Abstract
Restenosis, or arterial lumen re-narrowing, occurs in 30–50% of the patients undergoing angioplasty. Adaptive remodeling is the compensatory enlargement of the vessel size, and has been reported to prevent the deleterious effects of restenosis. Our previous studies have shown that elevated transforming growth factor (TGF-β) and its signaling protein Smad3 in the media layer induce adaptive remodeling of angioplastied rat carotid artery accompanying an increase of total collagen in the adventitia. In order to gain insights into a possible role of collagen in Smad3-induced adaptive remodeling, here we have investigated a mechanism of cell–cell communication between medial smooth muscle cells (SMCs) and adventitial fibroblasts in regulating the secretion of two major collagen subtypes. We have identified a preferential collagen-3 versus collagen-1 secretion by adventitial fibroblasts following stimulation by the conditioned medium from the TGF-β1-treated/Smad3-expressing medial smooth muscle cells (SMCs), which contained higher levels of CTGF and IGF2 as compared to control medium. Treating the TGF-β/ Smad3-stimulated SMCs with an siRNA to either CTGF or IGF2 reversed the effect of conditioned media on preferential collagen-3 secretion from fibroblasts. Moreover, recombinant CTGF and IGF2 together stimulated adventitial fibroblasts to preferentially secrete collagen-3 versus collagen-1. This is the first study to identify a preferential secretion of collagen-3 versus collagen-1 from adventitial fibroblasts as a result of TGF-β/Smad3 stimulation of medial SMCs, and that CTGF and IGF2 function together to mediate this signaling communication between the two cell types.
Keywords: Collagen type I and III, Adventitial fibroblasts, Medial smooth muscle cells, CTGF, IGF2
1. Introduction
Atherosclerosis produces a number of serious vascular disorders including heart attack, stroke, and renal failure depending upon the affected artery. Cardiovascular disease is the no. 1 cause of mortality in America, resulting in more than 800,000 deaths in 2005. Percutaneous transluminal coronary angioplasty (PTCA) is a common intervention used to open narrowed or obstructed arteries; over two million such interventions are carried out around the world each year. However, within 6 months after the treatment, restenosis or lumen re-narrowing occurs in up to 30–50% of the patients [1].
There are two components of restenosis, intimal hyperplasia or thickening of the neointima, and arterial remodeling which is reflected by a change in arterial size.
While intimal hyperplasia is an important contributor to restenosis, the patency or luminal diameter of the artery is also determined by permanent changes of arterial wall geometry, generally termed arterial remodeling. For example, if intimal plaque that develops following angioplasty narrows the artery lumen, the artery may then compensate for this with adaptive remodeling or overall arterial enlargement [2]. Alternatively, the luminal narrowing produced by intimal plaque may be made more severe by the process of constrictive remodeling, where the artery shrinks or constricts, exacerbating the degree of luminal stenosis. Growing evidence indicates that constrictive remodeling may be as important or even amore important contributor to restenosis than intimal hyperplasia [3]. Nevertheless, it is the combination of these two processes that determines the final degree of luminal narrowing.
There have been encouraging reports suggesting that prevention or reduction of constrictive remodeling can be attained through manipulation of the factors that influence ECM production in the arterial adventitia [4]. Our group has found that in vivo overexpression of Smad3, which is the canonical signaling protein of TGF-β, resulted in adaptive remodeling in the rat carotid artery following balloon angioplasty [5]. In these animals, Smad3 transgene produced an increase of luminal area by inducing adaptive remodeling, as measured by an enlargement of the external elastic lamina (EEL). Moreover, Smad3-induced adaptive remodeling was associated with elevated levels of connective tissue growth factor (CTGF) as well as an increase in total collagen in the adventitia [5]. Together these studies suggest that TGF-β through Smad3 promotes the secretion of CTGF (and other factors) from medial SMCs, which in turn stimulates the neighboring adventitial fibroblasts to produce collagen.
The mechanism of arterial remodeling, in particular adaptive remodeling, is poorly understood. However, it is known that ECM proteins in the adventitia, especially collagen, are an important component of this process [6]. Collagen is abundant in the ECM of the vasculature, especially the adventitia [7]. There have been conflicting reports regarding how collagen affects vascular remodeling. There are multiple subtypes of collagen, and collagen type 1 (CN-1) and type 3 (CN-3) are the most prevalent in the arterial wall [5]. Whereas CN-1 is rigid in nature, CN-3 is more elastic. Considering these characteristics, increased levels of CN-1 may promote constrictive remodeling, and increased levels of CN-3 may favor adaptive remodeling.
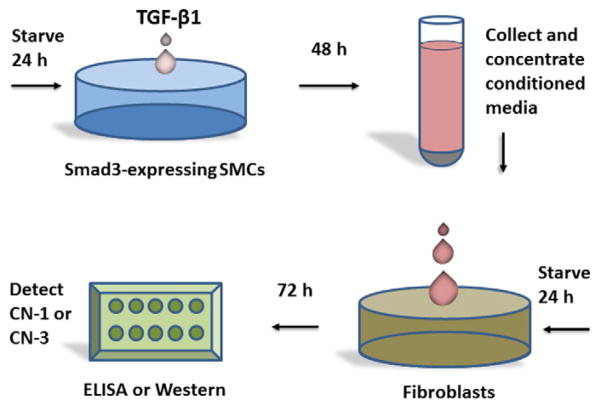
Thus, a possible role of collagen subtypes in regulating adaptive remodeling prompted us to determine the secretion of CN-3 and CN-1 from adventitial fibroblasts in response to the stimulation from Smad3-expressing and TGF-β-treated medial SMCs. We performed experiments employing two cell types to mimic a paracrine system (Fig. 1), so that we were able to stimulate Smad3-expressing SMCs with TGF-β and determine whether the resultant secreted factors from SMCs in turn stimulate adventitial fibroblasts to secrete CN-3 and CN-1 differentially.
Fig. 1.
Diagram of the in vitro experimental procedures to assess fibroblast collagen secretion in response to the stimulation of conditioned media from TGF-β1/Smad3-treated SMCs. TGF-β1 (5 ng) was added to Smad3-expressing medial SMCs that were previously starved for 24 h in the culture medium containing 0.5% serum. GFP-expressing SMCs served as control. After treatment for 48 h, conditioned media which contained factors produced by the TGF-β1/Smad3-treated SMCs, were collected, concentrated, and then added into the starved (for 24 h) rat aortic adventitial fibroblasts. Following a 72 h incubation, the quantity of CN-3 and CN-1 secreted from the fibroblasts (media)was assessed by either immunoblotting or ELISA. (CN, collagen).
We have found a greater increase of CN-3 versus CN-1 secreted from adventitial fibroblasts following stimulation by the conditioned media from the TGF-β1-treated/Smad3-expressing medial SMCs, and increased levels of two growth factors, CTGF and IGF2, in the conditioned media compared to control. While the use of siRNA for either CTGF or IGF2 reversed the conditioned media-induced preferential CN-3 secretion, recombinant CTGF and IGF2 together stimulated fibroblasts to preferentially secrete CN-3 versus CN-1, indicating that CTGF and IGF2 together mediated the inter-cellular signaling that promoted the differential CN-3 and CN-1 secretion from adventitial fibroblasts.
2. Methods
2.1. Cell culture
Aortic medial smooth muscle cells (MOVAS) were isolated and transduced with GFP or GFP-SMAD3 using retrovirus. Rat aortic adventitial fibroblasts were isolated and cultured according to our published method [8]. The cells of 3–7 passages were used for the experiments.
2.2. Stimulation of fibroblasts with the conditioned media from the Smad3-expressing and TGF-β1-treated SMCs
The in vitro experimental method is demonstrated in Fig. 1. SMCs expressing GFP (control) or GFP-Smad3 (for simplicity, hereafter denoted as Smad3) were starved for 24 h in the DMEM culture medium supplemented with 0.5% serum (Gibco). TGF-β1 was then added into the cell culture to a final concentration of 5 ng/ml [8]. After the stimulation with TGF-β1 for 48 h, the conditioned medium was collected, and concentrated with a Centricon spin column (Millipore). Adventitial fibroblasts were starved for 24 h (with 0.5% serum) and then incubated for 72 h with the concentrated medium from the SMC culture. The medium from the fibroblasts was then collected, and subjected to Western blotting or ELISA for measurement of secretion of collagen type 1 or 3 by the fibroblasts.
2.3. Immunoblotting
The samples taken from the fibroblast media (30 μg total protein each) were subjected to SDS-PAGE (10% gel) followed by electrotransfer of the proteins from the gel to the PVDF membrane. After blocking, a primary antibody (1:1000 dilution) against CN-3 or CN-1 (Fitzgerald) was incubated overnight with the blot, followed by another 60 min incubation with the secondary antibody (goat-anti-rabbit, 1:5000 dilution, Bio-Rad). The collagen bands were then visualized by applying enhanced chemiluminiscence (ECL) reagents to the blot and then scanning in an ImageQuant LAS 4000 mini biomolecular imager (GE Health Care Life Sciences). Quantification of CN-3 and CN-1 bands was performed using the Image J program.
2.4. Enzyme linked immunosorbent assay (ELISA)
In addition to Western blotting, ELISA was also performed to assess the abundance of CN-3 and CN-1 in fibroblast media, or the levels of CTGF (antibody from Sigma-Aldrich) and IGF2 (antibody from Abcam) in SMC-conditioned media. The primary antibodies were diluted according to the manufacturer’s instructions. A goat-anti-rabbit secondary antibody was used with a 1:200 dilution. The ELISA with secondary antibody but without primary antibody served as background control.
2.5. Knockdown of CTGF or IGF2 using siRNAs
SMCs were plated at ~50% confluence in DMEM containing 10% FBS in 6-well plates and incubated for 24 h. Cells were transfected in Opti-MEM I medium with 100 pmol of siRNA for CTGF or IGF2 (Santa Cruz) or the scramble control siRNA [8], using the RNAi Max transfection reagent, as described by the manufacturer’s protocol (Invitrogen, Carlsbad, Calif). After 6 h, the Opti-MEM I medium was replaced with DMEM containing 10% FBS and incubated with the cells for 12 h.
2.6. Rat carotid balloon injury and periadventitial CTGF application
Balloon injury of the left common carotid artery was performed in Male Sprague-Dawley rats (300–350 g) as previously described [5]. Briefly, after induction of anesthesia with isoflurane, a 2F balloon catheter was inserted through the left external carotid artery into the common carotid artery and insufflated three times with 2 atm of pressure. The external carotid artery was then ligated, and flow was re-established through the common and internal carotid arteries. 200 μL of pluronic gel containing 80 ng of CTGF was placed around the injured segment of the common carotid artery. Rats were euthanized 14 days after injury. The injured common carotid arteries were collected and fixed in 4% paraformaldehyde overnight for embedding in paraffin.
2.7. Immunohistochemistry
Paraffin-embedded common carotid arteries were cut into 5-μm sections, for immunostaining of CN-3 and CN-1, following a method described previously [5]. Antibody controls included species-matched IgG. For each animal, ten sections were sampled from the segment of collected carotid artery. Images were taken from six fields (×200) of each section. CN-3 or CN-1 staining was quantified using Image J in all images, the average and the standard error of the mean were calculated for each group.
3. Results
3.1. Conditioned media from TGF-β1/Smad3-treated SMCs result in differential secretion of CN-3 versus CN-1 from adventitial fibroblasts
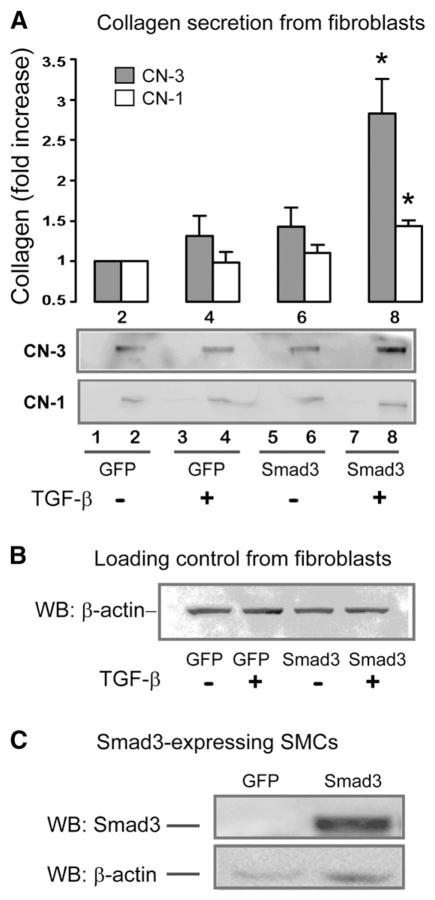
We have previously demonstrated that TGF-β through Smad3 stimulates adaptive remodeling following balloon injury of the rat common carotid artery, a process associated with an increase in total adventitial collagen [5]. In the current study, we have explored whether TGF-β/Smad3 stimulates SMCs to secrete factors that enhance collagen production from adventitial fibroblasts. Moreover, we have evaluated the type of collagen that is secreted, by comparing production of CN-3 which is more flexible, with that of CN-1 which is more rigid. Starved SMCs overexpressing Smad3 were treated with TGF-β1 (5 ng/ml) for 48 h. The medium was collected and incubated with rat adventitial fibroblasts for 72 h and the resultant supernatant analyzed by Western blotting or ELISA for CN-1 and CN-3 (Fig. 1). As demonstrated in Fig. 2, there was a 2.82±0.46 fold increase (p>0.05) in CN-3 secretion from fibroblasts stimulated with conditioned media from the Smad3-expressing and TGF-β1-treated SMCs as compared to control (GFP and no TGF-β1). In contrast, CN-1 secretion from fibroblasts increased by only 1.41±0.10 fold (p>0.05) in response to stimulation by the conditioned media from TGF-β/Smad3 treated SMCs. To rule out the possibility that conditioned media from SMC cultures contained CN-1 or CN-3, equal volumes of media derived from SMCs were analyzed by Western blotting. There was no discernible signal for either CN-1 or CN-3 in the samples from the SMC-conditioned media (Fig. 2A, lanes 1, 3, 5, and 7). Moreover, TGF-β added to the original SMC cultures had minimal effect on the secretion of CN-1 or CN-3 from fibroblasts (see Fig. 2A, lane 4).
Fig. 2. Measurement of differential secretion of CN-3 versus CN-1 from adventitial fibroblasts following stimulation with the conditioned media from TGF-β1/Smad3-treated SMCs.
A. CN-3 and CN-1 secretion from adventitial fibroblasts. These experiments were conducted as described in Fig. 1. After stimulation with the SMC-conditioned media for 72 h, samples were collected from fibroblast culture media and analyzed for CN-3 (black) or CN-1 (white) by Western blotting, as shown in lanes 2, 4, 6, and 8. The control samples of SMC conditioned media were loaded in lanes 1, 3, 5, and 7, respectively, indicating absence of detectable collagen signal. Lanes 2 and 4 represent stimulation with the conditioned media from the GFP-expressing SMCs without or with TGF-β1, respectively. Lanes 6 and 8 represent stimulation with conditioned media from the Smad3-expressing SMCs without or with TGF-β1, respectively. The quantitated data normalized by β-actin loading control (see B) are presented in the bar graph. Each bar represents a mean±SEM (at least 3 independent experiments). *p>0.05, as compared to GFP control.
B. Western blotting of β-actin as loading control. Shown are the cell lysate samples taken from the fibroblast cultures from which the media samples were analyzed for CN-3 and CN-1 secretion in A in lanes 2, 4, 6, and 8, respectively.
C. Western blotting of Smad3. The representative blot shows overexpression of Smad3 in Smad3-expressing SMCs as compared to the control of GFP-expressing SMCs.
We then repeated these experiments using ELISA for CN-1 and CN-3 (Fig. 5). We found a 1.56±0.1 fold (p>0.05) increase of CN-3 secretion from fibroblasts that were stimulated with conditioned media from the Smad3-expressing and TGF-β1-treated SMCs compared to the control (GFP, no TGF-β1). In contrast, CN-1 secretion increased by only 1.1±0.02 fold (p>0.05) in response to stimulation by similar conditioned media. These findings confirm that medial SMCs in response to TGF-β/Smad3, secreted a mediator that in a paracrine fashion stimulated adventitial fibroblasts to produce collagen. Moreover, fibroblasts, in response to this factor, secreted a higher ratio of more flexible CN-3 compared to less flexible CN-1. It is important to note that the preferential CN-3 versus CN-1 secretion from fibroblasts was not due to a direct stimulation of residual TGF-β, which possibly remained in the conditioned SMC medium, because the conditioned medium from the GFP control (Fig. 2A, lane 4, GFP+TGF-β) did not result in a significant change of CN-3 or CN-1.
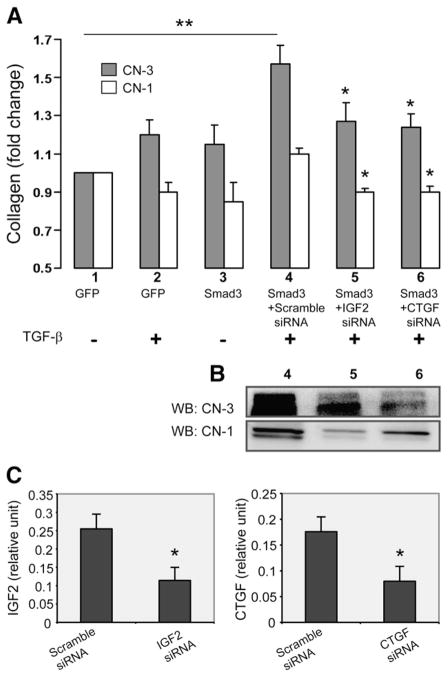
Fig. 5. Effect of CTGF or IGF2 siRNA applied to TGF-β/Smad3-treated SMCs on CN-3 versus CN-1 secretion from adventitial fibroblasts.
A. Measurement of CN-3 and CN-1 by ELISA. Experiments were performed as depicted in Fig. 1, except that respective siRNAs (100 pmol, 6 h) were used to knock down IGF2 or CTGF expression in the Smad3-expressing SMCs. Scramble siRNA (100 pmol) was used as negative control. At 72 h following the addition of the SMC-conditioned media, samples were taken from the fibroblast cultures and analyzed for CN-3 (black) and CN-1 (white) by ELISA. Each bar represents a mean±SEM (at least 3 independent experiments). Different conditions are labeled by numbers (1–6) under the respective bars. **p>0.05, as compared to no TGF-β/Smad3. *p>0.05, as compared to respective maximum CN-3 or CN-1 change (TGF-β/Smad3+scramble siRNA).
B. Western blotting of collagen. In addition to the ELISA measurements in A, CN-3 and CN-1 in the fibroblast media were also detected by immunobotting. Lanes 4, 5, and 6 correspond to the same conditions in A (4, 5, and 6), respectively.
C. Knockdown of IGF2 and CTGF by their respective siRNA, as measured by ELISA. The conditions of scramble siRNA and IGF2 siRNA correspond to those in 4 and 5 in A, respectively; the conditions of scramble siRNA and CTGF siRNA correspond to those in 4 and 6 in A, respectively.
3.2. TGF-β treatment of Smad3-expressing SMCs stimulates secretion of CTGF and IGF2
CTGF has multiple cellular effects including stimulation of a number of matrix proteins such as collagen. Moreover, it has been previously reported that IGF2 interacts with CTGF through an IGF binding domain enhancing matrix protein synthesis [9]. Consequently we hypothesized that CTGF and IGF2 might be the factors released by SMCs that in turn stimulate the secretion of collagen from adventitial fibroblasts.
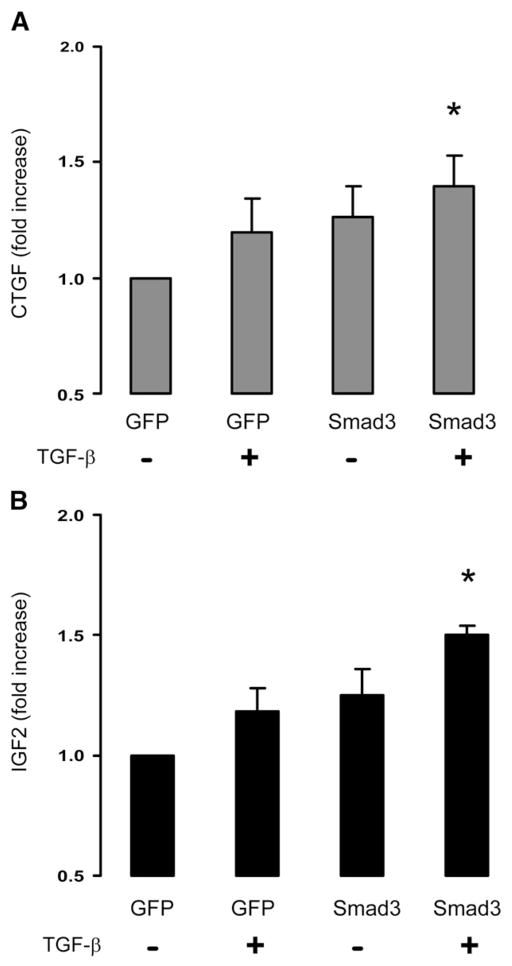
SMCs overexpressing Smad3 were incubated with TGF-β1 (5 ng/ml), and the medium was collected and analyzed for CTGF and IGF2 by ELISA (Fig. 3). CTGF secretion from the TGF-β/Smad3-treated SMCs increased by 1.4±0.1 fold (p>0.05), compared to the SMCs without Smad3 overexpression or TGF-β1 treatment (Fig. 3A). Moreover, IGF2 secretion from the TGF-β/Smad3-treated SMCs increased by 1.5±0.04 fold (p>0.05), compared to the SMCs without Smad3 overexpression or TGF-β1 treatment (Fig. 3B). These findings suggest that either CTGF or IGF2 or a combination of these two factors may mediate the effect of SMCs on adventitial collagen production.
Fig. 3.
Measurement of CTGF and IGF2 in the conditioned media of the Smad3-expressing and TGF-β1-treated SMCs. Conditioned media from the GFP-expressing SMCs without (Bar 1) or with (Bar 2) TGF-β1 treatment, or from the Smad3-expressing SMCs without (Bar 3) or with (Bar 4) TGF-β1 treatment, were analyzed for secretion of CTGF (A) or IGF2 (B) by ELISA. Each bar represents a mean±SEM (at least 3 independent experiments). *p>0.05, as compared to GFP control.
3.3. Stimulation of adventitial fibroblasts with CTGF and IGF2 enhances the secretion of CN-3 versus CN-1
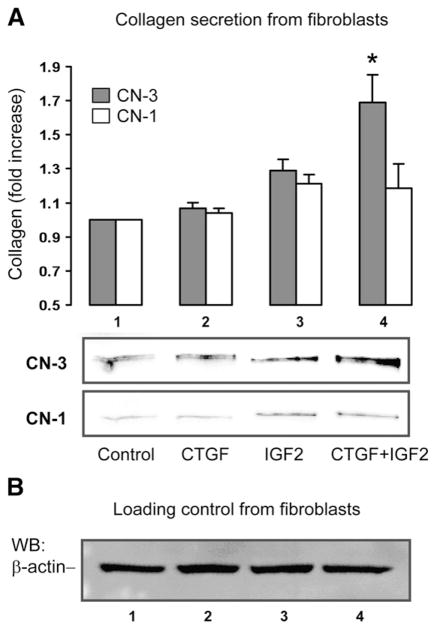
To further confirm the role of CTGF and/or IGF2 in stimulating collagen production by adventitial fibroblasts, we directly added recombinant CTGF and/or IGF2 to fibroblast cell cultures and incubated for 72 h, and then measured CN-3 and CN-1 secreted into the media using Western blotting. As shown in Fig. 4, CTGF alone had no effect on the secretion of either CN-1 or CN-3. Stimulation of fibroblasts with IGF2 alone produced a slight numerical increase in both CN-1 and CN-3, although these increases were not statistically significant. However, combining CTGF and IGF2 produced a 1.69±0.11 (p>0.05) fold increase in CN-3 and no significant change in CN-1. These data provide further evidence that CTGF and IGF2 may be the mediators that allow TGF-β/Smad3-stimulated SMCs to preferentially enhance CN-3 secretion by adventitial fibroblasts.
Fig. 4.
Measurement of CN-3 and CN-1 secretion from adventitial fibroblasts stimulated with recombinant CTGF and/or IGF2. Recombinant CTGF (100 ng/ml) or recombinant IGF2 (300 ng/ml) were added either alone (lanes 2 and 3, respectively) or together (lane 4) into starved (for 24 h) fibroblasts, and incubated for 72 h. A solvent equivalent was added to the control (lane 1). Fibroblast culture media were then analyzed by Western blotting for secretion of CN-3 and CN-1. The data presented in A are normalized using β-actin loading control (see B). Each bar represents a mean±SEM (at least 3 independent experiments). *p>0.05, as compared to GFP control.
3.4. SiRNA inhibition of CTGF and IGF-2 secretion from medial SMCs blocks CN-3 production by adventitial fibroblasts
To provide definitive proof that CTGF and IGF2 are the proteins that mediated CN-3 production by adventitial fibroblasts in response to TGF-β/Smad3 stimulated SMCs, we employed siRNAs to block the production of both of these proteins (Fig. 5). Following treatment of SMCs with TGF-β/Smad3 and siRNA, conditioned media were incubated with adventitial fibroblasts and CN-1 and CN-3 were measured. In the experimental group, SMCs were pre-incubated for 6 h with siRNAs to CTGF or IGF2 and in the control group pre-incubation was with scrambled siRNA. Compared with the scramble siRNA control, siRNAs for either CTGF or IGF2 respectively reduced the levels of these two proteins by ~60% in the SMC-conditioned media (Fig. 5C). Interestingly, either the siRNA to CTGF or the siRNA to IGF2 completely inhibited the ability of TGF-β1/Smad3-treated SMCs to stimulate the CN-3 secretion by adventitial fibroblasts, whereas scramble siRNA had no effect (Fig. 5A).
3.5. Periadventitial application of recombinant CTGF to the balloon-injured arteries enhances the production of adventitial CN-3 versus CN-1
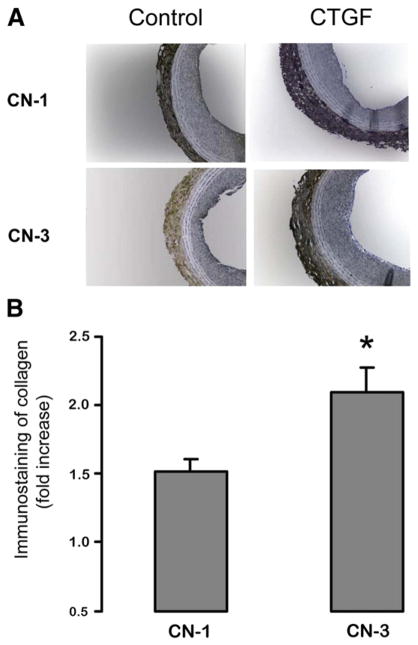
We have previously found that perivascular application of recombinant CTGF stimulated adaptive remodeling following angioplasty [5]. Recombinant CTGF was applied via pluronic gel following balloon injury of the rat common carotid artery. Animals were sacrificed at 14 days, followed by immunostaining for CN-1 and CN-3 on the carotid sections. We proposed that CTGF might produce this effect by stimulating fibroblasts to preferentially release CN-3. To explore a relationship between CTGF and CN-3 in vivo, we have immunostained CN-3 and CN-1 on the same carotid artery sections that were obtained from the previous study [5]. The results are shown in Fig. 6. Compared to controls without CTGF treatment, in animals treated with recombinant CTGF there was a 2.1±0.2 fold increase of CN-3 whereas the increase of CN-1 was only 1.5±0.1 fold. These data provide further evidence that CTGF may be at least one of the factors responsible for adaptive remodeling. It is likely that exogenous CTGF by interacting with endogenous IGF2, or IGF1, which are elevated following arterial injury [10,11], has stimulated preferential secretion of CN-3 versus CN-1 in the adventitia.
Fig. 6. Measurement of CN-3 and CN-1 in the injured rat carotid artery following periadventitial application of CTGF. Rat common carotid arteries were injured using a balloon catheter, and pluronic gel (200 μl) containing recombinant CTGF (CTGF, 80 ng) or PBS (Control) of equal volume was applied to the outside of arteries, as described in Methods [5]. Cross-sections of the arteries, harvested at 14 days post injury, were immunostained for either CN-1 or CN-3.
A. Representative CN-1 and CN-3 immunostaining of cross-sections from control or CTGF-treated arteries.
B. Quantification of adventitial CN-1 or CN-3 immunostaining. The effect of CTGF on adventitial CN-1 or CN-3 content is expressed as fold induction versus the control without CTGF treatment (n=6 rats, *p>0.05).
4. Discussion
TGF-β produced at the time of arterial injury has a profound effect on the development of restenosis. While TGF-β has been proven to enhance restenosis by stimulating intimal hyperplasia [12], there are also data from our laboratory [5] suggesting that in parallel, TGF-β through its signaling protein Smad3, stimulates adaptive remodeling which in part compensates for the lumen loss due to intimal hyperplasia. There are accumulating data suggesting that adaptive remodeling plays an important role in mitigating the effect of intimal hyperplasia on restenosis [7].
Our previous study [5] showed that adaptive remodeling induced by overexpression of Smad3 in the media was associated with elevated total CN levels in the adventitia, and the in vitro experiments confirmed that total CN secretion by adventitial fibroblasts was increased by stimulation of the conditioned media taken from the TGF-β/Smad3-treated medial SMCs. Here we have demonstrated that the increase of total CN was mainly accounted for by a preferential CN-3 versus CN-1 production. We have further uncovered that it was CTGF and IGF2, rather than CTGF alone, in the SMC-conditioned medium that promoted the preferential CN-3 secretion from the fibroblasts. Blocking studies with siRNAs provide further evidence that CTGF and IGF2 function as mediators by which TGF-β/Smad3-stimulated SMCs effect preferential secretion of CN-3 from adventitial fibroblasts. Thus, this study provides the first report of a preferential secretion of CN-3 versus CN-1 from adventitial fibroblasts as a result of TGF-β/Smad3 stimulation of medial SMCs, which is mediated by CTGF and IGF2 secreted from the stimulated SMCs.
Adaptive remodeling is a complex process and the mechanisms that lead to arterial expansion are not clear [7]. Collagen has long been suggested to play a critical role in this process, however, there have been conflicting reports regarding how collagen influences remodeling [7]. A possible explanation to this controversy is that the quantity of collagen is not the sole determinant for remodeling; but rather, changes in collagen type, cross-linking or conformation such as the compaction of collagen fibrils etc., collectively modulate the mechanical properties of the vessel wall and hence the final vessel geometry. There are many types of collagen with differing properties, among which CN-3 and CN-1 are most relevant to remodeling. Whereas CN-3 is elastic CN-1 is more rigid [13]. It is therefore reasonable to propose that relative levels of CN-3 versus CN-1 in the vessel wall may be an important contributor to the remodeling process; higher levels of CN-3 in the adventitia may allow the vessel to expand, thus favoring adaptive remodeling.
It has previously been shown that higher levels of CN-3 render the ECM more elastic. In the vasculature, CN-1 confers rigidity and tensile strength, while CN-3 promotes vascular elasticity [13]. The rigidity of the aorta has been found to be related to relative CN-1 and CN-3 abundance; decreased CN-3 may partially account for the increased stiffness in the aging male monkey aorta [13,14]. A more recent report suggests that stiffening in the aging aorta could be partially explained by an increase in CN-1 and a decrease in elastin [15]. Moreover, it has been reported that increased myocardial content of CN-1 produces maladaptive remodeling and reduced cardiac function due to pressure overload, whereas increased levels of CN-3 are associated with adaptive remodeling leading to improved cardiac function [16]. Further substantiating these findings, a recent study revealed that relief of pressure overload to the mouse left ventricle was accompanied by a collagen isoform shift with an increase of CN-3 levels versus CN-1 [17] resulting in improved cardiac function. These studies point to a beneficial effect of CN-3 versus CN-1 on the mechanical properties of the arterial wall.
Our previous studies suggest that CTGF is likely one of the signaling factors that mediates the effect of TGF-β/Smad3-stimulated SMCs on the fibroblast production of arterial collagen [5]. Here, our data further demonstrate that CTGF does not act alone, but rather in cooperation with IGF2, in mediating the paracrine effect of medial SMCs on fibroblast production of CN-3. The combination of CTGF and IGF2 added to the fibroblast culture raised levels of CN-3, whereas neither of these alone was able to produce this effect. Moreover, knocking down either CTGF or IGF2 in SMCs abolished the stimulatory effect on fibroblast CN-3 secretion, suggesting that the two factors together are required for preferential CN-3 production.
Our finding that IGF2 functions as a co-factor with CTGF in stimulating CN-3 secretion from fibroblasts is not unexpected. CTGF is a modular protein that is composed of multiple domains, which interact with various other proteins to play a critical role in cellular functions. Reports from Grotendorst et al. [9,18] have revealed that the N-terminal and C-terminal domains of CTGF mediate distinct biological effects by interacting with IGF2 and EGF respectively. Specifically, by binding to the N-terminal domain of CTGF, IGF2 stimulates myofibroblast transformation as well as collagen synthesis in rat kidney fibroblasts. These studies reveal that in normal rat kidney fibroblasts, an interaction between CTGF and IGF2 is necessary to mediate CN production. We have demonstrated that a similar interaction is necessary for the secretion of CN-3 from rat arterial adventitial fibroblasts. It is interesting to note that IGF2 and IGF1 are homologous proteins and structurally highly similar. Compared to IGF2, the function of IGF1 in the vascular system is better understood; it is known that IGF1 plays an important role in vascular cell proliferation and restenosis [10,11]. Interestingly, in addition to the reports indicating an important role of CTGF and IGF2 in collagen production by normal rat kidney fibroblasts [9,17], recombinant IGF1 in combination with CTGF, has also been reported to be a potent mediator of the production of CN-3 in human renal fibroblasts [19].
It is well documented that TGF-β is up-regulated in injured arteries [1]. We have previously observed that following arterial injury of the rat carotid artery, Smad3 expression is markedly elevated as well [20]. Furthermore, overexpression of Smad3 in the media of injured arteries produced adaptive remodeling as well as an increase of total collagen content in the adventitia [5]. We thus inferred that the activation of the TGF-β/Smad3 pathway stimulates medial SMCs to produce soluble factors that diffuse into the adventitia and stimulate collagen secretion from fibroblasts. In the current study, using an experimental system employing SMCs and fibroblasts derived from the media and adventitia respectively to mimic a paracrine communication between the two cell types, we have identified that TGF-β/Smad3-stimulated medial SMCs promoted adventitial fibroblast production of collagen, preferentially CN-3, an elastic subtype. Moreover, we have found that CTGF and IGF2 that were secreted from SMCs together mediated the stimulation of preferential CN-3 versus CN-1 secretion from fibroblasts. In addition, our in vivo experiments (Fig. 6) showed a consistent result of greater increase of CN-3 versus CN-1, in the adventitia in the rat carotid arteries treated with CTGF following balloon angioplasty.
In summary, our data have revealed a new finding that a greater increase of CN-3 versus CN-1 secretion was produced from adventitial fibroblasts and mediated by CTGF and IGF2 that were secreted from TGF-β-treated/Smad3-expressing medial SMCs. Considering that CN-3 promotes vascular elasticity by forming small fibers that mesh with CN-1 bundles [13], increased abundance of CN-3 in the adventitia may alter the ECM structure in favor of post-angioplasty adaptive remodeling. Thus our finding provides important implications into the mechanism of adaptive remodeling that can be induced through an approach of TGF-β/Smad3 activation. Further mechanistic studies are expected to produce useful knowledge for developing new therapeutic strategies for amelioration of restenosis.
Footnotes
The authors declare that no conflict of interest exists. This work was supported by a Public Health Service Grant R01-HL-068673 (to K.C.K.) from the National Heart, Lung, and Blood Institute.
References
- 1.Khan R, Agrotis A, Bobik A. Cardiovascular Research. 2007;74:223–234. doi: 10.1016/j.cardiores.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Kakuta T, Currier JW, Haudenschild CC, Ryan TJ, Faxon DP. Circulation. 1994;89:2809–2815. doi: 10.1161/01.cir.89.6.2809. [DOI] [PubMed] [Google Scholar]
- 3.Mintz GS, Popma JJ, Pichard AD, Kent KM, Salter LF, Chuang YC, Griffin J, Leon MB. Journal of the American College of Cardiology. 1996;27:1678–1687. doi: 10.1016/0735-1097(96)00083-6. [DOI] [PubMed] [Google Scholar]
- 4.Kingston PA, Sinha S, Appleby CE, David A, Verakis T, Castro MG, Lowenstein PR, Heagerty AM. Circulation. 2003;108:2819–2825. doi: 10.1161/01.CIR.0000097068.49080.A0. [DOI] [PubMed] [Google Scholar]
- 5.Kundi R, Hollenbeck ST, Yamanouchi D, Herman BC, Edlin R, Ryer EJ, Wang C, Tsai S, Liu B, Kent KC. Cardiovascular Research. 2009;84:326–335. doi: 10.1093/cvr/cvp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osherov AB, Gotha L, Cheema AN, Qiang B, Strauss BH. Cardiovascular Research. 2011;91:16–26. doi: 10.1093/cvr/cvr012. [DOI] [PubMed] [Google Scholar]
- 7.Goel SA, Guo LW, Liu B, Kent KC. Cardiovascular Research. 2012;96:363–371. doi: 10.1093/cvr/cvs276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suwanabol PA, Seedial SM, Zhang F, Shi X, Si Y, Liu B, Kent KC. American Journal of Physiology - Heart and Circulatory Physiology. 2012;302:H2211–H2219. doi: 10.1152/ajpheart.00966.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grotendorst GR, Duncan MR. The FASEB Journal. 2005;19:729–738. doi: 10.1096/fj.04-3217com. [DOI] [PubMed] [Google Scholar]
- 10.Delafontaine P, Song YH, Li Y. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:435–444. doi: 10.1161/01.ATV.0000105902.89459.09. [DOI] [PubMed] [Google Scholar]
- 11.Bayes-Genis A, Conover CA, Schwartz RS. Circulation Research. 2000;86:125–130. doi: 10.1161/01.res.86.2.125. [DOI] [PubMed] [Google Scholar]
- 12.Suwanabol PA, Kent KC, Liu B. Journal of Surgical Research. 2011;167:287–297. doi: 10.1016/j.jss.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu H, Depre C, Ghosh K, Resuello RG, Natividad FF, Rossi F, Peppas A, Shen YT, Vatner DE, Vatner SF. Circulation. 2007;116:669–676. doi: 10.1161/CIRCULATIONAHA.107.689208. [DOI] [PubMed] [Google Scholar]
- 14.Qiu H, Tian B, Resuello RG, Natividad FF, Peppas A, Shen YT, Vatner DE, Vatner SF, Depre C. Physiological Genomics. 2007;29:169–180. doi: 10.1152/physiolgenomics.00229.2006. [DOI] [PubMed] [Google Scholar]
- 15.Zhou RH, Vendrov AE, Tchivilev I, Niu XL, Molnar KC, Rojas M, Carter JD, Tong H, Stouffer GA, Madamanchi NR, Runge MS. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:745–755. doi: 10.1161/ATVBAHA.111.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber KT, Jalil JE, Janicki JS, Pick R. American Journal of Hypertension. 1989;2:931–940. doi: 10.1093/ajh/2.12.931. [DOI] [PubMed] [Google Scholar]
- 17.Bjornstad JL, Sjaastad I, Nygard S, Hasic A, Ahmed MS, Attramadal H, Finsen AV, Christensen G, Tonnessen T. European Heart Journal. 2011;32:236–245. doi: 10.1093/eurheartj/ehq166. [DOI] [PubMed] [Google Scholar]
- 18.Grotendorst GR, Rahmanie H, Duncan MR. The FASEB Journal. 2004;18:469–479. doi: 10.1096/fj.03-0699com. [DOI] [PubMed] [Google Scholar]
- 19.Lam S, van der Geest RN, Verhagen NA, van Nieuwenhoven FA, Blom IE, Aten J, Goldschmeding R, Daha MR, van Kooten C. Diabetes. 2003;52:2975–2983. doi: 10.2337/diabetes.52.12.2975. [DOI] [PubMed] [Google Scholar]
- 20.Tsai S, Hollenbeck ST, Ryer EJ, Edlin R, Yamanouchi D, Kundi R, Wang C, Liu B, Kent KC. American Journal of Physiology - Heart and Circulatory Physiology. 2009;297:H540–H549. doi: 10.1152/ajpheart.91478.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]