Abstract
Rationale
Preclinical experimental models of pathological aggressive behavior are a sorely understudied and difficult research area.
Objectives
How valid, reliable, productive and informative are the most frequently used animal models of excessive aggressive behavior?
Methods
The rationale, key methodological features, supporting data and arguments as well as their disadvantages and limitations of the most frequently used animal models for excessive aggressive behavior are summarized and their validity and reliability are evaluated.
Results
Excessive aggressive behavior is validly and reliably seen in (1) a proportion of feral-derived rats and selectively bred mice, (2) rats with compromised adrenal function resulting in a hypoglucocorticoid state, (3) a significant minority of mice, rats and monkeys after consumption of a moderate dose of alcohol, and (4) resident animals of various species after social instigation. Limitations of these procedures include restrictive animal research regulations, the requirement of expertise in surgical, pharmacological and behavioral techniques, and the behaviorally impoverished mouse strains that are used in molecular genetics research. Promising recent initiatives for novel experimental models include aggressive behaviors that are evoked by optogenetic stimulation and induced by the manipulation of early social experiences such as isolation rearing or social stress.
Conclusions
One of the most significant challenges for animal models of excessive, potentially abnormal aggressive behavior is the characterization of distinctive neurobiological mechanisms that differ from those governing species-typical aggressive behavior. Identifying novel targets for effective intervention requires increased understanding of the distinctive molecular, cellular and circuit mechanisms for each type of abnormal aggressive behavior.
Keywords: aggression, violence, animal models, glucocorticoids, genetics, alcohol, individual differences
1. Introduction
How well do current methods for the study of animal aggression translate to the human condition? This question interests psychopharmacologists and neuroscientists in general because animal models of aggression aim to capture cardinal features of violent outbursts and callous acts in humans and to identify targets for treatment. The rationale for studying animal aggression begins with the Darwinian thesis of the evolutionary history of emotional expressions and conflict resolution. Ethologists have mainly examined the distal and proximal causes, the ontogenetic and phylogenetic origins of aggressive behavior, and characterized the ultimate adaptive significance of the salient patterns of different types of aggression (Lorenz 1966; Marler 1976; Tinbergen 1968). Given the urgent need for rational treatments of pathological aggression (Comai et al. 2012a; Comai et al. 2012b), we focus here on escalated aggression that exceeds species-normative levels (i.e. out of proportion and control) or patterns (i.e. out of context), and examine the most frequently used and promising methods, primarily in rodents.
Escalated aggressive behavior is operationally defined by (1) being readily provoked (i.e. low threshold, short latency), (2) high rate, (3) intense and tissue-damaging nature, targeting vulnerable body parts, (4) attacks lacking normal structure (i.e. deficiency of signaling intentions by threats) and context (i.e. being unable to identify an opponent according to its nature, sex and locale), (5) failure to terminate aggressive bursts, (6) failure to respond to appeasement signals, and (7) insensitivity to long-term consequences (Haller and Kruk 2006; Miczek et al. 2004a; Nelson and Trainor 2007). Excessive aggressive behavior thus differs both quantitatively and qualitatively from normal species-typical adaptive aggressive behavior. These operational definitions for escalated aggressive behavior have so far only been developed for males, and escalated aggressive behavior in female models has only begun to be studied (da Veiga et al. 2011).
The clinical relevance of escalated aggressive behavior in experimental animals under controlled laboratory conditions is initially evaluated according to long established criteria for all animal models of psychopathologies (Kornetsky 1989; McKinney 1989). The most valuable models achieve homology between the experimental preparation and cardinal symptoms of the clinical condition in terms of phylogenetic and ontogenetic origins (construct validity), phenomenology (face validity) and response to clinically established treatments using clearly understood neurobiological mechanisms (predictive validity). In addition to these criteria, animal models need to be evaluated in terms of their stability and the reproducibility within and between laboratories (reliability). Current models refrain from claiming homology and restrict the focus on certain isomorphic signs and symptoms (Geyer and Markou 2002). In the case of excessive aggression, the phylogenetic and ontogenetic origins in humans and non-humans remain a matter of inference and mostly speculation (Kravitz and Huber 2003; Marler 1976). Analogous models of aggressive behavior are often validated by circular logic, namely the positive response to the very treatments that are unsatisfactory and in need of replacement.
2. Escalated aggressive behavior in unselected feral animals and selective breeding for escalated aggression
(a) Background
Although often considered an exclusively human proclivity, escalated and violent forms of aggression during social conflict have been documented consistently in a number of field studies in several vertebrate and invertebrate animal species (see Natarajan and Caramaschi 2010). However, reports of violent outbursts in animals under controlled laboratory conditions have been rather limited. This is consistent with epidemiological evidence that, in human and other primate populations, only a small fraction of individuals develop and express this abnormal and pathological behavior rarely, i.e., violence is a low frequency but high impact behavior.
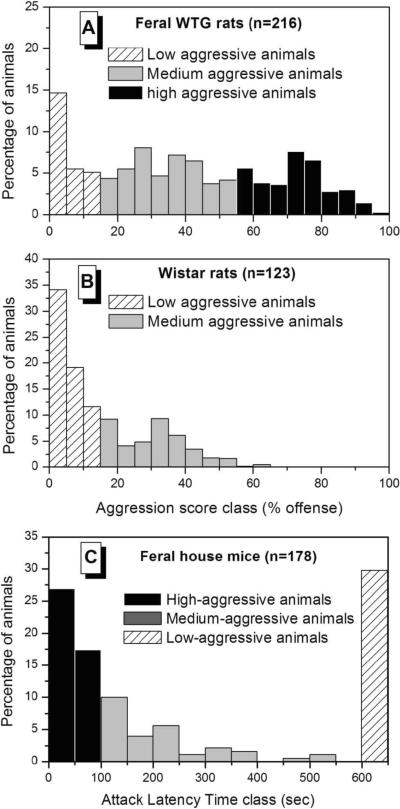
To further impede the preclinical evaluation of aggressive behavior, most laboratory rodent strains are very placid and docile compared to their wild ancestors. In virtually all commercially available laboratory mouse (>500) and rat (>250) strains today, the aggressive behavioral traits, including the putatively underlying molecular genetic components, are greatly compromised in terms of absolute level and variation. Most likely, this is the result of artificial selection for tame and tractable behavior during the century-long domestication process of these wild-caught animals, being kept, reared and bred in captivity (Barnett 1963; Boice 1973; de Boer et al. 2003; King 1939; Plyusnina et al. 2011). A classic example of this is the maintenance of docile characteristics long after selection for tameness in wild silver foxes even though selection is no longer applied, indicating that alleles that predispose to aggression have been removed from the population (Belyaev 1979). Consequently, to obtain appreciable levels of offensive aggression in these constitutionally placid laboratory strains, several procedural manipulations (see de Boer et al. 2009; Natarajan and Caramaschi 2010) have to be employed in order to enhance their tendency to display offensive aggressive behavior. Obviously, and validly so, some of these procedures have been adopted with the intent to mimic the conditions under which violent behavior in humans occurs. While these experimentally heightened levels of aggressive behavior may seem to resemble more intense forms when compared to the already low species-typical rates of aggression, they mostly still fall into the normative range when compared to the patterns and levels of their wild ancestors. Indeed, as shown in Figure 1, much higher levels and a broader range of innate and normal adaptive offensive aggression are displayed by feral or semi-natural populations of rats and mice compared to their highly domesticated laboratory-bred conspecifics. Interestingly, however, clear escalated aggressive and violent characteristics, as defined above, can be engendered in approximately 10% of medium to highly aggressive wild-derived rats that experience repeated victorious episodes of aggression (i.e. by permitting them to physically dominate other conspecifics).
Figure 1.
Frequency distribution of offensive resident-intruder aggression in a population of unselected feral Wild-Type Groningen rats (A) and in standard Wistar laboratory rats (B) as well as in a population of unselected feral house mice (C). The two rat strains differ considerably in the number of animals that will show aggressive behavior at all; note that the highly aggressive phenotype is absent in the domesticated rat strain. Note also that in the feral rodent populations, animals with an extreme high or low aggressive behavioral phenotype do not only coexist but are also encountered at a much higher rate than expected by chance, i.e., a bi-or tri-modally distributed pattern. This is in sharp contrast with the usually-encountered normal distribution patterns for most behavioral phenotypes in laboratory animals. (Adapted from van Oortmerssen and Bakker 1981)
Like humans, most individual rats respond to these repetitive social conflicts with appropriate and well-controlled functional forms of aggressive behavior, while only a small fraction demonstrate escalated aggression and become violently destructive. Much of the individual variation in aggressive behavior can be attributed to genetic variation. Heritability estimates for people are generally high, ranging between 35–70% (Hudziak et al. 2003; Rhee and Waldman 2002). Similar high heritability estimates for aggressive behavior have also been observed for several other species, including rodents (van Oortmerssen and Bakker 1981). Consequently, the response to artificial selection for either increased or decreased aggression is generally rapid in rodents, within three to four generations. Obviously, artificial selection represents a forward genetic approach that is limited mainly by the existing variation in the starting population.
Starting 50 years ago, three major independent selection experiments were conducted, resulting in three different strains of high- and low-aggressive mice (Miczek et al. 2001): (1) the Turku Aggressive (TA) and Turku Non Aggressive (TNA) lines originated from an outbred colony of Swiss albino laboratory mice (Lagerspetz 1964; Nyberg et al. 2004), (2) the aggressive Short Attack Latency (SAL) and low-aggressive Long Attack Latency (LAL) mice originated from a population of wild-trapped feral house mice (Sluyter et al. 2003; van Oortmerssen and Bakker 1981) and (3) the high-aggressive NC900 and low-aggressive NC100 mice derived from an ICR (Institute of Cancer Research) laboratory Swiss-Webster outbred stock (Cairns et al. 1983). Although these lines came from different genetic backgrounds and different laboratories with their own housing idiosyncrasies, and although selection criteria differed in terms of testing environment, type of test, type of opponent, the offensive aggression levels of these strains are generally comparable. In recent experiments Caramaschi et al. (2007) and Natarajan et al. (2009a; 2009b) evaluated males of all three strains in the same laboratory against the same type of opponent and found that the time spent on aggressive behavior was nearly similar across the different strains. The aggressive lines spent on average 30–40% of their time on attack, threat and chase, whereas the non-aggressive lines – with the exception of the TNA line – showed either no or only very few aggressive acts. However, the quality of the heightened aggressive behavior in SAL, TA and NC900 mice is not the same (Natarajan et al. 2009b); only SAL males displayed abnormal and pathological forms of attack. For instance, they attacked vulnerable body parts, females, immobilized intruders, and they disregarded submissive signals from their opponents (Natarajan et al. 2009b; Sluyter et al. 2003).
In addition to these behavioral features of escalated aggression, a number of `violent-specific' autonomic, endocrine and neurobiological alterations including low heart rates, low glucocorticoids, low brain serotonin levels, high 5-HT1A autoreceptor activity, and low serotonin reuptake transporter activity were observed in SAL mice but are not present in the other high-aggression mouse lines (Natarajan and Caramaschi 2010). Given the parallels between male SAL mice and humans who exhibit persistent and pervasive antisocial aggressive behavior, these artificially-selected SAL mice are an informative mouse model for investigations in the genetics, development and neuromolecular architecture of this problematic behavioral trait. The fact that only the artificially selected SAL mouse line, derived from wild-trapped animals, exhibits clear signs of escalated and violent aggressive behavior suggests the presence of alleles that predispose to escalated aggression in this strain of feral mice that have been lost in inbred and outbred strains of domesticated laboratory strains of mice.
In rats, artificial selection for high or low offensive aggressive behavior has not been executed so far. However, lines of tame and aggressive rats were developed by long-term selection of wild-derived gray rats for elimination and enhancement of defensive aggressiveness towards humans (Belyaev and Borodin 1982). Interestingly, when these lines of animals were tested for offensive aggression in a resident-intruder paradigm, the domesticated and tame line hardly displayed offensive aggression towards Wistar intruders, whereas in the aggressive line the levels of offensive aggression directed at conspecifics remained unchanged compared to the unselected animals (Naumenko et al. 1989; Plyusnina et al. 2011).
Selecting for particular behavioral or physiological responses may also result in indirect selection of other linked traits or response strategies. For example, Wistar rats selected for extremes in anxiety-related behavior in the elevated-plus maze test or in locomotor exploratory behavior in the open field test also exhibited considerable differences in offensive aggression in the resident-intruder aggression test (Neumann et al. 2010; Akil et al. 2007).
(b) Key methodological features
Most preclinical aggression research is conducted in territorial male resident rats or mice confronting a naive intruder conspecific, the so-called resident-intruder paradigm (Blanchard et al. 1977; Koolhaas et al. 1980; Miczek 1979; Miczek and O'Donnell 1978). This paradigm is based on the fact that an adult male will establish, and subsequently defend, a territory when given sufficient living space. Animals are therefore housed as residents in somewhat larger experimental observation cages (rats: 90×55×50 cm; mice:75×30×25 cm) than the standard plastic laboratory cages to allow proper display of species-typical offensive acts and postures directed toward unfamiliar conspecific intruder males. Territoriality is strongly enhanced in the presence of females or by accruing sexual experiences (Barnett et al. 1968). The intruder rats are standardized as much as possible in terms of strain, age and weight. In general, rats of a non-aggressive laboratory strain (e.g., Wistar) or slightly smaller rats from the same feral wild-type strain as the resident male are used as intruders.
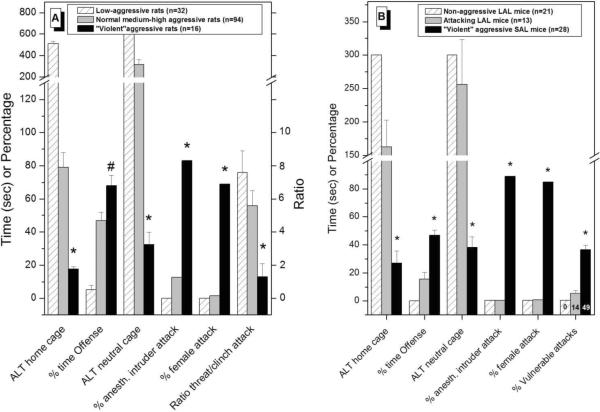
By recording the frequencies, durations, latencies and temporal and sequential patterns of all the salient behavioral acts and postures displayed by the combatants during these confrontations, detailed quantitative and qualitative analyses (ethograms) of resident's offensive aggressive behavior are obtained (Koolhaas et al. 1980; Miczek and de Boer 2005). After repeated resident-intruder confrontations, additional behavioral tests, analyses and criteria are conducted to delineate deviant from adaptive forms of aggression such as (1) steadily decreasing attack latencies (i.e., falling below 30 sec) and increasing levels of persistent aggressive displays with repeated testing are a first indicator of uncontrolled and escalated aggressive tendencies; (2) gradually disappearing sequences of investigatory acts and threatening postures before the consummatory clinch and biting attacks, as well as a concomitantly increasing ratio of vulnerable/non-vulnerable body region attacks are reliable indicators of out of control aggression; (3) the appearance of out of context aggression like attacking unfamiliar (estrus) female intruders, attacking anesthetized male intruders, and/or attacking male intruders in a novel environment with almost the same intensity as within their own home cage. Figure 2 depicts these normal and “violent” behavioral characteristics in resident feral rats and mice.
Figure 2.
A. Normal and violent aggressive behavioral characteristics in low-aggressive and medium-high aggressive WTG rats after multiple (>10) victorious experiences. B. Generally similar violent aggressive characteristics are observed in artificially-selected high-aggressive SAL mice after only 4 repetitive winning experiences. * indicates significant differences from the other two groups.
(c) Support for the Model
The broad individual variation in aggressive behavior and the unambiguous identification of an escalated aggressive phenotype in a relatively small proportion of these feral rats has a considerable degree of face validity to human violence and uncontrolled aggression.
Some construct validity of this model is achieved by the fact that the pathological aggressive phenotype is obtained in a minor fraction of genetically predisposed individuals upon positively reinforcing social experiences. Humans and various animal species demonstrate enhanced aggressive responding following previous victory and winning experiences (i.e., the so-called winner effect).
As in humans and other primate species, only the highly aggressive and violent feral subjects exhibit dysfunctional brain serotoninergic neurotransmission. The strong link between brain serotonergic dysfunction and impulsive aggressive and violent behaviors is one of the most frequently reported findings in biological psychiatry. Capturing this neurobiological hallmark of pathological aggression adds to the face validity and possibly to the construct validity of the model. In vivo microdialysis methods have begun to delineate whether these serotonergic dysfunctions are predisposing as antecedents to escalated aggression or are the consequences (van Erp and Miczek 2000).
(d) Disadvantages and Limitations
As a result of laboratory animal housing rules and specific hygiene conditions, the use of unconventionally housed and wild-derived rodent strains is difficult to implement. This seriously constrains the inter-laboratory reliability of the model.
Ethical and animal welfare issues regarding the harm and injury inflicted on the defeated intruder animals may seriously constrain the acceptance and adoption of this and other escalated aggression models.
While a range of pharmacological compounds effectively reduces aggressive behavior in wild-type rats that show adaptive levels of offensive aggressive behavior, the specific effectiveness of these compounds has not been demonstrated in pathological and escalated aggressive individuals.
3. Excessive aggression as a result of hypoglucorticoid status
(a) Background
The development of the hypoglucorticoid model was prompted by the discovery that violence in patients with antisocial personality disorder is accompanied by a marked hypoarousal in terms of glucocorticoid production, heart rate and skin conductance (Dolan et al. 2001; Raine and Mednick 1989; Virkkunen 1985). As glucocorticoids are genomically active steroid hormones with considerably constitutive activity (De Kloet et al. 1993), we hypothesized that the deficient functioning of this gene-controlling factor results in neural changes that contribute to the expression of violence (Haller et al. 2001). The involvement of genomic glucocorticoid mechanisms was indirectly supported by findings showing that conduct disorder-associated aggression was predicted by consistently low glucocorticoid levels, as measured over two years (McBurnett et al. 2000).
(b) Key methodological features
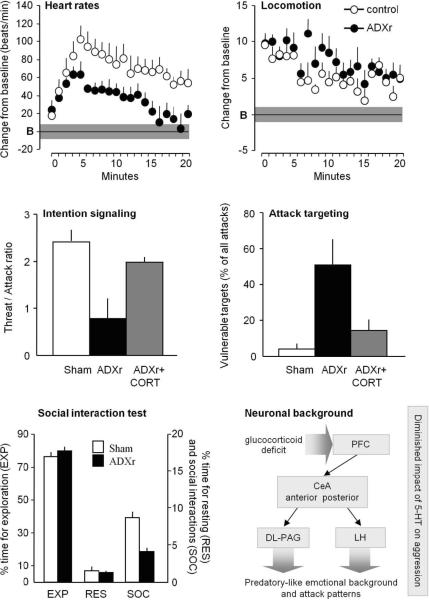
The experimental protocol typically begins with adrenalectomy to chronically reduce glucocorticoid production, followed by low level glucocorticoid replacement (ADXr; Haller et al. 2001). The adrenal gland is removed undamaged and in its entirety, to avoid the proliferation of tissue fragments and partial restoration of glucocorticoid production. Corticosterone replacement is achieved by implanting corticosterone pellets (100 mg, containing 25% corticosterone and 75% cholesterol compressed without additives) subcutaneously into the dorsal region of the rat's neck. Behavioral testing in rats is performed in large cages (60×40×50 cm) starting one week after surgery. Autonomic activity is evaluated by the biotelemetric measurement of heart rate during basal conditions and aggressive confrontations. The critical behavioral measures are attack targeting and the behavioral context of attacks, which are assessed separately from measurements of the behavioral repertoire during the attack episodes (Figure 3). This analysis requires the repeated replay of the digital record at various speeds to identify the target of the attack bite and the behavior preceding the attack (i.e. the behavioral context; see Videos 1 and 2). Attack targets considered vulnerable include the head, throat, abdomen, and paws, while the back and flanks are considered non-vulnerable. Attacks are considered “out of context” when preceded by resting, exploring, grooming, or sniffing, and are categorized as “signaled” when preceded by offensive threats. In many studies, we characterized intention signaling by calculating the attack/offense ratio (Halasz et al. 2002; Haller et al. 2001; Haller et al. 2004; Haller et al. 2007); more recently, we have specified the behavior that immediately preceded the attack (Toth et al. 2011; Toth et al. 2012).
Figure 3.
The impact of glucocorticoid deficiency on autonomic arousal, behavior, and the neural background of aggression. Upper panels: aggression-induced autonomic activation (left) and locomotion (right) during a 20 min-long aggressive encounter. B, baseline; the grey horizontal bar indicates the standard error of the baseline. Middle panels: intention signaling as expressed by the threat/offense ratio (left) and the share of vulnerable targets (right) in rats exposed to three aggressive encounters at three day intervals. Values represent the average of the three encounters. ADXr+CORT, acute corticosterone treatment before each encounter. Lower panels: the behavior of ADXr rats in the social interaction test (left) and the putative mechanism of glucocorticoid deficiency-associated aggression (right). ADXr did not change anxiety levels as shown by the elevated plus-maze test but reduced social interaction-induced heart rates (data not shown). PFC, prefrontal cortex; CeA, central amygdala; DLPAG, dorsolateral column of the periaqueductal gray; LH, lateral hypothalamus. For more details see Haller et al. 2001; Haller et al. 2004; Haller et al. 2007; Tulogdi et al. 2010.
(c) Support for the model
At present, this is the only laboratory model where abnormal manifestations of aggression are associated with marked hypoarousal. Its relevance is supported by the recent recognition in the DSM-5 (American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 5th edition) of the “callous-unemotional” specifier for Conduct Disorder (“childhood form” of antisocial personality disorder) and by recurring reports of hypoarousal in violent antisocial adults (see above).
The behavioral dysfunctions in ADXr rats can be considered analogous to several basic symptoms of violent antisocial personality disorder: callousness and rule breaking (dysfunctional attack targeting and absence of social signaling), unemotionality (reduced autonomic activation), and social deficits (behavior in the social interaction test).
Intriguingly, aggression in this model is associated with the marked activation of predation-related brain structures (Tulogdi et al. 2010). So-called unemotional aggression is frequently referred to as “predatory” in the psychiatric literature.
The etiological factor of behavioral dysfunctions is similar to that believed to underlie violence in conduct and antisocial personality disorders, namely a marked deficit in glucocorticoid production.
The effects of ADXr are consistent, robust, and durable and tolerate additional manipulations, e.g., surgeries for systemic or local brain injections or the implantation of biotelemetry emitters. This feature makes the model suitable for studies of the brain mechanisms of abnormal aggression, including optogenetic experiments.
This model differs profoundly from hyper-emotional aggression. Post-weaning social isolation, a putative laboratory model of social neglect-induced emotional problems, can lead to abnormal aggression that develops in conjunction with markedly increased autonomic, glucocorticoid and behavioral activation (Toth et al. 2011). The neural background of hypo- and hyperarousal-associated aggression showed qualitative differences (Toth et al. 2012). Thus, the two models may be used in tandem to decipher the impact of emotionality on aggression per se, and on its neural background.
(d) Disadvantages and Limitations
The model requires surgery, which is its major limitation. Although deficient glucocorticoid secretion is typical of individuals with violent antisocial disorder, in humans the deficit is not the result of surgical intervention. Thus, construct validity is questionable, even if the face validity of the model appears ensured, and several findings suggest predictive validity as well (Haller et al. 2007). Furthermore, the inter-laboratory reliability of the model is not yet known because the model has hardly been adopted or evaluated in other laboratories.
In humans, multiple forms of early maltreatment lead to glucocorticoid deficits and aggression in adulthood (Gunnar and Vazquez 2001); it remains to be seen whether this can be modeled in laboratory rodents and whether the behavioral symptoms and neural background of such interventions will be similar to those seen in ADXr rats.
The model is relatively easy to employ, but one needs skills in three areas: endocrinology, surgery, and behavioral observation – a combination that is not universally available.
Violence in antisocial personality disorder is often instrumental. This key feature has not yet been investigated in ADXr rats.
The model has not been tested in mice.
4. Alcohol-heightened Aggression
(a) Background
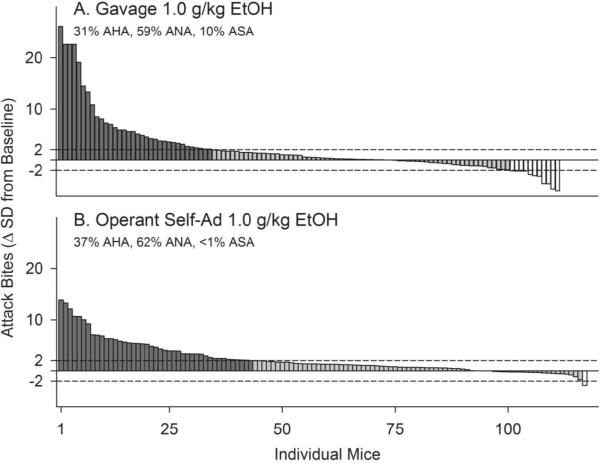
Two thirds of all incidences of violence involve alcohol consumption, either by the perpetrator or the victim (Roizen 1997). After decades of inconsistent studies reporting increases or decreases in aggressive behavior or no effect after treatment with alcohol (Brain 1986), reliable and reproducible methods have been developed to study escalated aggression after consumption of alcohol in a significant subgroup of individual mice, rats and monkeys (Figure 4; Miczek et al. 1997; Miczek et al. 2004b).
Figure 4.
Histogram representing the proportion and individual magnitude of alcohol-heightened aggression after gavage (A) or operant self-administration (B) of 1.0 g/kg alcohol. Dark vertical bars represent outbred CFW mice whose average frequency of attack bites after 1 g/kg alcohol exceeds their baseline levels of aggression by >2 SD (Alcohol-Heightened Aggression, or AHA). Gray vertical bars represent mice whose aggressive behavior is not significantly altered by 1 g /kg alcohol (Alcohol Non-heightened Aggression, or ANA), and white vertical bars represent mice whose aggressive behavior after 1 g/kg alcohol is reduced by >2 SD (Alcohol-Suppressed Aggression, or ASA). Dotted horizontal lines represent a 95% confidence interval, ±2 SD from average baseline.
(b) Key methodological features
A prerequisite for studying alcohol-heightened aggression is the consistent display of stable levels of aggressive behavior in a common species of laboratory animals. Since most laboratory animals are placid and are gentled by repeated handling, it is essential to create conditions under which purpose-bred laboratory rodents reliably display species-typical aggressive behavior (Miczek and de Boer 2005). These conditions comprise the selection of a well-characterized strain and species of rodents and housing animals in a species-appropriate environment that promotes territorial marking and pheromonal communication, preferably in a breeding pair. Baseline levels of aggressive behavior are typically established by repeated confrontations between the experimental animal and smaller intruders with no prior history of aggressive encounters. In mice and rats, the highest levels of aggressive behavior are seen when the confrontation occurs in the resident animal's home cage.
The key experimental manipulation consists of the administration of a moderate dose of ethanol such as 1 g/kg to an animal with a demonstrated capacity to display aggressive behavior. Initially, the resident mouse or rat self-administers a precise dose of ethanol according to the body weight of the animal. Self-administration of ethanol via the oral route is preferable over experimenter-administered alcohol on account of its superior face validity. Under appropriate conditions, most laboratory mice and rats will self-administer ethanol in a 6% w/v alcohol concentration and achieve a moderate dose (e.g., 1 g/kg) within less than 5 minutes.
The measurements are based on the precise analysis of video recordings of the salient acts of aggressive behavior in an experimentally controlled confrontation, typically a resident-intruder test conducted during the dark phase when these nocturnal species are most active (Video 3). Establishing the requisite degree of inter- and intra-observer reliability requires familiarity with the species-typical pattern of the strain and species under investigation.
(c) Support for the Model
Consumption of a moderate dose of alcohol can escalate aggressive behavior far beyond the normative levels for the species, although the abnormal nature of this behavior has not been adequately characterized.
Individual differences in the propensity to engage in alcohol-heightened aggressive behavior are readily captured in common laboratory rodent and non-human primate species, with good intra- and inter-laboratory replication.
Alcohol-heightened aggressive behavior is systematically dependent on dose and follows an orderly time course.
The oral route for alcohol consumption is an effective way to introduce the precise, individually adjusted amount of drug rapidly into the organism.
Voluntary and preferential alcohol self-administration in a brief bout can engender escalated aggressive behavior in an ensuing confrontation.
Quantitative analysis of alcohol-heightened aggressive behavior has revealed a failure to terminate aggressive bouts.
Alcohol-heightened aggression requires intact impulse flow and receptor regulation in the serotonin system which has been implicated in the neurobiology of impulsive aggressive behavior. Somatodendritic autoreceptors, pre- and post-synaptic receptors and transporter sites are targets for effective reduction of alcohol-heightened aggression.
(d) Disadvantages and Limitations
Specific methods of inducing alcohol consumption are required to engender voluntary alcohol consumption in significant amounts. Methods of forced alcohol exposure do not translate readily to the human condition.
So far, no biomarkers have been identified that predict which individuals will engage in alcohol-heightened aggressive behavior and which will not. Neither top-down nor bottom-up genetic strategies have successfully targeted a specific mechanism for alcohol-heightened aggression.
Some strains of mice, such as the C57BL/6, readily drink large amounts of alcohol, but exhibit low or inconsistent amounts of aggressive behavior in resident-intruder confrontations. It is unclear whether or not they engage in unambiguous alcohol-heightened aggression.
Current molecular biology techniques are being applied to mouse strains that do not consume alcohol in significant amounts and that display only remnants of species-typical aggressive behavior if any at all.
5. Social Instigation and Escalated Aggression
(a) Background
Impulsively violent patients are often characterized by their excessive aggressive response to a perceived provocation (Haspel 1995; Volavka 2002). As a matter of fact, the appearance of an unfamiliar individual may trigger aggressive acts and postures, attributed to fear of the unfamiliar or stranger, termed aggressive xenophobia (Holloway 1974; Marler 1976). In field work and experimental studies, the increase in aggressive arousal or “attack readiness” has been observed just prior to the introduction of an intruder into a resident's cage. This type of arousal has been claimed to be specific to aggressive confrontations (Berkowitz 1993; Lorenz 1966) and is not reflected as increased locomotion, feeding or sexual behavior (Lagerspetz and Hautojarvi 1967; Potegal and Tenbrink 1984). The instigation effect persists for some time, even after the instigating stimulus has been removed. Clinically, reducing aggressive arousal in patients with various psychiatric diagnoses by beta-blockers has proven successful and provides some pharmacological validation (Elliott 1977; Haspel 1995; Ratey et al. 1992; Yudofsky et al. 1990).
(b) Key methodological features
Briefly exposing an adult male or female animal to a potential rival that is nearby but inaccessible for a limited time prior to an unrestricted confrontation engenders aggressive behavior at high intensity and frequency (Figure 5), as originally described in mice (Lagerspetz 1969; Tellegen and Horn 1972). The social instigation protocol consists of two phases: First, the experimental subject, usually a resident mouse, rat or hamster, is confronted with a breeding opponent in its home cage behind a protective screen. The resident can hear, see and smell the instigator or provocateur, but cannot actively defend his territory and expel the rival mouse, rat or hamster (de Almeida et al. 2005; Potegal 1991). The precise duration of this initial phase depends on the species and strain of animals. In mice, rats or hamsters, the instigation phase is usually 5 min in duration, at the end of which the instigator is removed. Second, after a short interval of typically 1–5 minutes, the resident confronts an intruder in its home cage without any protective screen, as described above. The video recordings of these confrontations are analyzed in the same fashion as detailed for the other types of aggressive behavior, with a quantitative assessment of all salient acts and postures. The instigation effect is evident with the very first parameter, the significantly shortened latency to the first attack, followed by a very high frequency of attack bites and threats and lengthened bouts of aggressive behavior (Figure 5; de Almeida and Miczek 2002; Fish et al. 1999; Potegal 1991). The social instigation protocol has been applied with success in hamsters, rats, mice and fish (Centenaro et al. 2008; de Almeida and Miczek 2002; Fish et al. 1999; Heiligenberg 1974; Newman et al. 2012; Potegal and Tenbrink 1984). It also has been extended from male residents confronting male instigators and intruders to female rats (da Veiga et al. 2011; Veiga et al. 2007; Veiga et al. 2011).
Figure 5.
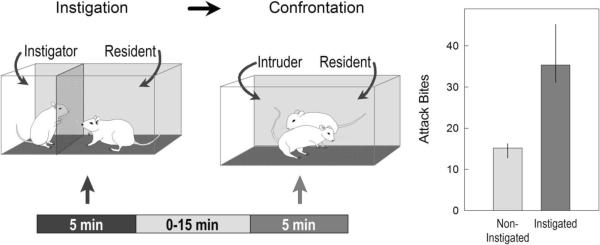
Aggression heightened by social instigation. For five minutes, a resident male mouse is exposed to an intruder male that is protected by a screen through which olfactory and visual cues are still available. After an interval, the resident attacks an unprotected intruder with greater frequency. Bars represent the median attack bites and vertical lines represent the inter-quartile range after control (light gray) and instigated (dark gray) conditions. (From Miczek et al. 2007b).
(c) Support for the Model
The social instigation procedure is relatively easy to implement, requiring no special equipment and no genetic, pharmacological or neurochemical manipulations.
Aggressive arousal as a result of social instigation has been readily quantified by measurement of salient autonomic and behavioral indices in clinical and preclinical settings.
The social instigation effect is seen in animal species that are commonly used in experimental laboratory research. The largest and most reliable effects have been reported in hamsters and outbred mice (de Almeida and Miczek 2002; Fish et al. 1999; Potegal 1991).
Escalated aggressive behavior is seen in socially instigated animals in locales other than their home cage.
Serotonergic compounds, particularly those acting on the 5-HT1A and 5-HT1B receptors, effectively reduce aggressive behavior that is escalated as a result of social instigation.
(d) Disadvantages and Limitations
It remains to be determined whether or not socially instigated animals display aggressive behavior that is not only quantitatively escalated, but shows also the characteristic features of abnormal aggression.
In order to obtain a significant social instigation effect, it is necessary to adjust the parameters to the species and strain of animals with considerable care. The social instigation effect seems to follow a biphasic function, with short, moderately intensive exposures resulting in a strong escalation of aggressive behavior, whereas prolonged and highly intense instigation produces exhaustion.
The neurobiology and psychopharmacology of instigation-induced aggressive arousal as distinct from other types of arousal await characterization.
All preclinical models of aggression-related psychopathologies as described in the DSMIV are characterized by the out-of-proportion and out-of context nature of aggressive behavior. For example, irritability and uncontrollable outbursts of aggression are diagnostic criteria for a number of disorders such as antisocial personality, borderline personality, depressive, intermittent explosive, oppositional-defiant, and post-traumatic stress disorders. The currently discussed preclinical models comprise behaviors that are analogous to those shown by patients. Proximate causes may be identified, and their neurobiological mechanisms can be studied in the “frustration” and “alcohol” models (see above). Similarly, the likelihood of a subgroup of feral animals to show abnormal aggression after winning experiences and the aggressive behavior of genetically selected mouse lines may help to understand the etiological factors and genetic mechanisms leading to this condition. The putative model of early social neglect-induced pathological aggression may serve the same purpose. The human relevance of hypoglucocorticoid model corresponds in part to particular symptoms of aggression in DSM-IV disorders and the callous-unemotional specifier of conduct disorder in DSM-V. Aggression associated with a restricted range of affect such as in post-traumatic stress disorder, physically cruelty to people (conduct disorder), reckless disregard for safety of self or others (antisocial personality disorder) are examples of such DSM-IV symptoms, while the delivery of attacks on vulnerable targets under conditions of low arousal may be considered behavioral analogs of callous-unemotional aggression as introduced by DSM-V. It is clear that none of the models discussed here are “disorder-models” but each is potentially able to provide insights into the mechanisms of psychopathologies that include aggressive behavior as described in the DSM.
6. Promising Developments
Transgenic mice have emerged as a most instructive research tool in psychopharmacology (Crawley et al. 1997). Specifically, newly developed techniques allow for brain region-specific induction or suppression of gene expression at specific time periods in the life of the mouse. A most promising novel method is to selectively silence the gene encoding the estrogen receptor alpha protein in the medial preoptic area of females resulting in decreased sexual behavior and intact aggressive behavior (Ribeiro et al. 2012). These genetic manipulations will be most useful to learn about the significance of specific gene products in the expression of excessive aggressive behavior. A prerequisite is the development of methods that engender excessive aggressive behavior in mouse strains that are most frequently used as the background for genetic mutations (e.g., 129Sv, C57BL/6).
The recently developed technique to genetically express light wavelength-sensitive membrane channels (opsins) in neurons and to subsequently activate them by light pulses (optogenetics) provides a novel tool for establishing a causal relationship between neural activity and various features of aggressive behavior. Optogenetic stimulation of the ventrolateral subdivision of the ventromedial hypothalamus has been reported to evoke biting attacks in mice (Lin et al. 2011). Previously, electrical stimulation of analogous hypothalamic structures has proven effective in eliciting attacks in cats and rats (Siegel et al. 1999). Electric and optogenetic stimulation differ in several important ways; (i) electric stimulation readily evokes attacks in rats and cats, but not in mice; (ii) mice do attack inanimate objects upon optogenetic stimulation; and (iii) the effective area of optogenetic stimulation is surprisingly restricted in mice, especially considering that the anterior hypothalamus, ventrolateral hypothalamus, the tuber cinereum, and the lateral hypothalamus are significant in the neural control of mouse aggression (Duncan et al. 2009; Haller et al. 2006; Hasen and Gammie 2006; Trainor et al. 2006). Although these species- or technique-related differences are difficult to reconcile at present, optogenetic stimulation per se and its combination with transgenic technologies promises to greatly enhance our understanding of brain mechanisms underlying species-typical and abnormal aggressive behavior.
One new animal model that mimics etiological factors of aggression-related psychopathologies relies on post-weaning social isolation. This putative model of early social neglect-induced pathological aggression results in high levels of aggression, attacks on vulnerable targets, sudden attacks, and ambivalence between offensive and defensive behavior (Toth et al. 2008). Like humans, rats show exacerbated autonomic and glucocorticoid stress responses in this model (Toth et al. 2011). Such models based on etiological factors await the exploration of therapeutic interventions.
Several experimental protocols can engender excessive aggression in animal species that are less commonly studied under experimentally controlled conditions in the psychopharmacology laboratory. These species are mostly studied only in the laboratory of the model's originator. For example, hamsters, voles, fish, and fruit flies offer important advantages in the neurobiological and pharmacological study of social and aggressive behavior (Delville et al. 2000; Ferris et al. 1997; Gobrogge et al. 2009; Miczek et al. 2007a; Ricci et al. 2005; Summers et al. 2005), but currently only begin to be characterized pharmacologically.
Epigenetic mechanisms. The lasting effects of winning experience in feral rats, as well as those of hypoglucocorticoid status, and post-weaning social isolation among the emerging models suggest that the underlying mechanisms involve neuronal plasticity associated with or caused by epigenetic changes. This assumption is supported by the impact of social status, adrenalectomy, and social environments on DNA methylation and/or histone acetylation (Azmitia et al., 1994; Bountra et al., 2011; Thayer et al., 2011; Tung et al., 2012). Moreover, growing up in aggressive social environments and frequent engagement in aggressive conflicts also induce epigenetic changes (Simmons et al., 2012; Tung et al., 2012) which may add to the pre-existing genetic differences seen in genetic lines selected for aggression.
Supplementary Material
The recording shows a short episode of an aggressive encounter between a sham-operated resident and a naive intruder. The attack episode was recorded in low light; the opponents were illuminated by the infrared light source of the camcorder. The biting attack starts by lateral or sideways threat, after which the resident bends over the intruder in an attempt to bite. Accidentally, however, the mouth of the resident comes close to the belly of the opponent, i.e. a vulnerable target (see the 4th second of the recording). The deliberate avoidance of vulnerable targets by control rats is depicted by the behavior of the resident, which quickly corrects the position of its mouth, and bites the left flank of the intruder. The intruder immediately submits. This reaction to the bite is indicative of a “hard bite”. Note that the resident avoids biting the belly of the submissive opponent, despite the fact that this body area is clearly exposed and easy to bite. At the end of the bite, the intruder recovers relatively quickly, and resumes the exploration of the environment.
The recording shows a short episode of an aggressive encounter between an ADXr resident and a naive intruder. The attack episode was recorded in low light; the opponents were illuminated by the infrared light source of the camcorder. At the start of the episode, both rats are in an upright posture, and reciprocally sniff each other. The resident suddenly delivers a soft bite targeting the nose of the intruder (i.e. a vulnerable area). Note that the intruder responds by upright freezing to the next approach by the resident, despite the fact that the bite was clearly soft. After a short episode of offense by the resident, the opponent falls back into submission, and is immediately bitten just above the right paw, at the border between the throat and abdomen (i.e. a vulnerable target). After this bite, the opponent remains in supine posture even after the resident stops keeping it down. Albeit not reported in earlier studies, vulnerable area attacks usually intimidate the intruder as shown by this recording.
The first segment of the video captures a resident-intruder confrontation in which the resident male displays species-typical aggressive behavior. Within seconds, the resident male initiates the attack with a bite to the posterior left flank of the intruder. Following the first attack bite, the intruder escapes and the resident pursues, attempting to nip at his hind flanks. The intruder turns to exhibit an upright defensive posture, but the resident bites several times, pauses briefly to reposition and aims the next bite at the posterior flanks of the intruder. After a short interval, the resident exhibits a sideways threat, which is rapidly followed by the delivery of two more attack bites. The resident walks away from the intruder, marking the end of the aggressive bout. Microanalysis of the resident’s species-typical aggressive behavior revealed a distinct bout of aggression and precise delivery of attack bites to the posterior flanks of the intruder.
The second segment of the video captures a confrontation in which the resident male mouse displays alcohol-heightened aggression after rapidly self-administering 1 g/kg of 6% ethanol solution (w/v). Following a brief latency, the resident delivers an initial bite to the intruder’s right forearm. As the intruder turns to escape, the resident pursues and bites the posterior flank of the intruder twice. The intruder reacts by displaying a prolonged defensive upright posture, and the resident circles the intruder to bite his flank again. Assuming a defensive position on his back, the intruder exposes his abdomen, which the resident bites repeatedly. To avoid attacks to his vulnerable underside, the intruder attempts to escape, which provokes the resident to pursue and bite the intruder’s hind flanks. After cornering the intruder, the resident pins the intruder and continues to bite the intruder’s abdomen and flanks. The intruder persists in his defensive display, and eventually resumes escape behavior. As before, the resident pursues while biting the hind flanks of the intruder. Finally, the resident walks away from the intruder to groom briefly, marking the end of the aggressive bout. From this analysis, it is evident that there are qualitative differences between species-typical and alcohol-heightened aggression. Alcohol-heightened aggression is characterized by prolonged, intense aggressive bouts and targeting attack bites at vulnerable regions.
Acknowledgments
This research was supported by NIH grants DA031734 and AA013983 to KAM.
References
- Azmitia EC, Liao B. Dexamethasone reverses adrenalectomy-induced neuronal de-differentiation in midbrain raphe-hippocampus axis. Ann N Y Acad Sci. 1994;746:180–193. doi: 10.1111/j.1749-6632.1994.tb39232.x. [DOI] [PubMed] [Google Scholar]
- Barnett SA. A study in behaviour. Aldine; Chicago: 1963. The rat. [Google Scholar]
- Belyaev DK. The Wilhelmine E. Key 1978 invitational lecture. Destabilizing selection as a factor in domestication. J Hered. 1979;70:301–308. doi: 10.1093/oxfordjournals.jhered.a109263. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. Its causes, consequences and control. Mc Graw Hill; New York: 1993. Aggression. [Google Scholar]
- Blanchard RJ, Blanchard DC, Takahashi T, Kelley MJ. Attack and defensive behaviour in the albino rat. Anim Behav. 1977;25:622–634. doi: 10.1016/0003-3472(77)90113-0. [DOI] [PubMed] [Google Scholar]
- Boice R. Domestication. Psychol Bull. 1973;80:215–230. doi: 10.1037/h0034893. [DOI] [PubMed] [Google Scholar]
- Bountra C, Oppermann U, Heightman TD. Animal models of epigenetic regulation in neuropsychiatric disorders. Curr Top Behav Neurosci. 2011;7:281–322. doi: 10.1007/7854_2010_104. [DOI] [PubMed] [Google Scholar]
- Brain PF. Alcohol and aggression. Croom Helm; London: 1986. [DOI] [PubMed] [Google Scholar]
- Cairns RB, MacCombie DJ, Hood KE. A developmental-genetic analysis of aggressive behavior in mice: I. Behavioral outcomes. J Comp Psychol. 1983;97:69–89. [PubMed] [Google Scholar]
- Caramaschi D, de Boer SF, Koolhaas JM. Differential role of the 5-HT1A receptor in aggressive and non-aggressive mice: an across-strain comparison. Physiol Behav. 2007;90:590–601. doi: 10.1016/j.physbeh.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Centenaro LA, Vieira K, Zimmermann N, Miczek KA, Lucion AB, de Almeida RM. Social instigation and aggressive behavior in mice: role of 5-HT1A and 5-HT1B receptors in the prefrontal cortex. Psychopharmacology. 2008;201:237–248. doi: 10.1007/s00213-008-1269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai S, Tau M, Gobbi G. The psychopharmacology of aggressive behavior: a translational approach. Part 1: Neurobiology. J Clin Psychopharmacol. 2012a;32:83–94. doi: 10.1097/JCP.0b013e31823f8770. [DOI] [PubMed] [Google Scholar]
- Comai S, Tau M, Pavlovic Z, Gobbi G. The psychopharmacology of aggressive behavior: a translational approach. Part 2: Clinical studies employing atypical antipsychotics, anticonvulsants, and lithium. J Clin Psychopharmacol. 2012b;32:237–260. doi: 10.1097/JCP.0b013e31824929d6. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- da Veiga CP, Miczek KA, Lucion AB, de Almeida RMM. Social instigation and aggression in postpartum female rats: role of 5-HT1A and 5-HT1B receptors in the dorsal raphé nucleus and prefrontal cortex. Psychopharmacology. 2011;213:475–487. doi: 10.1007/s00213-010-2083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida RMM, Ferrari PF, Parmigiani S, Miczek KA. Escalated aggressive behavior: Dopamine, serotonin and GABA. Eur J Pharmacol. 2005;526:51–64. doi: 10.1016/j.ejphar.2005.10.004. [DOI] [PubMed] [Google Scholar]
- de Almeida RMM, Miczek KA. Aggression escalated by social instigation or by discontinuation of reinforcement (“frustration”) in mice: inhibition by anpirtoline - a 5-HT1B receptor agonist. Neuropsychopharmacology. 2002;27:171–181. doi: 10.1016/S0893-133X(02)00291-9. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Caramaschi D, Natarajan D, Koolhaas JM. The vicious cycle towards violence: focus on the negative feedback mechanisms of brain serotonin neurotransmission. Front Behav Neurosci. 2009;3:52. doi: 10.3389/neuro.08.052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer SF, Van Der Vegt BJ, Koolhaas JM. Individual variation in aggression of feral rodent strains: a standard for the genetics of aggression and violence? Behav Genet. 2003;33:485–501. doi: 10.1023/a:1025766415159. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Oitzl MS, Joels M. Functional implications of brain corticosteroid receptor diversity. Cell Mol Neurobiol. 1993;13:433–455. doi: 10.1007/BF00711582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Dolan M, Anderson IM, Deakin JF. Relationship between 5-HT function and impulsivity and aggression in male offenders with personality disorders. Br J Psychiatry. 2001;178:352–359. doi: 10.1192/bjp.178.4.352. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Inada K, Farrington JS, Koller BH, Moy SS. Neural activation deficits in a mouse genetic model of NMDA receptor hypofunction in tests of social aggression and swim stress. Brain Res. 2009;1265:186–195. doi: 10.1016/j.brainres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott FA. Propranolol for the control of belligerent behavior following acute brain damage. Ann Neurol. 1977;1:489–491. doi: 10.1002/ana.410010516. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT1B receptor agonist CP-94,253. Psychopharmacology. 1999;146:391–399. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Markou A. The role of preclinical models in the development of psychotropic drugs. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology. The Fifth Generation of Progress. Lippincott William & Wilkins; Philadelphia: 2002. pp. 445–455. [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A. 2009;106:19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Halasz J, Liposits Z, Kruk MR, Haller J. Neural background of glucocorticoid dysfunction-induced abnormal aggression in rats: involvement of fear- and stress- related structures. Eur J Neurosci. 2002;15:561–569. doi: 10.1046/j.0953-816x.2001.01883.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Halasz J, Mikics E, Kruk MR. Chronic glucocorticoid deficiency-induced abnormal aggression, autonomic hypoarousal, and social deficit in rats. J Neuroendocrinol. 2004;16:550–557. doi: 10.1111/j.1365-2826.2004.01201.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Horvath Z, Bakos N. The effect of buspirone on normal and hypoarousal-driven abnormal aggression in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:27–31. doi: 10.1016/j.pnpbp.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Haller J, Kruk MR. Normal and abnormal aggression: human disorders and novel laboratory models. Neurosci Biobehav Rev. 2006;30:292–303. doi: 10.1016/j.neubiorev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Haller J, Toth M, Halasz J, de Boer SF. Patterns of violent aggression-induced brain c-fos expression in male mice selected for aggressiveness. Physiol Behav. 2006;88:173–182. doi: 10.1016/j.physbeh.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Haller J, van de Schraaf J, Kruk MR. Deviant forms of aggression in glucocorticoid hyporeactive rats: a model for `pathological' aggression? J Neuroendocrinol. 2001;13:102–107. doi: 10.1046/j.1365-2826.2001.00600.x. [DOI] [PubMed] [Google Scholar]
- Hasen NS, Gammie SC. Maternal aggression: new insights from Egr-1. Brain Res. 2006;1108:147–156. doi: 10.1016/j.brainres.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Haspel T. Beta-blockers and the treatment of aggression. Harv Rev Psychiatry. 1995;2:274–281. doi: 10.3109/10673229509017146. [DOI] [PubMed] [Google Scholar]
- Heiligenberg W. Processes governing behavioral states of readiness. Adv Study Behav. 1974;5:173–200. [Google Scholar]
- Holloway RL. Primate aggression, territoriality and xenophobia: a comparative perspective. Academic Press; New York: 1974. p. 515. [Google Scholar]
- Hudziak JJ, van Beijsterveldt CE, Bartels M, Rietveld MJ, Rettew DC, Derks EM, Boomsma DI. Individual differences in aggression: genetic analyses by age, gender, and informant in 3-, 7-, and 10-year-old Dutch twins. Behav Genet. 2003;33:575–589. doi: 10.1023/a:1025782918793. [DOI] [PubMed] [Google Scholar]
- King HD. Life processes in gray Norway rats during fourteen years in captivity. Amer Anat Mem. 1939;17:1–72. [Google Scholar]
- Koolhaas JM, Schuurman T, Wiepkema PR. The organization of intraspecific agonistic behaviour in the rat. Prog Neurobiol. 1980;15:247–268. doi: 10.1016/0301-0082(80)90024-6. [DOI] [PubMed] [Google Scholar]
- Kornetsky C. Animal models: promises and problems. In: Koob GF, editor. Animal models of depression. Birkhauser; Boston: 1989. pp. 18–29. [Google Scholar]
- Kravitz EA, Huber R. Aggression in invertebrates. Curr Opin Neurobiol. 2003;13:736–743. doi: 10.1016/j.conb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Lagerspetz K. Studies on the aggressive behaviour of mice. Ann Acad Sci Fenn. 1964;131:1–131. [Google Scholar]
- Lagerspetz K, Hautojarvi S. The effect of prior aggressive or sexual arousal on subsequent aggressive or sexual reactions in male mice. Scand J Psychol. 1967;8:1–6. doi: 10.1111/j.1467-9450.1967.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Lagerspetz KMJ. Aggression and aggressiveness in laboratory mice. In: Garattini S, editor. Aggressive behaviour. Excerpta Medica Foundation; Amsterdam: 1969. pp. 77–85. [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K. On aggression. Methuen; London: 1966. [Google Scholar]
- Marler P. On animal aggression: the roles of strangeness and familiarity. Am Psychol. 1976;31:239–246. doi: 10.1037//0003-066x.31.3.239. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Arch Gen Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- McKinney WT. Basis of development of animal models in psychiatry: An overview. In: Koob GF, editor. Animal models of depression. Birkhauser; Boston: 1989. pp. 3–17. [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology. 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Almeida RMM, Kravitz EA, Rissman EF, de Boer SF, Raine A. Neurobiology of escalated aggression and violence. J Neurosci. 2007a;27:11803–11806. doi: 10.1523/JNEUROSCI.3500-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, de Boer SF. Aggressive, defensive, and submissive behavior. In: Whishaw IQ, Kolb B, editors. The behavior of the laboratory rat: a handbook with tests. Oxford University Press; New York: 2005. pp. 344–352. [Google Scholar]
- Miczek KA, DeBold JF, Van Erp AMM, Tornatzky W. Alcohol, GABAA-benzodiazepine receptor complex, and aggression. In: Galanter M, editor. Recent developments in alcoholism: alcoholism and violence. Plenum Publishing Corp; New York: 1997. pp. 139–171. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Faccidomo S, de Almeida RMM, Bannai M, Fish EW, DeBold JF. Escalated aggressive behavior: new pharmacotherapeutic approaches and opportunities. Ann N Y Acad Sci. 2004a;1036:336–355. doi: 10.1196/annals.1330.021. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Faccidomo SP, Fish EW, DeBold JF. Neurochemistry and molecular neurobiology of aggressive behavior. In: Blaustein J, editor. Behavioral neurochemistry, neuroendocrinology and molecular neurobiology. Springer; New York: 2007b. pp. 285–336. [Google Scholar]
- Miczek KA, Fish EW, de Almeida RMM, Faccidomo S, DeBold JF. Role of alcohol consumption in escalation to violence. Ann N Y Acad Sci. 2004b;1036:278–289. doi: 10.1196/annals.1330.018. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Natarajan D, Caramaschi D. Animal violence demystified. Front Behav Neurosci. 2010;4:9. doi: 10.3389/fnbeh.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan D, de Vries H, Saaltink DJ, de Boer SF, Koolhaas JM. Delineation of violence from functional aggression in mice: an ethological approach. Behav Genet. 2009a;39:73–90. doi: 10.1007/s10519-008-9230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan D, de Vries H, de Boer SF, Koolhaas JM. Violent phenotype in SAL mice is inflexible and fixed in adulthood. Aggress Behav. 2009b;35:430–436. doi: 10.1002/ab.20312. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Neurosci Rev. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Newman EL, Chu A, Bahamon B, Takahashi A, DeBold JF, Miczek KA. NMDA receptor antagonism: escalation of aggressive behavior in alcohol-drinking mice. Psychopharmacology. 2012;224:167–77. doi: 10.1007/s00213-012-2734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg J, Sandnabba K, Schalkwyk L, Sluyter F. Genetic and environmental (inter)actions in male mouse lines selected for aggressive and nonaggressive behavior. Genes Brain Behav. 2004;3:101–109. doi: 10.1111/j.1601-183x.2003.0056.x. [DOI] [PubMed] [Google Scholar]
- Plyusnina IZ, Solov'eva MY, Oskina IN. Effect of domestication on aggression in gray Norway rats. Behav Genet. 2011;41:583–592. doi: 10.1007/s10519-010-9429-y. [DOI] [PubMed] [Google Scholar]
- Potegal M. Attack priming and satiation in female golden hamsters: Tests of some alternatives to the aggression arousal interpretation. Aggress Behav. 1991;17:327–335. [Google Scholar]
- Potegal M, Tenbrink L. Behavior of attack-primed and attack-satiated female golden hamsters (Mesocricetus auratus) J Comp Psychol. 1984;98:66–75. [Google Scholar]
- Raine A, Mednick SA. Biosocial longitudinal research into antisocial behavior. Rev Epidemiol Sante Publique. 1989;37:515–524. [PubMed] [Google Scholar]
- Ratey JJ, Sorgi P, O'Driscoll GA, Sands S, Daehler ML, Fletcher JR, Kadish W, Spruiell G, Polakoff S, Lindem KJ. Nadolol to treat aggression and psychiatric symptomatology in chronic psychiatric inpatients: a double-blind, placebo-controlled study. J Clin Psychiatry. 1992;53:41–46. [PubMed] [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychol Bull. 2002;128:490–529. [PubMed] [Google Scholar]
- Ribeiro AC, Musatov S, Shteyler A, Simanduyev S, Arrieta-Cruz I, Ogawa S, Pfaff DW. siRNA silencing of estrogen receptor-alpha expression specifically in medial preoptic area neurons abolishes maternal care in female mice. Proc Nat Acad Sci. 2012;109:16324–16329. doi: 10.1073/pnas.1214094109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci LA, Knyshevski I, Melloni J. Serotonin type 3 receptors stimulate offensive aggression in Syrian hamsters. Behav Brain Res. 2005;156:19–29. doi: 10.1016/j.bbr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Roizen J. Epidemiological issues in alcohol-related violence. In: Galanter M, editor. Recent developments in alcoholism. Plenum Press; New York: 1997. pp. 7–41. [DOI] [PubMed] [Google Scholar]
- Siegel A, Roeling TAP, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neurosci Biobehav Rev. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Simmons RK, Howard JL, Simpson DN, Akil H, Clinton SM. DNA methylation in the developing hippocampus and amygdala of anxiety-prone versus risk-taking rats. Dev Neurosci. 2012;34:58–67. doi: 10.1159/000336641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyter F, Arseneault L, Moffitt TE, Veenema AH, de Boer SF, Koolhaas JM. Toward an animal model for antisocial behavior: parallels between mice and humans. Behav Genet. 2003;33:563–574. doi: 10.1023/a:1025730901955. [DOI] [PubMed] [Google Scholar]
- Summers CH, Korzan WJ, Lukkes JL, Watt MJ, Forster GL, Overli O, Hoglund E, Larson ET, Ronan PJ, Matter JM, Summers TR, Renner KJ, Greenberg N. Does serotonin influence aggression? Comparing regional activity before and during social interaction. Physiol Biochem Zool. 2005;78:679–694. doi: 10.1086/432139. [DOI] [PubMed] [Google Scholar]
- Tellegen A, Horn JM. Primary aggressive motivation in three inbred strains of mice. J Comp Physiol Psychol. 1972;78:297–304. doi: 10.1037/h0032192. [DOI] [PubMed] [Google Scholar]
- Thayer ZM, Kuzawa CW. Biological memories of past environments: epigenetic pathways to health disparities. Epigenetics. 2011;6:798–803. doi: 10.4161/epi.6.7.16222. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. On war and peace in animals and man: An ethologist's approach to the biology of aggression. Science. 1968;160:1411–1418. doi: 10.1126/science.160.3835.1411. [DOI] [PubMed] [Google Scholar]
- Toth M, Halasz J, Mikics E, Barsy B, Haller J. Early social deprivation induces disturbed social communication and violent aggression in adulthood. Behav Neurosci. 2008;122:849–854. doi: 10.1037/0735-7044.122.4.849. [DOI] [PubMed] [Google Scholar]
- Toth M, Mikics E, Tulogdi A, Aliczki M, Haller J. Post-weaning social isolation induces abnormal forms of aggression in conjunction with increased glucocorticoid and autonomic stress responses. Horm Behav. 2011;60:28–36. doi: 10.1016/j.yhbeh.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Toth M, Tulogdi A, Biro L, Soros P, Mikics E, Haller J. The neural background of hyper-emotional aggression induced by post-weaning social isolation. Behav Brain Res. 2012;233:120–129. doi: 10.1016/j.bbr.2012.04.025. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Greiwe KM, Nelson RJ. Individual differences in estrogen receptor alpha in select brain nuclei are associated with individual differences in aggression. Horm Behav. 2006;50:338–345. doi: 10.1016/j.yhbeh.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulogdi A, Toth M, Halasz J, Mikics E, Fuzesi T, Haller J. Brain mechanisms involved in predatory aggression are activated in a laboratory model of violent intra-specific aggression. Eur J Neurosci. 2010;32:1744–1753. doi: 10.1111/j.1460-9568.2010.07429.x. [DOI] [PubMed] [Google Scholar]
- Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Gilad Y. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc Natl Acad Sci U S A. 2012;109:6490–6495. doi: 10.1073/pnas.1202734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Erp A, Miczek KA. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci. 2000;20:9320–9325. doi: 10.1523/JNEUROSCI.20-24-09320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oortmerssen GA, Bakker TCM. Artificial selection for short and long attack latencies in wild Mus musculus domesticus. Behav Genet. 1981;11:115–126. doi: 10.1007/BF01065622. [DOI] [PubMed] [Google Scholar]
- Veiga CP, Aranda BCC, Stein D, Franci CR, Miczek KA, Lucion AB, de Almeida RMM. Effect of social instigation and aggressiv behavior on hormone levels of lactating dams and adult male Wistar rats. Psychol Neurosci. 2011;4:103–113. [Google Scholar]
- Veiga CP, Miczek KA, Lucion AB, de Almeida RMM. Effect of 5-HT1B receptor agonists injected into the prefrontal cortex on maternal aggression in rats. Braz J Med Biol Res. 2007;40:825–830. doi: 10.1590/s0100-879x2006005000113. [DOI] [PubMed] [Google Scholar]
- Virkkunen M. Urinary free cortisol secretion in habitually violent offenders. Acta Psychiatr Scand. 1985;72:40–44. doi: 10.1111/j.1600-0447.1985.tb02568.x. [DOI] [PubMed] [Google Scholar]
- Volavka J. Neurobiology of Violence. Second Edition American Psychiatric Publishing; Arlington, VA: 2002. [Google Scholar]
- Yudofsky SC, Silver JM, Hales RE. Pharmacologic management of aggression in the elderly. J Clin Psychiatry. 1990;51(Suppl):22–28. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The recording shows a short episode of an aggressive encounter between a sham-operated resident and a naive intruder. The attack episode was recorded in low light; the opponents were illuminated by the infrared light source of the camcorder. The biting attack starts by lateral or sideways threat, after which the resident bends over the intruder in an attempt to bite. Accidentally, however, the mouth of the resident comes close to the belly of the opponent, i.e. a vulnerable target (see the 4th second of the recording). The deliberate avoidance of vulnerable targets by control rats is depicted by the behavior of the resident, which quickly corrects the position of its mouth, and bites the left flank of the intruder. The intruder immediately submits. This reaction to the bite is indicative of a “hard bite”. Note that the resident avoids biting the belly of the submissive opponent, despite the fact that this body area is clearly exposed and easy to bite. At the end of the bite, the intruder recovers relatively quickly, and resumes the exploration of the environment.
The recording shows a short episode of an aggressive encounter between an ADXr resident and a naive intruder. The attack episode was recorded in low light; the opponents were illuminated by the infrared light source of the camcorder. At the start of the episode, both rats are in an upright posture, and reciprocally sniff each other. The resident suddenly delivers a soft bite targeting the nose of the intruder (i.e. a vulnerable area). Note that the intruder responds by upright freezing to the next approach by the resident, despite the fact that the bite was clearly soft. After a short episode of offense by the resident, the opponent falls back into submission, and is immediately bitten just above the right paw, at the border between the throat and abdomen (i.e. a vulnerable target). After this bite, the opponent remains in supine posture even after the resident stops keeping it down. Albeit not reported in earlier studies, vulnerable area attacks usually intimidate the intruder as shown by this recording.
The first segment of the video captures a resident-intruder confrontation in which the resident male displays species-typical aggressive behavior. Within seconds, the resident male initiates the attack with a bite to the posterior left flank of the intruder. Following the first attack bite, the intruder escapes and the resident pursues, attempting to nip at his hind flanks. The intruder turns to exhibit an upright defensive posture, but the resident bites several times, pauses briefly to reposition and aims the next bite at the posterior flanks of the intruder. After a short interval, the resident exhibits a sideways threat, which is rapidly followed by the delivery of two more attack bites. The resident walks away from the intruder, marking the end of the aggressive bout. Microanalysis of the resident’s species-typical aggressive behavior revealed a distinct bout of aggression and precise delivery of attack bites to the posterior flanks of the intruder.
The second segment of the video captures a confrontation in which the resident male mouse displays alcohol-heightened aggression after rapidly self-administering 1 g/kg of 6% ethanol solution (w/v). Following a brief latency, the resident delivers an initial bite to the intruder’s right forearm. As the intruder turns to escape, the resident pursues and bites the posterior flank of the intruder twice. The intruder reacts by displaying a prolonged defensive upright posture, and the resident circles the intruder to bite his flank again. Assuming a defensive position on his back, the intruder exposes his abdomen, which the resident bites repeatedly. To avoid attacks to his vulnerable underside, the intruder attempts to escape, which provokes the resident to pursue and bite the intruder’s hind flanks. After cornering the intruder, the resident pins the intruder and continues to bite the intruder’s abdomen and flanks. The intruder persists in his defensive display, and eventually resumes escape behavior. As before, the resident pursues while biting the hind flanks of the intruder. Finally, the resident walks away from the intruder to groom briefly, marking the end of the aggressive bout. From this analysis, it is evident that there are qualitative differences between species-typical and alcohol-heightened aggression. Alcohol-heightened aggression is characterized by prolonged, intense aggressive bouts and targeting attack bites at vulnerable regions.