Abstract
Background
The primary analyses of the COMBINE study revealed significant naltrexone and Combined Behavioral Intervention (CBI) main effects on drinking outcomes but failed to find additional benefits of the combination of treatments. Investigating differences in patterns of adherence over time may shed light on the treatment effects in COMBINE. The goals of the study were to identify trajectories of medication adherence and participation in CBI, to estimate predictive and moderating effects of adherence trajectories on drinking outcomes and to characterize subjects in adherence trajectories. The results of these analyses may suggest approaches to improving adherence in order to ultimately improve treatment outcome.
Methods
We used a trajectory-based approach to identify patterns of treatment adherence separately for naltrexone, acamprosate and CBI adherence. Logistic regression and general linear models assessed associations among adherence trajectories, drinking outcomes and patient characteristics.
Results
Three trajectories of adherence were identified for each treatment: “excellent adherers”, “late non-adherers” and “early non-adherers” and there was good agreement among adherence trajectories with different treatments. “Excellent adherers” had significantly higher percent days abstinent (PDA) and lower percent heavy drinking days (PHDD). CBI significantly decreased PHDD for subjects on acamprosate in the “early non-adherers with medication” trajectory (p=0.01). Either naltrexone or acamprosate was associated with lower PHDD than placebo for “early non-adherers with CBI” (p<0.01). Receiving active medication decreased the likelihood to be in the excellent medication adherence trajectory. Younger age, greater drinking severity, dissatisfaction with the medicine and session frequency, adverse events and lack of benefit were related to less favorable medication adherence trajectories. “Excellent adherers with CBI” were significantly more satisfied with the CBI counselor.
Conclusions
Patterns of treatment adherence appear to be a participant characteristic. Individuals who fail to adhere early in treatment have worse outcomes regardless of treatment. However, treatment outcomes of participants who exhibit early problems with adherence to one treatment modality could potentially be improved by offering an alternative behavioral or pharmacologic treatment.
Keywords: medication adherence, compliance, naltrexone, acamprosate, therapy, side effects
1. Introduction
The COMBINE study was designed to assess the benefits of combining pharmacological treatment (naltrexone, acamprosate) and behavioral interventions (Medication Management (MM), Pettinati et al., 2004; the Combined Behavioral Intervention (CBI), Miller, 2004). The primary analyses (Anton et al., 2006) revealed that either naltrexone (+ MM) or CBI (+ placebo naltrexone + MM) improved outcomes compared to MM + placebo but there was no additional advantage of combining CBI with naltrexone. This finding was surprising because prior research had suggested added benefit of the combination (O’Malley et al., 1992; Heinala et al., 2001; Anton et al, 2005). Differences in treatment adherence with the combination of treatments and with the mono-therapies may provide an explanation for the lack of additive effect of the active treatments.
Complex pharmacotherapy dosing strategies are often associated with poorer treatment adherence (Claxton et al., 2001; Weiss, 2004). Zweben et al. (2008) found that the acamprosate + naltrexone group took significantly lower percent of the total prescribed pills than the naltrexone group and the placebo group. Acamprosate was also associated with lower treatment adherence than placebo in subjects who did not take naltrexone. These findings were in contrast with the primary adherence results evaluating main effects on summary adherence measures that did not find significant effects (Anton et al., 2006).
Unquestionably, higher levels of treatment participation have been shown to be associated with better outcomes (Volpicelli et al., 1997; Mattson et al. 1998; Chick et al., 2000). In COMBINE, more MM visits attended (Ernst et al., 2008) and medication adherence (defined as 80% or more of the total prescribed pills taken, Zweben et al., 2008) were associated with better drinking outcomes. Adherers had a significantly longer delay to the first day of heavy drinking compared to the non-adherent group if they did not receive CBI and received placebos; whereas there was not a significant difference between the adherent and non-adherent subjects on placebo if they received CBI (Zweben et al., 2008). The addition of CBI did not alter the differences between adherent and non-adherent groups who received naltrexone – the degree of naltrexone exposure was more influential.
While the study by Zweben et al. (2008) provides important insight about medication adherence, it uses a combined measure of adherence with naltrexone and acamprosate which does not provide information about adherence with either medication separately and the analysis uses traditional summary measures of adherence which mask temporal trends. In general, little work has examined temporal patterns of treatment participation with the exception of the well documented finding that dropout from treatment occurs early for many (Carroll, 1997). Since the same mean adherence could reflect early good adherence followed by poor adherence, or moderate adherence throughout, and since these patterns of adherence can have different effects on the outcome, it is important to analyze the temporal trends of adherence. Unlike predefined cutoffs based on historical data (e.g. 80% of pills taken, Zweben et al., 2008), a data-driven approach based on all longitudinal data on adherence can suggest cutoffs with greater discriminating power between subjects and can detect additional patterns of adherence with different treatment outcomes.
In the current study we used trajectory modeling of daily reports of medication adherence and of biweekly measures of treatment participation in CBI to better understand treatment effects in COMBINE. We hypothesized that there would be at least three adherence trajectories: “excellent adherers”, “progressive non-adherers”, and “non-adherers” for each treatment, that a trajectory of excellent adherence throughout would be associated with good treatment response across treatments and that combined treatments would yield less favorable treatment participation trajectories. We also hypothesized that the differences between active drug and placebo would be smaller among non-adherers or progressive non-adherers because of low drug exposure. We also explored patient characteristics to identify factors that may be related to different trajectories of adherence. The results of these analyses may suggest approaches to improving adherence in order to ultimately improve treatment outcome.
2. Methods
This is a secondary analysis of the COMBINE study (The Combine Study Group, 2001–2004; Anton et al., 2006) which enrolled 1,383 abstinent alcohol dependent patients across 11 US academic sites between January 2001 and January 2004. Participants’ median age was 44 years, 71% had at least 12 years of education, and 42% were married. Ethnic minorities comprised 23% of the sample. In addition to meeting diagnostic criteria for alcohol dependence, eligible participants were required to meet the following drinking criteria: an average weekly minimum of 14 drinks (females), or 21 drinks (males) with a minimum of two heavy drinking days (four or more drinks for females and five or more drinks for males per drinking day) within a 30-day period in the 90 days prior to the baseline screening; and abstinence for at least 96 consecutive hours but no more than 21 days prior to randomization. Briefly, exclusion criteria included psychiatric illness requiring medication; current drug dependence other than marijuana or nicotine; current need for or abuse of opioids, pregnancy and medical illnesses that posed safety concerns. Additional specific inclusion and exclusion criteria can be found in the main report (Anton et al., 2006) and additional information about the study methods and procedures is available in the publication by the Combine Study Research Group (2003). Eight groups (n=1226) received MM and either placebos, naltrexone, acamprosate, or naltrexone + acamprosate. Half of these groups also received the CBI. A ninth group that received CBI alone with no pills in order to examine placebo effects in secondary analyses is not included in this report. Medication adherence was assessed using pill counts at scheduled visits.
2.1. Medication adherence
Patients received their study medications during their MM sessions (Pettinati et al., 2004) scheduled to occur at weeks 0, 1, 2, 4, 6, 8, 10, 12 and 16. Naltrexone or its placebo was given once per day as 2 pills titrated up to 100mg per day. Acamprosate or its placebo was administered as 2 pills (500mg each) three times per day. Participants were required to return their used medication blister packs at each MM visit for a tablet count and discussion of adherence. Medication adherence was obtained for each day of the 16-week double-blind medication trial using Timeline Follow-back procedures (i.e., using self-report combined with review of returned blister packs). We coded an individual as adherent if they took one or more doses of that medication on a particular day.
2.2. Combined Behavioral Intervention adherence
CBI was designed to be a state-of-the-art individual outpatient psychotherapy for alcohol dependence (Miller et al., 2004). CBI merges well-supported treatment methods into an integrated approach using aspects of Cognitive Behavioral Therapy (Kadden et al., 1995), Twelve-Step Facilitation (Nowinski et al., 1992), Motivational Interviewing (Miller et al., 1992) and support system involvement (Azrin et al., 1982; Meyers and Smith, 1995). The number, frequency, and duration of CBI treatment sessions were negotiated between therapist and patient, within the bounds of a minimum of 12 sessions and a maximum of 20 sessions with a final visit at 16 weeks. CBI was delivered by licensed behavioral health specialists with at least master’s degrees in psychology, social work or counseling in individual sessions. Participants attended a median of 10 sessions and typically these sessions coincided with MM sessions to reduce participant burden. We created binary measures of CBI adherence for the following assessment periods (week 0–2, 3–4, 5–6, 7–8, 9–10, 11–12 and 13–16). During each of these periods each patient was expected to have attended at least one CBI appointment, hence the binary variable is 1 if they attended one or more CBI sessions in a particular period and 0 otherwise.
2.3. Reasons for Medication Non-adherence
Subjects were asked at all MM appointments about medication adherence. If they missed taking any pills, the primary reason for non-adherence was documented using the Medication Non-compliance Checklist Form developed for the COMBINE Study. The 21 possible response options were coded into six categories by two of the authors (SSO, DD): Forgetting (e.g., “forgot to take medications”, “ran out of pills”); Adverse Events (i.e., “physical side effects”, “mental side effects”); Perceived Benefit (e.g., “thought taking placebo”, “thought taking active medication but not helping”); Expectations (“meds not best treatment for alcoholism”, “uneasy about pill taking”); Medication regimen (“too many pills to take at one time”, “too many times each day to take pills”); and Alcohol-related intentional non-adherence (“wanted to drink or take illicit drugs and not mix pills”, “wanted to test the need for medications”). If a subject endorsed a particular reason for non-adherence for any period between MM visits during treatment he/she was coded as 1 for this reason for the entire treatment period.
2.4. Baseline characteristics
The baseline characteristics (described below) were selected based on a priori expectations of their relevance to adherence trajectories. They also had fairly complete data (more than 85% complete) and meaningful statistical distributions (categorical variables had cell counts of at least 5 in each cell and continuous variables had approximately normal distributions).
Demographic variables included sex (male, female), age, age of onset of alcohol dependence, marital status (married, not married), education (more than high school, high school or less), race (Black, Hispanic, Other, White), smoking status (current non-smoker, current smoker), employment status (employed, not employed), family history of alcoholism (yes, no).
Drinking behavior variables derived from the Form 90 (Miller, 1996) at baseline included drinks per drinking day, percent abstinent days, percent heavy drinking days and days of abstinence prior to randomization. Information about prior inpatient treatment or alcohol detoxification medications, legal history, and mental health problems during the prior 90 days was obtained on the Form90; history of alcohol withdrawal symptoms was obtained from the SCID-IV Alcohol Module (First et al., 1997); and current symptoms were obtained using the Clinical Withdrawal Assessment Scale-AR (Sullivan et al., 1989). Other clinical assessments included the Alcohol Dependence Scale (ADS; Skinner and Allen, 1982), the total score of the Drinker Inventory of Consequences (DrInC; Miller et al., 1995), the total score of Obsessive Compulsive Drinking Scale (Anton et al., 1995), the University of Rhode Island Change Assessment (URICA; McConnaughy, et al., 1983) and the Alcohol Abstinence Self-Efficacy total score (AASE; DiClemente, et al., 1994). Past history of any alcohol treatment and expectations about whether medications or behavioral treatment would be helpful were obtained from the Treatment Expectations and Experiences Questionnaire (TEE), which was developed for the COMBINE Study. Commitment to Abstinence was determined from the treatment goal question from the Thoughts about Abstinence Scale (Hall et al., 1990). A binary variable was computed based on the response “I want to quit using alcohol once and for all, to be totally abstinent, and never use alcohol ever again for the rest of my life” versus all others. Participants completed these assessments at one of the intake appointments (Combine Research Group, 2003).
2.5. Drinking Outcomes
Post-randomization drinking outcomes included percent days abstinent (PDA) and percent heavy drinking days (PHDD; heavy drinking was defined as four or more drinks for females and five or more drinks for males per drinking day) during the 16-week treatment period. These two variables were created based on the daily drinking data collected using the Timeline Follow Back Interview (TLFB) at each visit. The TLFB is the most comprehensive self-report measure and has good reliability and internal consistency on summary drinking measures (Sobell and Sobell, 1992, 1995).
2.6. Self-reported Benefit, Side Effects and Treatment Satisfaction
At the end of treatment, participants completed a question that asked them to describe their experience taking the medications. There were six response options that incorporated three levels of unwanted side effects (no unwanted side effects, some unwanted side effects, and a lot of unwanted side effects) and two levels of benefit (benefitted from taking the medications, did not benefit from taking the medications). Two variables were constructed from this question: Benefit and Side Effect Severity.
Participants also answered three questions regarding satisfaction with the medication therapy including satisfaction with the medications, the health care professional and the number of appointments with the health care professional. Each of these questions was coded as “very satisfied” vs. all other possible answers (e.g. “somewhat dissatisfied”, “not satisfied”) and the association of these questions with adherence trajectory membership were assessed.
Similar questions were asked regarding satisfaction with CBI including satisfaction with the counseling, the counselor, and the number of counseling sessions. Each of these questions was coded as “very satisfied” vs. all other possible answers (e.g. “somewhat dissatisfied”, “not satisfied”) combined in one category.
2.7. Analyzable sample and missing data
We excluded subjects for whom all drinking data were missing (n=6), all medication adherence data were missing (n=10), and daily medication adherence data could not be aligned with daily drinking data (n=36). Our final sample size was 1174 subjects. We also coded missing daily medication adherence data and missing biweekly CBI and MM adherence as non-adherence. All analyses regarding CBI adherence were restricted to the half of the sample that received CBI.
2.8. Trajectories of adherence
We used the approach of Nagin (1999) to identify adherence trajectories during the 16 weeks of the study. We considered naltrexone, acamprosate and CBI adherence separately. The models assumed fixed polynomial trends over time within each trajectory. The final models were obtained via model selection (number of trajectory classes and degree of the polynomial trends over time such as linear, quadratic, cubic) based on the Schwartz Bayesian criterion (BIC) and on having at least 5% of subjects in each trajectory class. Cubic models fit best for medication adherence while quadratic models fit best for CBI adherence. This modeling strategy allowed the data to guide the choice of the number of trajectories that best fit the data and to determine the shape of each trajectory. It also allowed estimation of the proportion of the population whose compliance corresponds most closely to each trajectory group. Classification accuracy was assessed using the entropy measure (Muthén, 2004) with values close to 1 indicating excellent classification of individuals in trajectory classes. For the analysis we used SAS PROC TRAJ (Jones et al., 2001). Posterior probabilities of membership in trajectory class were used to assign individuals to classes and to assess association among trajectory classes for adherence with each component of treatment (naltrexone, acamprosate, CBI). Weighted kappa measures assessed agreement among classes.
2.9. General modeling strategy
Backward elimination generalized logistic models were used to assess treatment effects on adherence trajectories, relationship between baseline characteristics and adherence trajectories, and relationship between side effects, treatment benefit, reasons for non-compliance and adherence trajectories. Interactions among predictor variables were considered whenever possible. Non-significant interactions and main effects were dropped from the models one at a time so that at each step the models were hierarchically well-formulated. Significance cutoff alpha=0.05 was used in all models. Similarly, backward elimination general linear models starting with all possible interactions among naltrexone, acamprosate, CBI and adherence trajectory were used to assess the predictive and moderating effects of adherence trajectories on percent days abstinent (PDA) and percent heavy drinking days (PHDD) during treatment (baseline to week 16). Pairwise comparisons were performed at Bonferroni corrected level of 0.01.
3. Results
3.1. Trajectories of adherence
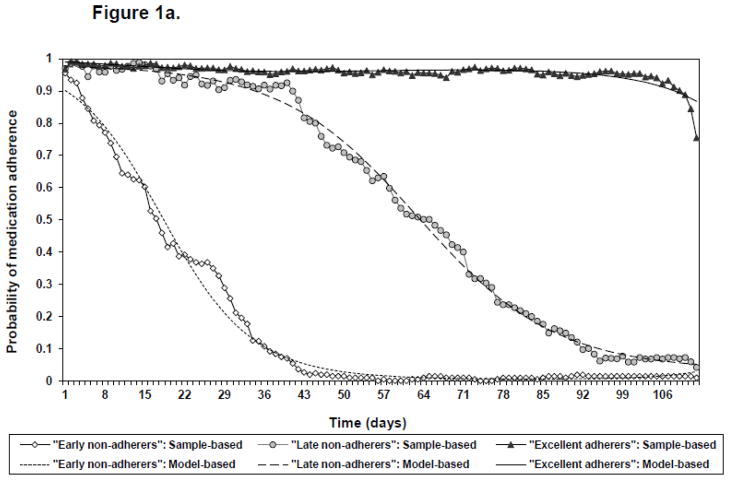
Three trajectory patterns with similar shapes over time were identified for all treatments. Agreement was excellent for adherence with naltrexone and acamprosate (weighted kappa = 0.97) and hence all subsequent analyses of medication adherence focused on naltrexone adherence (Figure 1A). The three medication adherence trajectories are “adherers with medication” (63.3%), “late non-adherers with medication” (18.5%) and “early non-adherers with medication” (18.2%). Median number of days subjects took pills were 110 days (minimum = 66, maximum = 112, interquartile range (IQR) = 106 to 112) for “adherers with medication”, 61 days (min. = 37, max. = 91, IQR = 51 to 75) for “late non-adherers with medication” and 17 days (min. = 1, max. = 41, IQR = 10 to 29) for “early non-adherers with medication”. Classification accuracy was excellent (entropy = 0.99) and average latent class probabilities confirmed the excellent separation of the identified classes (Table 1).
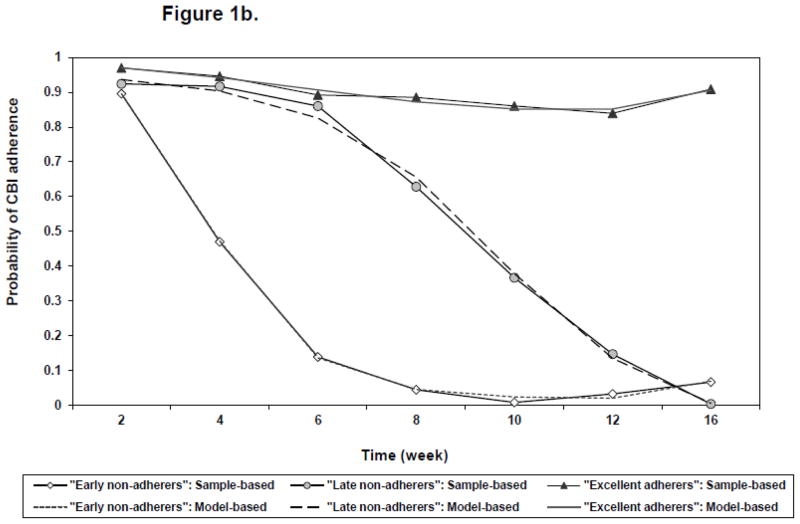
Figure 1.
Figure a: Three trajectories of medication adherence. a
Figure b: Three trajectories of CBI adherence. a
a Solid lines with symbols represent sample-based probabilities of adherence based on all subjects weighted by the posterior probability of trajectory membership. Dashed lines without symbols represent model-based probabilities of adherence over time for each trajectory group.
Table 1.
Average latent class probabilities in medication adherence trajectories.
| Trajectory Classification | Average probability T1: Early non-adherers |
Average probability T2: Late non-adherers |
Average probability T3: Adherers |
|---|---|---|---|
| T1: Early non-adherers | 0.997 | 0.003 | 0 |
| T2: Late non-adherers | 0.007 | 0.990 | 0.004 |
| T3: Adherers | 0 | 0.001 | 0.999 |
Agreement was good for medication adherence and adherence with CBI (weighted kappa = 0.58). The three groups of CBI adherence will be referred to as “adherers with CBI” (77.0%), “late non-adherers with CBI” (10.9%) and “early non-adherers with CBI” (12.1%) (Figure 1B). Classification accuracy was excellent (entropy =0.89) and average latent class probabilities showed very good separation of the identified classes (Table 2).
Table 2.
Average latent class probabilities in CBI adherence trajectories.
| Trajectory Classification | Average probability T1: Early non-adherers |
Average probability T2: Late non-adherers |
Average probability T3: Adherers |
|---|---|---|---|
| T1: Early non-adherers | 0.941 | 0.048 | 0.012 |
| T2: Late non-adherers | 0.112 | 0.774 | 0.115 |
| T3: Adherers | 0.001 | 0.013 | 0.986 |
3.2. Treatment effects on adherence trajectories
There were only significant main effects of naltrexone and acamprosate on medication adherence. Subjects on naltrexone were significantly less likely to be “excellent adherers with medication” than “early non-adherers with medication” (OR=0.73, 95% CI: (0.54, 0.99)). Subjects on acamprosate were significantly less likely to be “excellent adherers with medication” than “early non-adherers with medication” (OR=0.68, 95% CI: (0.50, 0.92)) and “late non-adherers with medication” (OR=0.69, 95% CI: (0.51, 0.94)). There were no significant effects for the association between any of the treatments and CBI adherence. Furthermore, there were no significant interactions between treatments on adherence trajectories and hence we did not find sufficient evidence that combined treatments were associated with higher likelihood of progressive non-adherence than the mono-therapies.
3.3. Predictive and moderating effects of adherence trajectories on drinking outcomes
3.3.1. Medication Trajectories
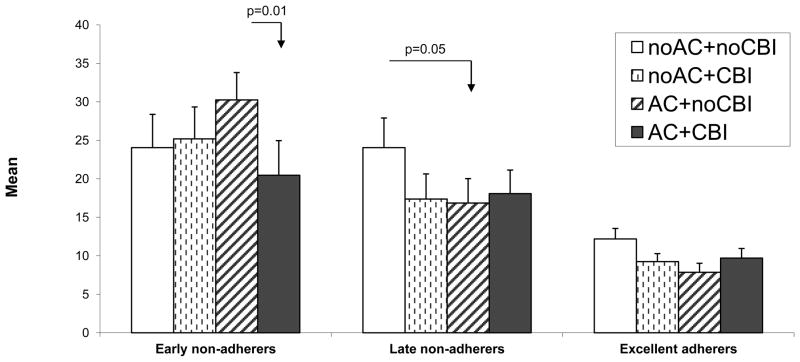
There were main effects of medication adherence trajectories on both PDA and PHDD (both p<0.0001) and a significant three-way interaction between medication adherence trajectory, acamprosate and CBI for PHDD (F(2,1158)=3.39, p=0.03, Figure 2). The “late non-adherers with medication” and “early non-adherers with medication” had significantly lower PDA and higher PHDD than the “adherers with medication” (all p-values < 0.0001). Also, “early non-adherers with medication” had significantly lower PDA and higher PHDD than “late non-adherers with medication” (p=0.002 and p=0.003 respectively). “Late non-adherers with medication” had significantly higher PHDD than “adherers with medication” in the following treatment conditions: 1) no CBI, placebo acamprosate (p = 0.004) and 2) CBI, placebo acamprosate (p = .009). CBI significantly decreased PHDD for “early non-adherers with medication” on acamprosate (p=0.01, Figure 2).
Figure 2.
Percent heavy drinking days by treatment and medication adherence trajectories.
3.3.2. CBI Trajectories
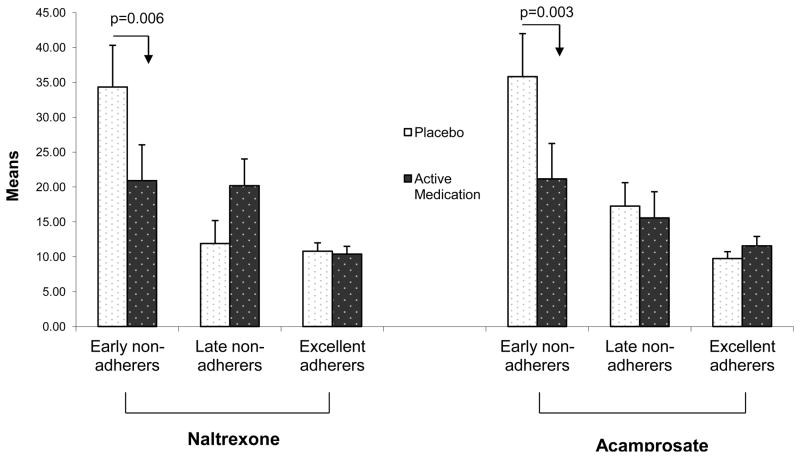
There were main effects of adherence trajectories with CBI on both drinking measures (p<0.0001). The “late non-adherers with CBI” and “early non-adherers with CBI” had significantly worse drinking outcomes than the “adherers with CBI” (p-values < 0.003). There was a significant two-way interaction between naltrexone and adherence with CBI and a significant two-way interaction between acamprosate and adherence with CBI on PHDD (F(2,583)=5.28, p=0.005 and F(2,583)=5.03, p=0.007 respectively). For “early non-adherers with CBI”, PHDD was significantly lower for subjects on naltrexone compared to placebo (p = .006) or for those who received acamprosate compared to placebo (p = .003, Figure 3).
Figure 3.
Percent heavy drinking days by treatment and CBI adherence trajectories
3.4. Reasons for non-adherence
The most frequently endorsed reason for medication non-adherence was forgetting to take medication (95% of “medication adherers”, 86% of “late non-adherers” and 63% of “early non-adherers” reported some non-adherence, p<.0001, Table 3). The second most-frequently endorsed reason was side effects (40% of “early non-adherers”, 28% of “late non-adherers” and 12% of “adherers”, p<.0001). Treatment expectancies were endorsed as reasons for non-adherence by 8% of the “early non-adherers”, 5% of “late non-adherers” and only 1% of the “adherers” (p<0.0001). Benefit was also associated with adherence membership category (p=0.02) but number of pills was not.
Table 3.
Stated reasons for medication non-compliance by medication adherence trajectory.
| Reason | Overall (n=955) | T1: Early non-adherers (n=145) | T2: Late non-adherers (n=195) | T3: Adherers (n=615) | p-value |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Forgetting | 843(88.27) | 91(62.76) | 167(85.64) | 585(95.12) | <.0001 |
| Adverse Events | 184(19.27) | 58(40) | 54(27.69) | 72(11.71) | <.0001 |
| Benefit | 47(4.92) | 11(7.59) | 15(7.69) | 21(3.41) | 0.015 |
| Expectancies | 29(3.04) | 12(8.28) | 9(4.62) | 8(1.3) | <.0001 |
| Number of pills | 31(3.25) | 7(4.83) | 9(4.62) | 15(2.44) | 0.17 |
| Other intentional nonadherence | 89(9.32) | 17(11.72) | 32(16.41) | 40(6.5) | 0.0001 |
Stated reasons for medication no-adherence were based on responses on the Medication Non-Compliance Checklist:
Adverse events = 1 if subject reported physical or mental side effects, 0 otherwise
Benefit = 1 if subject believed being cured, reported being impatient waiting for meds to work so took more than prescribed, thought taking active medication but not helping, or thought taking placebo; 0 otherwise
Forgetting = 1 if subject forgot to take medications, didn’t have access to medications, ran out of pills, lost pills or misunderstood instructions; 0 otherwise
Number of pills = 1 if subject reported that there are too many pills to take at one time or too many times each day to take pills; 0 otherwise
Expectancies = 1 if subject reported misconception about medications, being uneasy about pill taking, medications not best treatment for alcoholism or medications best for severe alcoholism but own case not severe enough; 0 otherwise
Other Intentional Non-adherence = 1 if subject wanted to drink or take illicit drugs and not mix pills or wanted to test need for medications; 0 otherwise
3.5. Predicting adherence trajectories from baseline characteristics
After backward elimination only age (χ2(2)=15.14, p=0.0005), alcohol dependence symptoms (χ2(2)=7.92, p=0.02) and percent heavy drinking days at baseline (χ2(2)=10.51, p=0.005) were significantly associated with medication adherence (Table 4). Similarly, only age (χ2(2)=12.9, p=0.0002) and drinks per drinking day (χ2(2)=8.43, p=0.01) were significantly associated with CBI adherence trajectories. Younger age and more severe alcohol dependence were associated with higher non-adherence.
Table 4.
Odds ratios and 95% confidence intervals for the relationship between baseline characteristics and membership in adherence trajectories.
| Naltrexone adherence: | “Early non-adherers” vs. “Adherers” | “Late non-adherers” vs. “Adherers” | “Early non-adherers” vs. “Late non-adherers” |
|---|---|---|---|
| Age (older vs. younger) | 0.971 (0.954, 0.989)* | 0.973 (0.956, 0.991)* | 0.998 (0.976, 1.021) |
| Alcohol dependence symptoms: (more vs. less) | 1.210 (1.045, 1.400)* | 1.135 (0.982, 1.311) | 1.066 (0.889, 1.278) |
| Percent days heavy drinking (more vs. less) | 0.992 (0.986, 0.999)* | 1.005 (0.999, 1.011) | 0.987 (0.980, 0.995)* |
| CBI adherence: | “Early non-adherers” vs. “Adherers” | “Late non-adherers” vs. “Adherers” | “Early non-adherers” vs. “Late non-adherers” |
|---|---|---|---|
| Age (older vs. younger) | 0.969 (0.939, 0.999)* | 0.949 (0.920, 0.980)* | 1.02 (0.980, 1.062) |
| Drinks per drinking day: (more vs. less) | 1.029 (0.997, 1.063) | 1.042 (1.012, 1.072)* | 0.988 (0.954, 1.023) |
Odds ratios denoted by stars correspond to significant effects at 0.05 level.
3.6. Comparison of reports of side effects and treatment benefit
Both reported benefit (χ2(2)=70.0, p<.0001) and experience of side effects (χ2(2)=49.3, p<.0001) were significantly associated with medication adherence (Table 5) but the interaction was not significant. “Early non-adherers” reported more side effects (59 out of 98, or 60.2% of subjects reported at least some side effects) and lack of benefit (54 out of 98, 55.1% reported lack of benefit). “Late non-adherers” compared to “adherers” reported significantly more often side effects (71 out of 139 or 51.1% vs. 296 out of 727 or 40.7%) and no benefit (41 out of 139 or 29.5% vs. 105 out of 727 or 14.4%). “Late non-adherers” compared to “adherers” were also significantly less satisfied with medication and number of sessions. “Early non-adherers” were significantly less satisfied with their health care professional than subjects in the other trajectories.
Table 5.
Odds ratios and 95% confidence intervals for the relationship between reported benefit, side effects and membership in adherence trajectories.
| Naltrexone Adherence | “Early non-adherers” vs. “Adherers” | “Late non-adherers” vs. “Adherers” | “Early non-adherers” vs. “Late non-adherers” |
|---|---|---|---|
| Benefit: | |||
| “no benefit” vs. “benefit” | 4.92 (2.91, 8.35)* | 2.10 (1.32, 3.26)* | 2.34 (1.27, 4.32)* |
| Side effects: | |||
| “a lot” vs. “none” | 10.41 (4.89, 22.16)* | 4.29 (2.03, 9.07)* | 2.43 (1.10, 5.37)* |
| “a lot” vs. “some’ | 5.97 (2.78, 12.84)* | 3.09 (1.45, 6.61)* | 1.93 (0.86, 4.36) |
| “some” vs. “none” | 1.74 (1.04, 2.93)* | 1.39 (0.93, 2.07) | 1.26 (0.69, 2.30) |
| Satisfaction with: | |||
| H|ealth professional: | |||
| “Not very” vs. “Very” | 2.35 (1.38, 4.01)* | 0.81 (0.47, 1.40) | 2.89 (1.50, 5.59)* |
| Number of sessions: | |||
| “Not very” vs. “Very” | 1.06 (0.60, 1.90) | 1.73 (1.12, 2.66)* | 0.62 (0.32, 1.18) |
| Medications: | |||
| “Not very” vs. “Very” | 1.68 (0.87, 3.26) | 1.61 (1.04, 2.49) | 1.05 (0.49, 2.21) |
| CBI adherence: | “Early non-adherers” vs. “Adherers” | “Late non-adherers” vs. “Adherers” | “Early non-adherers” vs. “Late non-adherers” |
|---|---|---|---|
| Benefit: | |||
| “no benefit” vs. “benefit” | 1.90 (0.76, 4.79) | 2.45 (1.23, 4.87)* | 0.78 (0.26, 2.30) |
| Satisfaction with counselor: | |||
| “Not very” vs. “Very | 2.74 (1.12, 6.68)* | 3.54 (1.81, 6.94)* | 0.77 (0.27, 2.21) |
Odds ratios denoted by stars correspond to significant effects at 0.05 level.
Only benefit and satisfaction with counselor were significantly associated with CBI adherence. “Late non-adherers” compared to “adherers” reported no benefit and dissatisfaction with their counselor significantly more often. “Early non-adherers” compared to “adherers” were also significantly less satisfied with their counselor.
4. Discussion
In summary, we identified trajectories reflecting excellent adherence and progressive non-adherence that differed according to timing of when non-adherence occurred. We did not identify a true “non-adherer” trajectory or a “progressive adherer” trajectory. Unlike predefined cutoffs (e.g. 80% of pills taken, Zweben et al., 2008), the trajectory approach uses all temporal information and selects cutoffs with greater discriminating power between subjects. Furthermore, this approach allows us to assess treatment effects in the context of different patterns of adherence. We demonstrated differential treatment outcomes for the three adherence trajectories and interactive effects of adherence trajectories and treatment on drinking outcomes. We further expanded on Zweben’s approach by considering naltrexone and acamprosate adherence separately and assessing agreement of adherence with different aspects of treatment.
We observed excellent agreement between adherence trajectories with naltrexone and with acamprosate and good agreement between trajectories of medication adherence and of adherence with behavioral treatment. Thus, it appears that adherence is a participant-level characteristic that is somewhat different for medication and behavioral treatments. Consistent with this view, adverse events associated with medications predicted medication adherence trajectories but did not predict CBI adherence.
Not surprisingly, active medications were associated with increased chance of membership in the early and late non-adherence trajectories and early non-adherence was associated with adverse events. Given that COMBINE tested a higher than standard dose of naltrexone (100mg vs. 50 mg daily) and of acamprosate (3 g vs. 2 g daily), lower doses could be considered to minimize adverse events and perhaps improve adherence. Perceived benefit was also related to medication non-adherence, although the relationship was not as strong as observed with adverse events. Notably, there was no interaction between side effects and reported benefit in the assessment of their association with adherence trajectories. Thus side effects and perceived benefit appear to affect medication adherence independently and both should be targeted in order to improve adherence and outcome.
Interestingly, adverse events did not predict CBI adherence. Instead, satisfaction with the CBI therapist was the strongest predictor. Less satisfaction with the medical care professional also predicted early non-adherence to medications. These findings are consistent with an extensive literature indicating that therapist effects, including therapeutic alliance, are a major determinant of treatment response in alcohol dependence (Project MATCH Research Group, 1993; Connors, et al., 1997; Meier, et al., 2005; Fuertes et al., 2006; Ernst et al., 2008). Similarly, the literature indicates that the therapeutic alliance between patient and provider may mediate adherence to and the outcomes of both behavioral and pharmacological interventions for alcohol, depression, and other psychiatric disorders (Krupnick et al., 1996; Dundon et al., 2008; Zeber et al., 2008; Byrne and Deane, 2011). Our data suggest that therapist effects may have an early effect on adherence to treatment.
As expected, excellent adherence was associated with good treatment response. We further demonstrated that early non-adherence was associated with significantly worse drinking outcomes than late non-adherence potentially due to lower involvement in beneficial aspects of treatment. We had also hypothesized that drug placebo differences would be smaller among progressive non-adherers. In contrast, we observed significant treatment effects for “early non-adherers”. Interestingly, for “early non-adherers with medication”, receiving CBI was associated with lower PHDD on acamprosate while for “early non-adherers with CBI” receiving either medication treatment was associated with lower PHDD. This suggests that patients who are unable to adhere with a particular treatment perhaps due to adverse events in the case of medications or due to dissatisfaction with their therapist in the case of CBI can still improve their outcome if they receive the alternative treatment. Thus our results have implications for the design of future studies on the effectiveness of switching or augmenting treatments. Early non-adherence might provide an early guidepost for switching treatments and inadequate response might continue to provide a later flag pointing to need to augment or switch therapies.
Our a priori hypothesis that combined treatments would yield less favorable treatment participation trajectories was not confirmed by our analysis. The combination treatments were not associated with higher chance of early or late non-adherence than the mono-therapies. Thus it seems unlikely that the failure of the original analyses of the COMBINE data to demonstrate increased effectiveness of the combination of treatments can be explained by decreased adherence.
Our finding of protective effect of CBI on PHDD among “early non-adherers with medication” who did not get acamprosate treatment is similar to the finding of Zweben et al. (2008) on time to first heavy drinking day. Also, similar to Zweben et al. (2008) differences in outcome were strongest between “early non-adherers with medication” and “adherers” in the absence of CBI. However, there were no significant interactions between naltrexone, CBI and adherence in our analyses. The differences in the discovered interactions may be due to the different strategies of categorizing adherence, to consideration of different outcomes and to consideration of adherence with single medication vs. adherence with both medications together.
Only a few pretreatment characteristics predicted adherence. Not surprisingly older age and lower severity of alcohol related problems were associated with better medication adherence. Since adverse events and perceived benefit reported during treatment were associated with adherence, future studies might consider obtaining a pretreatment measure of an individual’s medication adherence tendencies, such as the Medication Adherence Questionnaire (Morisky et al, 1986; Toll et al., 2007). Other reasons for non-adherence were less positive expectations about the medications and intentional non-adherence related to wanting to use alcohol or to test the need for medications. In these instances, patient education about the role of medications and their safety in combination with alcohol may be of benefit. Alternatively, medications with long duration of action could be considered as one means to reduce the influence of momentary increases in motivation to drink.
Our study has several limitations. First, our analyses are exploratory and need external validation. Second, despite adjustments it is possible that type I error may be inflated. Third, the trajectory-based analyses may lead to spurious findings if the population is homogeneous rather than heterogeneous. Fourth, the COMBINE Study had strict inclusion/exclusion criteria and study procedures, thus the results may not generalize to more complicated patients, to other medications or to treatment provided in more general practice settings.
Different trajectories may have occurred if we had electronic means of monitoring adherence (e.g. Krystal et al., 2001)), considered other medications such as disulfiram (Fuller et al., 1986) or focused on different patient populations. In particular, a “non-adherence” trajectory is expected to be observed in clinical practice where total non-adherence occurs because individuals prescribed a medication never fill their prescription (Osterberg and Blashke, 2005). In COMBINE, however, medication was dispensed at the first appointment and at no cost to participants. In addition, participants had volunteered to participate in a medication trial and the medication management therapy was designed to support and encourage adherence. While these factors limit the generalizability of the results, clinician support, monitoring of adherence and efforts to minimize barriers to adherence all make for good clinical practice.
In summary, as expected excellent adherence to medications or to behavioral interventions was associated with better treatment outcomes. In COMBINE, early non-adherence among alcohol dependent individuals was associated with the greatest risk of poor outcomes. Early non-adherers were younger suggesting that they may represent a target for adherence enhancing interventions (Reid et al., 2005; Heffner et al., 2010). Our approach highlights the potential value of combining medication and psychosocial treatment early in treatment. Specifically, the negative impact of early non-adherence to CBI was attenuated by medication and CBI mitigated the impact of early medication non-adherence. In addition to providing patients with information specific to the medications involved and exploring patients’ expectations about these medications, efforts to enhance adherence to medication and behavioral interventions should also be placed on clinician training to implement strategies that enhance the therapeutic alliance (Byrne and Deane, 2011).
Highlights.
Three adherence patterns with medication/behavioral treatment were identified.
Early non-adherers had worse drinking outcomes than late non-adherers and adherers.
The negative impact of early non-adherence to CBI was attenuated by medication.
CBI mitigated the impact of early non-adherence to medication on drinking outcome.
Negative outcomes of early non-adherers can be improved by an alternative treatment.
Acknowledgments
Role of Funding Sources: This work was supported by the National Institute on Alcohol Abuse and Alcoholism [R01AA017173, P50 AA012870, K05 AA014715, K05 AA14906], the State of Connecticut, Department of Mental Health and Addiction Services, and the following U.S. Veterans Administration Centers: Alcohol Research Center and National Center for PTSD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The author(s) acknowledge(s) that the reported results are, in whole or in part, based on analyses of the COMBINE Data Set. These data were collected as part of a multisite clinical trial of alcoholism treatments supported by a series of grants from the National Institute on Alcohol Abuse and Alcoholism, NIH, DHHS. This paper has not been reviewed or endorsed by NIAAA or the COMBINE Research Group and does not necessarily represent the opinions of its members or NIAAA, who are not responsible for the contents.
Footnotes
Contributors: Authors Gueorguieva and O’Malley designed the study, prepared the literature overview, evaluated results from statistical analyses and wrote the first draft of the manuscript. Authors Wu and Gueorguieva undertook the statistical analyses. Authors Donovan and Krystal participated in review of the results from statistical analyses, data interpretation and manuscript revisions. All authors contributed to and have approved the final manuscript.
Conflict of Interest: Dr. Krystal has served as a scientific consultant and/or on the Scientific Advisory Board to the following companies: Abbot Laboratories, Aisling Capital, LLC, Astellas Pharma Global Development, Inc, AstraZeneca Pharmaceuticals, Biocortech, Brint & Nicolini, Inc, Bristol-Myers Squibb, Easton Associates, Eisai, Inc, Eli Lilly and Co, Forest Laboratories, Inc, Gilead Sciences, Inc, GlaxoSmithKline, Janssen Pharmaceuticals, LoHocla Research Corporation, Lundbeck Research USA, Medivation, Inc, Merz Pharmaceuticals, MK Medical Communications, Mnemosyne Pharmaceuticals, Inc, Naurex, Inc, Pfizer Pharmaceuticals, F. Hoffmann-La Roche Ltd, SK Holdings Co., Ltd, Shire Pharmaceuticals, Sunovion Pharmaceuticals, Inc, Takeda Industries, Teva Pharmaceutical Industries, Ltd and Tetragenex Pharmaceuticals. He is a co-sponsor for two patents under review for glutamatergic agents targeting the treatment of depression. He has the following patent: Seibyl JP, Krystal JH, Charney DS. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia.
Dr. Stephanie O’Malley: member American College of Neuropsychopharmacogy workgroup, the Alcohol Clinical Trial Initiative, sponsored by Abott Laboratories, Alkermes, Eli Lilly & Company, GlaxoSmithKline, Johnson & Johnson Pharmaceuticals, Lundbeck and Schering Plough; partner, Applied Behavioral Research; contract, Nabi Biopharmaceuticals; medication donations, Pfizer, Inc.; Advisory Board, Gilead Pharmaceuticals, Lundbeck; consultant, Alkermes, GlaxoSmithKline, Brown University, University of Chicago, Scientific Panel of Advisors, Hazelden Foundation.
All other authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: A self rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcoholism: Clinical and Experimental Research. 1995;19:92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham P, Waid LR, Myrick H, Voronin K, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. Journal of Clinical Psychopharmacology. 2005;25(4):349–357. doi: 10.1097/01.jcp.0000172071.81258.04. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo D, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. Journal of the American Medical Association. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Azrin NH, Sisson RW, Meyers R, Godley M. Alcoholism treatment by disulfiram and community reinforcement therapy. Journal of Behavior Therapy and Experimental Psychiatry. 1982;13(2):105–112. doi: 10.1016/0005-7916(82)90050-7. [DOI] [PubMed] [Google Scholar]
- Byrne MK, Deane FP. Enhancing patient adherence: outcomes of medication alliance training on therapeutic alliance, insight, adherence, and psychopathology with mental health patients. International Journal of Mental Health Nursing. 2011;20(4):284–295. doi: 10.1111/j.1447-0349.2010.00722.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM. Enhancing retention in clinical trials of psychosocial treatments: Practical strategies. In: Onken LS, Blaine JD, Boren JJ, editors. Beyond the Therapeutic Alliance: Keeping the Drug Dependent Individual in Treatment. NIDA Research Monograph Series, Monograph No. 165, NIH Pub No. 97–4142. 1997. pp. 4–24. [PubMed] [Google Scholar]
- Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, et al. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol and Alcoholism. 2000;35(6):587–593. doi: 10.1093/alcalc/35.6.587. [DOI] [PubMed] [Google Scholar]
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication adherence. Clinical Therapeutics. 2001;23(8):1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- The COMBINE Study Research Group. Testing combined pharmacotherapies and behavioral interventions in alcohol dependence: Rationale and methods. Alcoholism: Clinical and Experimental Research. 2003;27:1107–1122. doi: 10.1097/00000374-200307000-00011. [DOI] [PubMed] [Google Scholar]
- Combine Research Group. The Combined Pharmacotherapies and Behavioral Interventions Study (COMBINE) (data files and documentation) Sponsored by the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, DHHS; 2001–2004. [Google Scholar]
- Connors GJ, Carroll KM, DiClemente CC, Longabaugh R, Donovan DM. The therapeutic alliance and its relationship to alcoholism treatment participation and outcome. Journal of Consulting & Clinical Psychology. 1997;65:588–598. doi: 10.1037//0022-006x.65.4.588. [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Carbonari JP, Montgomery RP, Hughes SO. The Alcohol Abstinence Self-Efficacy scale. Journal of Studies on Alcohol. 1994;55(2):141–148. doi: 10.15288/jsa.1994.55.141. [DOI] [PubMed] [Google Scholar]
- Dundon WD, Pettinati HM, Lynch KG, Xie H, Varillo KM, Makadon C, Oslin DW. The therapeutic alliance in medical-based interventions impacts outcome in treating alcohol dependence. Drug and Alcohol Dependence. 2008;95:230–236. doi: 10.1016/j.drugalcdep.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst DB, Pettinati HM, Weiss RD, Donovan DM, Longabaugh R. An intervention for treating alcohol dependence: relating elements of Medical Management to patient outcomes with implications for primary care. Annals of Family Medicine. 2008;6(5):435–40. doi: 10.1370/afm.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (SCID-CV) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism. Journal of the American Medical Association. 1986;256(11):1449–1455. [PubMed] [Google Scholar]
- Fuertes JN, Mislowack A, Bennett J, Paul L, Gilbert TC, Fontan G, Boylan LS. The physician-patient working alliance. Patient Education and Counseling. 2006;66:29–36. doi: 10.1016/j.pec.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW Vivitrex Study Group. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: A randomized controlled trial. Journal of the American Medical Association. 2005;29(13):1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Hall SM, Havassy BE, Wasserman DA. Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. Journal of Consulting and Clinical Psychology. 1990;59:175–181. doi: 10.1037//0022-006x.58.2.175. [DOI] [PubMed] [Google Scholar]
- Heffner JL, Tran GQ, Johnson CS, Barrett SW, Blom TJ, Thompson RD, Anthenelli RM. Combining motivational interviewing with compliance enhancement therapy (MI-CET): development and preliminary evaluation of a new, manual-guided psychosocial adjunct to alcohol-dependence pharmacotherapy. Journal of Studies on Alcohol and Drugs. 2010;71(1):61–70. doi: 10.15288/jsad.2010.71.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinala P, Alho H, Kiianmaa K, Lonnqvist J, Kuoppasalmi K, Sinclair JD. Targeted use of naltrexone without prior detoxification in the treatment of alcohol dependence: a factorial doubleblind, placebo-controlled trial. Journal of Clinical Pharmacology. 2001;21:287–92. doi: 10.1097/00004714-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Jones B, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research. 2001;29:374–393. [Google Scholar]
- Kadden RP, Carroll K, Donovan D, Cooney N, Monti P, Abrams D, Litt M, Hester R. Project MATCH Monograph Series. Vol. 3. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 1995. Cognitive-Behavioral Coping Skills Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence. [Google Scholar]
- Krupnick JL, Sotsky SM, Simmens S, Moyer J, Elkin I, Watkins J, Pilkonis PA. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532–539. doi: 10.1037//0022-006x.64.3.532. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. New England Journal of Medicine. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Mattson ME, Del Boca FK, Carroll KM, Cooney NL, DiClemente CC, Donovan D, et al. Adherence with treatment and follow-up protocols in project MATCH: predictors and relationship to outcome. Alcoholism: Clinical and Experimental Research. 1998;22(6):1328–1339. [PubMed] [Google Scholar]
- McConnaughy EN, Prochaska JO, Velicer WF. Stages of change in psychotherapy: Measurement and sample profiles. Psychotherapy: Theory, Research and Practice. 1983;20:368–375. [Google Scholar]
- Meier PS, Barrowclough C, Donmall MC. The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction. 2005;100(3):304–316. doi: 10.1111/j.1360-0443.2004.00935.x. [DOI] [PubMed] [Google Scholar]
- Meyers RJ, Smith JE. Clinical Guide to Alcohol Treatment: The Community Reinforcement Approach. Guilford Press; New York: 1995. [Google Scholar]
- Miller WR. Form 90: A structured assessment interview for drinking and related behaviors. Vol. 5. Bethesda, MD: U.S. Department of Health and Human Services; 1996. [Google Scholar]
- Miller WR, Zweben A, Diclemente CC, Rychtarik RG. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Vol. 2. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 1992. [Google Scholar]
- Miller WR, Tonigan JS, Longabaugh R. Test manual. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 1995. Drinker Inventory of Consequences (DrInC): An instrument for assessing adverse consequences of alcohol abuse. [Google Scholar]
- Miller WR, editor. Combined Behavioral Intervention manual: A clinical research guide for therapists treating people with alcohol abuse and dependence. Vol. 1. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2004. [Google Scholar]
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- Muthén BO. Mplus Technical Appendices. Los Angeles, CA: Muthén & Muthén; 2004. Appendix 8. [Google Scholar]
- Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychological Methods. 1999;4:139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Nowinski J, Baker S, Carroll K. Twelve step facilitation therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Vol. 1. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 1992. [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS. Naltrexone and coping skills therapy for alcohol dependence: A controlled study. Archives of General Psychiatry. 1992;49(11):881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Osterberg L, Blashke T. Adherence to medication. New England Journal of Medicine. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Weiss RD, Miller WR, Donovan DM, Ernst DB, Rounsaville BJ. Medical Management (MM) treatment manual: A clinical research guide for medically trained clinicians providing pharmacotherapy as part of the treatment for alcohol dependence. Vol. 2. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2004. [Google Scholar]
- Project MATCH Research Group. Therapist effects in three treatments for alcohol problems. Psychotherapy Research. 1998;8:455–474. [Google Scholar]
- Reid SC, Teesson M, Sannibale C, Matsuda M, Haber PS. The efficacy of compliance therapy in pharmacotherapy for alcohol dependence: a randomized controlled trial. Journal of Studies on Alcohol. 2005;66(6):833–841. doi: 10.15288/jsa.2005.66.833. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: Measurement and validation. Journal of Abnormal Psychology. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring alcohol consumption: Psycosocial and biological methods. New Jersey: Human Press; 1992. [Google Scholar]
- Sobell LC, Sobell MB. Alcohol consumption measures. In: Allen JP, Columbus M, editors. Assessing alcohol problems: A guide for clinician and researchers. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1995. pp. 55–73. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: The revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-AR) British Journal of Addictions. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Toll BA, McKee SA, Martin J, Jatlow P, O’Malley SS. Factor structure and validity of the Medication Adherence Questionnaire (MAQ) with cigarette smokers trying to quit. Nicotine and Tobacco Research. 2007;9:597–605. doi: 10.1080/14622200701239662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Rhimes KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP. Naltrexone and alcohol dependence. Role of subject adherence. Archives of General Psychiatry. 1997;54:737–742. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- Weiss RD. Adherence to pharmacotherapy in patients with alcohol and opioid dependence. Addiction. 2004;99(11):1382–1392. doi: 10.1111/j.1360-0443.2004.00884.x. [DOI] [PubMed] [Google Scholar]
- Zeber JE, Copeland LA, Good CB, Fine MJ, Bauer MS, Kilbourne AM. Therapeutic alliance perceptions and medication adherence in patients with bipolar disorder. Journal of Affective Disorders. 2008;107:53–62. doi: 10.1016/j.jad.2007.07.026. [DOI] [PubMed] [Google Scholar]
- Zweben A, Pettinati HM, Weiss RD, Youngblood M, Cox CE, Mattson ME, Gorrochurn P, Ciraulo D. Relationship between medication adherence and treatment outcomes: the COMBINE study. Alcoholism: Clinical and Experimental Research. 2008;32(9):1661–1669. doi: 10.1111/j.1530-0277.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]