Abstract
This study examined the effect of two-weeks infusion of angiotensin-II (Ang-II; 175 ng/kg/min) via minipump in rats (n=7) upon the mean arterial blood pressure (mBP) and heart rate (HR) response to an acute stress as compared to rats infused with saline (n=7). The acute stress was produced by a classical aversive conditioning paradigm: a 15 sec. tone (CS+) followed by a half second tail shock. Baseline mBP in Ang-II infused rats (167.7 ± 21.3 mm Hg; mean ± SD) significantly exceeded that of controls (127.6 ± 13.5 mm Hg). Conversely, baseline HR in the Ang-II infused rats (348 ± 33) was significantly lower than controls (384 ± 19 bpm). The magnitude of the mBP increase during CS+ did not differ between groups, but the HR slowing during CS+ in the Ang-II infused rats (−13.2 ± 8.9 bpm) was significantly greater than that seen in controls (−4.2 ± 5.5 bpm). This augmented bradycardia may be inferentially attributed to an accentuated increase in cardiac parasympathetic activity during CS+ in the Ang-II infused rats. The mBP increased above baseline immediately post-shock delivery in controls, but fell in the Ang-II infused rats, perhaps because of a ‘ceiling effect’ in total vascular resistance. This classical conditioning model of ‘acute stress’ differs from most stress paradigms in rats in yielding a HR slowing concomitant with a pressor response, and this slowing is potentiated by Ang-II.
Keywords: classical Pavlovian conditioning, anxiety, hypertension, renin angiotensin system (RAS)
INTRODUCTION
The autonomic nervous system (ANS) and the renin-angiotensin system (RAS) are major determinants of the cardiovascular response to acute ‘emotional stress’, and over-activity in either or both systems triggered by such stress has been implicated in the development of cardiovascular dysfunction (e.g., Mancia, et al., 2006; Mayorov, 2011). The majority of studies of the effects of acute emotional stress in rodent experiments use models that induce pressor responses and, in most cases, a tachycardia. In fact, however, the heart rate (HR) response, whether acceleratory or deceleratory, depends upon the nature of the stress and upon the strain of rat (Paré, 1993). Inhibition of the RAS in these studies generally diminishes the sympathetic arousal normally attendant upon such stressors (summarized in Mayorov, 2011, Table 1). Reports of the effects of RAS manipulation upon the HR response to stress, however, vary (e.g., compare Lee, et al., 2004 and Davern, et al., 2009).
Table 1.
Changes in mean arterial blood pressure and heart rate during CS+ and CS− in saline (S) infused rats (n=7) vs. angiotensin-II (Ang-II) infused rats (n=7)
| CS+ | CS− | |||||||
|---|---|---|---|---|---|---|---|---|
| ΔmBP (mm Hg) |
ΔHR (bpm) | ΔmBP (mm Hg) |
ΔHR (bpm) | |||||
| S | Ang-II | S | Ang-II | S | Ang-II | S | Ang-II | |
| pk C1 | +6.3± 3.2 |
+5.7± 3.3 |
+5.2 ±3.8 |
+11.2 ±6.4 |
+5.2± 2.0 |
+4.1± 2.3 |
+8.1± 3.3 |
+11.6 ±6.5 |
| avg C2 | +1.7± 2.4 |
+2.4± 2.1 |
−4.2± 5.5 |
−13.2± 8.9* |
+0.3± 0.8 |
+0.6± 1.3 |
−3.1± 3.5 |
−4.1± 5.6 |
| post- shock |
6.5± 4.0 |
−4.0± 6.2* |
+26 ±24 |
+18 ±8 |
N/A | N/A | N/A | N/A |
See Figure 1 for definitions of pk C1, avg C2 and post-shock. No shock was delivered post-CS−, so a value is inappropriate. The values shown in Table 1 may differ from those read directly from Figure 1 because the individual rat’s changes in mBP or in HR, particularly for pkC1, were attained at somewhat different times after tone onset across rats.
= p < 0.05, saline vs. Ang-II
We have reported (Randall, et al., 1993, 1994; Li, et al., 1998) that classical aversive conditioning, produced by following a 15 sec. pulsed tone (i.e., the conditional stimulus or CS+) with a ½ sec. tail shock in Sprague-Dawley (S-D) rats, produces a moderate pressor response with modest HR slowing, while the same paradigm in the spontaneously hypertensive rat (SHR) evokes a large increase in mean arterial pressure (mBP) and a large concomitant bradycardia (Li, et al., 1997). The fact that HR slowed significantly less in the borderline hypertensive rat (BHR) on a high salt diet than it did in the same strain on a low salt diet for a similar change over baseline in renal sympathetic nerve activity (SNA) implicates the RAS as an influence in the nature of the HR change (Brown, et al., 1999). The goal of the present study is to determine the effect of sustained exposure to angiotensin-II (Ang-II) via chronic infusion upon the magnitudes of the mBP and HR changes evoked by classical aversive conditioning in the S-D rat. We report that increases in mBP during CS+ were not statistically different in rats infused with Ang-II (175 ng/min/kg for 2 weeks) via osmotic minipump vs. those infused with saline, but that the concomitant bradycardia was significantly greater in the rats infused with Ang-II.
METHODS
Animals
Sprague-Dawley male rats obtained from Harlan Industries (Indianapolis, IN) constituted a group ultimately to receive angiotensin-II (n=7) or saline (n=7). Each animal was allowed free access to water and regular rat chow except during the time of behavioral training and testing. The protocol was performed in accordance with the guidelines for animal experimentation described in the “Guiding Principles for Research Involving Animals and Human Beings” (American Physiological Society, 2002), and was approved by the University of Kentucky Animal Care and Use Committee.
Drug Protocol
The animals were anesthetized with sodium pentobarbital (65 mg/kg IP) two weeks prior to the behavioral stress tests in preparation for surgical implantation of the mini-pump for drug administration. Aseptic techniques appropriate for rodent survival surgery were used. An Alzet osmotic mini-pump (Duret Corp., Cupertino, CA) filled with Ang-II (175ng/kg/min) was inserted under the skin over the dorsal chest (n=7). Control rats (n=7) received a minipump filled with normal saline.
Behavioral conditioning to stressful stimulus
Animals were behaviorally trained using classical (i.e., Pavlovian) conditioning according to a previously described technique (Randall, et al., 1993, 1994). Each rat was trained in the behavioral paradigm commencing one week after insertion of a mini-pump. Briefly, the animals were habituated for 1-2 hours daily for several days to handling and restraint in a comfortable cloth sock. Each rat was then exposed daily to five trials of a 15-second pulsed tone (CS+; on for 0.064 s, off for 0.16 s) and another five trials of an uninterrupted tone (i.e., non-pulsed; CS−) that was otherwise identical to the pulsed-sound. CS+ and CS− were presented in pseudorandom pairs (e.g., …CS+, CS−,CS−, CS+…). On the initial training day only the last (i.e., 5th) pulsed tone was reinforced with the 0.5 sec. shock (i.e., to establish that the tone itself was initially neutral); the shock was delivered via bipolar electrodes held by a plastic cuff placed around the subject’s tail prior to each day’s trials. The shock was adjusted to the lowest value that caused the rat to flinch and squeak which was usually 0.2 mA and never exceeded 0.3 mA. Training continued for two additional days, but with each CS+ shock reinforced. A minimum of five minutes was allowed between presentations of successive trials to allow the studied variables to return to baseline. Each rat was removed from the sock at the end of each daily session and was returned to the animal quarters.
Catheter Implant Surgery
The animals were re-anesthetized, as above, two weeks after pump implantation and a Teflon catheter (0.012 inch interior diameter) was implanted in the abdominal aorta by way of the femoral artery. The distal end of the catheter was tunneled under the skin, exited at the shoulders, and run through a flexible wire tether stabilized at the nape of the neck. The distal end was also glued to a piece of PE 50 plastic tubing to allow coupling to a pressure transducer during data acquisition. After surgery, each animal was individually housed, supplied with food and water, with its movement only slightly restricted by the flexible tether. Each animal was allowed 48 hours recuperation before behavioral testing commenced.
Data Acquisition
BP was recorded by connecting the arterial catheter to a pressure transducer (Cobe model CDX-III). The pressure signal was recorded on a Grass model 7 polygraph. Data were acquired and analyzed using software developed by ViiSoftware (Lexington, KY). During data sessions mean arterial BP and HR were calculated from the pulsatile BP signal digitized at 500 Hz. Individual files were created for each conditioning trial starting 15 seconds before tone onset and terminating 15 seconds after the end of a tone so that each 45-s recording covered the pretone baseline, tone, and recovery periods. Data were recorded for five trials of each tone on each of two days. The individual digital data files for 10 CS+ trials (and for 10 CS− trials) from a single subject were ensemble averaged to yield a single file depicting the conditional cardiovascular response for that animal; we have called this process a “high resolution analysis” (Randall, et al., 1993, 1994). For each rat the first component of the conditional response (C1) was read from the high resolution file as the peak value of mBP between 16-18 sec. (individual pkC1, that is, within the first 3 sec. of the tone’s sounding). The second component (C2) for each rat was taken as the average between 20-29 sec. The unconditional response (UR) for that rat was the peak value between 30.5-33 sec., where the shock was presented at t = 30 sec. Finally, a group average, or ensemble (cf. Figure 1), was constructed by averaging the high resolution files for each condition (e.g., saline trials or Ang-II trials) for each tone (i.e., CS+ or CS−) across all rats in a given treatment. This process reliably assesses the cardiovascular response pattern to each tone (Randall, et al., 1993).
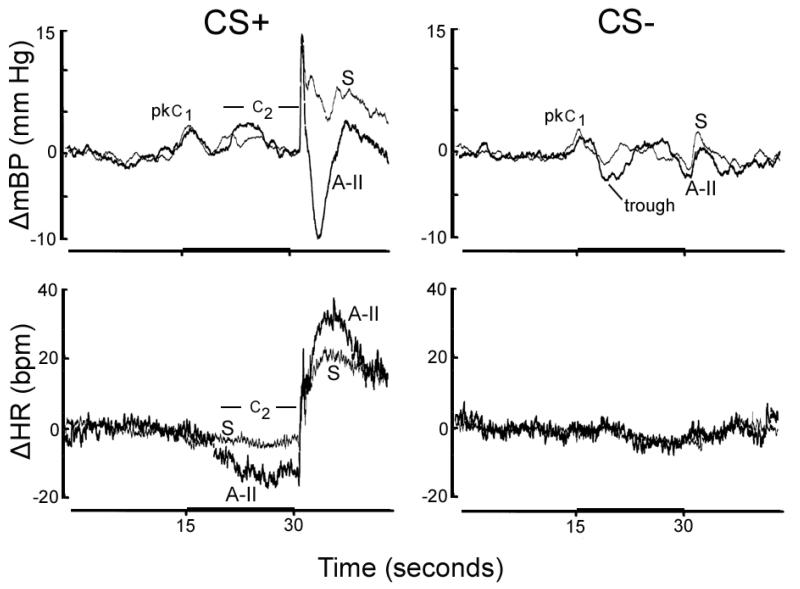
Figure 1.
Group average change (Δ) vs. baseline in mean arterial blood pressure (mBP) and in heart rate (HR) during 30 sec. pulsed tone (CS+; dark bar on time scale) followed by half second tail shock (left panels) and during 30 sec. steady tone (CS−) never followed by shock (right panels) in rats infused with saline (S; thin lines) and those infused for 2 weeks with angiotensin-II (Ang-II; 175ng/kg/min; thick lines). Amplitude of changes in mBP during CS+ did not differ between groups (upper left panel), but HR slowed significantly more in the Ang-II vs. saline group (lower left). Mean BP dropped transiently post-shock in Ang-II infused rats while mBP was elevated in saline-infused group. The transient drop in pressure following the initial ‘on response’ for CS− (i.e., trough) was accentuated in the Ang-II infused rats.
Data analysis
The amplitude of the BP and HR conditional response was calculated relative to the beat-by-beat average BP (or HR) during the pre-tone baseline period. We defined pretone BP to be the average mean pressure between 0-14 sec. The quantitative nature of the conditional response was summarized by averaging the individual values (see above) for pkC1, C2 and UR across rats to yield a group average (Table 1). Data were tested by analysis of variance (ANOVA) followed by post hoc t-tests, when appropriate. Statistical significance was accepted for p < 0.05. All results are shown as mean ± SD.
RESULTS
Baseline mBP and HR
Baseline mean arterial BP was 127.6 ± 13.5 mm Hg during the 15 sec. prior to CS+ in the saline-infused rats. The corresponding value in the Ang-II infused rats of 167.7 ± 21.3 significantly exceeded that of the saline-infused controls (Student’s t12 = 4.207). Baseline HR in the saline group was 384 ± 19 bpm which was significantly (t12 = 2.510) greater than the 348 ± 33 bpm in the Ang-II infused rats.
Conditional mBP and HR Responses in Saline vs. Ang-II Infused Rats
Figure 1 presents the group (n=7, each) ensemble average change (Δ; relative to baseline) in mBP (top row) and in HR (bottom row) during CS+ (left) and CS− (right). In each case the tone was presented during the dark bar on the time axis (i.e., between 15 and 30 sec.). The thicker tracings are for rats treated with angiotensin-II; lighter tracings present the saline (S) group. The peak increase in mBP during the initial C1 component of the conditional response (i.e., pkC1 in Fig. 1) did not differ across groups in response to either CS+ or CS− (Table 1). (Recall that the steady CS− tone is the same frequency as the pulsed CS+ tone; it, therefore, evokes an initial C1 increase in mBP since the subject is initially unable to determine which trial is underway (Randall, et al., 1993, 1994).) The HR value in Table 1 for C1 is the change recorded concomitantly with the peak C1 mBP increase; for CS+ the difference approached significance (t12 = 2.108; p=0.057).
The change in mBP during C2 for CS+ trials did not differ between groups whether assessed as the average value during the last ~10 seconds of the tone (Table 1), or as the peak value during the interval (Saline-infused: + 5.9 ± 2.4 mm Hg; Ang-II infused: +7.4 ± 4.0 mm Hg). Conversely, as illustrated in Figure 1 (lower left) the HR slowing during C2 was significantly larger in the Ang-II infused rats as compared to the saline-infused controls (see also Table 1).
The saline-infused rats maintained an elevated mBP during the seconds immediately following the sharp spike coincident with shock delivery (Figure 1 and Table 1). Conversely, the Ang-II infused rats experienced a clear, if transient, drop in mBP during this same interval. The difference was statistically significant (t12 = 3.765). Both groups showed a sustained tachycardia post-shock.
The mBP response to CS− includes an initial, short-lived pressor response analogous to C1, followed by a drop below baseline designated as “trough” in Figure 1 (upper right panel). The magnitude of this decrease at its nadir in the Ang-II infused rats (−6.5 ± 3.7 mm Hg) was significantly (t12 = 2.165) larger than in the saline-infused controls (−3.1 ± 2.6 mm Hg). There was no sustained change in mBP or in HR during CS− in either group, indicating that animals in both groups discriminated between CS+ and CS− (Randall, et al., 1993, 1994).
DISCUSSION
As expected for unanesthetized S-D rats infused with 175ng/kg/min of Ang-II via osmotic minipump (Cassis, et al., 1998), baseline mBP was elevated, but, perhaps unexpectedly, neither the amplitude of the initial, transient mBP increase (i.e., C1), nor that of the later, more sustained C2 component was significantly altered vs. the saline-infused controls. Conversely, HR slowed significantly more during the C2 component of the conditional response in the Ang-II infused rats as compared to the controls. In fact, while the HR change during CS+ in the saline-infused rats closely resembled that reported elsewhere in the S-D rat (Randall, et al., 1993, 1994), the bradycardia seen in the rats infused with Ang-II qualitatively resembled that seen in the SHR (Li, et al., 1997). The saline-infused controls maintained an elevated mBP post-shock, while pressure dropped below baseline in the Ang-II infused rats. Finally, the drop in mBP below baseline in CS− trials that follows the C1 mBP increase was more pronounced in the Ang-II infused rats vs. controls. The text that follows considers possible explanations for these observations in light of what is known about the mediation of the pattern of changes that comprise the conditional mBP and HR responses.
The CS+ tone elicits a sequential series of changes in SNA, mBP and HR (Brown, et al., 1999; Li, et al., 1997; Randall, et al., 1994). The initial event is an abrupt (for S-D, latency from tone onset = 0.16 ± 0.03 sec.), intense (4.09 ± 1.02 times average baseline), but transient (duration: 0.58 ± 0.12 sec) ‘sudden burst’ (SB) in SNA. The SB is quickly followed by the initial C1 BP increase (peak increase at 1.6 ± 0.2 sec. after beginning of CS+ tone) onset. The C1 BP increase ‘maps’ to changes in SNA (Burgess, et al., 1997). This C1 increase in mBP results essentially exclusively from an increase in peripheral vascular resistance since stroke volume and cardiac output are unchanged (Li, et al., 1998). Because the amplitudes of the peak C1 pressor event did not differ between the Ang-II vs. saline-infused rats in the present study, we infer that the smooth muscle contractile response to the sudden burst in SNA which occurs in response to tone onset also did not differ between groups.
The SB is immediately followed by a transient drop in SNA below baseline which we termed the ‘quiet period’ (QP); the decrease in mBP following the initial C1 peak is closely associated with this transient drop in SNA (Randall, et al., 1994). The associated drop in mBP is eliminated following sino-aortic denervation (SAD; Willingham, et al., 2004); we therefore tentatively attribute the QP / mBP-decrease to action of the baroreflex in response to the C1 BP increase (Randall, et al., 1994), though recordings of SNA during CS+ before vs. after SAD are required to substantiate this hypothesis. Since the frequency of the CS+ and CS− tones is the same, an animal cannot know at first sounding whether the trial is a shock-reinforced CS+ (i.e., pulsed tone) or a ‘safe’ CS− trial (non-pulsed tone). Therefore, a similar sequence occurs in the initial response to CS−, excepting that the magnitude of the QP decrease in SNA during the CS− significantly exceeds the magnitude of the decrease observed during CS+ (Randall, et al., 1994). It is noteworthy, therefore, that in the present study the decrease in mBP in CS− that follows the C1 event was significantly larger in the Ang-II infused vs. saline-infused rats. This suggests a more robust reflex HR slowing to the C1 mBP increase in the Ang-II infused rats, even though the magnitude of that mBP increase was similar across groups.
SNA increases during the last 10 sec. of the CS+ tone to an average of 1.24 ± 0.14 (i.e., in Sprague-Dawley) of baseline; this change in SNA for CS+ is accompanied in S-D by a modest, but statistically significant, C2 increase in BP that averages 3 ± 3 mm Hg (Randall, et al., 1994). The changes in mBP again ‘map’ onto the changes in SNA (Burgess, et al., 1997). The C2 mBP increase is largely secondary to a sustained elevation in stroke volume and cardiac output (Li, et al., 1998). The amplitude of the concomitant bradycardia differs across rat strains: it is small, or absent in S-D rats (Randall, et al., 1993, 1994), moderately large in the borderline hypertensive rat on low salt diet (Brown, et al., 1999), and very large in the SHR, which also has a large C2 pressor response (Li, et al., 1997). We have reported in the Zucker lean rat that this bradycardia is modestly attenuated by administration of a β-adrenergic antagonist, but it is virtually eliminated by administration of atropine (El-Wazir, et al., 2008). Therefore, the differences we describe here in the HR component of the cardiovascular conditional response between the two groups of rats can be interpreted most parsimoniously in terms of an action of Ang-II that enhances parasympathetic slowing of HR. In turn, we posited that “…one could reasonably ascribe this to the baroreflex secondary to the MAP increase. (Li, 1997, p. 153).” If so, the larger amplitude of the decrease in HR during C2 in the Ang-II infused rats vs. the saline-infused rats, despite the near similarity in the corresponding mBP increases, may again most reasonably be ascribed to a greater baroreflex response in the Ang-II infused rats.
The interactions of Ang-II, the sympathetic nervous system and baroreflex have been extensively studied (reviewed in Reid, 1992; Head, et al., 2002). In particular, Ang-II given intravenously (10 ng/kg/min) to unanesthetized rabbits reportedly increased mBP without changing HR, but reset the baroreflex to higher pressure with no change in gain (Reid and Chou, 1990). Head, et al. (2002) conclude from their own work, and their review of the literature, that “…in situations where there are increased excitatory and decreased inhibitory inputs to the RVLM brainstem, such as barodenervation, acute stress, hypertension or heart failure, the sympathoexcitatory Ang(iotensin) system within the brainstem clearly becomes very important.” (quoted from p. 1057). At least under the conditions of our experiment, where Ang-II had been infused for 2 weeks via a subdural osmotic minipump, there was little evidence that the sympathetically mediated pressor responses evoked by CS+ were significantly enhanced.
In contrast to interactions of Ang-II with the sympathetic nervous system, interactions with the parasympathetic system per se have been less extensively examined. Lumbers, et al. (1979), however, pursued an early study in which they recorded activity of single cardiac efferent fibers in anesthetized dogs during pressor responses produced by bolus injections of phenylephrine (PE) or inflation of an aortic balloon, vs. those produced by Ang-II infusion (2-25 μg). They reported that the first two interventions increased vagal discharge in each of 8 fibers recorded from 8 anesthetized dogs. Conversely, Ang-II pressor responses were associated with a fall in vagal discharge (three fibers), little change (4 fibers) or an increase, but an increase smaller than occurred in response to PE (1 fiber). In a recent and elegant study in anesthetized rabbits with SAD and vagotomy, Kawada, et al. (2009) stimulated the peripheral end of the right vagus with vs. without background iv Ang-II administration (10 ng/kg/min). They reported that the gain of the transfer function from vagal stimulation to HR slowing decreased significantly with concomitant Ang-II administration vs. control (i.e., no Ang-II). They interpreted the findings to suggest that the elevated Ang-II inhibited acetylcholine release from the vagal nerve axons. In the human, Ang-II administration to healthy volunteers reduced HR spectral power centered around 0.25 Hz, thought to be attributable primarily to parasympathetic activity, to a greater degree than observed during equi-pressor doses of PE (Townend, et al., 1995). Moreover, heart failure patients treated with an angiotensin-converting enzyme inhibitor experienced increased HR variability, indices of parasympathetic tone (Binkley, et al., 1993). Conversely, in our study rats infused with Ang-II demonstrated increased HR slowing associated with the C2 mBP elevation, which, based upon our earlier work (El-Wazir, et al., 2008), is attributable to a greater increase in parasympathetic tone during CS+.
The effects of systemic or intracerebroventricular (icv) exposure to Ang-II, or to agents that inhibit the effects of endogenous Ang-II, on the HR (and BP) responses to non-Pavlovian conditioning models of acute stress have been reported by others. In an earlier study Saiki, et al. (1997) reported that icv administration of Ang-II receptor antagonists, both non-selective and AT1-receptor selective, attenuated the pressor and tachycardic responses normally attendant upon restraint stress in WKY and SHR, thereby indicating that central Ang-II has a generally excitatory role in sympathetic response to stress. A more recent study in mice, Lee, et al. (2004) used a cage-switch stress paradigm (placing a male mouse in a cage previously occupied by a different male mouse) and reported that pretreatment of the stressed animal with captopril in the drinking water attenuated the usual pressor response, but had no effect upon the tachycardia seen in the control state. They suggest that Ang-II played an important role in mediating the hypertensive response, probably via an action through β1 receptors. Davern, et al. (2009) studied the cage-switch cardiovascular response of mice genetically deficient in the Ang-II AT1-receptor (AT1A−/−) vs. AT1A+/+. Baseline mBP was decidedly lower in the AT1A−/− vs. controls, but there was no between-group HR difference. The magnitudes of the mBP and HR increases during stress in the AT1A−/− were significantly smaller than the controls; activity was also less in the test mice, but the differences in mBP and HR changes persisted during quiet periods. These investigators concluded that activation of central AT1A receptors during stress normally facilitates the autonomic manifestations of the stress. We are unaware of similar studies of the effects of Ang-II manipulations upon HR slowing during stress.
One additional between group comparison seems worthy of note: the saline infused rats maintained mBP above baseline during the interval immediately after cessation of shock, as presented as ‘post-shock’ in Table 1, but mBP fell in the Ang-II infused rats. In Sprague-Dawley rats, the elevation in mBP is attributable to an increase in peripheral vascular resistance; in fact, stroke volume and cardiac output drop precipitously from the values they have immediately prior to shock delivery (Li, et al., 1998). The present data imply, therefore, that the vasculature in the Ang-II infused rats was unable to create the sudden and large increase in vasomotor tone required for the mBP elevation, perhaps because vascular resistance was already high secondary to the drug treatment.
Our observations of an unchanged pressor response with an accentuated HR slowing during CS+ must be interpreted cautiously in light of generally opposite published reports. Note, however, that the conditional pressor response in the Ang-II infused rats occurs superimposed upon a significantly increased baseline mBP so that, as mentioned immediately above, we may be dealing with a ‘ceiling effect’ whereby any additional pressor response to stress was constrained in our rats. Nonetheless, in the conditioning paradigm it seems likely that the baroreflex remains active, and may, in fact, orchestrate a larger increase in cardiac parasympathetic activity in response to a given change in mBP. Perhaps the system was operating on a portion of the mBP/HR relationship where the slope (i.e., ΔHR/ΔmBP), or gain, was greater.
In summary, chronic infusion of Ang-II via osmotic minipump elevated baseline mBP and modestly decreased the corresponding HR. The amplitude of the increase in mBP during acute stress was unchanged, but the HR slowing was significantly enhanced such that it resembled in character that seen in the SHR. The most likely explanation for the larger conditioned bradycardia is an enhancement of cardiac parasympathetic activity during CS+, perhaps via an increased baroreflex response to the conditional pressor responses. Irrespective, our findings clearly demonstrate the importance of looking at a variety of behavioral situations when seeking an understanding of the roles of any given regulatory system in producing ‘stress-induced’ cardiovascular responses.
ACKNOWLEDGEMENTS
Supported by grants RO1 NS39774 and HL073085 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this study were conducted as an undergraduate research project by Richard Hoyt at Asbury University, Wilmore, KY; he received his M.D. degree in 2006 from the University of Kentucky, and is currently Major, United States Air Force. Richard Speakman received his bachelors degree from Asbury University and his M.D. degree in 2006 from the University of Kentucky, and is currently Major, United States Air Force.
REFERENCES
- American Physiological Society Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002;283:R281–R283. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- Binkley PF, Haas GJ, Starling RC, Nunziata E, Hatton PA, Leier CF, Cody RJ. Sustained augmentation of parasympathetic tone with angiotensin-converting enzyme inhibition in patients with congestive heart failure. J Am Coll Cardiol. 1993;21:655–661. doi: 10.1016/0735-1097(93)90098-l. [DOI] [PubMed] [Google Scholar]
- Brown DR, Li S-G, Lawler JE, Randall DC. Sympathetic control of BP and BP variability in borderline hypertensive rats on high- vs. low-salt diet. Am J Physiol Regul Integr Comp Physiol. 1999;277:R650–R657. doi: 10.1152/ajpregu.1999.277.3.R650. [DOI] [PubMed] [Google Scholar]
- Burgess DE, Hundley JC, Li S, Randall DC, Brown DR. Multifiber renal sympathetic nerve activity recordings predict mean arterial blood pressure in unanesthetized rat. Am J Physiol Regul Integr Comp Physiol. 1997;273:R851–R857. doi: 10.1152/ajpregu.1997.273.3.R851. [DOI] [PubMed] [Google Scholar]
- Cassis LA, Marshall DE, Fettinger MJ, Rosenbluth B, Lodder RA. Mechanisms contributing to angiotensin II regulation of body weight. Am J Physiol Endocrinol Metab. 1998;274:E867–E876. doi: 10.1152/ajpendo.1998.274.5.E867. [DOI] [PubMed] [Google Scholar]
- Davern PJ, Chen D, Head GA, Chavez CA, Walther T, Mayorov DM. Role of angiotensin II type 1A receptors in cardiovascular reactivity and neuronal activation after aversive stress in mice. Hypertension. 2009;54:1262–1268. doi: 10.1161/HYPERTENSIONAHA.109.139741. [DOI] [PubMed] [Google Scholar]
- El-Wazir YM, Li S-G, Smith R, Silcox DL, Brown DR, Randall DC. Parasympathetic Response to Acute Stress is Attenuated in Young Zucker Obese Rats. Autonomic Neuroscience: Basic and Clinical. 2008;143:33–39. doi: 10.1016/j.autneu.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head GA, Saigusa T, Mayorov DN. Angiotensin and baroreflex control of the circulation. Brazilian J Med Biol Res. 2002;35:1047–1059. doi: 10.1590/s0100-879x2002000900005. [DOI] [PubMed] [Google Scholar]
- Kawada T, Mizuno M, Shimizu S, Uemura K, Kamiya A, Sugimachi M. Angiotensin II disproportionally attenuates dynamic vagal and sympathetic heart rate controls. Am J Physiol Heart Circ Physiol. 2009;296:H1666–H1674. doi: 10.1152/ajpheart.01041.2008. [DOI] [PubMed] [Google Scholar]
- Lee DL, Webb RC, Brands MW. Sympathetic and angiotensin-dependent hypertension during cage-switch stress in mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1394–R1398. doi: 10.1152/ajpregu.00306.2004. [DOI] [PubMed] [Google Scholar]
- Li S-G, Lawler JE, Randall DC, Brown DR. Sympathetic nervous activity and arterial pressure responses during rest and acute behavioral stress in SHR vs. WKY rats. J. Autonom. Nerv. Sys. 1997;62:147–154. doi: 10.1016/s0165-1838(96)00119-1. [DOI] [PubMed] [Google Scholar]
- Li S-G, Randall DC, Brown DR. Roles of cardiac output and peripheral resistance in mediating blood pressure response to stress in rats. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1065–R1069. doi: 10.1152/ajpregu.1998.274.4.R1065. [DOI] [PubMed] [Google Scholar]
- Lumbers ER, McCloskey DI, Potter EK. Inhibition by angiotensin II of baroreceptor-evoked activity in cardiac vagal efferent nerves in the dog. J Physiol (London) 1979;294:69–80. doi: 10.1113/jphysiol.1979.sp012915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia G, Dell’Oro R, Quarti-Trevano F, Scopelliti F, Grassi G. Angiotensin-sympathetic system interactions in cardiovascular and metabolic disease. J Hypertens. 2006;24(suppl 1):S51–S56. doi: 10.1097/01.hjh.0000220407.84363.fb. [DOI] [PubMed] [Google Scholar]
- Mayorov DM. Brain angiotensin AT1 receptors as specific regulators of cardiovascular reactivity to acute psychoemotional stress. Clin Exp Pharm Physiol. 2011;38:126–135. doi: 10.1111/j.1440-1681.2010.05469.x. [DOI] [PubMed] [Google Scholar]
- Paré WP. Passive-avoidance behavior in Wistar-Kyoto (WKY), Wistar and Fischer-344 rats. Physiol Behav. 1993;54:845–852. doi: 10.1016/0031-9384(93)90291-m. [DOI] [PubMed] [Google Scholar]
- Randall DC, Brown DR, Brown LV, Kilgore JM, Behnke MM, Moore SK, Powell KR. Two-component arterial blood pressure conditional response in rat. Integrative Physiol. Behav. Sci. 1993;28:258–269. doi: 10.1007/BF02691243. [DOI] [PubMed] [Google Scholar]
- Randall DC, Brown DR, Brown LV, Kilgore JM. Sympathetic nervous activity and arterial blood pressure control in conscious rat during rest and behavioral stress. Am J Physiol Regul Integr Comp Physiol. 1994;267:R1241–R1249. doi: 10.1152/ajpregu.1994.267.5.R1241. [DOI] [PubMed] [Google Scholar]
- Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol Endocrinol Metab. 1992;262:E763–E778. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- Reid IA, Chou L. Analysis of the action of angiotensin II on the baroreflex control of heart rate in conscious rabbits. Endocrinol. 1990;126:2749–2756. doi: 10.1210/endo-126-5-2749. [DOI] [PubMed] [Google Scholar]
- Saiki Y, Watanabe T, Tan N, Matsuzaki M, Nakamura S. Role of central ANG II receptors in stress-induced cardiovascular and hyperthermic responses in rats. Am J Physiol Regul Integr Comp Physiol. 1997;272:R26–R33. doi: 10.1152/ajpregu.1997.272.1.R26. [DOI] [PubMed] [Google Scholar]
- Townsend JN, Muzahim A-A, West JN, Littler WA, Coote JH. Modulation of cardiac autonomic control in humans by angiotensin II. Hypertension. 1995;25:1270–1275. doi: 10.1161/01.hyp.25.6.1270. [DOI] [PubMed] [Google Scholar]
- Willingham A, Williams D, Brown L, Brown D, Cassis L, Silcox D, Anigbogu C, Randall D. Arterial Blood Pressure Response to an Acute Stress in Rat following Sino-aortic Denervation. FASEB J. 2004;18:A673–A674. abstract. [Google Scholar]