Abstract
Interleukin-17 (IL-17), a proinflammatory cytokine produced by CD4+ Th17 cells, has been associated with the pathogenesis of several autoimmune diseases including uveitis. The fate of IL-17 during HIV/AIDS, however, remains unclear, and a possible role for IL-17 in the pathogenesis of AIDS-related diseases has not been investigated. Toward these ends, we performed studies using a well-established animal model of experimental murine cytomegalovirus (MCMV) retinitis that develops in C57/BL6 mice with retrovirus-induced immunosuppression (MAIDS). After establishing baseline levels for IL-17 production in whole splenic cells of healthy mice, we observed a significant increase in IL-17 mRNA levels in whole splenic cells of mice with MAIDS of 4-weeks (MAIDS-4), 8-weeks (MAIDS-8), and 10-weeks (MAIDS-10) duration. In contrast, enriched populations of splenic CD4+ T cells, splenic macrophages, and splenic neutrophils exhibited a reproducible decrease in levels of IL-17 mRNA during MAIDS progression. To explore a possible role for IL-17 during the pathogenesis of MAIDS-related MCMV retinitis, we first demonstrated constitutive IL-17 expression in retinal photoreceptor cells of uninfected eyes of healthy mice. Subsequent studies, however, revealed a significant decrease in intraocular levels of IL-17 mRNA and protein in MCMV-infected eyes of MAIDS-10 mice during retinitis development. That MCMV infection might cause a remarkable downregulation of IL-17 production was supported further by the finding that systemic MCMV infection of healthy, MAIDS-4, or MAIDS-10 mice also significantly decreased IL-17 mRNA production by whole splenic CD4+ T cells. Based on additional studies using IL-10 −/− mice infected systemically with MCMV and IL-10 −/− mice with MAIDS infected intraocularly with MCMV, we propose that MCMV infection downregulates IL-17 production via stimulation of suppressor of cytokine signaling (SOCS)-3 and interleukin-10.
Keywords: Interleukin-17, Interleukin-10, Suppressor of cytokine signaling-3, AIDS, Human Cytomegalovirus, Murine Cytomegalovirus, MAIDS, Retinitis
1. Introduction
CD4+ T-helper (Th) cells play a significant role in mediating immune responses to protect the host against foreign invaders. Mosmann and Coffman originally proposed that CD4+ Th cells could be divided into two subsets, Th1 and Th2, based on their unique patterns of secreted cytokines and their corresponding functions [1–3]. Th1 cells induce cell-mediated immunity, induce delayed-type hypersensitivity (DTH), and protect the host against intracellular pathogens through the release of interferon (IFN)-γ and interleukin-2 (IL-2) [1, 4]. In comparison, Th2 cells induce extracellular immunity and the allergic response, including the production of immunoglobulin E, via IL-4, IL-6, and IL-10 production [1, 4].
A more recently recognized CD4+ Th cell subset, Th17 cells, secretes a unique set of cytokines that includes IL-17A (IL-17) as well as tumor necrosis factor-α, IL-22, and IL-6 [5–8]. Differentiation of the Th17 linage is dependent upon the secretion of IL-6 and transforming growth factor-β from antigen-presenting cells (APCs) [5–7, 9]. The Th17 lineage is stabilized by IL-23 secretion from APCs [5–7, 9]. A pro-inflammatory cytokine, IL-17 functions by binding to its receptor, IL-17RA, that is expressed on a wide range of cells [8, 10] and thereby stimulates a number of immune responses including the recruitment of macrophages and neutrophils [8, 10, 11]. Significantly, IL-17 has been associated with cellular damage that occurs during autoimmune diseases including rheumatoid arthritis and multiple sclerosis [6, 8, 12, 13]. Recent work by Luger and colleagues [14, 15] as well as others [16–18] has also linked IL-17 to uveitis, a leading cause of blindness in the United States [19].
Another sight-threatening disease of the eye, cytomegalovirus retinitis [20, 21], is caused by human cytomegalovirus (HCMV), an enveloped, DNA-containing, β-herpesvirus that infects up to 80% of the population worldwide [22]. Productive infection by HCMV of several cell types that include monocytes/macrophages, CD34+ bone marrow cells, immature dendritic cells, and endothelial cells results in the coordinated synthesis of virus-encoded proteins in a cascade fashion following initial attachment and penetration of the host cell via virus-encoded envelope glycoproteins [reviewed in 23]. Primary infection with HCMV is typically asymptomatic in the immunologically normal person, but the virus nonetheless establishes a latent infection in monocytes and bone marrow cells [22–24]. In patients who are immunosuppressed for solid organ or bone marrow transplantation or who are immunocompromised by HIV/AIDS, however, HCMV can reactivate and induce a sight-threatening retinitis [24]. Prior to development of antiretroviral therapy (ART), AIDS-related HCMV retinitis caused vision loss and blindness in up to 46% of HIV/AIDS patients [20, 25, 26]. While the availability to ART has significantly reduced the number of AIDS-related cases of HCMV retinitis in the United States, incidence remains at 33% and 20% in Africa and Thailand, respectively, due to inaccessibility to ART [27, 28]. In addition, while numerous studies have elucidated the basic clinical and histopathological characteristics of AIDS-related HCMV retinitis, significant gaps remain in our understanding of disease pathogenesis.
Although Th17 cells have been implicated in the pathogenesis of various autoimmune diseases, the role of IL-17 during HIV-induced immunosuppression remains controversial. Brenchley and co-workers [29] found that Th17 cells were lost in mucosa of the gastrointestinal tract of HIV-infected patients, but remained intact in simian immunodeficiency virus-infected sooty mangabey monkeys. In contrast, other studies have found that IL-17 secretion from Th17 cells was increased during HIV infection [30, 31]. To more clearly elucidate the role of Th17 cells in HIV infection as well as the potential contribution(s) of IL-17 to the pathogenesis of AIDS-related HCMV retinitis, we performed a series of studies to test the hypotheses that systemic IL-17 is increased during retrovirus-induced immunosuppression, and that this systemic increase in IL-17 production plays a role in the pathogenesis of cytomegalovirus retinitis within the ocular compartment.
We tested these hypotheses using a mouse model of murine cytomegalovirus (MCMV) retinitis that develops in mice with MAIDS, a retrovirus-induced immunodeficiency syndrome with features that closely mimic HIV/AIDS in humans [25]. Importantly, mice with MAIDS exhibit lymphadenopathy, polyclonal B-cell activation, hypergammaglobulinemia, and a retrovirus-induced shift in cytokine production from a dominant Th1 response to a dominant Th2 response prior to susceptibility to experimental MCMV retinitis [25, 32, 33], thereby making this an attractive animal model to investigate IL-17 in the context of MCMV retinal disease development.
We report herein that while IL-17 mRNA and protein levels increased in mice with MAIDS, CD4+ T cells were not the sole source of IL-17 during retrovirus-induced immunosuppression. Surprisingly, however, IL-17 mRNA and protein levels were dampened in MCMV-infected eyes of MAIDS mice that were susceptible to retinitis, a result that was also observed following systemic MCMV infection of both healthy mice and MAIDS mice. Decreased levels of IL-17 correlated with increased mRNA levels of IL-10 and suppressor of cytokine signaling (SOCS)-3 during MCMV infection. Systemic MCMV infection of healthy IL-10 −/− mice also resulted in a partial recovery of IL-17 protein levels, and IL-10 −/− MAIDS mice remained susceptible to MCMV retinitis at levels equivalent to wildtype MAIDS mice following subretinal MCMV infection. Taken together, our results suggest that while IL-17 plays no direct role in the pathogenesis of MAIDS-related MCMV retinitis, MCMV infection remarkably downregulates IL-17 production by CD4+ T cells through the upregulation of IL-10 and potentially SOCS-3.
2. Methods and Materials
2.1. Animals
Wild-type female C57/BL6 mice and female IL-10 −/− mice on a C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, Maine). Mice were housed in pathogen-free conditions, allowed unlimited access to food and water, and maintained in alternating 12 hr light/dark cycles. All animal procedures were conducted in accordance with Georgia State University Institutional Animal Care and Use Committee (IACUC) policies and the Association for Research in Vision and Ophthalmology (ARVO) statement for Use of Animals in Ophthalmic and Vision Research.
2.2. Viruses
Stocks of MCMV (Smith strain, ATCC, Manassas, VA) and murine retrovirus mixture (LP-BM5) (kindly provided by the AIDS Research and Reference Reagent Program, Germantown, MD) were prepared and stored as previously described [32].
2.3. Induction of MAIDS
MAIDS was induced in three-week-old wild-type C57BL/6 mice or IL-10 −/− mice by intraperitoneal injection using the LP-BM5 retrovirus mixture as described previously [32]. Mice with MAIDS of 4-weeks (MAIDS-4), 8-weeks (MAIDS-8), and 10-weeks duration (MAIDS-10) were used throughout this investigation.
2.4. MCMV infection of mice
2.4.1. Systemic MCMV infection
Mice with MAIDS and healthy age-matched mice were systemically infected with a sublethal dose of MCMV [1×104 plaque forming units (PFU)] by intraperitonal inoculation.
2.4.2. Subretinal MCMV infection
Retinal disease was induced in mice with MAIDS by subretinal infection with MCMV as described previously [34]. Briefly, 2 μl of 1 × 104 PFU of MCMV contained within maintenance medium was injected subretinally into the left eye. To control for the mechanics of the injection procedure, 2 μl of maintenance media alone was injected subretinally into the contralateral eye that served as control.
2.5. Splenic CD4+ T cell, macrophage, and Gr-1+ cell isolation and enrichment
Whole spleens were collected from euthanized MAIDS mice with or without systemic MCMV infection as well as from euthanized healthy, age-matched mice with or without systemic MCMV. Immediately after removal, whole spleens were placed in Dulbecco’s minimal essential medium (DMEM) (Cellgro, Manassas, VA), teased apart, and pushed through a 210-μm nylon mesh screen. The resulting splenic cell suspension was treated with Gey’s solution (Sigma Aldrich, St. Louis, MO) for lysis of red blood cells, and processed for enrichment of individual splenic cell populations according to the protocol provided by Miltenyi Biotec (Cambridge, MA). Enrichment of splenic CD4+ T cells was accomplished by the addition of CD4+ microbeads (Miltenyi Biotec) followed by the addition of anti-CD4+ FITC-labeled antibody (Miltenyi Biotec) to the splenic cell suspension. Enrichment of splenic macrophages was accomplished by the addition of anti-F4/80-PE antibody for 10 min in the dark at 4°C (eBioscience, San Diego, CA) followed by the addition of anti-PE microbeads (Miltenyi Biotec) to the splenic cell suspension. Enrichment of GR-1+ cells (including neutrophils) was accomplished by the addition of anti-Gr-1-PE antibody (Miltenyi Biotec) for 10 min in the dark at 4°C followed by the addition of anti-PE microbeads (Miltenyi Biotec) to the splenic cell suspension. Following incubation of all cell suspensions for 10 min in the dark at 4°C and an additional wash with 1 ml of MACS buffer [phosphate buffered saline (PBS) (Cellgro, Manassas, VA), 2 mM ethylenediaminetetraacetic acid (EDTA) (Sigma Aldrich, St. Louis, MO), and 0.5% bovine serum albumin (BSA) (Fisher Scientific, Pittsburgh, PA)], all cell suspensions were applied to magnetic columns as described in the manufacturer’s instructions (Miltenyi Biotec), and the resulting eluents containing the labeled cell populations were sorted via flow cytometry (BD FACS Aria III, BD Biosciences, San Jose, CA) to achieve an enrichment of > 90%.
2.6. Total RNA extraction
Total RNA was extracted from whole splenic cells, enriched populations of splenic cells, and whole eyes using Trizol (Invitrogen, Grand Island, NY) coupled with the PureLink RNA mini kit (Invitrogen) as per manufacturer’s instructions. Extracted total RNA was stored at −80 °C.
2.7. Quantitative real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay
Extracted total RNA was subjected to reverse transcription following the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen) protocol. cDNA was stored at −20 °C. The resulting cDNAs from whole splenic cells, enriched splenic CD4+ T cells, enriched splenic macrophages, enriched splenic Gr-1-expressing cells, and ocular tissues from all animal groups were subjected to quantitative real-time RT-PCR assay to determine the level of transcription specific for IL-17 (Sense: 5′ CCT GGC GGC TAC AGT GAA G 3′; Antisense: 5′ TTT GGA CAC GCT GAG CTT TG 3′) (Integrated DNA Technologies, Coralville, IA), IL-23 (Sense: 5′ GGT TGA GCG GAA T 3′; Antisense: 5′ AGG GAG TGG GAA C 3′) (Integrated DNA Technologies), IL-6 (Qiagen, Valencia, CA), and IL-10 (Qiagen). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Qiagen) served as the endogenous control. Briefly, 1.2 μl of cDNA was added to a reaction mixture of 15 μl of Power SYBR Green Master Mix (Applied Biosystems, Carlsbad, CA), 1.5 μl of forward and reverse primers for each gene, 9.9 μl of double-distilled water, and 0.9 μl of DMSO (Sigma, St. Louis, MO) for a total volume of 30 μl per reaction. Parameters for each quantitative real-time RT-PCR assay cycle were 15 min at 95 °C, 15 sec at 94 °C, 31 sec at 55 °C, 35 sec at 72 °C for a total of 45 cycles. Transcription levels were determined utilizing 7500 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA) and average threshold cycles (Ct) were determined using the 7500 Fast Real-Time PCR software.
2.8. IL-17 and IL-10 protein quantification by ELISA
Whole eyes and whole spleens collected from all animal groups were stored individually in liquid nitrogen, thawed, individually homogenized in 1 ml of PBS containing a protease inhibitor cocktail (Sigma), and stored individually at −20 C. Homogenates were thawed, sonicated, clarified by centrifugation, and subjected to ELISA for quantification of IL-17 or IL-10 protein using a commercially available kit provided by eBioscience (San Diego, CA). Total protein amounts were determined using the Bradford Protein Assay (BioRad, Hercules CA).
2.9. Immunohistochemical staining of ocular tissues
Whole eyes collected from all animal groups were immediately fixed in 10% buffered formalin (Electron Microscopy Sciences, Hatfield PA) for at least 48 hrs. Formalin-fixed eyes were then frozen in O.C.T. medium (Thermo Scientific, Rochester, NY) and cut using a Shandon Special Motorized Electronic Cryotome (Thermo Scientific) into 8-μm sections that were placed onto positively charged microscope slides (Thermo Scientific). Immunohistochemical staining was performed by incubating slides with eye sections in 10 mM sodium citrate (Sigma Aldrich) for 10 min at room temperature followed by a 5-min rinse with PBS (Cellgro) at room temperature. Immunohistochemical staining for IL-17 in retinal tissues was accomplished by reacting slides with rehydrated eye sections with polyclonal rabbit anti-hIL-17 IgG (1:50) (Santa Cruz Biotechnology, Santa Cruz, CA) or with polyclonal normal rabbit IgG (1:400) (control, Santa Cruz Biotechnology), processed using the rabbit ABC Staining System (Santa Cruz Biotechnology), and stained with Vector Red alkaline phosphatase substrate (Vectastain ABC-AP Kit, Vector Laboratories, Burlingame, CA). Slides were counterstained with DAPI (Vector Laboratories).
Double immunohistochemical staining for the identification of IL-17 producing retinal cells was accomplished by incubating rehydrated eye sections with rabbit-anti-hIL-17 IgG (1:50) (Santa Cruz Biotechnology) and goat anti-hRhodopsin IgG (1:200) for the identification of retinal rod cells (Santa Cruz Biotechnology) or goat anti-hOpsinSW IgG for the identification of retinal cone cells (1:200) (Santa Cruz Biotechnology) at 4 °C overnight in a humidified atmosphere. Following three washes for 5 min with PBS, retinal sections were reacted with secondary antibodies, donkey anti-goat DyLight488 (1:300) (Jackson ImmunoResearch Laboratories INC, West Grove, PA) and chicken anti-rabbit DyLight594 (1:300) (Jackson ImmunoResearch Laboratories INC) and incubated in the dark at room temperature for 1 hr in a humidified atmosphere. Sections were washed three times with PBS, mounted with medium containing DAPI (Vector Laboratories). All stained sections were viewed at 200× or 400× magnification.
2.10. Statistical analysis
All quantitative data from real-time RT-PCR assay and ELISA were expressed as means ± standard error of the mean or standard deviation, respectively. Statistical analysis was performed in this investigation using the Wilcoxon Rank Sum Test or Student T-test. A p-value of < 0.05 was considered significant.
3. Results
3.1. IL-17 protein production in whole splenic cells and whole eyes of healthy C57BL/6 mice
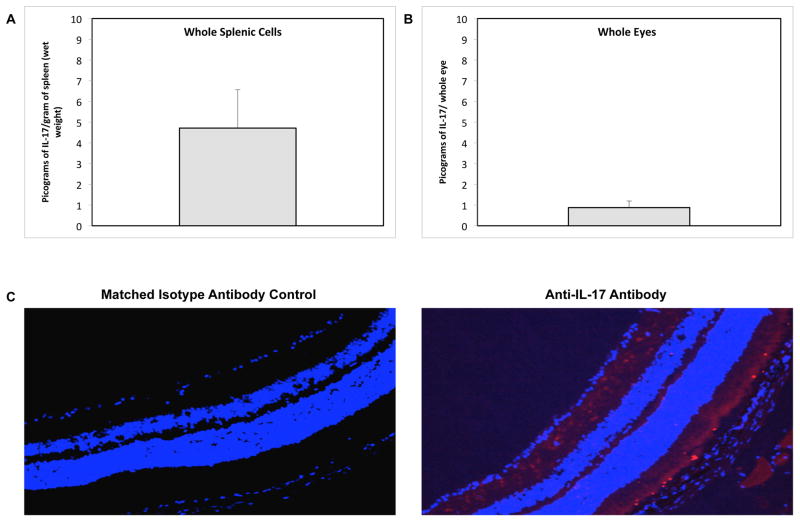
We first sought to determine baseline levels of expression of IL-17 protein in the spleens and eyes of C57BL/6 mice during health. Detectable amounts of IL-17 protein were found in whole splenic cell and whole eye homogenates from healthy animals (Fig. 1A and Fig. 1B). ELISA showed 4.73 ± 1.84 pg of IL-17 per gram of spleen (wet weight) and 0.87 ± 0.31 pg of IL-17 per whole eye. Moreover, visualization of IL-17 protein by immunohistochemical staining of the posterior segment of healthy whole eyes revealed constitutive expression of IL-17 confined to the photoreceptor layer of the neurosensory retina (Fig. 1C). Thus, IL-17 was not only produced within the spleen of healthy mice, but was also produced by cells of the neurosensory retina of healthy mice. Further analysis of the retinal cell types producing IL-17 revealed that both photoreceptor cell types, rods and cones, constitutively express IL-17 during health (Fig 2).
Figure 1.
Detection and quantification of IL-17 protein in whole splenic cells and whole eyes of healthy C57BL/6 mice. (A) IL-17 protein in whole splenic cells of adult healthy C57BL/6 mice as determined by ELISA [pg of IL-17 per gram of spleen (wet weight] (n = 10). Bars = Standard Deviation of 1 experiment. (B) IL-17 protein in whole eyes of adult healthy C57BL/6 mice as determined by ELISA [pg of IL-17 per whole eyes] (n = 10). Bars = Standard Deviation of 1 experiment. (C) Detection of IL-17 in cells of a representative retinal tissue section collected from an adult healthy mouse. Formalin-fixed cytosections were reacted with anti-IL-17 antibody (red) or an isotype-matched normal antibody (control). Nuclei were counterstained with DAPI (blue) (200×).
Figure 2.
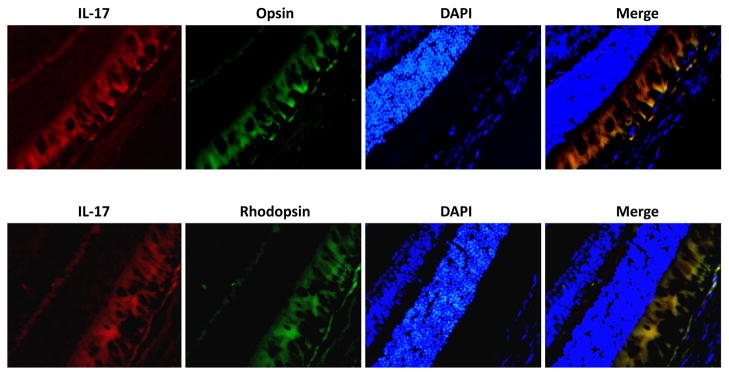
Detection of IL-17 protein in photoreceptor cells of healthy C57BL/6 mice. Formalin-fixed cytosections were reacted with anti-IL-17 antibody (red) and anti-OpsinSW antibody (green) or anti-Rhodopsin antibody (green). Nuclei were counterstained with DAPI (blue) (400×).
3.2. IL-17 mRNA and protein production in whole splenic cells and whole eyes of C57BL/6 mice during progression of MAIDS
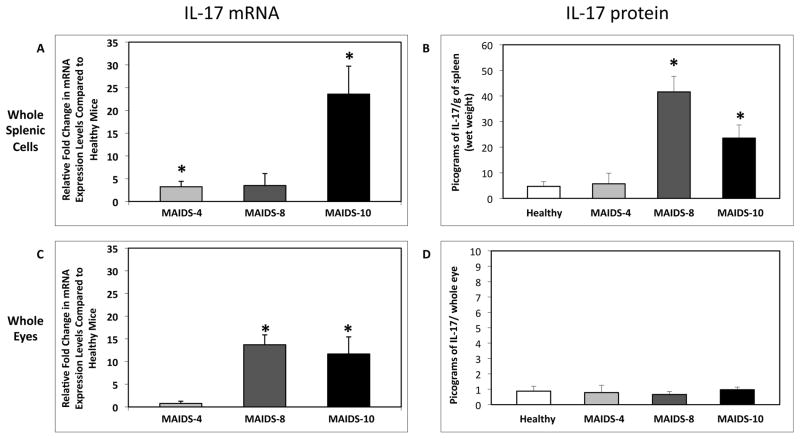
After establishing baseline levels of IL-17 expression in the spleen and eyes of healthy C57BL/6 mice, we next investigated possible changes in IL-17 mRNA and protein production during the progression of MAIDS in order to clarify the fate of IL-17 during retrovirus-immunosuppression. Whole splenic cells and whole eyes were collected from C57BL/6 mice with MAIDS of 4-weeks (MAIDS-4), 8-weeks (MAIDS-8), or 10-weeks (MAIDS-10) duration, and quantified for IL-17 levels. Progression of MAIDS was associated with a significant (p ≤ 0.03) increase in IL-17 mRNA levels in whole splenic cells, with the levels peaking in MAIDS-10 animals (Fig. 3A). This increase in IL-17 mRNA was reflected in a significant (p ≤ 0.001) increase in IL-17 protein production in whole splenic cells of MAIDS-8 and MAIDS-10 animals (41.5 ± 6.1 and 23.5 ± 5.13 pg per gram of spleen (wet weight), respectively) (Fig. 3B).
Figure 3.
IL-17 mRNA and protein levels in whole splenic cells and whole eyes of C57BL/6 mice during the progression of MAIDS. Comparison of (A) IL-17 mRNA levels of whole splenic cells collected from groups of MAIDS-4, MAIDS-8, and MAIDS-10 mice (n = 5), and (C) IL-17 mRNA levels of whole eyes collected from groups of MAIDS-4, MAIDS-8, and MAIDS-10 mice (n = 5). Levels (fold-change) of IL-17 mRNA were determined by quantitative RT-PCR assay. Bars = Standard Error of the Mean of 3 independent experiments. Asterisks indicate statistical significance. Comparison of (B) IL-17 protein levels of whole splenic cells collected from groups of MAIDS-4, MAIDS-8, and MAIDS-10 mice (n = 5), and (D) IL-17 protein levels of whole eyes collected from groups of MAIDS-4, MAIDS-8, and MAIDS-10 mice (n = 5). Levels (fold-change) of IL-17 mRNA were determined by quantitative RT-PCR assay. Protein levels were determined by ELISA. Bars = Standard Deviation of 1 experiment.
MAIDS progression was also associated with a significant (p ≤ 0.0006) increase in IL-17 mRNA levels in whole eyes of MAIDS-8 and MAIDS-10 animals (Fig. 3C), but ocular IL-17 protein levels in these animals did not differ from IL-17 levels in the eyes of healthy mice (Figure 3D) possibly due to the degradation of IL-17 mRNA shortly after transcription or the storage of IL-17 mRNA within cytoplasmic vesicles such that the IL-17 mRNA is not translated into protein. It is noteworthy that previous work has shown MAIDS-8 and MAIDS-10 animals are susceptible to MCMV retinitis [25, 32]. The elevated levels of IL-17 mRNA and protein in whole splenic cells during the progression of MAIDS suggested that numbers of IL-17-producing Th17 cells may be increased during progression of retrovirus-induced immunosuppression, but that this increase may not necessarily be found within the ocular compartment.
3.3. IL-17 mRNA levels in enriched populations of splenic CD4+ T cells, splenic macrophages, and splenic Gr-1-expressing cells (neutrophils) during progression of MAIDS
We next sought to determine the cellular source of splenic IL-17 production in MAIDS-8 and MAIDS-10 animals. Known cellular sources of IL-17 that included populations of splenic CD4+ T cells, splenic macrophages, and splenic Gr-1-expressing cells (neutrophils) [10, 13, 35] were enriched by flow cytometry (purity > 90%) from the whole spleens of MAIDS mice and quantified for IL-17 mRNA levels. Unlike whole splenic cells, however, the progression of MAIDS was associated with decreased IL-17 mRNA levels in splenic CD4+ T cells (Fig. 4). Splenic macrophages and splenic Gr-1-expressing cells (neutrophils) also exhibited similar yet significant (p ≤ 0.05) decreases in IL-17 mRNA levels, and therefore contributed little to overall expression of IL-17 mRNA within the whole spleen (Fig. 4). Decreased IL-17 mRNA from CD4+ T cells during MAIDS progression suggested that retrovirus-induced immunosuppression might alter essential Th17 cell differentiation factors including IL-23 and IL-6.
Figure 4.
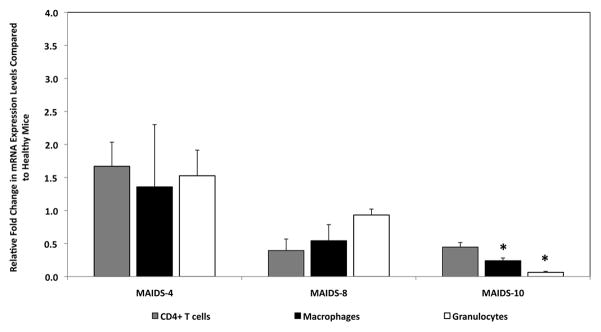
IL-17 mRNA levels of enriched populations of splenic CD4+ T cells, splenic macrophages, and splenic Gr-1+ cells (neutrophils) from C57BL/6 mice during the progression of MAIDS. IL-17 mRNA levels for enriched populations (> 90% purity) of splenic CD4+ T cells (gray bars), splenic macrophages (black bars), and splenic Gr-1+ cells (neutrophils) (white bars) of whole splenic cells collected from groups of MAIDS-4, MAIDS-8, and MAIDS-10 mice (n = 5). Levels (fold-change) of IL-17 mRNA were determined by quantitative RT-PCR assay. Bars = Standard Error of the Mean of 3 independent experiments. Asterisks indicate statistical significance.
3.4. IL-6 and IL-23 mRNA production in whole splenic cells during progression of MAIDS
Since IL-6 and IL-23 from APCs are required for the differentiation of the mouse Th17 lineage [5–7, 9], we measured IL-6 and IL-23 mRNA levels during retrovirus-induced immunosuppression to address the discrepancy observed in IL-17 mRNA expression levels between whole splenic cells and purified populations of splenic CD4+ T cells during MAIDS progression. Whole splenic cells from MAIDS mice exhibited IL-6 and IL-23 mRNA levels equivalent to those seen in healthy age-matched control mice (Fig. 5). Thus, the decrease in IL-17 mRNA production observed in CD4+ T cells during MAIDS progression was not due to the unavailability of the essential linage factors IL-6 or IL-23. Our findings (Figs. 4 and 5) therefore suggest that other cellular sources might contribute to the overall systemic increase in IL-17 production during MAIDS progression.
Figure 5.
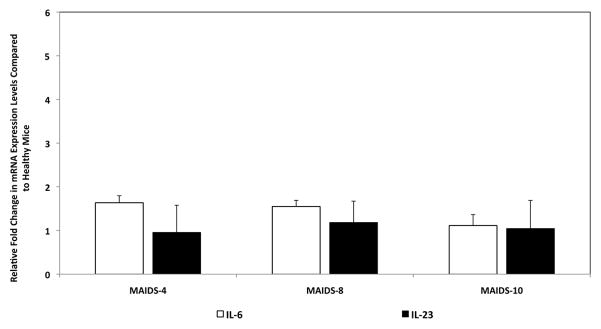
IL-6 and IL-23 mRNA levels of whole splenic cells from C57BL/6 mice during the progression of MAIDS. IL-6 and IL-23 mRNA levels of whole splenic cells collected from groups of MAIDS-4, MAIDS-8, and MAIDS-10 mice (n = 5). Levels (fold-change) of IL-6 and IL-23 mRNA were determined by quantitative RT-PCR assay. Bars = Standard Error of the Mean of 3 independent experiments. Asterisks indicate statistical significance.
3.5. IL-17 mRNA levels in MCMV-infected eyes of retinitis-resistant MAIDS-4 mice and MCMV-infected eyes of retinitis-susceptible MAIDS-10 mice
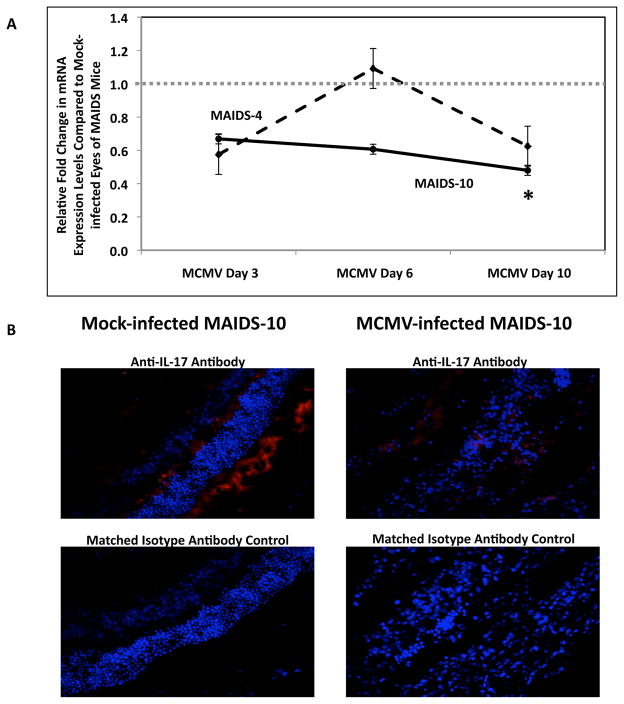
We next sought to determine whether the increased systemic levels of IL-17 observed in MAIDS mice contributed to the pathogenesis of MCMV-related retinitis. We have previously reported that while MCMV-infected eyes of MAIDS-4 and MCMV-infected eyes of MAIDS-10 mice have equivalent amounts of infectious virus at 10 days after subretinal MCMV infection, MAIDS-4 animals are resistant to MCMV retinitis whereas MAIDS-10 animals are susceptible to MCMV retinitis [36, 37]. Because MAIDS-4 and MAIDS-10 mice exhibit such divergent pathogenic outcomes following subretinal MCMV infection, we compared eyes from MAIDS-4 and MAIDS-10 mice inoculated subretinally with MCMV or maintenance medium only (control) on days 3, 6, and 10 post-infection for amounts of IL-17 mRNA. Subretinal MCMV infection of eyes of MAIDS-4 mice did not result in increased IL-17 mRNA levels when compared to mock-infected eyes of MAIDS-4 mice (Fig. 6A). In sharp contrast, MCMV-infected eyes of MAIDS-10 mice exhibited a significant (p ≤ 0.05) decrease in IL-17 mRNA levels on day 10 when compared to mock-infected eyes of MAIDS-10 mice (Fig. 6A).
Figure 6.
IL-17 mRNA levels in MCMV-infected eyes of MAIDS-4 mice versus MAIDS-10 mice and detection of IL-17 protein in MCMV-infected eyes of MAIDS-10 mice. (A) Comparison of IL-17 mRNA levels in MCMV-infected eyes of MAIDS-4 mice (dashed line) versus MCMV-infected eyes of MAIDS-10 mice (solid line) collected at 3, 6, or 10 days after subretinal MCMV infection (n = 5). Levels (fold-change) of IL-17 mRNA were determined by quantitative RT-PCR assay. Bars = Standard Error of the Mean of 2 independent experiments. Asterisks indicate statistical significance. (B) Detection of IL-17 in cells of representative retinal tissue sections collected from eyes of MAIDS-10 mice at 10 days after subretinal MCMV infection or mock infection. Formalin-fixed cytosections were reacted with anti-IL-17 antibody (red) or an isotype-matched normal antibody (control). Nuclei were counterstained with DAPI (blue) (200×).
Due to the significant decrease in IL-17 mRNA levels observed in MCMV-infected eyes of MAIDS-10 mice, we wished to quantify intraocular IL-17 protein in these animals. IL-17 protein levels were decreased in MCMV-infected eyes of MAIDS-10 mice, although not significantly, when compared to contralateral mock-infected eyes (data not shown). Immunohistochemical staining also revealed that IL-17 protein was detectable in cells of the neurosensory retina of eyes of both MCMV-infected and mock-infected MAIDS-10 mice (Fig 6B), but IL-17 production in the MCMV-infected eyes of MAIDS-10 mice was dampened when compared to mock-infected eyes. Taken together (Fig 6A and 6B), these unexpected results suggest that IL-17 plays no direct role in the pathogenesis of MAIDS-related MCMV retinitis, but that MCMV infection may downregulate IL-17 production within the ocular compartment during MAIDS.
3.6. Effect of systemic MCMV infection on IL-17 mRNA levels in splenic CD4+ T cells during health and MAIDS
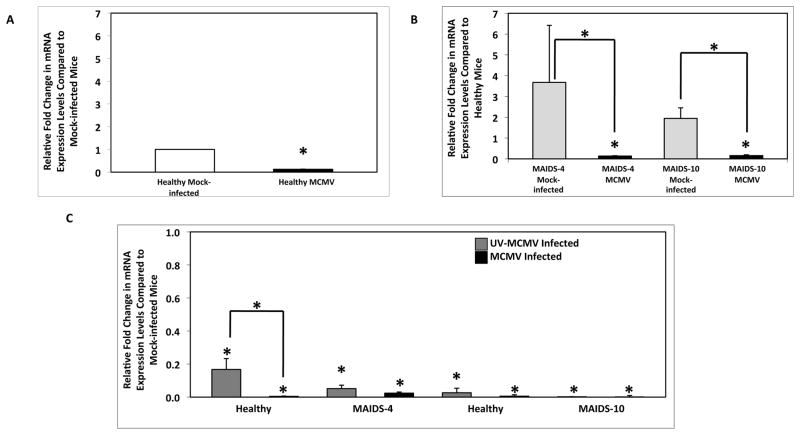
The observation of significantly decreased IL-17 mRNA levels and dampened protein levels in MCMV-infected eyes of MAIDS-10 mice led us to investigate the possibility that MCMV infection might lead to a preferential downregulation of IL-17 from CD4+ Th17 cells systemically. IL-17 mRNA levels were quantified in enriched splenic CD4+ T cells from groups of healthy mice or MAIDS mice infected systemically by intraperitoneal injection with a sublethal dose of MCMV for 6 days. MCMV infection of all animals resulted in a significant (p ≤ 0.01) decrease in IL-17 mRNA levels in splenic CD4+ T cells when compared to mock-infected controls (Figs. 7A and 7B). Whole splenic cell populations from all animal groups also exhibited a significant (p ≤ 0.008) decrease in IL-17 mRNA levels (data not shown). Thus, systemic MCMV infection did indeed result in a significant downregulation of IL-17 mRNA production from CD4+ T cells.
Figure 7.
IL-17 mRNA levels in splenic CD4+ T cells of healthy C57LB/6 mice, MAIDS-4 mice, and MAIDS-10 mice following systemic MCMV infection. IL-17 mRNA levels of splenic CD4+ T cells collected from (A) groups of healthy C57BL/6 mice (n = 5) and (B) groups of MAIDS-4 and MAIDS-10 mice (n = 5) at 10 days after intraperitoneal inoculation with MCMV or mock-infected. Levels (fold-change) of IL-17 mRNA were determined by quantitative RT-PCR assay. Bars = Standard Error of the Mean of 3 independent experiments. Asterisks indicate statistical significance. (C) IL-17 mRNA levels of splenic CD4+ T cells collected from MAIDS-4 mice, MAIDS-10 mice, and their age-matched healthy controls inoculated with either UV-inactivated MCMV or infectious MCMV. Levels (fold-change) of IL-17 mRNA were determined by quantitative RT-PCR assay. Bars = Standard Error of the Mean of 1 experiment. Asterisks indicate statistical significance.
In order to determine whether productive MCMV infection was required to downregulate IL-17 mRNA production in CD4+ T cells, groups of healthy mice or MAIDS mice were systemically inoculated with either UV-inactivated MCMV or infectious MCMV for 6 days. IL-17 mRNA levels in splenic CD4+ T cells were significantly (p ≤ 0.05) reduced in all animals inoculated with UV-inactivated MCMV (Fig. 7C), but IL-17 mRNA levels were further reduced when animals were inoculated with infectious MCMV (Fig. 7C). These results suggest that MCMV structural protein(s) (tegument proteins and/or glycoproteins) as well as one or more virus-induced proteins produced during active virus replication alter the host cell response to decrease IL-17 production during systemic MCMV infection.
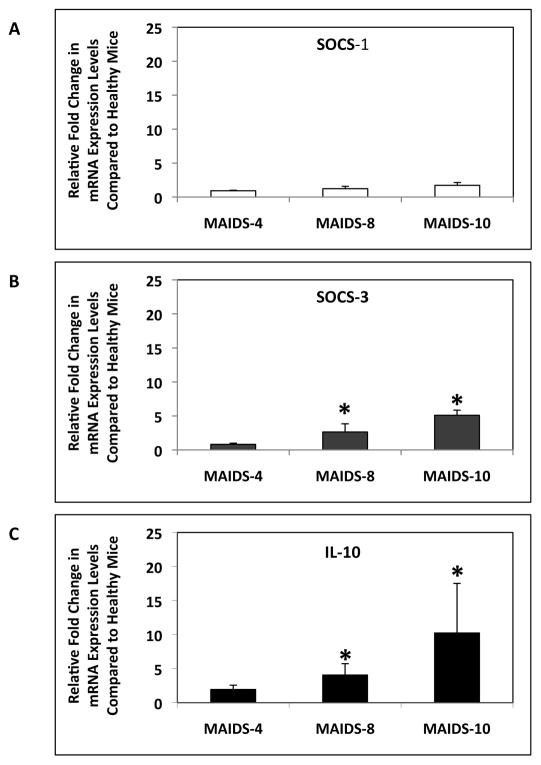
3.7. SOCS-3, SOCS-1, and IL-10 mRNA expression levels in whole splenic cells of mice with MAIDS
We next sought to investigate the possible mechanism(s) by which IL-17 mRNA production was downregulated in CD4+ T cells during MAIDS progression as well as during MCMV infection. Previous studies by Chen and colleagues [38] identified the SOCS-3 protein as a negative regulator of IL-17 in CD4+ T cells. Additionally, loss of the SOCS-1 protein leads to defective Th17 cell differentiation [39, 40]. Although both SOCS-1 and SOCS-3 are increased during HIV infection [41, 42], we investigated whether SOCS-3 mRNA levels were increased during retrovirus-induced immunosuppression and/or whether SOCS-1 mRNA levels were decreased, thereby leading to a decrease in IL-17 mRNA expression from CD4+ T cells during MAIDS progression. Whereas SOCS-1 mRNA levels in whole splenic cells of MAIDS animals remained equivalent to mRNA levels of healthy mice (Fig. 8A), SOCS-3 mRNA levels were significantly (p ≤ 0.05) increased during MAIDS progression (Fig. 8B). Importantly, increased SOCS-3 mRNA levels correlated with decreased IL-17 mRNA levels in splenic CD4+ T cells seen during MAIDS progression (Fig. 4).
Figure 8.
SOCS-1, SOCS-3, and IL-10 mRNA levels in whole splenic cells of C57BL/6 mice during the progression of MAIDS. (A) SOCS-1 mRNA levels, (B) SOCS-3 mRNA levels, and (C) IL-10 mRNA levels of whole splenic cells collected from groups of MAIDS-4, MAIDS-8, and MAIDS-10 mice (n = 5). Levels (fold-change) of SOCS-1 mRNA, SOCS-3 mRNA, and IL-10 mRNA were determined by quantitative RT-PCR assay. Bars = Standard Error of the Mean of 2 independent experiments. Asterisks indicate statistical significance.
Recent work has also determined that IL-10 negatively regulates IL-17 secretion from Th17 cells [43, 44]. We therefore sought to determine if IL-10 mRNA expression during MAIDS progression was also increased. Whole splenic exhibited a significant (p ≤ 0.03) increase in IL-10 mRNA production during MAIDS progression (Fig. 8C). In particular, levels of IL-10 mRNA were high in whole splenic cells of retinitis-susceptible MAIDS-8 and MAIDS-10 animals [25, 32]. The increase in IL-10 mRNA levels in whole splenic cells of MAIDS-8 mice and MAIDS-10 mice correlated with the decrease in IL-17 mRNA levels observed in CD4+ T cells during MAIDS progression (Fig. 4).
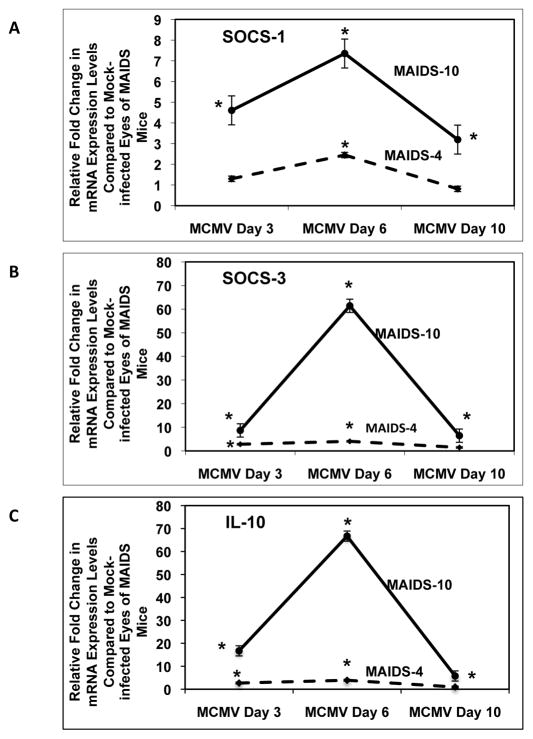
3.8. SOCS-1, SOCS-3, and IL-10 mRNA levels in MCMV-infected eyes of retinitis-resistant MAIDS-4 mice and MCMV-infected eyes of retinitis-susceptible MAIDS-10 mice
To determine whether SOCS-3 and IL-10 production were also negatively influencing the expression of IL-17 during the onset and progression of retinitis in the eyes of MAIDS mice, MCMV-infected and mock-infected eyes from MAIDS-4 and MAIDS-10 mice were compared for amounts of SOCS-3 and IL-10 mRNA. mRNA levels of the required Th17 lineage differentiation factors SOCS-1 and IL-6 [5, 39] were also examined. While SOCS-3 mRNA levels were indeed significantly (p ≤ 0.03) increased in MCMV-infected eyes of MAIDS-4 mice on day 6, MCMV-infected eyes of MAIDS-10 mice exhibited a far greater increase (p ≤ 0.007) in SOCS-3 mRNA levels when compared with MCMV-infected eyes of MAIDS-4 mice (Fig. 9B). Similarly, IL-10 mRNA levels in MCMV-infected eyes of MAIDS-10 mice exhibited a dramatic 12-fold and ~60-fold (p ≤ 0.01) increase in IL-10 mRNA on days 3 and 6 postinfection, respectively, when compared with mRNA levels in MCMV-infected eyes of MAIDS-4 mice (Fig. 9C). SOCS-1 (Fig. 9A) and IL-6 (data not shown) mRNA levels mirrored those observed for SOCS-3 and IL-10, with mRNA levels of both molecules being significantly (p ≤ 0.03) increased in MCMV-infected eyes of MAIDS-10 mice when compared to MCMV-infected eyes of MAIDS-4 mice. It is noteworthy that peak mRNA levels of SOCS-3 and IL-10 in the MCMV-infected eyes of MAIDS-10 mice preceded the decrease in IL-17 mRNA levels observed in the eyes of these animals during retinitis development (Fig 6A), suggesting that SOCS-3 and/or IL-10 contribute to the downregulation of IL-17 during ocular MCMV infection.
Figure 9.
SOCS-1, SOCS-3, and IL-10 mRNA levels in MCMV-infected eyes of MAIDS-4 mice versus MCMV-infected eyes of MAIDS-10 mice. (A) SOCS-1 mRNA levels, (B) SOCS-3 mRNA levels, and (C) IL-10 mRNA levels of MCMV-infected eyes of MAIDS-4 mice (dashed line) versus MCMV-infected eyes of MAIDS-10 mice (solid line) collected at 3, 6, or 10 days after subretinal MCMV infection (n = 5). Levels (fold-change) of SOCS-1 mRNA, SOCS-3 mRNA, and IL-10 mRNA were determined by quantitative RT-PCR assay. Bars = Standard Error of the Mean of 2 independent experiments. Asterisks indicate statistical significance.
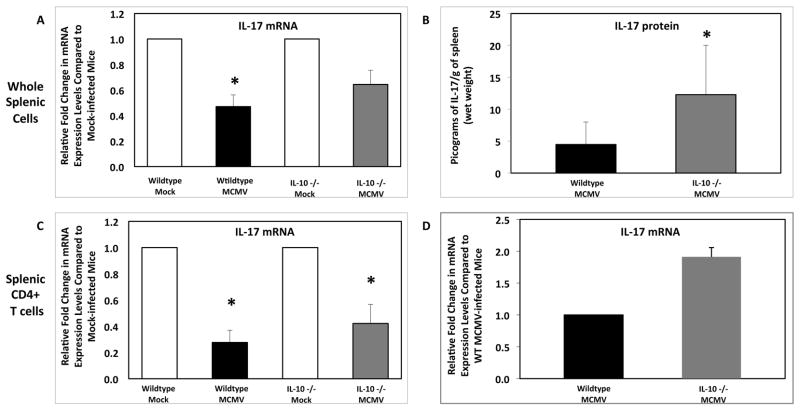
3.9. Effect of systemic MCMV infection on IL-17 mRNA and protein levels in whole splenic cells and splenic CD4+ T cells of wildtype mice and IL-10 −/− mice without MAIDS
Since we demonstrated that IL-10 mRNA levels were upregulated in whole splenic cells of MAIDS animals (Fig. 8C) as well as in the eyes of retinitis-susceptible MAIDS animals following subretinal MCMV infection (Fig. 9C) and that this increase in IL-10 mRNA correlated with the decrease in IL-17 mRNA observed in CD4+ T cells, we investigated whether knockout of IL-10 would restore IL-17 levels. Wildtype mice without MAIDS and IL-10 −/− mice without MAIDS were systemically infected with a sublethal dose of MCMV for 6 days. Systemic MCMV infection of wildtype mice resulted in a significant (p ≤ 0.03) decrease in IL-17 mRNA levels in whole splenic cells (Fig. 10A), a finding that confirmed our previous findings (Fig 7A). However, while IL-17 mRNA levels were dampened in splenic cells of systemically infected IL-10 −/− mice (Fig. 10A), IL-17 protein levels were significantly (p ≤ 0.05) increased in whole splenic cells of these mice [12.28± 7.7 pg of IL-17 per gram of spleen (wet weight)] when compared to infected wildtype mice [4.47± 3.5 pg of IL-17 per gram of spleen (wet weight)] (Fig. 10B). While the production of IL-17 mRNA in splenic CD4+ T cells was significantly (p ≤ 0.03) reduced in both animal groups (Fig. 10C), IL-17 mRNA levels in infected IL-10 −/− animals were ~2-fold higher (Fig. 10D). These results suggest that IL-10 knockout partially restored IL-17 levels during systemic MCMV infection.
Figure 10.
IL-17 mRNA and protein levels in whole splenic cells and splenic CD4+ T cells of wildtype mice and IL-10 −/− mice following systemic MCMV infection. Comparison of IL-17 mRNA levels of (A) whole splenic cells, (C) and (D) splenic CD4+ T cells collected from wildtype mice (n = 5) and IL-10 −/− mice (n = 5) at 6 days following intraperitoneal MCMV infection. Levels (fold-change) of IL-17 mRNA were determined by quantitative RT-PCR assay. Bars = Standard Error of the Mean of 2 independent experiments. Comparison of IL-17 protein levels of (B) whole splenic cells collected from wildtype mice (n = 5) and IL-10 −/− mice (n = 5) at 10 days following intraperitoneal MCMV infection. Protein levels were determined by ELISA. Bars = Standard Error of the Mean of 2 independent experiments. Asterisks indicate statistical significance.
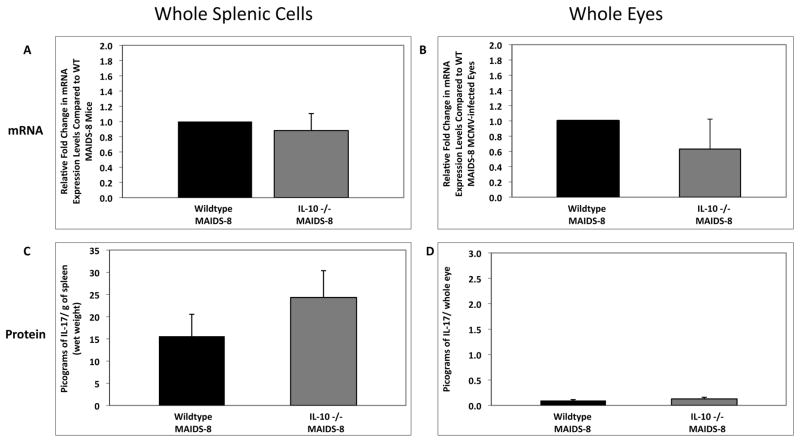
3.10. IL-17 mRNA and protein levels in whole splenic cells and eyes of wildtype MAIDS-8 mice and IL-10 −/− MAIDS-8 mice following subretinal MCMV inoculation
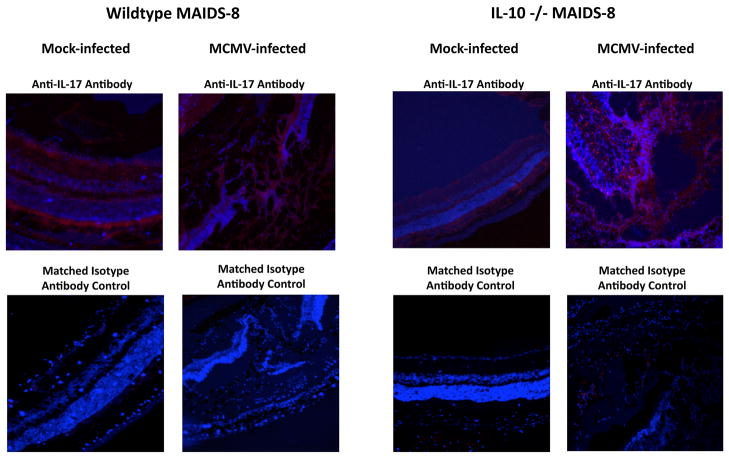
Since we demonstrated that systemic MCMV infection of IL-10 −/− mice without MAIDS resulted in a partial restoration of IL-17 mRNA and protein levels, we next wanted to determine whether subretinal MCMV infection of retinitis-susceptible IL-10 −/− MAIDS-8 mice also resulted in partial recovery of IL-17 mRNA and protein levels, which protected mice from retinitis development. While IL-17 mRNA levels did not increase in either whole splenic cells or whole eyes of IL-10 −/− MAIDS-8 mice following subretinal MCMV inoculation (Fig. 11A and 11B), IL-17 protein levels were consistently elevated in both splenic cells and eyes of the IL-10 −/− MAIDS-8 animals (Fig. 11C and 11D). Ocular tissues collected from IL-10 −/− MAIDS-8 mice and analyzed for IL-17 protein via immunohistochemistry exhibited an enhancement in IL-17 protein production when compared to MCMV-infected eyes of wildtype MAIDS-8 mice (Fig. 12), although both groups remained equally susceptible to MCMV retinitis (data not shown). As observed during systemic MCMV infection (Fig. 10), these results suggest that partial restoration of IL-17 was not sufficient to protect MAIDS mice against MCMV retinitis.
Figure 11.
IL-17 mRNA and IL-17 protein levels in whole splenic cells and MCMV-infected eyes of wildtype MAIDS-8 mice and IL-10 −/− MAIDS-8 mice following subretinal MCMV infection. Comparison of IL-17 mRNA levels in (A) whole splenic cells and (B) MCMV-infected eyes of groups of wildtype MAIDS-8 mice (n = 10) and IL-10 −/− MAIDS-8 mice (n = 10) at 10 days after subretinal MCMV infection. Levels (fold-change) of IL-17 mRNA were determined by quantitative RT-PCR assay. Bars = Standard Error of the Mean of 1 experiment. Comparison of IL-17 protein levels in (C) whole splenic cells and (D) MCMV-infected eyes of groups of wildtype MAIDS-8 mice (n = 10) and IL-10 −/− MAIDS-8 mice (n = 10) at 10 days after subretinal MCMV infection. Protein levels were determined by ELISA. Bars = Standard Error of the Mean of 1 experiment. All comparisons showed no statistical significance.
Figure 12.
Detection of IL-17 protein in MCMV-infected eyes of wildtype MAIDS-8 mice and IL-10 −/− MAIDS-8 mice. Detection of IL-17 in cells of representative retinal tissue sections collected from eyes of wildtype MAIDS-8 mice and IL-10 −/− MAIDS-8 mice at 10 days after subretinal MCMV infection or mock infection. Formalin-fixed cytosections were reacted with anti-IL-17 antibody (red) or an isotype-matched normal antibody (control). Nuclei were counterstained with DAPI (blue) (200×).
4. Discussion
The present study used a clinically relevant mouse model of MAIDS-related MCMV retinitis (i) to determine the fate of IL-17 during retrovirus-induced immunosuppression and (ii) to determine whether IL-17 contributes to the pathogenesis of HCMV retinitis in patients with AIDS. Whereas IL-17 expression was detected within spleen and eyes of healthy mice, IL-17 production increased systemically during MAIDS progression. Surprisingly, MCMV-infected eyes of retinitis-susceptible MAIDS-10 mice, but not MCMV-infected eyes of retinitis-resistant MAIDS-4 mice, exhibited significant decrease in IL-17 production. IL-17 mRNA production was also decreased in whole splenic cells and in splenic CD4+ T cells of both healthy mice and MAIDS mice upon systemic MCMV infection. This decrease was associated with increased production of SOCS-3 and IL-10 mRNAs during MCMV infection. Knockout of IL-10 in MAIDS mice only partially restored IL-17 levels and failed to protect mice against MCMV retinitis.
4.1. IL-17 production in splenic cells and eyes of C57BL/6 mice during health
IL-17 was detected at low levels in the spleens of healthy mice, an observation consistent with that of Brenchley and coworkers [29] who detected low levels of IL-17-expressing CD4+ T cells in the peripheral blood of healthy human patients. IL-17 was also detected, albeit at lower levels than spleen, in cells of the neurosensory retina of healthy mice. Further investigation determined that both rod and cone cells of the photoreceptor layer of the neurosensory retina constitutively expressed IL-17 during health, although the biological consequence(s) of IL-17 production by these unique cells remain unclear. To our knowledge, ours is the first study to identify resident IL-17-expressing cells within the ocular compartment of healthy mice. In addition to retinal photoreceptors cells, retinal pericytes and/or microglia may also produce IL-17 due to their ability to secrete inflammatory cytokines [45–47].
4.2. IL-17 production in splenic cells and eyes of C57BL/6 mice during progression of MAIDS
Our findings to determine the fate of IL-17 in whole splenic cells during the progression of MAIDS agreed with previous studies [31, 48] that showed that IL-17 production is increased in patients during HIV infection. It was surprising, however, that MAIDS progression was also associated with a corresponding decrease in IL-17 mRNA levels in enriched splenic CD4+ T cells, a finding in agreement with those of Brenchley and coworkers [29] in HIV-infected persons. Other known cellular sources of IL-17 such as splenic macrophages and splenic Gr-1-expressing cells (neutrophils) contributed little to the overall IL-17 mRNA expression during MAIDS progression. It is therefore likely that CD4+ T cells, macrophages, and neutrophils in combination with other known sources of IL-17 such as natural killer T cells (NKTs), CD8+ T cells, and gamma-delta (γδ) T cells [9, 49] may all collectively contribute to the overall systemic increase of IL-17 observed during MAIDS.
4.3. IL-17 production and susceptibility to MCMV retinitis during MAIDS
Intraocular IL-17 production has been associated with uveitis, an inflammatory disease of the retina and uvea. Disease etiology is autoimmune, with uveitis patients exhibiting immune responses to the ocular antigens retinal arrestin and/or interphotoreceptor retinoid-binding protein [19]. Use of experimental autoimmune uveitis (EAU) in mice has also defined the effector cells involved in retinal and uveal tissue destruction during disease pathogenesis [14, 15, 19]. Originally thought to be solely a Th1-mediated disease, uveitis is also induced through Th17 cells and IL-17 secretion [14, 15, 19]. Neutralization of IL-17 through passive transfer of anti-IL-17 antibody mitigates EAU severity significantly [15, 17, 18], whereas adoptive transfer of retinal antigen-specific Th17 cells induces EAU development in naïve mice [15, 18]. Because IL-17 is involved in uveitis pathogenesis, we sought to determine whether increase in IL-17 levels seen in MAIDS mice played a role in susceptibility to MCMV retinitis. Whereas retinitis-resistant MAIDS-4 mice did not exhibit changes in IL-17 mRNA levels following subretinal MCMV inoculation, MCMV-infected eyes of retinitis-susceptible MAIDS-10 mice surprisingly exhibited a significant decrease in IL-17 mRNA levels. Ocular IL-17 protein levels and IL-17 immunohistochemical staining were also dampened in MCMV-infected eyes of retinitis-susceptible animals. We therefore conclude that IL-17 plays no direct role in increased susceptibility to MCMV retinitis during MAIDS. It is possible, however, that IL-17 acts indirectly to increase susceptibility to MCMV retinitis through its ability to activate neutrophils and macrophages [8, 10, 49, 50], key players in MCMV-related retinitis pathogenesis [37]. On the other hand, IL-17 may play a protective role in MCMV retinitis development during MAIDS because IL-17 was downregulated in retinitis-susceptible MAIDS-10 mice. One approach to test these hypotheses will be to perform parallel studies using IL-17A −/− mice when available.
4.4. IL-17 production in splenic CD4+ T cells of healthy mice and MAIDS mice following systemic MCMV infection
The surprising observation of significantly reduced IL-17 mRNA and dampened protein levels during ocular MCMV infection of MAIDS-10 mice led us to investigate whether MCMV also downregulated IL-17 production from CD4+ T cells systemically. In agreement with this hypothesis was our finding that systemic MCMV infection resulted in a significant reduction of IL-17 mRNA levels in whole splenic cells and splenic CD4+ T cells from both healthy mice and MAIDS mice. Experiments using UV-inactivated virus also suggested that active MCMV infection was not an absolute requirement for reduction of IL-17 mRNA in CD4+ T cells, although productive MCMV replication did result in a more profound decrease in IL-17 mRNA levels. Taken together, these results suggest that structural viral proteins as well as virus-induced proteins might work in concert to contribute to the downregulation of IL-17 in CD4+ T cells. HCMV pp65 tegument protein has been shown to alter the host immune response early during infection by blocking the interferon response and inhibiting the NK cell activity through direct interaction with its receptor [51–53]. Because MCMV encodes a pp65 homolog, M83/84 [54, 55], the M83/84 tegument protein of MCMV might also serve to downregulate IL-17 production in MCMV-infected cells. Several structural MCMV glycoproteins similary exhibit immune evasion properties. Glycoproteins (gp) gp34, gp48, and gp40 decrease MHC I protein expression on virus-infected cells [55–59] leading to decreased CD4+ T cell and CD8+ T cell activation. In addition, the MCMV protein m155 inhibits expression of the CD4+ T cell and CD8+ T cell stimulator protein CD40 in virus-infected monocytes/macrophages and dendritic cells [57, 60, 61]. The combined action of these virus-encoded proteins and glycoproteins to decease CD4+ T-cell activation might account for the reduction in IL-17 production from CD4+ T cells observed during MCMV infection of healthy mice and MAIDS mice.
4.5. Proposed mechanism of IL-17 downregulation during MCMV infection
The observation that IL-17 was decreased in CD4+ T cells during MAIDS progression as well as during ocular and systemic infection of retinitis-susceptible MAIDS animals led us to explore possible mechanisms by which retrovirus-induced immunosuppression might downregulate IL-17 production by CD4+ T cells. Recent studies have implicated both SOCS-3 and IL-10 as negative regulators of IL-17 secretion [35, 43, 44]. It is therefore possible that increases in one or both of these proteins would cause the IL-17 mRNA decrease observed by us in CD4+ T cells during MAIDS progression. SOCS-3 is induced by cytokine signaling [39] and negatively regulates Th17 cell differentiation by suppressing STAT3 activation of IL-6 and IL-23 receptors on Th17 cells [38, 39]. Herein, we have shown that SOCS-3 mRNA levels were significantly increased during retrovirus-induced immunosuppression with mRNA levels peaking in MAIDS-10 mice when most susceptible to MCMV retinitis [25, 32]. Importantly, the increase in SOCS-3 mRNA levels corresponded with a decrease in IL-17 mRNA levels observed in CD4+ T cells during MAIDS progression, thereby suggesting a potential explanation for downregulation in IL-17 mRNA from CD4+ T cells during MAIDS progression.
While little is known about the potential induction of SOCS-3 or SOCS-1 during MCMV infection, a number of viruses (HIV, herpes simplex virus type 1, influenza virus, and respiratory syncytial virus) exploit SOCS proteins to evade host cell immune responses [41, 62, 63]. We propose that MCMV acts in a similar manner. While we observed increases in both SOCS-1 and SOCS-3 mRNA levels in the MCMV-infected eyes of MAIDS-4 mice and MAIDS-10 mice, levels were far greater in MCMV-infected eyes of retinitis-susceptible MAIDS-10 mice. Importantly, the increase in ocular SOCS-3 mRNA levels preceded the decrease in IL-17 mRNA observed in MCMV-infected eyes of MAIDS-10 mice on day 10. It is therefore possible that the action of MCMV tegument proteins and/or glycoproteins upregulate SOCS-3 for efficient viral replication and/or for evasion of host immune responses, which, in turn, results in the downregulation of IL-17.
IL-10 is an anti-inflammatory cytokine secreted by numerous immune cells. It regulates IL-17 secretion by binding to its receptor on Th17 cells [43, 44, 64]. We demonstrated that IL-10 mRNA levels were significantly increased during MAIDS progression, and peaked in retinitis-susceptible MAIDS-10 mice [25, 32]. Similar to SOCS-3, the increase in IL-10 mRNA levels corresponded with decreased IL-17 mRNA levels observed in CD4+ T cells during MAIDS progression. Systemic MCMV infection also induces expression of IL-10 in both MCVM-infected CD4+ T cells and macrophages [65, 66]. Increased production of IL-10 during MCMV infection leads to reduction of MHC class II on virus-infected cells and subsequently a decreased host response [66]. We observed a significant increase in IL-10 mRNA levels in MCMV-infected eyes of MAIDS-10 mice that were susceptible to MCMV retinitis. As IL-10 mRNA levels increased during MCMV retinitis development, IL-17 mRNA levels decreased. It is therefore possible that MCMV orchestrates the downregulation of IL-17 through the induction of IL-10 along with SOCS-3.
Systemic MCMV infection of IL-10 −/− mice without MAIDS resulted in an increase in IL-17 mRNA and protein production in whole splenic cells and elevated IL-17 mRNA expression levels in splenic CD4+ T cells. In comparison, subretinal MCMV infection of MAIDS-8 IL-10 −/− mice led to elevated levels of IL-17 protein in whole splenic cells and in MCMV-infected eyes, but this partial recovery was not sufficient to protect MAIDS-8 mice against MCMV retinitis development. These results suggest that knockout of IL-10 results only in a partial restoration of IL-17 expression levels. Perhaps other proteins such as SOCS-3 may contribute to the downregulation of IL-17 during MCMV infection.
In conclusion, we have demonstrated that IL-17 plays no direct role in the pathogenesis of MAIDS-related MCMV retinitis. Remarkably, however, systemic MCMV infection of mice with MAIDS resulted in downregulation of IL-17 production from CD4+ T cells, possibly through the actions of specific MCMV viral proteins that lead to the upregulation of SOCS-3 and IL-10 during infection (Fig. 13). SOCS-3 and IL-10 may work in a synergistic manner to downregulate IL-17 from CD4+ T cells, although the direct role of SOCS-3 in downregulation of IL-17 during MCMV infection remains unclear. Lastly, while partial restoration of IL-17 through knockout of IL-10 did not reduce the frequency and/or severity of MCMV retinitis in MAIDS animals, it remains to be determined whether full restoration of IL-17 from Th17 cells during MAIDS plays a protective role in resistance to MCMV retinitis.
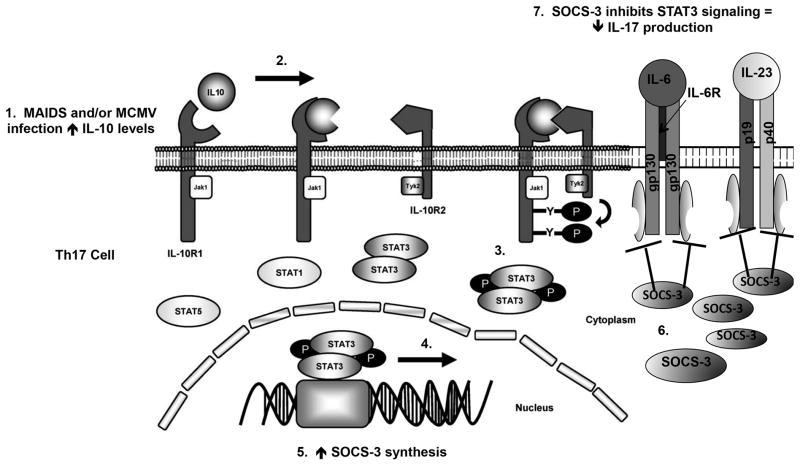
Figure 13. Proposed mechanism for downregulation of IL-17 in Th17 cells by MCMV.
MCMV infection stimulates IL-10 production during health and retrovirus-induced immunosuppression. (1) IL-10 binds to its receptor on Th17 cells that (2) induces activation of Janus kinase-1 (Jak1) and tyrosine kinase-2 (Tyk2) that (3) leads to the phosphorylation of signal transducer and activator of transcription-3 (STAT3). Phosphorylated STAT3 translocates to the nucleus where (4) it initiates SOCS-3 transcription that (5) results in increased SOCS-3 mRNA synthesis. Following translocation to the cytoplasm, (6) SOCS-3 mRNA is translated into SOCS-3 protein. (7) SOCS-3 protein ultimately inhibits STAT3 signaling at the IL-6 and IL-23 receptors on Th17 cells resulting in decreased IL-17 production. Adapted from [39, 67]
Highlights.
IL-17 is expressed constitutively within retinal tissues of healthy C57BL/6 mice.
IL-17 levels in whole splenic cells increase during the progression of MAIDS.
Cell populations other than CD4+ T cells contribute to IL-17 production during MAIDS.
MCMV infection downregulates IL-17 production during health and MAIDS.
MCMV infection may downregulate IL-17 production via stimulation of SOCS-3 and IL-10.
Acknowledgments
We thank the Biology Core Facilities, Department of Biology, and Georgia State University for assistance with real-time RT-PCR analysis and flow cytometry. This work has been supported in part by NIH/NEI Grant EY010568, NIH/NEI Core Grant P30EY006360, and Fight for Sight, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 2.Cherwinski HM, Schumacher JH, Brown KD, Mosmann TR. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–44. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 4.Wan YY. Multi-tasking of helper T cells. Immunology. 2010;130:166–71. doi: 10.1111/j.1365-2567.2010.03289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–7. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuzawa-Carballeda J, Vargas-Rojas MI, Cabral AR. Autoimmune inflammation from the Th17 perspective. Autoimmun Rev. 2007;6:169–75. doi: 10.1016/j.autrev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;120:247–54. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 8.Annunziato F, Cosmi L, Romagnani S. Human and murine Th17. Curr Opin HIV AIDS. 2010;5:114–9. doi: 10.1097/COH.0b013e32833647c2. [DOI] [PubMed] [Google Scholar]
- 9.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 11.Stockinger B, Veldhoen M, Martin B. Th17 T cells: Linking innate and adaptive immunity. Semin Immunol. 2007 doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Lovett-Racke AE, Yang Y, Racke MK. Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis? Biochim Biophys Acta. 2011;1812:246–51. doi: 10.1016/j.bbadis.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 14.Luger D, Caspi RR. New perspectives on effector mechanisms in uveitis. Semin Immunopathol. 2008;30:135–43. doi: 10.1007/s00281-008-0108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caspi R. Autoimmunity in the immune privileged eye: pathogenic and regulatory T cells. Immunol Res. 2008;42:41–50. doi: 10.1007/s12026-008-8031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 18.Peng Y, Han G, Shao H, Wang Y, Kaplan HJ, Sun D. Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2007;48:4153–61. doi: 10.1167/iovs.07-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120:3073–83. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabs DA. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc. 1995;93:623–83. [PMC free article] [PubMed] [Google Scholar]
- 21.Jabs DA. Cytomegalovirus retinitis and the acquired immunodeficiency syndrome--bench to bedside: LXVII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2011;151:198–216. doi: 10.1016/j.ajo.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knipe DM, Howley PM. Fields Virology. (5) 2007;2:2701–72. [Google Scholar]
- 23.Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22:76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddehase MJ. Cytomegaloviruses: Molecular Biology and Immunology. Norfolk: Caister Academic Press; 2006. [Google Scholar]
- 25.Dix RD, Cousins SW. AIDS-related cytomegalovirus retinitis: lessons from the laboratory. Curr Eye Res. 2004;29:91–101. doi: 10.1080/02713680490504641. [DOI] [PubMed] [Google Scholar]
- 26.Gallant JE, Moore RD, Richman DD, Keruly J, Chaisson RE. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. The Zidovudine Epidemiology Study Group. J Infect Dis. 1992;166:1223–7. doi: 10.1093/infdis/166.6.1223. [DOI] [PubMed] [Google Scholar]
- 27.Heiden D, Ford N, Wilson D, Rodriguez WR, Margolis T, Janssens B, et al. Cytomegalovirus retinitis: the neglected disease of the AIDS pandemic. PLoS Med. 2007;4:e334. doi: 10.1371/journal.pmed.0040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart MW. Optimal management of cytomegalovirus retinitis in patients with AIDS. Clin Ophthalmol. 2010;4:285–99. doi: 10.2147/opth.s6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–35. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue FY, Merchant A, Kovacs CM, Loutfy M, Persad D, Ostrowski MA. Virus-specific interleukin-17-producing CD4+ T cells are detectable in early human immunodeficiency virus type 1 infection. J Virol. 2008;82:6767–71. doi: 10.1128/JVI.02550-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maek ANW, Buranapraditkun S, Klaewsongkram J, Ruxrungtham K. Increased interleukin-17 production both in helper T cell subset Th17 and CD4-negative T cells in human immunodeficiency virus infection. Viral Immunol. 2007;20:66–75. doi: 10.1089/vim.2006.0063. [DOI] [PubMed] [Google Scholar]
- 32.Dix RD, Cray C, Cousins SW. Mice immunosuppressed by murine retrovirus infection (MAIDS) are susceptible to cytomegalovirus retinitis. Curr Eye Res. 1994;13:587–95. doi: 10.3109/02713689408999892. [DOI] [PubMed] [Google Scholar]
- 33.Morse HC, 3rd, Giese N, Morawetz R, Tang Y, Gazzinelli R, Kim WK, et al. Cells and cytokines in the pathogenesis of MAIDS, a retrovirus-induced immunodeficiency syndrome of mice. Springer Semin Immunopathol. 1995;17:231–45. doi: 10.1007/BF00196167. [DOI] [PubMed] [Google Scholar]
- 34.Atherton SS, Newell CK, Kanter MY, Cousins SW. Retinitis in euthymic mice following inoculation of murine cytomegalovirus (MCMV) via the supraciliary route. Curr Eye Res. 1991;10:667–77. doi: 10.3109/02713689109013858. [DOI] [PubMed] [Google Scholar]
- 35.Gu Y, Yang J, Ouyang X, Liu W, Li H, Bromberg J, et al. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol. 2008;38:1807–13. doi: 10.1002/eji.200838331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dix RD, Cousins SW. Susceptibility to murine cytomegalovirus retinitis during progression of MAIDS: correlation with intraocular levels of tumor necrosis factor-alpha and interferon-gamma. Current eye research. 2004;29:173–80. doi: 10.1080/02713680490504876. [DOI] [PubMed] [Google Scholar]
- 37.Chien H, Dix RD. Evidence For Multiple Cell Death Pathways During Development of Experimental Cytomegalovirus Retinitis in Mice with Retrovirus-induced Immunosuppression: Apoptosis, Necroptosis, and Pyroptosis. Journal of Virology. 2012 doi: 10.1128/JVI.01275-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8137–42. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–65. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka K, Ichiyama K, Hashimoto M, Yoshida H, Takimoto T, Takaesu G, et al. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-gamma on STAT3 and Smads. J Immunol. 2008;180:3746–56. doi: 10.4049/jimmunol.180.6.3746. [DOI] [PubMed] [Google Scholar]
- 41.Akhtar LN, Benveniste EN. Viral exploitation of host SOCS protein functions. J Virol. 2011;85:1912–21. doi: 10.1128/JVI.01857-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akhtar LN, Qin H, Muldowney MT, Yanagisawa LL, Kutsch O, Clements JE, et al. Suppressor of cytokine signaling 3 inhibits antiviral IFN-beta signaling to enhance HIV-1 replication in macrophages. Journal of immunology. 2010;185:2393–404. doi: 10.4049/jimmunol.0903563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huber S, Gagliani N, Esplugues E, O’Connor W, Jr, Huber FJ, Chaudhry A, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3 and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–65. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–78. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. Journal of leukocyte biology. 2004;75:388–97. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 46.Chen L, Yang P, Kijlstra A. Distribution, markers, and functions of retinal microglia. Ocul Immunol Inflamm. 2002;10:27–39. doi: 10.1076/ocii.10.1.27.10328. [DOI] [PubMed] [Google Scholar]
- 47.Crane IJ, Liversidge J. Mechanisms of leukocyte migration across the blood-retina barrier. Seminars in immunopathology. 2008;30:165–77. doi: 10.1007/s00281-008-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yue FY, Merchant A, Kovacs CM, Loutfy M, Persad D, Ostrowski MA. Virus-specific interleukin-17-producing CD4+ T cells are detectable in early human immunodeficiency virus type 1 infection. Journal of Virology. 2008;82:6767–71. doi: 10.1128/JVI.02550-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–62. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 50.Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin Immunol. 2007;19:353–61. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Miller-Kittrell M, Sparer TE. Feeling manipulated: cytomegalovirus immune manipulation. Virol J. 2009;6:4. doi: 10.1186/1743-422X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalejta RF. Tegument proteins of human cytomegalovirus. Microbiol Mol Biol Rev. 2008;72:249–65. doi: 10.1128/MMBR.00040-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnon TI, Achdout H, Levi O, Markel G, Saleh N, Katz G, et al. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol. 2005;6:515–23. doi: 10.1038/ni1190. [DOI] [PubMed] [Google Scholar]
- 54.Cranmer LD, Clark CL, Morello CS, Farrell HE, Rawlinson WD, Spector DH. Identification, analysis, and evolutionary relationships of the putative murine cytomegalovirus homologs of the human cytomegalovirus UL82 (pp71) and UL83 (pp65) matrix phosphoproteins. J Virol. 1996;70:7929–39. doi: 10.1128/jvi.70.11.7929-7939.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kattenhorn LM, Mills R, Wagner M, Lomsadze A, Makeev V, Borodovsky M, et al. Identification of proteins associated with murine cytomegalovirus virions. J Virol. 2004;78:11187–97. doi: 10.1128/JVI.78.20.11187-11197.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loewendorf A, Benedict CA. Modulation of host innate and adaptive immune defenses by cytomegalovirus: timing is everything. J Intern Med. 2010;267:483–501. doi: 10.1111/j.1365-2796.2010.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loewendorf AI, Steinbrueck L, Peter C, Busche A, Benedict CA, Kay-Jackson PC. The mouse cytomegalovirus glycoprotein m155 inhibits CD40 expression and restricts CD4 T cell responses. J Virol. 2011;85:5208–12. doi: 10.1128/JVI.02178-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mocarski ES., Jr Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 2002;10:332–9. doi: 10.1016/s0966-842x(02)02393-4. [DOI] [PubMed] [Google Scholar]
- 59.Wagner M, Gutermann A, Podlech J, Reddehase MJ, Koszinowski UH. Major histocompatibility complex class I allele-specific cooperative and competitive interactions between immune evasion proteins of cytomegalovirus. J Exp Med. 2002;196:805–16. doi: 10.1084/jem.20020811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–72. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol. 2009;21:265–72. doi: 10.1016/j.smim.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yokota S, Yokosawa N, Okabayashi T, Suzutani T, Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 confers efficient viral replication. Virology. 2005;338:173–81. doi: 10.1016/j.virol.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 63.Frey KG, Ahmed CM, Dabelic R, Jager LD, Noon-Song EN, Haider SM, et al. HSV-1-induced SOCS-1 expression in keratinocytes: use of a SOCS-1 antagonist to block a novel mechanism of viral immune evasion. Journal of immunology. 2009;183:1253–62. doi: 10.4049/jimmunol.0900570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. Journal of immunology. 2008;180:5771–7. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 65.Humphreys IR, de Trez C, Kinkade A, Benedict CA, Croft M, Ware CF. Cytomegalovirus exploits IL-10-mediated immune regulation in the salivary glands. J Exp Med. 2007;204:1217–25. doi: 10.1084/jem.20062424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Redpath S, Angulo A, Gascoigne NR, Ghazal P. Murine cytomegalovirus infection down-regulates MHC class II expression on macrophages by induction of IL-10. J Immunol. 1999;162:6701–7. [PubMed] [Google Scholar]
- 67.Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–44. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]