SUMMARY
Several recent publications indicate that the maximum stimulation of muscle protein fractional synthetic rate occurs with intake of 20 to 30 gms protein. This finding has led to the concept that there is a maximal anabolic response to protein intake with a meal, and that the normal amount of protein eaten with dinner will generally exceed the maximally-effective intake of protein.
However, protein breakdown has not been taken into account when evaluating the anabolic response to protein intake. Protein anabolism occurs only when protein synthesis exceeds breakdown protein breakdown.
Higher protein intakes when protein synthesis is maximized is characterized by suppressed protein breakdown and via that mechanism leads to a greater anabolic response. This explains why when net protein synthesis is measured, the relationship between amino acid availability and net gain remains linear, without any apparent plateau of effect at higher levels of availability.
We conclude that there is no practical upper limit to the anabolic response to protein or amino acid intake in the context of a meal.
Keywords: protein, anabolic response, meal, muscle protein synthesis
INTRODUCTION
The principal nutritional goal of a protein-rich meal is to induce an anabolic state in which muscle protein synthesis exceeds the rate of breakdown. Several recent publications, including our own (1), indicate that the maximum stimulation of muscle protein fractional synthetic rate (FSR) occurs with intake of 20 to 30 gms protein. This finding has led to the concept that there is a maximal anabolic response to protein intake with a meal, and that the normal amount of protein eaten with dinner will generally exceed the maximally-effective intake of protein (2). However, the extent of muscle protein anabolism (the anabolic response) is not simply the response of muscle protein FSR, but rather the net balance between the response of FSR and the rate of protein breakdown. Account has generally not been taken of protein breakdown when evaluating the anabolic response to protein intake. It is also important that many previous protein dose-response studies have considered the response to ingestion of pure amino acids, an isolated protein, or protein occurring in a particular food source (eg meat or milk) rather than in the context of a complete meal. We will review the anabolic response to protein or amino acid intake in isolation and in the context of a complete meal in order to address the question of whether there is a maximal anabolic response to protein intake with a meal.
MEASUREMENT OF THE ANABOLIC RESPONSE
When specific amino acid isotopes are infused as tracers in plasma or consumed with a meal, all proteins in the body will incorporate the labeled amino acids at rates that reflect the fractional synthetic rate (FSR) of the protein. The amount of tracer incorporated in a measured amount of time, corrected for the enrichment of the tracer in the precursor pool, enables calculation of the FSR for the protein in the tissue sampled. In the case of muscle, specific proteins (e.g. myofibrillar protein) may be isolated from the tissue and the synthesis rate calculated separately.
Muscle FSR only identifies the synthesis rate of muscle protein. All proteins in the body are in a constant state of turnover, meaning that there is simultaneous synthesis and breakdown. The net gain in muscle protein over time, or the anabolic response, is calculated as the difference between the rate of synthesis and the rate of protein breakdown. Although it is possible to measure the muscle fractional protein degradation rate (muscle FBR), this is not a very common measurement as it requires a more complicated experimental protocol, additional blood sampling, and complicated calculations (3), especially during feeding. An alternative approach to calculating muscle protein synthesis and breakdown is to measure the balance of specific amino acids across the leg or arm. This approach requires arterial and venous catheterization and measurement of tissue blood flow, and consequently over recent years has been used infrequently in human subjects. Furthermore, the reliability of this method to measure protein breakdown is limited in the non-steady state that exists after ingestion of amino acids or a protein meal (4). A three pool arterial-venous (a-v) model that also includes a tissue biopsy improves accuracy and enables an estimation of protein breakdown in the non-steady state (5).
Whole body protein synthesis and breakdown can also be measured in response to protein meals. This approach uses the addition of isotopes to the meal and the infusion of isotopes into the blood stream and collection of blood and/or exhaled CO2. Commonly used approaches are the addition of phenylalanine/leucine to the meal (taking into account absorption kinetics, either as a free amino acid or within a labeled protein), infusion of labeled tyrosine and phenylalanine and collection of blood or the infusion of labeled leucine and collection of blood and exhaled air. Although the measurement of whole body protein synthesis and breakdown is theoretically limited by the fact that all of the tissues are included in the measurement of net protein synthesis, during feeding it is often assumed that muscle protein synthesis and breakdown comprise a large portion of the whole body response above the basal value. There are technical advantages to determining whole body protein synthesis and breakdown, because no muscle biopsies are required and frequent blood sampling enables reasonable calculation of both synthesis and breakdown during amino acid or protein absorption.
Measurement of muscle FSR is technically and theoretically the most straight forward technique with which to assess the response of muscle protein synthesis to a protein meal, but it is limited because it gives us only half of the information needed to determine the anabolic response to a protein meal. The anabolic response can only be determined when synthesis and breakdown are measured simultaneously.
Which approach provides the best measure of the anabolic response?
The anabolic response is traditionally equated with muscle anabolism, so the measurement of protein synthesis and breakdown directly in muscle is obviously important in determining the nature and magnitude of the anabolic response. However, measurement of protein synthesis and breakdown at the whole body level is also of value when evaluating the anabolic response. The principal factor differentiating the responses to different types or amounts of protein is the resulting change in amino acid concentrations and profiles in the blood. However, not all of the amino acids absorbed from the digestion of protein appear directly in peripheral blood. Following ingestion of intact protein some of the digested amino acids are retained in the splanchnic area (splanchnic extraction). This retention mainly takes place in the gut (6), which can function as a labile protein pool that the body uses as a temporarily storage pool of essential amino acids (7). The gut has a high protein turnover rate and is therefore capable of rapidly retaining amino acids for protein synthesis, then releasing those amino acids over time for eventual incorporation into protein in other tissues, in particular muscle. Measurement of the acute response of muscle protein metabolism to intake of protein may therefore underestimate the total anabolic response over time. The total anabolic response from a nutritional standpoint is determined by the total gain of body protein, and for this reason, the determination of the whole body response is important. The ideal scenario is to measure both the whole body and muscle protein synthesis and breakdown, thereby enabling the determination of the anabolic response both at the whole body level and in muscle.
What is the mechanism of net anabolism?
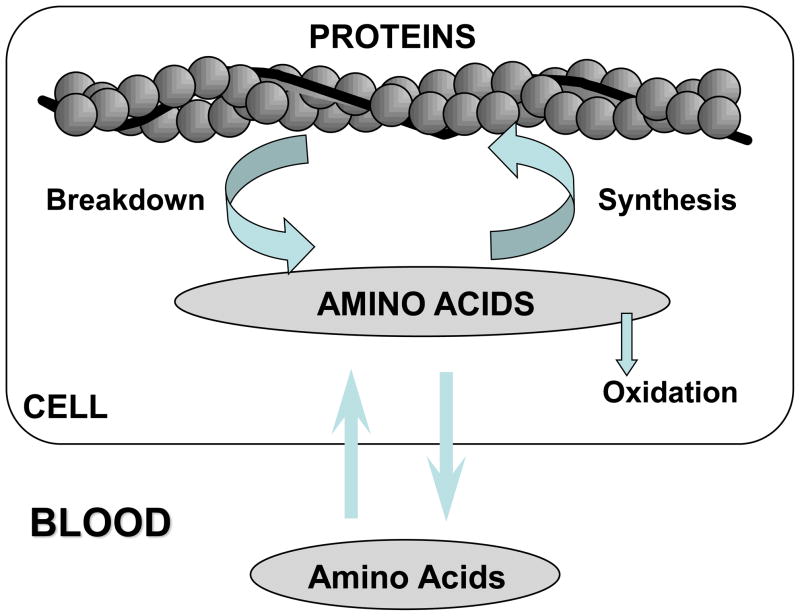
The net balance between muscle protein synthesis and breakdown distinguishes the catabolic state (breakdown exceeds synthesis) from the anabolic state (synthesis exceeds breakdown). The relationship between muscle protein turnover and plasma and intracellular essential amino acids is shown schematically in Figure 1. The synthesis of new protein is derived from the intracellular pool of amino acids (8). The intracellular pool of essential amino acids (amino acids not produced in significant quantities in the body) is the most important determinant of protein synthesis as the non-essential amino acids are readily available (9). These amino acids are derived from protein breakdown and inward flux from plasma. An anabolic response requires that the inward flux of amino acids exceeds the rate of oxidation or transamination of those amino acids within the muscle. In the post-absorptive state there is a net efflux of amino acids from the muscle into the blood. The absorption of digested protein causes an increase in plasma amino acid concentrations that result in an accelerated inward flux into muscle. The increased availability of intracellular essential amino acids stimulates the synthesis of new protein, as reflected by the measurement of muscle protein FSR.
Figure 1.
The intracellular pool of amino acids is the precursor pool for muscle protein synthesis. These amino acids can be derived from either protein breakdown or inward transport of amino acids from plasma. In addition to being incorporated into protein, intracellular amino acids can be oxidized or released into plasma.
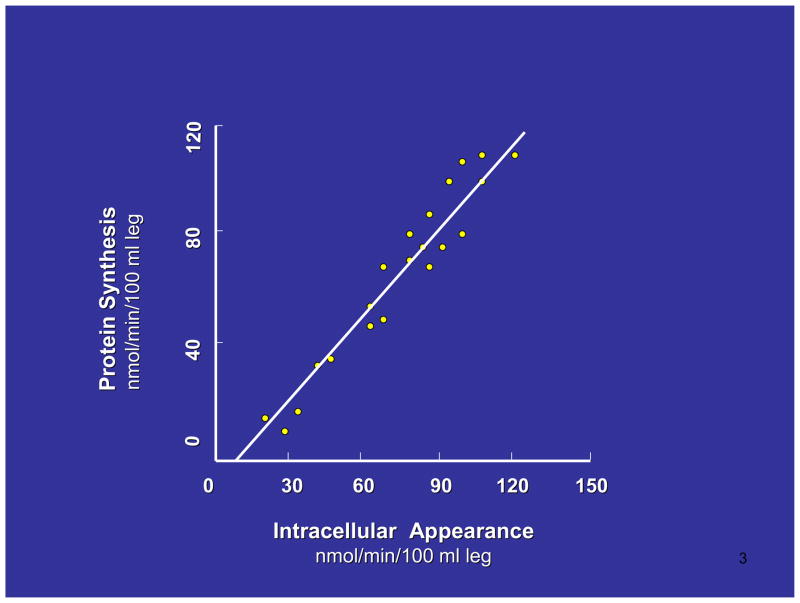
The schematic representation in Figure 1 illustrates that changes in breakdown alone can never induce a shift from the catabolic state to the anabolic state, since some of the amino acids released from protein breakdown are oxidized or transaminated and therefore not available for reincorporation into protein. On the other hand, the rate of protein breakdown will always be linked to some extent to the rate of synthesis because of the contribution of amino acids from breakdown to the intracellular pool of amino acids. In fact under most conditions, the majority of the intracellular pool of amino acids is derived from protein breakdown rather than inward flux from plasma. Thus, when considering the role of amino acid availability in regulating the rate of muscle protein synthesis it is necessary to take account of not only the amino acids from plasma, but also the amino acids that appear in the intracellular pool as a consequence of protein breakdown. In a wide variety of circumstances we have found a direct linear relationship between the total rate of appearance of essential amino acids into the intracellular pool and the rate of muscle protein synthesis (Figure 2). Thus, it is clear that the measurement of protein synthesis can only be interpreted in the context of the anabolic response when considered in relation to concurrent changes in the rate of breakdown, and the total rate of appearance of amino acids intracellularly.
Figure 2.
The relationship between muscle protein synthesis (protein synthesis minus breakdown) and the rate of intracellular appearance of amino acids. Rates were determined in human subjects using a three pool model of leg protein metabolism. Taken from (23).
WHY WAS THE CONCEPT OF MAXIMAL ANABOLIC RESPONSE DEVELOPED?
The fundamental observation that has led to the concept of a maximal anabolic response to protein intake is that increases in amino acid concentrations in plasma are directly related to the stimulation of muscle protein synthesis up to a point, whereupon further increases in plasma amino acid concentrations do not translate to greater increases in protein synthesis. When the rate of influx of amino acids from plasma into muscle is modest relative to the rate of protein synthesis, the intracellular concentrations of amino acids remain relatively constant (10). Once the maximal rate of synthesis is achieved, the intracellular concentrations of amino acids begin to rise as a function of further increases in plasma amino acid availability (10). The concentrations of the intracellular amino acids presumably rise above the post-absorptive levels because the maximal rate of incorporation into protein has been reached. However, the point at which intracellular amino acid concentration begin to increase does not necessarily coincide with the maximal anabolic response. Rather, the anabolic response may continue to increase as protein intake increases, even without the further stimulation of protein synthesis. This response can occur if, as appears to be the case (9), further increases in intracellular amino acid concentrations provide a signal to limit the rate of protein breakdown.
The role of insulin
Insulin is an anabolic hormone that is released at an increased rate in response to ingestion of carbohydrate. Ingestion of certain proteins and amino acids can also stimulate insulin release, particularly when eaten in large quantities. In the physiological range insulin inhibits protein breakdown, and as a result lowers free amino acid levels in both the plasma and intracellular spaces (11). Although insulin is an anabolic hormone that activates the signals that initiate protein synthesis, an increase in insulin concentration without a concomitant increase in amino acid concentrations may not affect or even decrease the rate of protein synthesis both in muscle and at the whole body level due. This is because the decreased availability of amino acids resulting from the suppression of protein breakdown overrides any stimulatory effect of insulin on protein synthesis (11).
In the setting of increased insulin and decreased endogenous amino acid concentrations, ingestion of protein will cause a significantly lower peak response of the plasma amino acids concentrations than if the same amount of protein was ingested in the absence of an insulin response. Also, insulin stimulates gut tissue net protein synthesis that as a result causes less amino acids to appear in the portal vein (12). As a consequence, the rate of protein synthesis will likely increase to a smaller extent in the setting of increased insulin concentration than if there was no insulin response, even though the total anabolic response may be greater because of the simultaneous suppression of protein breakdown. Because of the reduction in the concentrations of endogenous amino acids secondary to an increase in insulin concentration, the apparent maximal synthetic response to protein intake may not reflect the true capacity for synthesis because of limitations in total amino acid availability. More importantly, the synthetic response in the setting of elevated insulin does not reflect the maximal anabolic response. The role of insulin in suppressing protein breakdown is particularly important when considering the response to protein intake in the context of a complete meal, in which the carbohydrate can result in a significant insulin response.
Is there an upper limit to the anabolic response to protein intake with a meal?
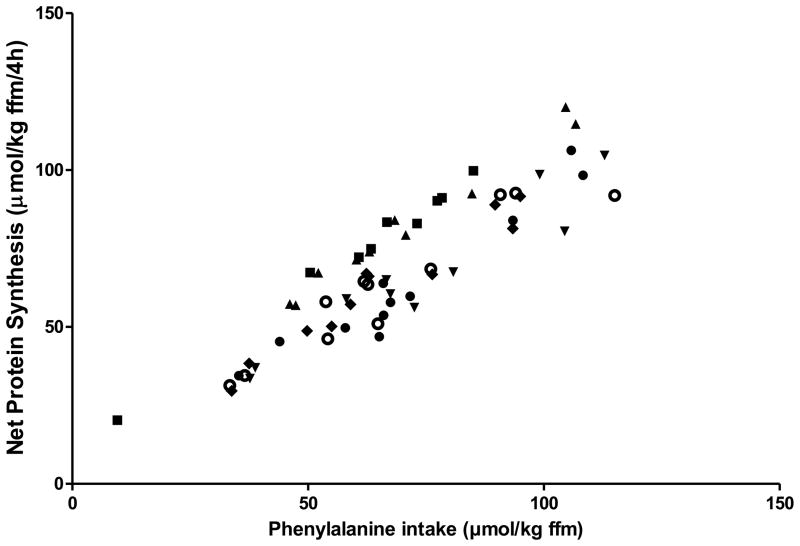
The theory described above predicts that there would be no practical limit to the anabolic response to protein intake with a meal. With greater and greater carbohydrate and protein intake a progressively greater insulin response will result, with subsequent suppression of protein breakdown, and thus a greater anabolic response. Results from our recent studies (Figure 3) and studies of others (13–18) in which whole body protein synthesis and breakdown have been measured suggest that in fact the theory is correct and that this relation remains even at high protein intakes. It is important to note that in all studies in which net protein synthesis has been measured, the relationship between amino acid availability and net gain is linear, with no plateau of effect at higher levels of availability. From these data we must conclude that there is no practical upper limit to the anabolic response to protein or amino acid intake in the context of a meal.
Figure 3.
Relation between the intake of essential amino acids (represented by the phenylalanine intake) and net protein synthesis after a bolus meal with a protein mixture with added carbohydrates in a group of male and females between 45 and 70 years of age in the healthy condition and with disease such as chronic renal failure, COPD and Cancer. All data are expressed per kg fat-free mass (ffm). Between 240 to 330 μmol phenylalanine is present in 1 gram protein. With an average of about 50 kg of ffm/subject, 100 μmol phenylalanine/kg ffm represents about 15 to 20 gram of protein in the bolus drink.
DO THE METABOLIC STUDIES OF THE ANABOLIC RESPONSE ACCURATELY PREDICT THE TRUE ANABOLIC RESPONSE OVER DAYS AND WEEKS?
We therefore have to conclude that there is no limit to the increase in the net anabolic response to higher and higher amounts of protein intake. While on the surface this observation seems questionable, the same observation has been made repeatedly using the N-balance approach and more modern stable isotope indicator methods also suggest continued protein accretion at intakes greater than the requirement level (13–18). A variety of studies substantiate that the tracer methods to quantify acute responses of protein synthesis and breakdown accurately predict changes over time in lean mass in tightly controlled conditions (19, 20). It is much harder to control all potentially important variables in long-term outcome studies than in acute metabolic studies, and consequently there are no prospective studies of which we are aware in which the responses of physiological outcome variables were measured in response to progressively increasing levels of protein intake. Several studies in older individuals have documented that supplementation of the diet with amino acids or protein increases lean body mass and function. Importantly, we are aware of no studies that show that higher levels of protein intake fail to increase lean mass or muscle function over time.
CONCLUSION AND IMPLICATIONS
The normal dinner includes more than 30 gms of protein, which is generally agreed to be sufficient to elicit the maximal stimulation of muscle protein synthesis. Consequently, it has been proposed that the maximal total stimulation of muscle protein synthesis over the day will be achieved by spreading protein intake out as opposed to eating most daily protein at dinner, which is currently the most common approach (2, 21). This hypothesis assumes that the nutritional goal of protein intake is the stimulation of muscle protein FSR. However, the anabolic response is a function not only of the rate of protein synthesis, but also of the rate of protein breakdown. We believe that the total anabolic response to protein intake, particularly in the context of a complete meal that stimulates an insulin response, is therefore incorrectly characterized by exclusive reliance on the FSR measurement. Extrapolation of the FSR response to the conclusion that there is a maximal amount of protein in a meal that can stimulate net protein anabolism is not justified. In our opinion, the preponderance of evidence indicates that the more protein in a meal, the more anabolism will be observed. This perspective is supported by a recent publication in this journal (22) in which consumption of 80% of the 1.5 gram protein/kg BW/day in a single meal was more anabolic than spreading the same amount of protein intake throughout the day. If the limit to the maximal anabolic response of 30 gram/meal, the approach of providing most protein in one meal would have been unsuccessful in achieving the optimal anabolic respone. The practical implication of this conclusion is that protein nutrition can be improved by increasing the protein intake at breakfast and lunch and maintaining a high amount of protein intake with dinner, or increasing the amount of protein eaten with dinner if that is more convenient. Alternatively, replacing low quality proteins with high quality proteins, containing higher levels of a balanced essential amino acid mixture, will additionally stimulate protein anabolism.
Acknowledgments
The project described was supported by Award Number R01GM084447 from the National Institute of General Medical, by Award Number R01HL095903 from the National Heart, Lung, and Blood Institute, and P30AG028717 from the National Institute of Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of General Medical Sciences, the National Heart, Lung, and Blood Institute or the National Institute of Aging of the National Institutes of Health.
Footnotes
STATEMENT OF AUTHORSHIP
The authors together wrote the manuscript.
POTENTIAL CONFLICTS OF INTEREST/DISCLOSURES.
N. Deutz has no conflict of interest. R. Wolfe is a member of the Abbott Research Advisory Board and the Essentient, LLC, Advisory Board, and has received funding for research grants and served as a consultant for the National Cattleman’s Beef Association.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. Journal of the American Dietetic Association. 2009 Sep;109(9):1582–6. doi: 10.1016/j.jada.2009.06.369. Epub 2009/08/25.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009 Jan;12(1):86–90. doi: 10.1097/MCO.0b013e32831cef8b. Epub 2008/12/06.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang XJ, Chinkes DL, Wolfe RR. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. Am J Physiol Endocrinol Metab. 2002 Oct;283(4):E753–64. doi: 10.1152/ajpendo.00053.2002. Epub 2002/09/10.eng. [DOI] [PubMed] [Google Scholar]
- 4.Katsanos CS, Chinkes DL, Sheffield-Moore M, Aarsland A, Kobayashi H, Wolfe RR. Method for the determination of the arteriovenous muscle protein balance during non-steady-state blood and muscle amino acid concentrations. Am J Physiol Endocrinol Metab. 2005 Dec;289(6):E1064–70. doi: 10.1152/ajpendo.00141.2005. Epub 2005/08/11.eng. [DOI] [PubMed] [Google Scholar]
- 5.Biolo G, Gastaldelli A, Zhang XJ, Wolfe RR. Protein synthesis and breakdown in skin and muscle: a leg model of amino acid kinetics. Am J Physiol. 1994 Sep;267(3 Pt 1):E467–74. doi: 10.1152/ajpendo.1994.267.3.E467. Epub 1994/09/01.eng. [DOI] [PubMed] [Google Scholar]
- 6.Deutz NE, Ten Have GA, Soeters PB, Moughan PJ. Increased intestinal amino-acid retention from the addition of carbohydrates to a meal. Clin Nutr. 1995 Dec;14(6):354–64. doi: 10.1016/s0261-5614(95)80053-0. Epub 1995/12/01.eng. [DOI] [PubMed] [Google Scholar]
- 7.Soeters PB, de Jong CH, Deutz NE. The protein sparing function of the gut and the quality of food protein. Clin Nutr. 2001 Apr;20(2):97–9. doi: 10.1054/clnu.2000.0376. Epub 2001/05/01.eng. [DOI] [PubMed] [Google Scholar]
- 8.Martini WZ, Chinkes DL, Wolfe RR. The intracellular free amino acid pool represents tracer precursor enrichment for calculation of protein synthesis in cultured fibroblasts and myocytes. J Nutr. 2004 Jun;134(6):1546–50. doi: 10.1093/jn/134.6.1546. Epub 2004/06/03.eng. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe RR, Miller SL. Amino acid availability controls muscle protein metabolism. Diabetes, nutrition & metabolism. 1999 Oct;12(5):322–8. Epub 2000/03/31.eng. [PubMed] [Google Scholar]
- 10.Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. The Journal of physiology. 2003 Oct 1;552(Pt 1):315–24. doi: 10.1113/jphysiol.2003.050674. Epub 2003/08/12.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfe RR, Volpi E. Handbook of Physiology Section The endocribe system. Oxford University press; 2001. Insulin and protein metabolism; pp. 735–57. [Google Scholar]
- 12.Deutz NEP, Ten Have GAM, Soeters PB, Moughan PJ. Increased intestinal amino-acid retention from the addition of carbohydrates to a meal. Clinical Nutrition. 1995;14(6):354–64. doi: 10.1016/s0261-5614(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 13.Elango R, Humayun MA, Ball RO, Pencharz PB. Protein requirement of healthy school-age children determined by the indicator amino acid oxidation method. Am J Clin Nutr. 2011 Dec;94(6):1545–52. doi: 10.3945/ajcn.111.012815. [DOI] [PubMed] [Google Scholar]
- 14.Humayun MA, Elango R, Ball RO, Pencharz PB. Reevaluation of the protein requirement in young men with the indicator amino acid oxidation technique. Am J Clin Nutr. 2007 Oct;86(4):995–1002. doi: 10.1093/ajcn/86.4.995. [DOI] [PubMed] [Google Scholar]
- 15.Reeds PJ, Garlick PJ. Protein and amino acid requirements and the composition of complementary foods. J Nutr. 2003 Sep;133(9):2953S–61S. doi: 10.1093/jn/133.9.2953S. Epub 2003/09/02.eng. [DOI] [PubMed] [Google Scholar]
- 16.Walrand S, Short KR, Bigelow ML, Sweatt AJ, Hutson SM, Nair KS. Functional impact of high protein intake on healthy elderly people. Am J Physiol Endocrinol Metab. 2008 Oct;295(4):E921–8. doi: 10.1152/ajpendo.90536.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JM, et al. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012 Apr 15;302(8):E992–9. doi: 10.1152/ajpendo.00517.2011. [DOI] [PubMed] [Google Scholar]
- 18.Rand WM, Young VR, Scrimshaw NS. Change of urinary nitrogen excretion in response to low-protein diets in adults. Am J Clin Nutr. 1976 Jun;29(6):639–44. doi: 10.1093/ajcn/29.6.639. [DOI] [PubMed] [Google Scholar]
- 19.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, et al. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab. 2004 Sep;89(9):4351–8. doi: 10.1210/jc.2003-032159. Epub 2004/09/10.eng. [DOI] [PubMed] [Google Scholar]
- 20.Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR. Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab. 2003 Jan;284(1):E76–89. doi: 10.1152/ajpendo.00234.2002. Epub 2002/10/22.eng. [DOI] [PubMed] [Google Scholar]
- 21.Layman DK. Dietary Guidelines should reflect new understandings about adult protein needs. Nutr Metab (Lond) 2009;6:12. doi: 10.1186/1743-7075-6-12. Epub 2009/03/17.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouillanne O, Curis E, Hamon-Vilcot B, Nicolis I, Chretien P, Schauer N, et al. Impact of protein pulse feeding on lean mass in malnourished and at-risk hospitalized elderly patients: A randomized controlled trial. Clin Nutr. 2012 Aug;30(0) doi: 10.1016/j.clnu.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe RR. Protein supplements and exercise. Am J Clin Nutr. 2000 Aug;72(2 Suppl):551S–7S. doi: 10.1093/ajcn/72.2.551S. [DOI] [PubMed] [Google Scholar]