an orphan G-protein coupled receptor) and ER-X [38, 39]. Although both types of ER transduce estrogen signals into a large variety of physiological responses in various organs, the nuclear ERs initiate the biological events in a slow motion such as in hours or even days, while the cell membrane ERs triggers an intracellular signaling cascade response in a much fast manner such as in seconds. In combination of tissue and cell-specific distribution of ERs, it is critical to understand the function and ER signaling in physiological and pathological condition during ageing.
ERα and ERβ
ERα and ERβ colocalize in many cell types, including the endothelium, epithelium, muscle, bone, cartilage, hematopoietic cells, neurons, and glia (Table 1), although each receptor exhibits a distinct pattern of tissue-specific distribution throughout the body. Although it is unclear how these patterns are dictated, it is known that ERα and ERβ are encoded by separate genes (ESR1 and ESR2, respectively) located on different chromosomes [40–44], and there are biological functional cross-talking between presence of the two receptors in certain cell types and tissues [45]. Is also known that some age-related changes of ERα and ERβ expression are tissue-specific and we will discuss in details for each age-related diseases later in the review.
Table 1.
Tissue-specific distribution of ERsa
| ER subtypes | Distribution in peripheral tissues (a) |
|---|---|
| ER α | primarily expressed in the uterus, epididymis, bone, breast, liver, kidney, white adipose tissue, stroma of prostate, theca and interstitial cells of ovary, and leydig cells of testes |
| ER β | primarily expressed in the colon, testis, bone marrow, vascular endothelium, lung, bladder, epithelium of prostate, granulosa cells of ovary |
| GPR30 | detected in adrenal medulla, renal pelvis and ovary |
| ER-X | enriched in the uterus and lung of the postnatal rodent; almost undetectable in the normal adult |
| ER subtypes | Distribution in the brain (b) | |||
|---|---|---|---|---|
| intense | moderate | weak | Absent | |
| ER α | Amygdala; Bed nucleus of the stria terminals; Periqueductal gray; Preoptic area. | Allocortex; Hypothalamus; Locus coeruleus; Spinal trigeminal nucleus | Hippocampus; Raphe nuclei; Zona incerta. | Anterior tegmental nucleus; Cerebellum; Globus pallidus; Inferior olive nucleus; Isocortex; Ponti nenuclei; Thalamus; Substantial nigra; Superior olive nucleus; Ventral tegmental area. |
| ER β | Amygdala; Bed nucleus of the stria terminals; Raphe nuclei; Substantial nigra. | Allocortex; Globus pallidus; Hippocampus; Locus coeruleus; Preoptic area; Ventral tegmental area; | Anterior tegmental nucleus; Hypothalamus; Inferior olive nucleus; Isocortex; Periqueductal gray; Pontine nuclei; Spinal trigeminal nucleus; Superior olive nucleus; Thalamus. | Cerebellum; Zona incerta. |
| GPR30 | Allocortex; Anterior tegmental nucleus; Cerebellum; Hippocampus; ypothalamus; Isocortex; Locus coeruleus; Pontine nuclei; Preoptic area; Spinal trigeminal nucleus; Superior olive nucleus. | Periqueductal gray. | Amygdala; Bed nucleus of the stria terminals; Raphe nuclei; Substantial nigra (reticular); | Ventral tegmental area |
| ER-X | enriched in the fetal baboon brain and the neocortex of the postnatal rodent, ; almost undetectable in the normal adult | |||
ERα is primarily expressed in the gonadal organs (uterus, ovary, prostate, testes, and breast), but is also present at lower levels in other tissues such as bone, liver, kidney, adipose tissue, and brain. ERβ is primarily expressed in non-gonadal tissues – colon, bone marrow, vascular endothelium, lung, bladder, and brain [46]. In the brain, both ERα and ERβ are widely distributed and are expressed in both neuronal and non-neuronal cell types. Although overlapping expression of ERα and ERβ has been observed in many brain regions, as summarized in table 1, the spatiotemporal distribution patterns of ERα and ERβ are distinct, as are the levels of expression. ERα is the most abundant subtype in the hypothalamus and amygdala, key regions involved with the neuroendocrine system, and helps to regulate the autonomic nervous system and emotional reactions. ERβ levels are highest in the hippocampus, claustrum, and cerebral cortex, and are lower in the hypothalamus [47, 48].
Gene knockout studies in mice and pharmacological studies with ER subtype-selective agonists in vivo have shown a significant correlation between the tissue distribution of ERα or ERβ and their functions [46]. ERα plays an essential role in the neuroendocrine and reproductive systems. By contrast, Studies using ERα and ERβ knockout mice have shown that ERα, but not ERβ, is required for both negative and positive feedback regulation of gonadotropin-releasing hormone neuron firing by estrogen [49]. ERβ is primarily involved in mood and cognitive activities; treatment with E2 or an ERβ agonist induced anti-anxiety-like behavior in wild-type mice, but not ERβ knockout animals, and cognitive activity is significantly reduced in ERβ knockout mice compared with wild-type mice [50]. However, there is a great functional overlap between ERα and ERβ in various tissues. For example, both ERα and ERβ are indispensable for normal ovarian function and estrogen-mediated cardioprotective actions [51, 52].
GPR30 and ER-X
GPR30, an orphan G protein coupled receptor, has been identified by several independent groups as membrane estrogen receptor which can trigger rapid estrogen non-genomic signaling independent of ERα and ERβ [53]. However, because CPR30 not only binds to estrogens but also other substances such as chemokine [54], whether or not GPR30 is a genuine novel estrogen receptor remains controversial. Furthermore, studies showed that higher concentration of E2 was needed to activate GPR30 under certain experimental condition [55] while some protection of E2 in mitochondrial and pancreatic islets are unrelated to GPR30 [56, 57]. GPR30 is expressed in many brain regions, including the hippocampus, cortex, hypothalamus, specific nuclei within the midbrain, and the Purkinje layer of the cerebellum, as well as the adrenal medulla, renal pelvis, and ovaries [38, 58]. ER-X expression is highly regulated during development, and has been shown to be enriched in the fetal baboon brain and in the neocortex, uterus, and lungs of - postnatal rodents [59]. The expression of ER-X peaks 7–10 days after birth and declines over the first postnatal month. In adults, ER-X expression is almost undetectable, but re-emerges after ischemic injury or in animal models of AD [59].
Estrogen Signaling Pathways
Although most estrogen-mediated signaling pathways are ER dependent, ER-independent signaling mechanisms exist. There are two different, but inter-related, ER-dependent mechanisms, which are classified as either “genomic” and “nongenomic” [60], based on whether the end result of ER signaling is transcription regulation. Despite these traditional classifications, recent studies have implicated some “nongenomic” pathways in gene transcription. In addition to being either genomic or nongenomic, ER-dependent pathways can initiate either in the nucleus or at the plasma membrane. Unlike ER-dependent pathways, instead of binding to estrogen receptors, estrogen initiates the ER-independent signaling pathways through regulating enzymatic activities or interacting with non-sex-steroid-hormone-nuclear-receptors in certain cells [61, 62]. Each of these regulatory mechanisms has been observed in the context of cell- or tissue-specific estrogen activities.
Pathway 1: ER-dependent, nuclear-initiated estrogen signaling
The major transcriptional effects of estrogen are nuclear-initiated, and are mediated by interactions with the nuclear receptors ERα and ERβ. ERα and ERβ share significant sequence homology, and some conservation of domain function; both ERα and ERβ serve as nuclear ligand-activated transcription factors that stimulate or repress target genes transcription [46, 63]. Despite these similarities, ERα and ERβ are structurally and functionally distinct in many aspects: ligand recognition, receptor activation, recruitment of co-regulators, and of the sets of target genes they regulate in a time- and tissue- dependent manner. For example, in vascular smooth muscle cells, the expression of inducible nitric oxide synthase is enhanced by ERβ and repressed by ERα [64].
As illustrated in figure 3, the DNA-binding domains (DBDs), which have important roles in receptor dimerization and the binding of specific DNA sequences, are the regions of greatest conservation between ERα and ERβ, with 97% homology. This is consistent with the fact that both ERs bind to estrogen responsive elements (EREs) with similar selectivity and affinity [65]. The N-terminal activation function-1 (AF-1) domain, which binds to the primary transcription machinery directly or via co-regulators, is the most divergent region between the two receptors, indicating that ERα and ERβ can signal through different intracellular pathways. The C-terminal E/F region, which contains the ligand binding domain (LBD) and AF-2 domain, is important for ligand binding-induced structural changes to the conformation of nuclear ERs, which transforms the ERs to an active state [46]. Activated ERs form homodimers (ERα/ERα, ERβ/ERβ) or heterodimers (ERα/ERβ) and translocate from the plasma to the nucleus (Figure 4). There, they recruit transcriptional machinery and other cofactors to specific DNA target sequences, designated EREs, of estrogen-responsive gene promoters, which results in regulation of ERE-containing genes [66].
Figure 3. Schematic diagram of the two human ERs, ERα and ERβ.
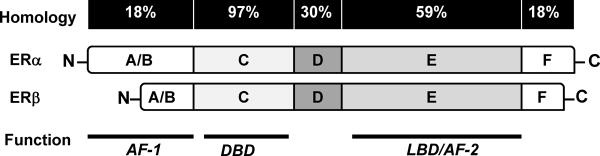
Both receptors consist of six functional domains, including the domain A/B at the N-terminal for protein–protein interactions and transcriptional activation of target-gene expression, the domain C for DNA-binding domain (DBD), domain D for the nuclear translocation signal, and domain E/F at the C-terminal for ligand-binding domain (LBD) and the ligand-dependent activation function AF-2. The percentage homology between the two receptors is indicated.
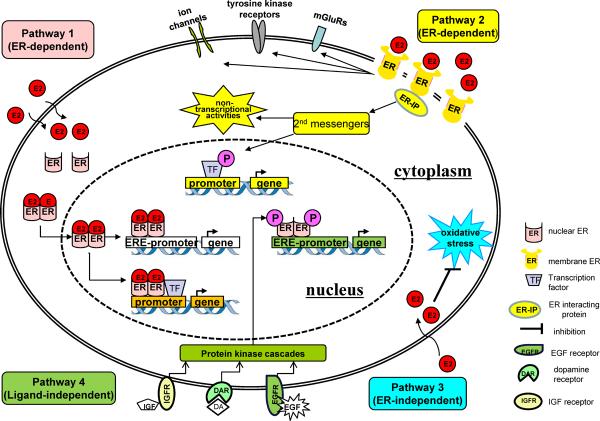
Figure 4. Signaling pathway mediated by E2 and ERs.
There are 4 estrogen and estrogen receptor signaling pathways. Pathway 1: The nuclear-initiated estrogen signaling mediated through classical ERs leads to the transcriptional changes in estrogen-responsive genes with or without EREs. Pathway 2: The membrane initiated estrogen signaling leads to diverse cytoplasmic effects, including the regulation of membrane-based ion channels, regulation of second messenger systems, and modification of transcription factors or other membrane receptors. Pathway 3: Estrogen can also exert antioxidant effects in an ER-independent manner. Pathway 4: Ligand-independent genomic actions. Growth factors (GF) activate protein-kinase cascades, leading to phosphorylation (P) and activation of nuclear ERs at EREs.
In addition to acting directly through EREs, ligand-activated nuclear ERs can regulate transcription by a nonclassical pathway through ER-DNA indirect association such as interacting with and influencing the activity of other transcription factors, such as stimulating protein-1 (SP-1), activator protein 1 (AP-1), nuclear factor κB (NF-κB), and c-jun [67]. Roughly 35% of the categorized human primary E2-responsive genes are transcripted via the ER-indirect DNA association [68]. Among the transcription factors involved in this pathway, SP-1 is the most predominant mediator of ER-DNA indirect interaction [68] and is involved in several E2-promoted genes, such as the low-density lipoprotein (LDL) receptor [69], and endothelial nitric oxide sinthase (eNOS) [70]. In addition, recent genomic studies found enriched AP-1 motifs within the regions bound by ER through mapping ER-binding sites [71–73], suggesting an extensive crosstalk between ER and AP-1 at genomic level. The ER-DNA indirect association plays a critical role in various biological events. For example, ERa-AP1 promotes breast cancer cell proliferation through co-regulating E2F1 and cyclin D1, genes involved in cell cycle [74, 75]. The outcome from ER-DNA indirect interaction can be opposite even with same transcription factor, such as ERα activates, whereas ERβ inhibits, AP-1 dependent transcription of cyclin D1 in the presence of E2 in Hela cells [76]. Together suggest an ER subtype-specific manner of the ER-DNA indirect association even ERα and ERβ interact with same transcription factor.
The ER-dependent nuclear signaling pathways change during ageing. Studies found a significant reduction of expression and alternation of p68 RNA helicase, which interacts directly with ERα AF-1 domain, in mice brain during ageing [77]. p68 RNA helicase acts as a transcriptional coactivator specific for the AF-1 domain of ERα in human [78]. The reduction of the expression level and the interaction with ERα of p68 RNA helicase in aged mice suggest the decline of ER-dependent signaling pathway in estrogen-mediated brain functions during aging [77].
Pathway 2: ER-dependent, membrane-initiated estrogen signaling
An alternative ER-dependent mechanism is the “membrane-initiated” pathway, in which estrogen-induced signaling is initiated in the membrane or cytoplasm and the downstream effects are only partially dependent on translation or transcription (see figure 4, pathway 2) [79]. This pathway is mainly responsible for a series of acute, rapid estrogen actions that occur much faster than transcriptional processes and play critical roles in the nervous system, skeleton, liver, and other tissues [80]. As shown in figure 4, membrane associated ERs mediate the rapid effects of estrogens through at least two mechanisms: crosstalk with other membrane receptors or activation of a variety of cytoplasmic signaling cascades. Previous studies have shown that membrane-initiated estrogen signaling involves modifying several growth factors, neurotransmitter receptors, tyrosine kinase receptors, and insulin-like growth hormone receptors [81]. For example, E2 activates membrane associated ERα and ERβ to interact with metabotropic glutamate receptors (mGluRs), leading to the activation of mGluR signaling without glutamate [82]. Studies showed that the membrane-associated ER pathway can also be mediated via the activation of different protein kinase cascades, such as MAPK/ERK, PI3K/AKT, cyclic AMP, PKA, PKC and the tyrosine kinases pathways [83].
Although ERα and ERβ are classical nuclear receptors, recent studies demonstrate that ERα and ERβ are expressed on the cell membrane in the brain by ultrastructural studies [84], and some of these estrogen-induced rapid effects can be triggered by ERα- or ERβ-specific ligands and diminished by knocking out ERα or ERβ [46]. Both nuclear- and membrane-associated ERα or ERβ are encoded by the same genes, respectively [85]. ERα and ERβ can be translocated to the plasma membrane by different post-transcriptional modifications [86]. A highly conserved motif within the C-terminal LBD domain of the receptors is responsible for this translocation [87].
Estrogen can also exert rapid effects through several novel membrane ERs in the CNS, including ER-X as well as G protein-coupled receptors, such as GPR30 and Gq-mER [58, 59]. ER-X is a high-affinity, saturable plasma membrane-associated ER enriched in the brain, uterus and lungs, and functionally distinct from ERα and ERβ [59]. Studies found that ERα can inhibit ERK activation, while ER-X promoted tyrosine phosphorylation of ERK1/ERK2, and activated the MAPK/ERK pathway [88]. GPR30 is classified as a membrane-associated ER as it mediates estrogen-induced G-protein activation [58]. GPR30 is located in the plasma membrane, Golgi apparatus and endoplasmic reticulum [89]. GPR30 can mediate the rapid activation of several signaling cascades in response to estrogen, such as PI3K and calcium signaling [58].
Pathway 3: the ER-independent pathway
Although the majority of the biological actions of estrogen are mediated through ERs, recent studies have demonstrated that estrogens can exert antioxidant effects and suppress oxidative stress in an ER-independent pathway [90]. Instead of binding to ER and triggering ER-dependent signaling, estrogen can regulate enzymatic activities or interact with non-sex-steroid-hormone-nuclear-receptors to protect against cell damages. For example, recent studies found that estrogens effectively prevent pro-oxidant stress by preventing ROS release from damaged mitochondria with independent of any known ERs [62]. The estrogen anti-oxidative action is related to the phenolic A ring of estrogens which is an intrinsic antioxidant and provides antioxidant/redox cycling activity in neurons that complements other neuroprotective activities of estrogens [62]. A large body of evidence from neurons and neuronal cell lines has demonstrated that E2 possesses specific antioxidant properties and suppresses oxidative stress induced by hydrogen peroxide, superoxide anions and other pro-oxidants [91]. Furthermore, using estrogen receptor knockout technology, studies found that E2 can promote breast cancer in female mice with deletion of endogenous ERα and ERβ, suggesting an ER-independent effects of E2 on tumor formation which likely occur via estrogen metabolites which does not bind to ERs [92]. In addition, vascular effects of estrogen are mediated via non-sex-hormone-nuclear receptor such as PPARγ-regulation in the vascular compartment might also be independent from ERs [61].
Pathway 4: Ligand-independent activation of ER
In addition to the estrogen-dependent ER pathway, recent evidence has shown that, in the normal physiology of animals, ERs can also be activated in a ligand-independent manner by a variety of factors, including neurotransmitters such as dopamine [93], growth factors such as epidermal growth factor (EGF) and insulin-like growth factor-1 (IGF-1) [94, 95], activators of particular intracellular signaling pathways, such as protein kinase C [96], protein kinase A [97], MAPK [98], phosphatidylinositol 3-kinase [99]. The ligand-independent pathway activation is largely related to phosphorylation of ERs by cellular protein kinase. For example, EGF can trigger the phosphorylation of Ser 118 in the AF-1 domain of ERα and activate the transcriptional activity of ERα [100]. Likewise, protein kinase A can also cause phosphorylation of Ser 236 in the DNA binding domain of ERα and regulate dimerization of the receptor [101]. For example, recent data suggests that dopamine can regulate expression of progestin receptors within restricted regions of the developing rat brain by activating estrogen receptors in a ligand-independent manner [102]. Furthermore, ERα is responsible for mediating leptin-induced cell proliferation in a ligand-independent manner in ovarian cancer cells [103].
Estrogen and ERs in age–related diseases
As age-related changes of endogenous estrogen production as well as estrogen receptor expression can be different depending on types of cell or tissue in human body, it is important to understand the tissue-specific roles of estrogen and estrogen receptors in age-related diseases. For example, reduction of endogenous estrogen levels increases risk of bone fracture, cardiovascular disease and Alzheimer's disease in postmenopausal women. Studies showed that estrogen therapy plays osteoprotective roles in both osteoporotic humans and rodents [104], while whether estrogen therapy can protect against heart disease or AD remain controversial [105]. Although it is unclear why the effeteness of estrogen therapy differs among those age-related diseases, literatures showed that healthy cells such as neurons response to estrogen treatment with positive and beneficial outcome while cells with unhealthy condition such as neurological healthy is compromised, estrogen explore might accelerate the disease process [106]. This bias of estrogen action is also confirmed in recent studies which showed that levels of local tissue-specific estrogen and estrogen receptors contribute the response to estrogen therapy in postmenopausal women [30, 107].
Cardiovascular Diseases
Cardiovascular disease (CVD) is the leading cause of death in both sexes. The incidence of CVD is much lower in premenopausal women, who usually have lower blood pressure and a much lower risk of developing the disease, compared with age-matched men. However, this advantage for women gradually disappears after menopause with the cessation of ovarian function and reductions in estrogen levels; eventually the risk of CVD in postmenopausal women becomes higher than age-matched men [108]. In animal models of CVD, young females have lower rates of vascular injury, a slower progression to heart failure, and lower mortality than males, but these differences can be reduced or eliminated by estrogen deficiency or ovariectomy [109].
Some of the beneficial effects of estrogen that protect the cardiovascular system are mediated through ER-dependent mechanisms. For example, in mice, the effects of estrogens mediated by ERα can improve vascular function and reduce atherosclerosis [110]. Some studies also showed that estrogen can prevent cardiac fibrosis by blocking the fibroblast to myofibroblast transition via interactions with ERβ [111]. However, unlike ERβ plays an important neuroprotective role in the central nervous system, in the heart, ERα appears to play a prominent role in most of the other tissues. The critical role of ERa in heart is evidenced by findings from another study showed a increased the ratio of ERβ/ERα in both vascular endothelium and smooth muscle in aged female mice causes the reversal in the antioxidant effect of estrogen to a pro-oxidant profile and is responsible for the increased oxidative stress during aging [112]. In addition, estrogen can reduce ischemia and reperfusion injury and preserve cardiac function via GPR30 and transactivating epidermal growth factor receptors [113]. The effect of ERs on the cardiovascular function might be a tissue-specific since studies have found that a down-regulation of ERβ expression in aged human brain while both ERα and ERβ plays neuroprotective roles in against age-related cognitive decline [114, 115].
However, there is conflicting evidence concerning the effects of estrogen treatment on CVD. Data from the Heart and Estrogen/Progestin Replacement Study (HERS-I, and the follow-up HERS-II) failed to confirm the protective effects on the heart [105], and the Women's Health Initiative (WHI) combined estrogen-progestin trial reported an increased risk for coronary heart disease in patients receiving estrogen supplements [116]. Questions remain regarding the effectiveness of the estrogen formulations used, dosing regimens, and routes of administration in those clinical trials, making it important to resolve the differences between the beneficial effects observed in the laboratory and the absence of such effects in clinical settings, at least as they pertain to estrogen-based therapeutic strategies for treating menopause-related cardiovascular risk factors.
Osteoporosis
Postmenopausal women have a higher risk of developing osteoporosis, and the prevalence of osteoporosis in females increases significantly in postmenopausal populations after ovarian function is lost, and continues to increase with age throughout the postmenopausal period [117]. Similarly, female rodents develop an osteoporotic phenotype after ovariectomy, and lower circulating estrogen levels were found to be associated with increased rates of bone loss [118]. Bone density has been reported to increase with estrogen treatment in a dose- and duration-dependent manner, which then prevents osteoporotic fractures [119].
Estrogen protects women from osteoporosis by regulating bone metabolism. Osteroporosis is characterized by a progressive loss of bone tissue and is associated with low bone mass, compromised microarchitecture of the bone, and increased risk of fractures; estrogens have been shown be osteoprotective by inhibiting high bone turnover and preventing bone loss in both osteoporotic humans and rodents [104]. Although both ERα and ERβ expressed in bone, the molecular role of bone receptors in the osteoprotection induced by estrogen remains unclear. It has been suggested that estrogens protect bone loss by inhibition of bone resorption mediated by the osteoclastic ERα, through the shortened lifespan of osteoclasts in female mice [120]. In addition, estrogen is also promoting bone formation by increasing osteoblast. To study the cell- and tissue-specific ER in bone formation, a recent study using a mouse model with osteoblast-specific ERα inactivation and found significant bone loss in female by reducing bone formation in females, not in males [121]. While ERα can modulate gene transcriptional through classic (ERE biding) and nonclassic (non-ERE binding) pathways (see figure 4, pathway 1), studies using gene knockout and knockin technologies in mice demonstrated that ERα-mediated osteroprotective actions is modulated through ERE biding signaling pathway in mice [122], suggesting a tissue-specific ER signaling in the bone turnover. As we mentioned above, ER can also be activated through ligand-independent signaling (figure 4, pathway 4). Indeed, recent studies found that mice with ovariectomy or overexpression of ERE did not change the mechanic loading-induced upregulation of osteogenic activity. Furthermore, mice with ERα gene knockout or specific inactivation of AF-1 in ERα showed a reduced response to mechanic loading-induced osteogenic activity [123], suggesting a ligand-independent pathway such as ERα-AP1 interaction, involved in the bone formation. Together, estrogen prevents bone loss and promotes bone formation through interactions with ERα with classic ER-dependent and ligend-independent signaling pathways. The bone-specific estrogen and ERα signaling pathways provides a potential strategy of new anti-osteoporotic drug development, such as a ideal selective estrogen receptor modulators for the prevention and treatment of postmenopausal osteoporosis and vertebral fractures with less side effects on uterus or breast where ERα also highly expressed.
Alzheimer's disease
AD is both the most common neurodegenerative disease and the most common form of dementia. It is characterized by brain-specific senile plaques composed of aggregated amyloid β (Aβ) peptide and neurofibrillary tangles [124]. In both genders, epidemiological studies show an increased risk for AD with the age-related loss of sex steroid hormones. It is also commonly reported that AD is more prevalent in postmenopausal women relative to age-matched men [125]. The precipitous loss of ovarian estrogens and progestogens at menopause has been presumed to account for the increased AD susceptibility in women, but recent studies in both animals and humans suggest that depletion of brain-derived estrogen, rather than the circulating estrogen, is a more direct and significant risk factor for developing AD [30].
A number of observational studies have suggested that estrogen replacement therapy (ERT) might protect postmenopausal women against AD and age-associated cognitive decline [126, 127]. However, the results from the Women's Health Initiative Memory Study (WHIMS) do not support the use of ERT to reduce the risk of AD in postmenopausal women older than 65 years [128]. Other randomized clinical trials in younger women offer a possible explanation for this discrepancy. For example, the Cache County Study reported that AD risk was only reduced in women using ERT long-term compared to short-term [129], suggesting that early initiation of ERT, most likely nearer the time of menopause, is essential for the beneficial effects on cognitive function in postmenopausal women.
As mentioned above, one of the major arguments regarding these controversial findings is the timing of ERT. The complications associated with ERT may include hormone responsiveness with age, that is, during the menopause transition, neural sensitivity to sex steroid hormones may weaken, indicating a “critical period” around the time of menopause in which ERT needs to be prescribed to protect cognitive function [130, 131]. Early ERT around menopause would allow the body to maintain hormone responsiveness by regulating hormone levels before the irreversible, age-related hormone fluctuations begin. It is also related to the condition of neuronal healthy since the epidemiological observation analysis showed a reduction in risk of AD in women who had estrogen therapy started at the time of menopause while estrogen increase risk of AD in those women began estrogen therapy at 10–15 years after menopause or who already developed AD [106, 129, 132–134]. Furthermore, our recent studies not only confirmed with the benefit effect of early ERT, but also showed that the level of brain estrogen level determines the effect of estrogen therapy in protect against AD pathology in animal model [107].
The level of expression, subcellular distribution and activities of ERα and ERβ can change during aging, which lead to the likely net outcome of a differential response to estrogen in the aging brain. The age-related expression of ERα and ERβ has been studied in rodent cerebral cortex and the result showed that ERα and ERβ undergo differential changes in expression during aging: ERα level did not change with aging while ERβ level decreases signicantly with advancing age in both sexes [114]. In female rats, the age-related decrease in ERβ expression in the cortex is correlated with a decline in cognitive function during aging [115]. The age-related changes of ERs are also evidenced by the subcellular distribution of ERα and ERβ, such as a significant attenuation of both ERα and ERβ expression in spine synapse complexes in hippocampal CA1 region in aged female rats compared to young rats [135, 136], which is correlated to the decreased ability of E2 in promoting spine density in the hippocampus during aging [137]. However, the changes of ERs observed in the brain during ageing is a tissue-specific event since, instead of decrease of ERβ expression, a gradual increase in ERβ expression was observed in uterine arteries during aging even 10 years after of menopause onset, while there is only a slight increase in ERα expression during aging [138]. The age-related elevation of ERβ expression in uterine arteries of the postmenopausal women is significant positive associated with the proinflammatory effects of estrogen.
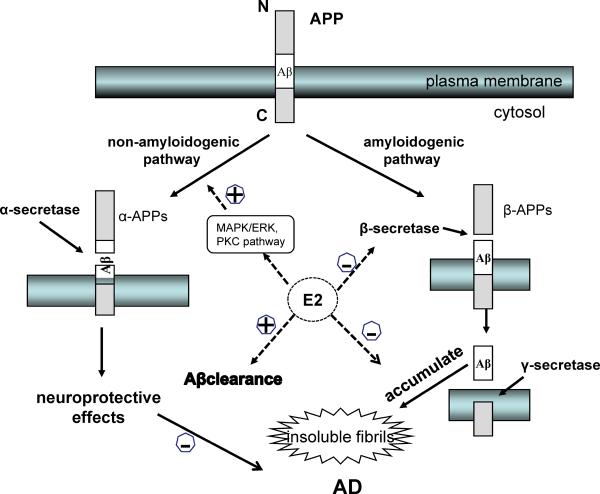
The accumulation of Aβ, a proteolytic byproduct of amyloid precursor protein (APP) metabolism, is an important aspect of AD pathology [124]. APP is processed by two competing pathways, the amyloidogenic pathway through β-secretase (BACE1) and γ-secretase, which produce β-APPs and Aβ40/Aβ42 peptides, and the predominant non-amyloidogenic pathway via α-secretase, which produces neuroprotective α-APPs and several non-amyloidogenic peptides [139]. Estrogens can reduce Aβ production by favoring the non-amyloidogenic pathway through activation of MARK/ERK, inhibiting the amyloidonenic pathway by reducing the levels of BACE1, and promoting Aβ clearance by stimulating microglial phagocytosis and degradation as well as regulating levels of major enzymes involved in Aβ degradation [88, 140].
Other mechanisms also contribute to the protective roles of estrogens in the context of AD. For example, estrogens can regulate the Bcl-2 protein family by increasing the expression of antiapoptotic Bcl-xL and Bcl-w, and suppressing the expression of proapoptotic Bim to protect against neuronal loss induced by Aβ-mediated toxicity [141]. Estrogens can also decrease the level of hyperphosphorylated tau (a major component of neurofibrillary tangles) by modulating the kinases and phosphatases involved in tau phosphorylation, such as GSK-3β, Wnt, or PKA pathways [142].
Notably, estrogen can enhance neural plasticity and affect related cognitive functions as well as regenerative potential of brain. As reviewed by Brinton [143], the benefit effects of estrogen on neural plasticity occur at three levels, such as cellular, morphological and synaptic function levels. Studies demonstrated that E2 can increase neurogenesis in various brain regions such as dentate gyrus of hippocampus [144]. These estrogen-induced newly generated neurons in the hippocampus contribute to brain region-specific type of learning and memory [145, 146]. In addition, E2 can also rapidly increase dendritc spine number or dendritic spine contacts in hippocampus, themedial amygdala and hypothalamus, and thus enhance performance on a hippocampal-dependent memory task in monkeys [147, 148]. Furthermore, E2 is a potent and efficacious potentiator of synaptic transmission in hippocampal system [149]. Together, E2 plays important roles in promoting neurogenesis and neuronal plasticity in order to keep healthy cognitive function and protect against cognitive decline in females during aging.
Parkinson's disease
PD is the second most common neurodegenerative disease, after AD. In contrast to higher incidence of AD, epidemiological observations found lower risk of PD in women. One might expect is the difference in the age at onset of the diseases. The average age at onset of PD is 60 years old with 10% of PD are younger than 40 years old. In addition, recent neuropathology evidence suggest a 5–20 years long preclinical period before the motor manifestations in PD patients [150]. In AD, the most common onset of the disease is at age of 65 or older which is further away from the onset of menopause. The estrogen protection of PD and AD is also evidenced by findings of women with early age at menopause have higher risk of dementia [151] and a delayed onset of PD in women with higher number of pregnancies, longer fertile life and longer cumulative length of pregnancies [152]. Furthermore, estrogen replacement therapy showed improvement of motor symptoms in female PD patients [153]. Together, it might explain the epidemiological observations of lower incidence of PD and higher risk of AD in women and the protective role of estrogens.
While ERa, ERb and GPR30 all been identified in the striatum and substantia nigra, the most affect brain regions in PD [154], ERα and ERβ could have distinct roles in neuroprotection against insult-induced dopamine cell death. Studies found that ERα is the dominant receptor involved in neuroprotection in PD, while ERβ plays little role in neuroprotection [155, 156]. Compare to wild type control mice, mice with genetic depletion of ERα, not for ERβ depletion, are more vulnerable to insult-induced cell toxicity and 17β-estradiol failed to rescue the dopamine neuronal death caused by the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in PD model, suggesting an ER-specific role in PD pathology.
In addition to the ER-dependent PD pathology, two major ER-dependent signaling pathways are also involved in the neuroprotective roles of estrogen against PD [157]. One is the extracellular signal-regulated kinase (ERK1/2) pathway and the other is the phosphatidylinositol 3 kinase (PI3K)/Akt pathway. In PD animal model studies, estrogen treatment protect against cell death and inhibitor of the PI3K/Akt pathway (LY294002) blocked the survival effects of estrogen in dopamine neurons, while inhibition of the MAPK/ERK signaling was ineffective [158]. The mechanism by which ERs activate ERK1/2 and Akt signaling involves multiple interactions with signaling proteins, such as calveolin proteins, G proteins, Src, and the p85a regulatory subunit of PI3K [159]. These two pathways converge downstream to some common targets. For example, both pathways can inhibit the pro-apoptotic protein Bad through phosphorylation [160]. Furthermore, the activation of ERK1/2 and PI3K/Akt pathway can also activate various transcription factors such as cAMP-response element-binding protein (CREB), which finally induces the expression of target genes such as Bcl-2 [161, 162].
Together, maintaining reproductive level of endogenous estrogen in females might prevent women to develop PD. Recent animal studies further demonstrated that ERα plays neuroprotection in PD via estrogen-dependent and --independent signaling pathways. These newly reported evidences further revealed molecular mechanisms of tissue- and disease-specific effects of estrogen on PD.
Conclusion
Although the roles of estrogens in gonadal organs are well understood, recent studies have begun to demonstrate that localized estrogen production plays tissue-specific roles, with or without dependency on circulating estrogen. The cell- and tissue-specific actions of estrogen and ERs are directly involved various age-related diseases. Nevertheless, it remains unclear how the local synthesis of estrogens and their respective receptor functions are controlled in normal aging. We believe that estrogens are no longer just sex hormones, but important therapeutic targets for preventing diseases as disparate as osteoporosis, heart disease, and neurodegeneration.
Figure 5. Estrogen prevents the accumulation of Aβ.
Estrogen can reduce Aβ production by favoring the non-amyloidogenic pathway of APP processing through activation of the MAPK/ERK or PKC pathways, while simultaneously promoting Aβ clearance in several ways, such as stimulating microglial phagocytosis and degradation as well as regulating levels of major enzymes involved in Aβ degradation.
Acknowledgment
This work was supported by grants from the Alzheimer's Association IIRG-07-59510, American Health Assistance Foundation Grant G2006-118, NIH R01AG032441-01 and R01AG025888. We also thank Alex Bishop, Jon Reed and Balaji Jothishankar for editing and proof reading.
References
- 1.Rao ML, Kolsch H. Effects of estrogen on brain development and neuroprotection--implications for negative symptoms in schizophrenia. Psychoneuroendocrinology. 2003;28(Suppl 2):83–96. doi: 10.1016/s0306-4530(02)00126-9. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, et al. Estrogen Facilitates Spinal Cord Synaptic Transmission via Membrane-bound Estrogen Receptors: IMPLICATIONS FOR PAIN HYPERSENSITIVITY. J Biol Chem. 2012;287:33268–33281. doi: 10.1074/jbc.M112.368142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarate S, et al. Estrogens induce expression of membrane-associated estrogen receptor alpha isoforms in lactotropes. PLoS One. 2012;7:e41299. doi: 10.1371/journal.pone.0041299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkhauser M. [Treatment of pain in estrogen deficiency] Arch Gynecol Obstet. 1996;259(Suppl 1):S74–79. [PubMed] [Google Scholar]
- 5.Kotov A, et al. Regulation of estrogen activity by sulfation in human Ishikawa endometrial adenocarcinoma cells. J Steroid Biochem Mol Biol. 1999;68:137–144. doi: 10.1016/s0960-0760(99)00022-9. [DOI] [PubMed] [Google Scholar]
- 6.Guo H, et al. Lipocalin 2 deficiency alters estradiol production and estrogen receptor signaling in female mice. Endocrinology. 2012;153:1183–1193. doi: 10.1210/en.2011-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santen RJ, et al. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev. 2009;30:343–375. doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]
- 8.Simpson ER, et al. Aromatase expression in health and disease. Recent Prog Horm Res. 1997;52:185–213. discussion 213–184. [PubMed] [Google Scholar]
- 9.Toda K, et al. Transcriptional regulation of the human aromatase cytochrome P450 gene expression in human placental cells. J Steroid Biochem Mol Biol. 1995;53:181–190. doi: 10.1016/0960-0760(95)00032-u. [DOI] [PubMed] [Google Scholar]
- 10.Harada N, et al. Tissue-specific expression of the human aromatase cytochrome P-450 gene by alternative use of multiple exons 1 and promoters, and switching of tissue-specific exons 1 in carcinogenesis. Proc Natl Acad Sci U S A. 1993;90:11312–11316. doi: 10.1073/pnas.90.23.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlier TD, et al. Human and quail aromatase activity is rapidly and reversibly inhibited by phosphorylating conditions. Endocrinology. 2011;152:4199–4210. doi: 10.1210/en.2011-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balthazart J, et al. Phosphorylation processes mediate rapid changes of brain aromatase activity. J Steroid Biochem Mol Biol. 2001;79:261–277. doi: 10.1016/s0960-0760(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 13.Carreau S, et al. Aromatase and estrogens in man reproduction: a review and latest advances. Adv Med Sci. 2008;53:139–144. doi: 10.2478/v10039-008-0022-z. [DOI] [PubMed] [Google Scholar]
- 14.Misso ML, et al. Adipose aromatase gene expression is greater in older women and is unaffected by postmenopausal estrogen therapy. Menopause. 2005;12:210–215. doi: 10.1097/00042192-200512020-00016. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, et al. Sex steroid synthesis in human skin in situ: the roles of aromatase and steroidogenic acute regulatory protein in the homeostasis of human skin. Mol Cell Endocrinol. 2012;362:19–28. doi: 10.1016/j.mce.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe M, et al. Aromatase expression in a human osteoblastic cell line increases in response to prostaglandin E(2) in a dexamethasone-dependent fashion. Steroids. 2007;72:686–692. doi: 10.1016/j.steroids.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Janssen JM, et al. Estradiol formation by human osteoblasts via multiple pathways: relation with osteoblast function. J Cell Biochem. 1999;75:528–537. doi: 10.1002/(sici)1097-4644(19991201)75:3<528::aid-jcb16>3.3.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Carreau S, et al. Role of estrogens in spermatogenesis. Front Biosci (Elite Ed) 2012;4:1–11. doi: 10.2741/e356. [DOI] [PubMed] [Google Scholar]
- 19.Hillier SG, et al. Follicular oestrogen synthesis: the `two-cell, two-gonadotrophin' model revisited. Mol Cell Endocrinol. 1994;100:51–54. doi: 10.1016/0303-7207(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 20.Stricker R, et al. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med. 2006;44:883–887. doi: 10.1515/CCLM.2006.160. [DOI] [PubMed] [Google Scholar]
- 21.Khosla S, et al. Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: a population-based study. J Clin Endocrinol Metab. 1997;82:1522–1527. doi: 10.1210/jcem.82.5.3946. [DOI] [PubMed] [Google Scholar]
- 22.Do Rego JL, et al. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30:259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Naftolin F, et al. Aromatization of androstenedione by the diencephalon. J Clin Endocrinol Metab. 1971;33:368–370. doi: 10.1210/jcem-33-2-368. [DOI] [PubMed] [Google Scholar]
- 24.Naftolin F, et al. Aromatization of androstenedione by limbic system tissue from human foetuses. J Endocrinol. 1971;51:795–796. doi: 10.1677/joe.0.0510795. [DOI] [PubMed] [Google Scholar]
- 25.Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol. 2008;29:1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright CL, et al. Cellular mechanisms of estradiol-mediated sexual differentiation of the brain. Trends Endocrinol Metab. 2010;21:553–561. doi: 10.1016/j.tem.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azcoitia I, et al. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–147. doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Gatson JW, et al. Aromatase is increased in astrocytes in the presence of elevated pressure. Endocrinology. 2011;152:207–213. doi: 10.1210/en.2010-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honda S, et al. Behavioral analysis of genetically modified mice indicates essential roles of neurosteroidal estrogen. Front Endocrinol (Lausanne) 2011;2:40. doi: 10.3389/fendo.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue X, et al. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer's disease animal model. Proc Natl Acad Sci U S A. 2005;102:19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yague JG, et al. Aromatase expression in the normal and epileptic human hippocampus. Brain Res. 2010;1315:41–52. doi: 10.1016/j.brainres.2009.09.111. [DOI] [PubMed] [Google Scholar]
- 32.Gottfried-Blackmore A, et al. Brain microglia express steroid-converting enzymes in the mouse. J Steroid Biochem Mol Biol. 2008;109:96–107. doi: 10.1016/j.jsbmb.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carswell HV, et al. Brain aromatase expression after experimental stroke: topography and time course. J Steroid Biochem Mol Biol. 2005;96:89–91. doi: 10.1016/j.jsbmb.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Hojo Y, et al. Hippocampal synthesis of sex steroids and corticosteroids: essential for modulation of synaptic plasticity. Front Endocrinol (Lausanne) 2011;2:43. doi: 10.3389/fendo.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kancheva R, et al. Neuroactive steroids in periphery and cerebrospinal fluid. Neuroscience. 2011;191:22–27. doi: 10.1016/j.neuroscience.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 36.Rosario ER, et al. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer's disease. Neurobiol Aging. 2011;32:604–613. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genazzani AR, et al. Estrogen, cognition and female ageing. Hum Reprod Update. 2007;13:175–187. doi: 10.1093/humupd/dml042. [DOI] [PubMed] [Google Scholar]
- 38.Levin ER. Plasma membrane estrogen receptors. Trends Endocrinol Metab. 2009;20:477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maggiolini M, Picard D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J Endocrinol. 2010;204:105–114. doi: 10.1677/JOE-09-0242. [DOI] [PubMed] [Google Scholar]
- 40.Greene GL, et al. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- 41.Green S, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 42.Kuiper GG, et al. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gosden JR, et al. Localization of the human oestrogen receptor gene to chromosome 6q24----q27 by in situ hybridization. Cytogenet Cell Genet. 1986;43:218–220. doi: 10.1159/000132325. [DOI] [PubMed] [Google Scholar]
- 44.Enmark E, et al. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 45.Couse JF, et al. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 46.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 47.Shughrue PJ, et al. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 48.Osterlund MK, et al. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. J Clin Endocrinol Metab. 2000;85:3840–3846. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- 49.Christian CA, et al. Classical estrogen receptor alpha signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology. 2008;149:5328–5334. doi: 10.1210/en.2008-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walf AA, et al. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krege JH, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mammary Gland Biol Neoplasia. 1997;2:323–334. doi: 10.1023/a:1026339111278. [DOI] [PubMed] [Google Scholar]
- 53.Thomas P, et al. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 54.Catusse J, et al. Attenuation of CXCR4 responses by CCL18 in acute lymphocytic leukemia B cells. J Cell Physiol. 2010;225:792–800. doi: 10.1002/jcp.22284. [DOI] [PubMed] [Google Scholar]
- 55.Pedram A, et al. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 56.Bopassa JC, et al. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;298:H16–23. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tiano JP, et al. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents beta cell failure in rodent models of type 2 diabetes. J Clin Invest. 2011;121:3331–3342. doi: 10.1172/JCI44564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Revankar CM, et al. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 59.Toran-Allerand CD, et al. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 61.Tiyerili V, et al. Estrogen improves vascular function via peroxisome-proliferator-activated-receptor-gamma. J Mol Cell Cardiol. 2012;53:268–276. doi: 10.1016/j.yjmcc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 62.Richardson TE, et al. Estrogen prevents oxidative damage to the mitochondria in Friedreich's ataxia skin fibroblasts. PLoS One. 2012;7:e34600. doi: 10.1371/journal.pone.0034600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kininis M, et al. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol. 2007;27:5090–5104. doi: 10.1128/MCB.00083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsutsumi S, et al. Differential regulation of the inducible nitric oxide synthase gene by estrogen receptors 1 and 2. J Endocrinol. 2008;199:267–273. doi: 10.1677/JOE-07-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hewitt SC, et al. Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. J Biol Chem. 2010;285:2676–2685. doi: 10.1074/jbc.M109.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gottsch ML, et al. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29:9390–9395. doi: 10.1523/JNEUROSCI.0763-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O'Lone R, et al. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- 69.Li C, et al. Requirement of Sp1 and estrogen receptor alpha interaction in 17beta-estradiol-mediated transcriptional activation of the low density lipoprotein receptor gene expression. Endocrinology. 2001;142:1546–1553. doi: 10.1210/endo.142.4.8096. [DOI] [PubMed] [Google Scholar]
- 70.Chambliss KL, Shaul PW. Rapid activation of endothelial NO synthase by estrogen: evidence for a steroid receptor fast-action complex (SRFC) in caveolae. Steroids. 2002;67:413–419. doi: 10.1016/s0039-128x(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 71.Carroll JS, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, et al. The genome landscape of ERalpha- and ERbeta-binding DNA regions. Proc Natl Acad Sci U S A. 2008;105:2604–2609. doi: 10.1073/pnas.0712085105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao C, et al. Genome-wide mapping of estrogen receptor-beta-binding regions reveals extensive crosstalk with transcription factor activator protein-1. Cancer Res. 2010;70:5174–5183. doi: 10.1158/0008-5472.CAN-09-4407. [DOI] [PubMed] [Google Scholar]
- 74.Dahlman-Wright K, et al. Interplay between AP-1 and estrogen receptor alpha in regulating gene expression and proliferation networks in breast cancer cells. Carcinogenesis. 2012;33:1684–1691. doi: 10.1093/carcin/bgs223. [DOI] [PubMed] [Google Scholar]
- 75.Stender JD, et al. Estrogen-regulated gene networks in human breast cancer cells: involvement of E2F1 in the regulation of cell proliferation. Mol Endocrinol. 2007;21:2112–2123. doi: 10.1210/me.2006-0474. [DOI] [PubMed] [Google Scholar]
- 76.Liu MM, et al. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem. 2002;277:24353–24360. doi: 10.1074/jbc.M201829200. [DOI] [PubMed] [Google Scholar]
- 77.Ghosh S, Thakur MK. Interaction of estrogen receptor-alpha transactivation domain with nuclear proteins of mouse brain: p68 RNA helicase shows age- and sex-specific change. J Neurosci Res. 2009;87:1323–1328. doi: 10.1002/jnr.21948. [DOI] [PubMed] [Google Scholar]
- 78.Endoh H, et al. Purification and identification of p68 RNA helicase acting as a transcriptional coactivator specific for the activation function 1 of human estrogen receptor alpha. Mol Cell Biol. 1999;19:5363–5372. doi: 10.1128/mcb.19.8.5363. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 80.Chimento A, et al. 17 beta-estradiol activates rapid signaling pathways involved in rat pachytene spermatocytes apoptosis through GPR30 and ER alpha. Mol Cell Endocrinol. 2010;320:136–144. doi: 10.1016/j.mce.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 81.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayashi S, Yamaguchi Y. Estrogen signaling pathway and hormonal therapy. Breast Cancer. 2008;15:256–261. doi: 10.1007/s12282-008-0070-z. [DOI] [PubMed] [Google Scholar]
- 84.Milner TA, et al. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- 85.Razandi M, et al. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 86.Levin ER. Cell localization, physiology, and nongenomic actions of estrogen receptors. J Appl Physiol. 2001;91:1860–1867. doi: 10.1152/jappl.2001.91.4.1860. [DOI] [PubMed] [Google Scholar]
- 87.Pedram A, et al. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 88.Singh M, et al. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsuda K, et al. Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci Lett. 2008;441:94–99. doi: 10.1016/j.neulet.2008.05.108. [DOI] [PubMed] [Google Scholar]
- 90.Haas MJ, et al. Estrogen-dependent inhibition of dextrose-induced endoplasmic reticulum stress and superoxide generation in endothelial cells. Free Radic Biol Med. 2012;52:2161–2167. doi: 10.1016/j.freeradbiomed.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 91.Behl C, Holsboer F. The female sex hormone oestrogen as a neuroprotectant. Trends Pharmacol Sci. 1999;20:441–444. doi: 10.1016/s0165-6147(99)01392-9. [DOI] [PubMed] [Google Scholar]
- 92.Yue W, et al. Effects of estrogen on breast cancer development: Role of estrogen receptor independent mechanisms. Int J Cancer. 2010;127:1748–1757. doi: 10.1002/ijc.25207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Power RF, et al. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science. 1991;254:1636–1639. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- 94.Ignar-Trowbridge DM, et al. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci U S A. 1992;89:4658–4662. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klotz DM, et al. Requirement of estrogen receptor-alpha in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem. 2002;277:8531–8537. doi: 10.1074/jbc.M109592200. [DOI] [PubMed] [Google Scholar]
- 96.Patrone C, et al. Cross-coupling between insulin and estrogen receptor in human neuroblastoma cells. Mol Endocrinol. 1996;10:499–507. doi: 10.1210/mend.10.5.8732681. [DOI] [PubMed] [Google Scholar]
- 97.Schreihofer DA, et al. Ligand-independent activation of pituitary ER: dependence on PKA-stimulated pathways. Endocrinology. 2001;142:3361–3368. doi: 10.1210/endo.142.8.8333. [DOI] [PubMed] [Google Scholar]
- 98.Kato S, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 99.Martin MB, et al. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology. 2000;141:4503–4511. doi: 10.1210/endo.141.12.7836. [DOI] [PubMed] [Google Scholar]
- 100.Kato S, et al. Molecular mechanism of a cross-talk between oestrogen and growth factor signalling pathways. Genes Cells. 2000;5:593–601. doi: 10.1046/j.1365-2443.2000.00354.x. [DOI] [PubMed] [Google Scholar]
- 101.Chen D, et al. Phosphorylation of human estrogen receptor alpha by protein kinase A regulates dimerization. Mol Cell Biol. 1999;19:1002–1015. doi: 10.1128/mcb.19.2.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Olesen KM, Auger AP. Dopaminergic activation of estrogen receptors induces fos expression within restricted regions of the neonatal female rat brain. PLoS One. 2008;3:e2177. doi: 10.1371/journal.pone.0002177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi JH, et al. Estrogen receptor alpha pathway is involved in leptin-induced ovarian cancer cell growth. Carcinogenesis. 2011;32:589–596. doi: 10.1093/carcin/bgq276. [DOI] [PubMed] [Google Scholar]
- 104.Frenkel B, et al. Regulation of adult bone turnover by sex steroids. J Cell Physiol. 2010;224:305–310. doi: 10.1002/jcp.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hulley S, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 106.Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li R. Brain Endogenous Estrogen Levels Determine Responses to Estrogen Replacement Therapy via Regulation of BACE1 and NEP in Female Alzheimer's Transgenic Mice. Mol Neurobiol. 2012 doi: 10.1007/s12035-012-8377-3. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reckelhoff JF, Maric C. Sex and gender differences in cardiovascular-renal physiology and pathophysiology. Steroids. 2010;75:745–746. doi: 10.1016/j.steroids.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 109.Dent MR, et al. Gender differences in apoptotic signaling in heart failure due to volume overload. Apoptosis. 2010;15:499–510. doi: 10.1007/s10495-009-0441-8. [DOI] [PubMed] [Google Scholar]
- 110.Kulpa J, et al. Rapid Changes in Cardiac Myofilament Function following the Acute Activation of Estrogen Receptor-Alpha. PLoS One. 2012;7:e41076. doi: 10.1371/journal.pone.0041076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pedram A, et al. Estrogen receptor-beta prevents cardiac fibrosis. Mol Endocrinol. 2010;24:2152–2165. doi: 10.1210/me.2010-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Novensa L, et al. Aging negatively affects estrogens-mediated effects on nitric oxide bioavailability by shifting ERalpha/ERbeta balance in female mice. PLoS One. 2011;6:e25335. doi: 10.1371/journal.pone.0025335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prossnitz ER, Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol Cell Endocrinol. 2009;308:32–38. doi: 10.1016/j.mce.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sharma PK, Thakur MK. Expression of estrogen receptor (ER) alpha and beta in mouse cerebral cortex: effect of age, sex and gonadal steroids. Neurobiol Aging. 2006;27:880–887. doi: 10.1016/j.neurobiolaging.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 115.Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rossouw JE, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 117.Stevenson JC. A woman's journey through the reproductive, transitional and postmenopausal periods of life: impact on cardiovascular and musculo-skeletal risk and the role of estrogen replacement. Maturitas. 2011;70:197–205. doi: 10.1016/j.maturitas.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 118.Nelson HD. Menopause. Lancet. 2008;371:760–770. doi: 10.1016/S0140-6736(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 119.Stevenson JC. HRT, osteoporosis and regulatory authorities Quis custodiet ipsos custodes? Hum Reprod. 2006;21:1668–1671. doi: 10.1093/humrep/del043. [DOI] [PubMed] [Google Scholar]
- 120.Imai Y, et al. Minireview: osteoprotective action of estrogens is mediated by osteoclastic estrogen receptor-alpha. Mol Endocrinol. 2010;24:877–885. doi: 10.1210/me.2009-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maatta JA, et al. Inactivation of estrogen receptor alpha in bone-forming cells induces bone loss in female mice. FASEB J. 2012 doi: 10.1096/fj.12-213587. [DOI] [PubMed] [Google Scholar]
- 122.Chokalingam K, et al. Examination of ERalpha Signaling Pathways in Bone of Mutant Mouse Models Reveals the Importance of ERE-Dependent Signaling. Endocrinology. 2012;153:5325–5333. doi: 10.1210/en.2012-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Windahl S, et al. Estrogen receptor-alpha is required for the osteogenic response to mechanical loading in a ligand-independent manner involving its activation function 1 but not 2. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 125.Vina J, Lloret A. Why women have more Alzheimer's disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J Alzheimers Dis. 2010;20(Suppl 2):S527–533. doi: 10.3233/JAD-2010-100501. [DOI] [PubMed] [Google Scholar]
- 126.Yaffe K, et al. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 127.Henderson VW. Oestrogens and dementia. Novartis Found Symp. 2000;230:254–265. doi: 10.1002/0470870818.ch18. discussion 265–273. [DOI] [PubMed] [Google Scholar]
- 128.Resnick SM, et al. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clin Trials. 2004;1:440–450. doi: 10.1191/1740774504cn040oa. [DOI] [PubMed] [Google Scholar]
- 129.Zandi PP, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 130.Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA. 2002;288:2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- 131.Taylor HS, Manson JE. Update in hormone therapy use in menopause. J Clin Endocrinol Metab. 2011;96:255–264. doi: 10.1210/jc.2010-0536. [DOI] [PubMed] [Google Scholar]
- 132.Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 133.Shumaker SA, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 134.Shumaker SA, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 135.Adams MM, et al. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Waters EM, et al. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011;1379:86–97. doi: 10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Adams MM, et al. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci U S A. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Novella S, et al. Effects of estrogen on vascular inflammation: a matter of timing. Arterioscler Thromb Vasc Biol. 2012;32:2035–2042. doi: 10.1161/ATVBAHA.112.250308. [DOI] [PubMed] [Google Scholar]
- 139.Selkoe DJ, et al. The role of APP processing and trafficking pathways in the formation of amyloid beta-protein. Ann N Y Acad Sci. 1996;777:57–64. doi: 10.1111/j.1749-6632.1996.tb34401.x. [DOI] [PubMed] [Google Scholar]
- 140.Toran-Allerand CD, et al. Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- 141.Pike CJ. Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer's disease. J Neurochem. 1999;72:1552–1563. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- 142.Zhang QG, et al. Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J Neurosci. 2008;28:8430–8441. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30:212–222. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao C, et al. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 145.Sisti HM, et al. Neurogenesis and the spacing effect: learning over time enhances memory and the survival of new neurons. Learn Mem. 2007;14:368–375. doi: 10.1101/lm.488707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aimone JB, et al. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 147.Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- 148.Rapp PR, et al. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim MT, et al. 17beta-Estradiol potentiates field excitatory postsynaptic potentials within each subfield of the hippocampus with greatest potentiation of the associational/commissural afferents of CA3. Neuroscience. 2006;141:391–406. doi: 10.1016/j.neuroscience.2006.03.075. [DOI] [PubMed] [Google Scholar]
- 150.Savica R, et al. When does Parkinson disease start? Arch Neurol. 2010;67:798–801. doi: 10.1001/archneurol.2010.135. [DOI] [PubMed] [Google Scholar]
- 151.Coppus AM, et al. Early age at menopause is associated with increased risk of dementia and mortality in women with Down syndrome. J Alzheimers Dis. 2010;19:545–550. doi: 10.3233/JAD-2010-1247. [DOI] [PubMed] [Google Scholar]
- 152.Yadav R, et al. A case control study of women with Parkinson's disease and their fertility characteristics. J Neurol Sci. 2012;319:135–138. doi: 10.1016/j.jns.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 153.A randomized pilot trial of estrogen replacement therapy in post-menopausal women with Parkinson's disease. Parkinsonism Relat Disord. 2011;17:757–760. doi: 10.1016/j.parkreldis.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 154.Brailoiu E, et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 155.Morissette M, et al. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 156.Al Sweidi S, et al. Oestrogen receptors and signalling pathways: implications for neuroprotective effects of sex steroids in Parkinson's disease. J Neuroendocrinol. 2012;24:48–61. doi: 10.1111/j.1365-2826.2011.02193.x. [DOI] [PubMed] [Google Scholar]
- 157.Bourque M, et al. Signaling pathways mediating the neuroprotective effects of sex steroids and SERMs in Parkinson's disease. Front Neuroendocrinol. 2012;33:169–178. doi: 10.1016/j.yfrne.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 158.Quesada A, et al. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson's disease. Dev Neurobiol. 2008;68:632–644. doi: 10.1002/dneu.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marino M, et al. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2006;7:497–508. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhu Y, et al. Transforming growth factor-beta 1 increases bad phosphorylation and protects neurons against damage. J Neurosci. 2002;22:3898–3909. doi: 10.1523/JNEUROSCI.22-10-03898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Parcellier A, et al. PKB and the mitochondria: AKTing on apoptosis. Cell Signal. 2008;20:21–30. doi: 10.1016/j.cellsig.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 162.Hetman M, Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur J Biochem. 2004;271:2050–2055. doi: 10.1111/j.1432-1033.2004.04133.x. [DOI] [PubMed] [Google Scholar]