Abstract
As a biochemical model, Manduca sexta has substantially contributed to our knowledge on insect innate immunity. The RNA-Seq approach was implemented in three studies to examine tissue immunotranscriptomes of this species. With the latest and largest focusing on highly regulated process- and tissue-specific genes, we further analyzed the same set of data using BLAST2GO to explore functional aspects of the larval fat body (F) and hemocyte (H) transcriptomes with (I) or without (C) immune challenge. Using immunity-related sequences from other insects, we found 383 homologous contigs and compared them with those discovered based on relative abundance changes. The major overlap of the two lists validated our previous research designed for gene discovery and transcript profiling in organisms lacking sequenced genomes. By concatenating the contigs, we established a repertoire of 232 immunity-related genes encoding proteins for pathogen recognition (16%), signal transduction (53%), microbe killing (13%), and others (18%). We examined their transcript levels along with attribute classifications and detected prominent differences in nine of the thirty level 2 gene ontology (GO) categories. The increase in extracellular proteins (155%) was consistent with the highly induced synthesis of defense molecules (e.g., antimicrobial peptides) in fat body after the immune challenge. We identified most members of the putative Toll, IMD, MAPK-JNK-p38, and JAK-STAT pathways and small changes in their mRNA levels. Together, these findings set the stage for on-going analysis of the M. sexta immunogenome.
Keywords: insect immunity, hemolymph proteins, RNA-Seq, gene discovery, transcript profiling
1. Introduction
Insects possess a pristine form of the metazoan antimicrobial defense known as innate immunity (Hultmark, 1993), together with a facet of adaptive immunity via phagocyte-mediated immune memory (Pham et al., 2007). However, they lack the luxury of B and T cell-mediated adaptive immunity found in vertebrates (Agaisse, 2007). Insect immunity, comprising humoral and cellular responses, is rapid and effective in identifying and eliminating invading pathogens and parasites (Brey and Hultmark, 1998; Jiang et al., 2010; Lemaitre and Hoffmann, 2007). The general process of insect immunity, before deploying killing mechanisms, consists of pathogen recognition via specific binding molecules (Kurata et al., 2006; Sansonetti, 2006; Yu et al., 2002), signal transduction and modulation via plasma serine proteinases and serine proteinase inhibitors (Gillespie et al., 1997; Kanost, 1999; Kanost et al., 2001; Marmaras and Lampropoulou, 2009), and receptor-mediated intracellular signaling via Toll (Valanne et al., 2011), IMD (Silverman and Maniatis, 2001), JNK (Ramet et al., 2002a), JAK-STAT (Baeg et al., 2005; Hou and Perrimon, 1997; Kisseleva et al., 2002), and MAPK-JNK-p38 (Han et al., 1998; Ragab et al., 2011) pathways. Signal transduction regulates both humoral and cellular immune responses. The former includes various antimicrobial peptides (AMPs) (Engstrom, 1999; Jiang, 2008), complement-like molecules (Aoun et al., 2011), and proteins involved in enzyme cascades that regulate melanin formation (Jang et al., 2008; Kanost and Gorman, 2008), which are synthesized and released into the plasma to entrap and kill invading pathogens or parasites (Gillespie et al., 1997; Hoffmann, 2003). In contrast, cellular immunity takes place in hemocytes and is comprised of phagocytosis, nodulation, and encapsulation (Fauvarque and Williams, 2011; Lavine and Strand, 2002; Strand, 2008; Zhuang et al., 2005).
Innate immunity plays a role in making insects the most diverse and abundant group of metazoans in the world (Chapman et al., 2006; Hultmark, 2003). This makes the immune system worth investigating in its own right. On the other hand, the common ancestry and similarities among insects and mammals make insects excellent model organisms (Hoffmann and Reichhart, 1997; Hultmark, 1993, 2003). These permit discovering evolutionary roots and features of animal immunity (Hoffmann et al., 1999; Khush and Lemaitre, 2000; Vilmos and Kurucz, 1998) and allow functional comparisons between diverse metazoan systems to identify shared and unique aspects of innate immunity (Khush and Lemaitre, 2000; Rolff and Reynolds, 2009; Wajant and Scheurich, 2004).
The advent of microarrays and next generation sequencing technologies coupled with bioinformatics tools has generated a large amount of immunotranscriptome data from insects with known genome sequence, such as Drosophila sp. (De Gregorio et al., 2001; Irving et al., 2001; Sackton et al., 2007), Anopheles gambiae (Christophides et al., 2002), Apis mellifera (Evans et al., 2006), Aedes aegypti (Waterhouse et al., 2007), Tribolium castaneum (Zou et al., 2007), Bombyx mori (Tanaka et al., 2008), and Acyrthosiphon pisum (Gerardo et al., 2010). Most of the immunotranscriptomic studies so far, for insects without sequenced genomes, lack quantitative levels of transcripts (Altincicek and Vilcinskas, 2007; Vogel et al., 2011; Zhang et al., 2010). As a member of economically important lepidopterans, Manduca sexta has been studied extensively in the field of insect physiology for decades (Jiang et al., 2010). Despite its prominent role, the M. sexta genome sequence is not yet published. Recently, transcriptomes of fat body, hemocytes, and midgut, in which many immunity-related genes are expressed, were determined using 454 pyrosequencing and Sanger sequencing technology (Pauchet et al., 2010; Zhang et al., 2011; Zou et al., 2008). The quantitative nature of the most recent study allowed us to analyze immune inducible and tissue specific gene expression. Although genome- and homology-independent discovery of new genes is possible, stringent thresholds set in the exploration hindered complete immunotranscriptomic analysis (Zhang et al., 2011). Therefore, the current work intended to extend the analysis by identifying most of the immunity-related genes in M. sexta, as a step towards the annotation of its immunogenome.
2. Methods and Materials
2.1. Construction, sequencing, and assembling of cDNA libraries
Insect rearing, bacterial injection, RNA isolation, cDNA synthesis, and library sequencing were described previously (Zhang et al., 2011). Briefly, fat body (F) and hemocytes (H) were prepared as controls (C) from sixty naïve larvae (5th instar, day 3) for total RNA isolation and mRNA purification. Similarly, the same tissues were obtained from sixty induced (I) larvae (5th instar, day 3, injected with a mixture of bacteria 24 h before) for mRNA isolation and cDNA synthesis. After the CF, CH, IF, and IH cDNA libraries were separately run on a 454 GS-FLX pyrosequencer, reads were assembled to 19,020 CIFH contigs. For each contig, numbers of the CF, CH, IF, and IH reads incorporated were extracted from the Newbler Assembler output and tabulated using Microsoft Excel. As the tissues were pooled from sixty insects, read numbers are expected to faithfully represent the naïve and induced states of fat body and hemocytes.
2.2. Homologous sequence search, GO mapping, annotation and InterProScan search
The contigs were analyzed using the BLAST2GO software (Conesa et al., 2005; Gotz et al., 2008). In search for homologous sequences, the non-redundant protein database at NCBI was searched using BLASTX (Altschul et al., 1990) with a cutoff E-value of 10−15. The BLAST hits were mapped to their corresponding GO annotations using the gene ontology database and several additional data files (Gotz et al., 2008). Subsequent annotation of contigs, to link information on cellular component (CC), molecular function (MF), and biological process (BP), was done by applying the annotation rule to all the GO terms. However, certain evidence code weights were changed from their default values to: EXP = IDA = IPI = 5, IMP = IGI = 4, and IEP = 3. Annotations were examined to remove broad or level 1 annotation. Additionally, the GO term known as auxin biosynthesis process was removed from the list of GO terms as the process does not exist in insects. Annex-based GO term augmentation was performed afterwards to, firstly, obtain extra annotations and, secondly, further validate annotations (Gotz et al., 2008; Myhre et al., 2006). Protein domain and signal peptide were predicted using InterProScan (Quevillon et al., 2005), which enabled further sequence annotation (Gotz et al., 2008). In order to obtain more refined annotations, level 1 annotation removal and Annex-based GO term augmentation were repeated.
2.3. Local BLASTX, domain search, and multiple sequence alignment
We downloaded immunity-related genes from D. melanogaster (462 genes from FlyBase using the keyword “immunity”), B. mori (205 genes from Tanaka et al. (2008)), and A. mellifera (184 genes from Evans et al. (2006)). Amino acid sequences of these genes were incorporated into a sequence database for local BLASTX analysis of the CIFH contigs. Domain prediction was performed in parallel search runs using batchwise domain search web utilities of web CD-search tool, Pfam and InterProScan. Sequence alignments and manual curation of the alignments were performed using MUSCLE (Edgar, 2004) implemented in MEGA 5 (Tamura et al., 2011).
2.4. Calculation of relative abundance of transcripts under immune challenge
Since each contig was assembled from reads in the four libraries, normalized read numbers (NRNs) were calculated as: actual reads number in library X × (LNFCF + LNFIF + LNFCH + LNFIH)/LNFx, where X is CF, IF, CH, or IH. Library normalization factors (LNFs) for CF (825), CH (3,980), IF (1,618), and IH (3,352) are the sums of read numbers for rpS2-rpS5, rpL4 and rpL8 in the corresponding libraries (Zhang et al., 2011). NRNs were then used to calculate relative abundance (RAx/y = NRNx/NRNy). When a particular reads number was zero, an adjusted reads number (ARNx/y = actual read # in library X × LNFy/LNFx) was calculated instead. When multiple contigs encode a single gene, the particular contigs were concatenated and their NRNs summed in individual libraries for calculating RA or ARN values of the gene.
2.5. Statistical analysis
Statistical differences in numbers of contigs or total normalized reads from a GO category were analyzed by Student’s t-test. For instance, p values of immune inducibility (IC comparison) were derived from sums of number of contigs of IF and IH versus those of CF and CH. IC comparisons were also performed using sums of NRNs of IF and IH versus those of CF and CH. Similarly, p values of tissue specificity (FH comparison) were derived from sums of number of contigs and sums of NRNs of CF and IF versus those of CH and IH.
Percentage increases in numbers of contigs from a GO category were calculated as: I/C = [(sum of contig numbers of IF and IH − sum of contig numbers of CF and CH)/lower of the sums of contig numbers of the two groups] × 100 and F/H = [(sum of contig numbers of CF and IF − sum of contig numbers of CH and IH)/lower of the sums of contig numbers of the two groups] × 100. Similar calculations were performed using sums of NRNs from CF, IF, CH, and IH. Percentage increases in numbers of reads from a specific tissue (e.g., fat body) were calculated as I/CF = [(sum of IF NRNs − sum of CF NRNs)/lower sum of the two NRNs] × 100.
Data generated in above steps were merged, and mining of specific data were performed using SQL scripts in Microsoft Access, and default functions in Microsoft Excel.
3. Results
3.1. Distribution of M. sexta immunity-related genes
Here we report the repertoire of and changes in transcripts involved in multiple facets of innate immunity in M. sexta, such as pathogen recognition, signal transduction/modulation, and hemocyte adhesion. We identified 129 additional immunity-related genes (i.e. 204 contigs) in this study, apart from 103 highly regulated genes (i.e. 179 contigs) found in the previous study (Zhang et al., 2011). Taken together, genes for intracellular signal transduction account for 31% of the entire set; extracellular signaling molecules and their modulators make up 22% (Fig. 1). Gene products for pathogen recognition constitute 16%, whereas highly induced AMPs represent 13% of the total.
Fig. 1. Distribution of 232 M. sexta immunity-related genes.
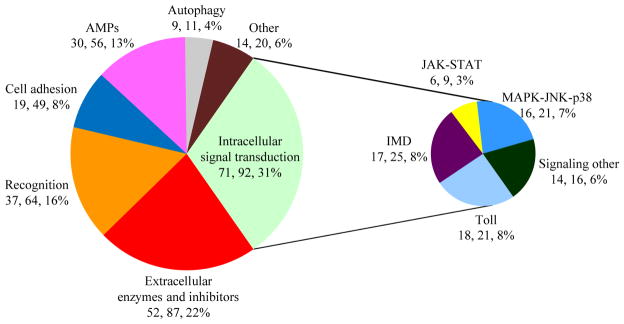
The pie chart shows gene number, contig number, and percentage of genes in each functional category relative to the entire set. The category of “intracellular signal transduction” is further divided into pathways (right).
3.2. Global changes in level 2 GO categories
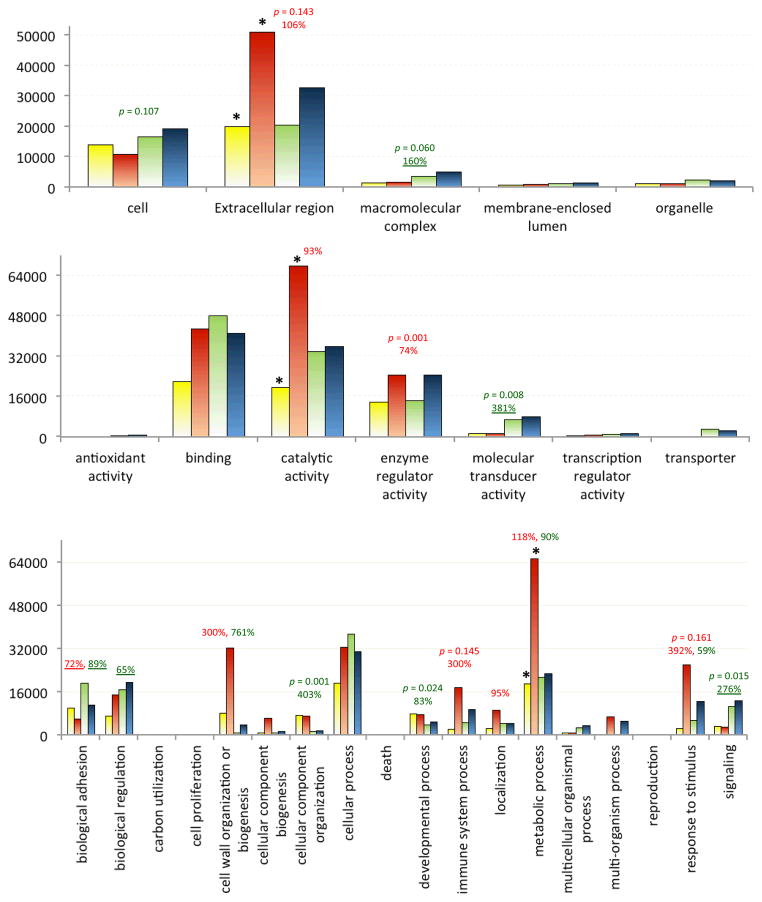
At GO level 2 (Gotz et al., 2008), expression of immunity-related genes is variable in fat body and hemocytes from naïve (C) and injected (I) larvae in terms of cellular component (CC), molecular function (MF) and biological process (BP). Since total numbers of the identified genes in each category do not significantly change between control and induced fat body (F) or hemocytes (H) (data not shown), we took advantage of the known read numbers for each contig in our datasets (Zhang et al., 2011), calculated summation of normalized read numbers (NRNs) for each gene (some concatenated from two or more contigs), and compared the sums of CF, IF, CH, and IH NRNs in each of level 2 GO categories (Fig. 2). In twelve of the thirty categories, their totals of all NRN sums were lower than 10% of the single highest NRN sum in the respective CC (51,074), MF (67,960), or BP (65,296) group and, therefore, omitted for statistical analysis. Five of the remaining eighteen had significant differences (t < 0.05): enzyme regulator activity (MF, p = 0.001, IC), molecular transducer activity (MF, p = 0.008, FH), cellular component organization (BP, p = 0.001, FH), developmental process (BP, p = 0.024, FH), and signaling (BP, p = 0.015, FH). Differences in the following five groups are less pronounced but worth mentioning, since level 2 GO terms are so general that a higher p value (e.g., 0.05~0.20) may still reflect important changes: cell (CC, p = 0.107, FH), extracellular region (CC, p = 0.143, IC), macromolecular complex (CC, p = 0.060, FH), immune system (BP, p = 0.145, IC), and response to stimulus (BP, p = 0.161, IC).
Fig. 2. Expression analysis of cellular components (CC, top), molecular functions (MF, middle), and biological processes (BP, bottom) at GO level 2.
The bar graph is generated using data from the sum of NRNs for each annotated gene. Each GO term is comprised of four values, each from a particular library (CF: yellow, IF, orange, CH: green, and IH: blue). Bar height represents sum of sums of NRNs in a library within a specific GO group. p value (< 0.20) and percentage (>50%) increase or decrease (underlined) of immune inducibility (red, IF-IH vs. CF-CH) and tissue specificity (green, CF-IF vs. CH-IH) are indicated on the top. Of the eleven GO categories in which NRN sums have >50% differences in either or both tissues, three increase most dramatically and are marked with “*”.
We further inspected percentage changes of NRNs in the eighteen level 2 GO categories. When IC and FH comparisons were performed, we observed >50% changes in the following fifteen categories: extracellular region (CC, I > C: 106%), macromolecular complex (CC, H > F: 160%), catalytic activity (MF, I > C: 93%), enzyme regulator activity (MF, I > C: 74%), molecular transducer activity (MF, H > F: 381%), biological adhesion (BP, C > I: 72%, H > F: 89%), biological regulation (BP, H > F: 65%), cell wall organization or biogenesis (BP, I > C, 300%; F > H, 767%), cellular component organization (BP, F > H, 403%), developmental process (BP, F > H: 83%), immune system (BP, I > C: 300%), localization (BP, I > C: 95%), metabolism (BP, I > C: 118%, F > H: 90%), response to stimulus (BP, I > C: 392%, F > H: 59%), and signaling (BP, H > F: 276%).
While differences were observed in more categories between fat body and hemocytes, it is perhaps more interesting from the perspective of immunity to document major increases in total NRNs in either tissue before and after the immune challenge. Therefore, we studied the dataset and detected over 50% changes in extracellular region (CC, F: 155%, H: 59%), binding (MF, F: 95%), catalytic activity (MF, F: 243%), enzyme regulator activity (MF, F: 77%; H: 71%), biological regulation (BP, F: 111%), cell wall organization or biogenesis (BP, F: 297%; H: 329%), cellular process (BP, F: 69%), immune system (BP, F: 756%, H: 99%), localization (BP, F: 265%), metabolism (BP, F: 246%), and response to stimulus (BP, F: 1015%, H: 126%). The most dramatic increases in NRN occurred in the categories of extracellular region (CC, F: 31,038, 155%) and catalytic activity (MF, F: 48,149, 243%). The increase in extracellular protein transcripts was consistent with the highly induced synthesis of defense molecules (e.g., AMPs) in fat body after the immune challenge.
3.3. Pathogen recognition
Pathogen detection is essential in subsequent measures taken to counteract the invasion. In insects, recognition proteins sense the pathogen presence by binding to their surface components known as pathogen-associated molecular patterns. We previously reported highly regulated β-1,3-glucan recognition proteins (βGRPs) and peptidoglycan recognition proteins (PGRPs) among others (Zhang et al., 2011). Here we report sixteen new genes coding for putative pattern recognition proteins: leureptin-2, Dscam, thioester-containing protein (TEP)-1 and -2, galectin-2 and -4, nimrod A, Draper, PGRP-L2, -L5, -LC, -S2, βGRP-3 and -4, immulectin (IML)-3a and - 3b (Table S1).
Apart from a role in pathogen recognition, these proteins are involved in phagocytosis as well as activation of signaling cascades. Leureptin (Zhu et al., 2010), Dscam (Watson et al., 2005), TEPs (Blandin and Levashina, 2004), galectins (Pace and Baum, 2004), nimrod A, Draper (Fauvarque and Williams, 2011) and IML-3 promote phagocytosis whereas PGRPs, βGRPs and IML-3 activate signaling cascades (Jiang et al., 2010). Among phagocytosis promoters, Dscam, nimrod A, and Draper showed mRNA level increases of >1.5-fold while leureptin and IML-3b transcript levels were up-regulated more than two-fold. In contrast, galectin-4 showed more than two-fold down-regulation in hemocytes. Transcript levels of galectin-2, TEP-1, and TEP-2 were low and their changes small. Draper and nimrod A showed preferred expression in hemocytes, whereas leureptins were highly expressed in fat body. Among activators of signaling cascades, PGRP-S5 levels were highly up-regulated in fat body while PGRP-L2 was down-regulated by two-fold. PGRP-L5 and βGRP-3 mRNA levels did not change much after the immune challenge. Unlike βGRPs involved in immunity, βGRP-4 contains a signal peptide and a GH16 domain with the catalytic residues (Glu, Asp, and Glu) but no RGD motif.
3.4. Extracellular enzymes and their regulation
Many members of the serine proteinase family have been cloned and characterized from M. sexta. Some comprise an extracellular enzyme system that leads pathogen recognition to killing mechanisms. These proteinases are sequentially activated and later down-regulated by inhibitors in the plasma (Jiang et al., 2005). We have identified contigs encoding putative signal mediators and modulators (Table S2), including nineteen hemolymph proteinases (HPs), prophenoloxidase-activating proteinases (PAPs), scolexin, serine proteinase homologs (SPHs), Zn proteinase, twelve serpins and two other proteinase inhibitors, as well as enzymes involved in melanization (e.g. punch, Phe hydroxylase, Tyr hydroxylase, dopa decarboxylase, phenoloxidase) (Jiang et al., 2010; Krishnakumar et al., 2000; Zhang et al., 2011).
In M. sexta, a serine proteinase cascade produces active phenoloxidase (PO) that catalyzes the formation of quinones and melanin (Kanost et al., 2004). HP14, an initiator of the pathway, was up-regulated along with the next cascade component, HP21 (Wang and Jiang, 2007). Both genes were predominantly expressed in fat body. Up-regulation of HP14 transcription occurred in fat body and hemocytes and this was true for HP21 only in hemocytes. Another branch of the proPO activation system stems from HP6. HP6 mRNA was slightly up-regulated in both tissues whereas proHP8 (an HP6 substrate) was mainly synthesized in fat body. Transcripts of proPAP1 (another HP6 substrate) showed a 3.3-fold up-regulation in fat body. Five M. sexta serpins are known to regulate the proteinase system at multiple steps (Jiang et al., 2010). Serpin-1 transcript level is high (NRN >7000) in fat body and does not change after immune challenge. Serpin-3, -4, and -6 mRNA levels experienced more than two-fold increases. Serpin-3 and -4 transcripts increased only in fat body and serpin-6 mRNA was up-regulated in hemocytes. PO is one member of the enzyme system for melanization. Other members include up-regulated Phe hydroxylase and slightly up-regulated GTP cyclohydrolase I (i.e. punch). In addition, we have identified HP2, HP3 (IH/CH: 0.5), HP4 (IH/CH: 0.8), HP5 (IH/CH: 5.8), HP12 (IF/CF: 1.3), HP13 (IH/CH: 1.0), HP15 (IH/CF: 6.5), HP20 (IF/CF: 4.4), SPH2, serpin-7 (IF/CF 2.0), and serpin-11.
3.5. Signal transduction via major signaling pathways
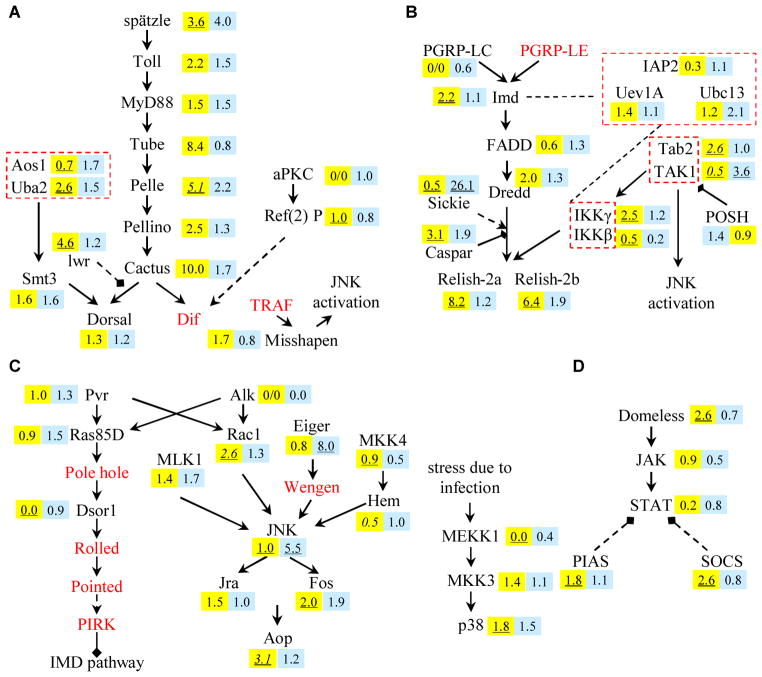
The Toll, IMD, JAK-STAT, JNK, and p38 signal transduction pathways (Table 1) govern the production of effector molecules to eliminate pathogens (Fig. 3) and, hence, have been in the limelight of insect innate immunity research (Boutros et al., 2002; Dostert et al., 2005; Han et al., 1998; Kallio et al., 2005; Kim and Kim, 2005). We have identified orthologs of the pathway members and assume their functions and modes of action are conserved among insects.
Table 1.
Members of the intracellular signaling pathways
| Gene name | Domain(s) | Contig(s) | nCF | nCH | nIF | nIH | nIF:nCF | nIH:nCH |
|---|---|---|---|---|---|---|---|---|
| Toll pathway | ||||||||
| Spätzle | 2287 | 11.8 | 68.8 | 42.3 | 274.1 | 3.6 | 4.0 | |
| Toll | 5599, 6893, 14282 | 106.64 | 164.6 | 235.6 | 247.9 | 2.2 | 1.5 | |
| MyD88 | Death, TIR | 864 | 118.5 | 137.5 | 181.2 | 201.2 | 1.5 | 1.5 |
| Tube | Death, PK | 1313 | 23.7 | 127.7 | 199.4 | 102.1 | 8.4 | 0.8 |
| Pelle | Death, PK | 2038 | 0.0 | 17.2 | 60.4 | 37.9 | 5.1 | 2.2 |
| Pellino | 292 | 71.1 | 147.4 | 175.2 | 189.6 | 2.5 | 1.3 | |
| Cactus | ANK-2 | 1044, 3381, 15574 | 118.5 | 260.3 | 1190.2 | 452.0 | 10.0 | 1.7 |
| Lesswright | UBC | 4591 | 11.8 | 167.0 | 54.4 | 198.3 | 4.6 | 1.2 |
| aPKC | PK, PK C-domain | 5708, 7433 | 0.0 | 39.3 | 0.0 | 37.9 | 0/0 | 1.0 |
| Ref(2)P | PBI, ZZ Zinc finger | 5971 | 23.7 | 78.6 | 24.2 | 64.2 | 1.0 | 0.8 |
| Rel/Dorsal | Rel domain | 2384 | 82.9 | 198.9 | 108.7 | 242.0 | 1.3 | 1.2 |
| ECSIT | ECSIT | 4177 | 0.0 | 14.7 | 6.0 | 20.4 | 0.5 | 1.4 |
| Tollip-d | CUE | 4949 | 71.1 | 0.0 | 72.5 | 0.0 | 1.0 | 0/0 |
| Tollip-v | C2, CUE | 731 | 59.2 | 311.9 | 48.3 | 344.1 | 0.8 | 1.1 |
| Smt3 | Sumo | 7946 | 59.2 | 167.0 | 96.7 | 262.5 | 1.6 | 1.6 |
| Aos1 | Aos1-SUMO | 5438 | 35.5 | 56.5 | 24.2 | 93.3 | 0.7 | 1.7 |
| Uba2 | Uba2-SUMO | 890 | 11.8 | 140.0 | 30.2 | 210.0 | 2.6 | 1.5 |
| Misshapen | PK, CNH | 289 | 106.6 | 311.9 | 181.2 | 259.5 | 1.7 | 0.8 |
|
| ||||||||
| IMD pathway | ||||||||
| IMD | Death | 2368 | 35.5 | 61.4 | 78.5 | 67.1 | 2.2 | 1.1 |
| FADD | Death, DID | 342 | 876.8 | 122.8 | 531.6 | 157.5 | 0.6 | 1.3 |
| Dredd | Caspase (Peptidase-C14) | 1615, 14535, 15028 | 71.1 | 149.8 | 139.0 | 201.2 | 2.0 | 1.3 |
| IAP2 | BIR/Inhibitor of apoptosis | 1174, 7327, 8290, 9234 | 106.6 | 98.2 | 30.2 | 110.8 | 0.3 | 1.1 |
| Ubc13/ben | UBC | 2901 | 82.9 | 117.9 | 96.7 | 247.9 | 1.2 | 2.1 |
| Uev1A | UBC | 3326 | 154.0 | 368.4 | 217.5 | 411.2 | 1.4 | 1.1 |
| TAK1 | PTK | 8422 | 0.0 | 4.9 | 6.0 | 17.5 | 0.5 | 3.6 |
| Tab2 | CUE | 1637 | 0.0 | 44.2 | 30.2 | 43.7 | 2.6 | 1.0 |
| IKKβ | PK | 5609 | 11.8 | 14.7 | 6.0 | 2.9 | 0.5 | 0.2 |
| IKKγ | 1049 | 11.8 | 93.3 | 30.2 | 107.9 | 2.6 | 1.2 | |
| Relish-2A | PEST | 15531, 15532 | 11.8 | 61.4 | 96.7 | 72.9 | 8.2 | 1.2 |
| Relish-2B | Rel homology domain | 4802 | 23.7 | 103.2 | 151.0 | 192.5 | 6.4 | 1.9 |
| Ntf2 | NTF2 | 6033, 8947 | 118.5 | 169.5 | 157.1 | 332.4 | 1.3 | 2.0 |
| Serpent | ZnF-GATA | 4249, 17496 | 485.8 | 582.1 | 163.1 | 501.6 | 0.3 | 0.9 |
| Sickie | 5128, 7157 | 35.5 | 2.5 | 18.1 | 64.2 | 0.5 | 26.1 | |
| Caspar | UBX | 2428 | 11.8 | 27.0 | 36.2 | 52.5 | 3.1 | 1.9 |
| POSH | SH3 | 1777, 5429 | 47.4 | 338.9 | 66.5 | 309.1 | 1.4 | 0.9 |
|
| ||||||||
| MAPK pathway with JNK and p38 branches | ||||||||
| Eiger | TNF | 1020 | 497.6 | 9.8 | 380.6 | 78.7 | 0.8 | 8.0 |
| Cdc42 | Cdc42 | 647 | 130.3 | 579.6 | 114.8 | 621.1 | 0.9 | 1.1 |
| Dsor1 | PK | 6185 | 11.8 | 34.4 | 0.0 | 29.2 | 0.0 | 0.9 |
| Rac1 | Rac1 | 3605 | 0.0 | 27.0 | 30.2 | 35.0 | 2.6 | 1.3 |
| Ras85D | Ras | 73, 132, 205, 1185 | 651.7 | 1350.8 | 555.8 | 1991.7 | 0.9 | 1.5 |
| MLK1 | PK, PTK, SH3 | 1825, 1841 | 59.2 | 117.9 | 84.6 | 198.3 | 1.4 | 1.7 |
| MEKK1 | PK | 1947 | 11.8 | 49.1 | 0.0 | 20.4 | 0.0 | 0.4 |
| Licrone/MKK3 | PK-MKK3-6 | 2351 | 82.9 | 108.1 | 114.8 | 122.5 | 1.4 | 1.1 |
| p38 | PK | 7214 | 23.7 | 63.9 | 42.3 | 93.3 | 1.8 | 1.5 |
| MKK4 | PK-MKK4 | 3655 | 35.5 | 63.9 | 30.2 | 29.2 | 0.9 | 0.5 |
| Hem | PK-MKK7 | 4608 | 0.0 | 24.6 | 6.0 | 23.3 | 0.5 | 1.0 |
| JNK | PK | 3082 | 23.7 | 7.4 | 24.2 | 40.8 | 1.0 | 5.5 |
| FOS | bZIP | 4904 | 23.7 | 95.8 | 48.3 | 177.9 | 2.0 | 1.9 |
| Jra | bZIP | 13290, 13291 | 71.1 | 186.7 | 108.7 | 186.6 | 1.5 | 1.0 |
| Aop | SAM-PNT, ETS | 1136 | 0.0 | 125.3 | 36.2 | 154.6 | 3.1 | 1.2 |
| MASK | ANK, KH-I | 225, 4036 | 106.6 | 206.3 | 157.1 | 265.4 | 1.5 | 1.3 |
| JAK-STAT pathway | ||||||||
| Domeless | SH2 | 7557, 9588 | 11.8 | 27.0 | 30.2 | 17.5 | 2.6 | 0.7 |
| JAK/Hopscotch | PTK, SH2, B41 | 20 | 272.5 | 498.6 | 241.7 | 250.8 | 0.9 | 0.5 |
| STAT | SH2, Protein | 2221, 14109 | 106.6 | 135.1 | 24.2 | 102.1 | 0.2 | 0.8 |
| PIAS | Interacting, MIZ/SP RING | 602 | 23.7 | 113.0 | 42.3 | 119.6 | 1.8 | 1.1 |
| SOCS | SH2, SOCS box | 1187, 6886 | 11.8 | 159.6 | 30.2 | 125.4 | 2.6 | 0.8 |
| Stam | VHS | 13543 | 0.0 | 19.6 | 6.0 | 20.4 | 0.5 | 1.0 |
Genes reported by Zhang et al. (2011) are underlined. nCF, nCH, nIF and nIH are normalized read numbers and, for genes with two or more contigs, they represent the total values. When nCF = 0, adjusted NRN for nIF (italics) is calculated as nIF × 825/9775; when nCH = 0, adjusted NRN for nIH (italics) is calculated as nIH × 3980/9775.
Fig. 3. Identification and profiling of transcripts involved in the Toll (A), IMD (B), JAK-STAT (C) and MAPK-JNK-p38 (D) signal transduction pathways.
The intracellular signaling processes, based mostly on Drosophila research, are described in the text assuming the pathways are conserved among insects. Genes that are not found in our dataset are shown in red. Immune inducibilities (i.e., NRN ratios or ARNs) in fat body (yellow) and hemocytes (blue) are indicated near the corresponding genes. Underlined number or 0/0 denotes low RNCF or RNCH (0 ~ 4) and, hence, less reliable NRN ratio.
3.5.1. Toll pathway
Components of Gram-positive bacteria and fungi activate the Drosophila Toll pathway through a cytokine, Spätzle (Lemaitre et al., 1996; LeMosy et al., 1999) (Fig. 3A). M. sexta Spätzle-1B (An et al., 2010) had a four-fold increase in mRNA levels in both tissues. The Toll receptor showed 2.2- and 1.5-fold up-regulation in fat body and hemocytes, respectively. All members of a complex formed after the Toll activation (MyD88, Tube, and Pelle) (Weber et al., 2003) were up-regulated in both tissues. The increase in MsMyD88 mRNA level was small (1.5-fold) while MsPelle transcripts elevated 5.1-fold in fat body. MsCactus was highly up-regulated in fat body and slightly in hemocytes after the injection but MsDorsal transcript abundance did not change significantly.
Sumoylation plays a regulatory role in immunity by covalent modification of proteins in the NF-κB signaling pathways (Mabb and Miyamoto, 2007). We have identified one ubiquitin-conjugating (Ubc) protein similar to Drosophila Lesswright or Ubc9, a conjugating enzyme that stabilizes Cactus (Table 1) (Abraham, 2007; Huang et al., 2005). MsLesswright mRNA showed 4.3-fold up-regulation in fat body whereas Smt3, a possible activator of Dorsal (Bhaskar et al., 2002; Xu et al., 2010), had a 1.6-fold increase in both tissues. Uba2 and Aos1, whose transcript levels showed slight increase of 1.5-fold in hemocytes, may activate Smt3 (Bhaskar et al., 2000).
Besides the aforementioned components, Pellino, Tollip-d, Tollip-v, TRAF, atypical PKC (aPKC), Ref(2)P, and ECSIT are associated with the Toll pathway as well (Valanne et al., 2011). We identified all their transcripts but TRAF. MsPellino mRNA level increased 2.5-fold in fat body after the challenge, other five members had <1.5-fold changes in mRNA levels. MsTollip-d was preferentially expressed in fat body whereas MsTollip-v mRNA was abundant in hemocytes.
3.5.2. IMD pathway
Diaminopimelic acid-PG of Gram-negative bacteria activates the IMD pathway via PGRP-LC, -LE, and IMD in Drosophila (Choe et al., 2002; Gottar et al., 2002; Kaneko et al., 2004 and 2006; Ramet et al., 2002b) (Fig. 3B). Members of a putative IMD-FADD-Dredd complex in M. sexta showed a two-fold up-regulation in fat body except for FADD (IF/CH: 0.6) (Table 1). MsIAP2 mRNA decreased more than three-fold in fat body. Functionally critical MsUev1 and MsUbc13 showed a constitutive level of expression. Despite its key role in the IMD pathway, MsTAK1 mRNA level was low. The TAK1 partner Tab2, however, had a 2.6-fold increase in transcript level in fat body. The Drosophila IKK complex may cleave Relish’s ankyrin (ANK) repeats and cause it to translocate into the nucleus (Stoven et al., 2003). Transcript levels of MsIKKβ were low but MsIKKγ’s increased more than two-fold in fat body. MsRelish expression was also highly up-regulated in fat body.
A homolog of DmSerpent, which activates AMP gene expression by binding to cognate cis regulatory elements (Petersen et al., 1999), was down-regulated more than three-fold in fat body. In contrast, DmNtf-2 prevents Dorsal, Dif, or Relish from nuclear translocation when mutated (Bhattacharya and Steward, 2002), and its homolog showed two-fold up-regulation in hemocytes (Table 1). Among regulators of the IMD pathway, Sickie, Caspar, and POSH homologs are found: DmSickie induces the Dredd-mediated Relish cleavage (Foley and O’Farrell, 2004) and MsSickie transcript level is down-regulated in fat body but highly up-regulated in hemocytes; DmCaspar inhibits IMD signaling (Lee and Ferrandon, 2011) and MsCaspar mRNA level is up-regulated in both tissues; DmPOSH governs the IMD pathway activation and termination as well as JNK pathway activation via regulation of TAK1 degradation (Lee and Ferrandon, 2011) and MsPOSH did not show any marked change in transcript levels.
3.5.3. MAPK-JNK-p38 pathway
In Drosophila, components of the Ras/MAPK pathway (Fig. 3D) activate JNK and p38, down-regulate the IMD pathway, and induce lamellocyte formation and hemocyte proliferation (Dong et al., 2002; Lee and Ferrandon, 2011; Ragab et al., 2011). M. sexta PDGF/VEGF receptor (PVR) and Alk receptor, which may trigger the MAPK pathway and Rac1 activation (Zettervall et al., 2004), did not show much change in transcript levels. The Alk mRNA level was particularly low and limited to naïve hemocytes (Table S6). Among members of the MAPK pathway were Cdc42, Dsor1, Rac1, and Ras85D. Except for a two-fold up-regulation of MsRac1 in fat body and slight increase of MsRas85D in hemocytes, changes in mRNA levels were small. Among identified members that may trigger p38 branch of the MAPK pathway were homologs of DmLicrone/MKK3 and DmMEKK1 (Han et al., 1998; Inoue et al., 2001). Both MsMKK3 and MsMEKK1 showed two-fold down-regulation in hemocytes despite slight up-regulation of p38 mRNA levels in both tissues.
JNK, a branch of the MAP kinase pathway, mediates stress-related responses and controls AMP gene expression (Ragab et al., 2011). In Drosophila, TAK1, Rac1, mixed-lineage kinases (MLKs), or MKK4 initiates the JNK pathway (Gallo and Johnson, 2002; Park et al., 2004; Silverman et al., 2003; Williams et al., 2006). MsMLK1 showed a slight increase in hemocytes, while MsMKK4 mRNA level decreased two-fold (Table 1). We identified all the members of the JNK branch. MsJNK mRNA was highly up-regulated in hemocytes, FOS and Jra had a less than two-fold up-regulation in fat body, and FOS mRNA level increased 1.9-fold in hemocytes. Contig 3082 encodes a part of MsJNK that has a protein kinase domain. JNK activates the transcription factor Aop (Anterior open) that mediates lamellocyte formation in Drosophila (Zettervall et al., 2004) and MsAop exhibited an up-regulation in fat body. Multiple ankyrin repeats single KH domain (MASK) is involved in signal relaying during the above processes (Bokoch, 2005; Hall, 1998; Kleino et al., 2005), and MsMASK is slightly up-regulated in fat body and hemocytes.
3.5.4. JAK-STAT pathway
The Drosophila JAK-STAT pathway is involved in antiviral immune responses (Dostert et al., 2005) (Fig. 3D). We identified all members of this pathway, except for the cytokine Unpaired (upd). All of them contain corresponding functional domains and are highly similar in structure to their homologs in B. mori. Most of them had lower mRNA levels in fat body than hemocytes. MsJAK (Hopscotch) showed a two-fold down-regulation in hemocytes.
3.6. Hemocyte adhesion
During an infection, usually non-adherent hemocytes tend to aggregate to trigger cellular immune responses against invading pathogens (Lavine and Strand, 2002). We identified three hemocyte-specific integrin α subunits, two β subunits, and one integrin-linked protein kinase (Table S3). Integrin α subunit mRNAs showed minimal changes except for α1, which was down-regulated in fat body. Integrin β subunits and the kinase were slightly up-regulated in hemocytes. We identified two other cell surface molecules, neuroglian and tetraspanin, that contribute to the integrin-mediated hemocyte aggregation (Nardi et al., 2006; Zhuang et al., 2007a; Zhuang et al., 2007b). Both proteins were highly expressed in hemocytes. Neuroglian had a more than 1.5-fold up-regulation in hemocytes while tetraspanin is slightly up-regulated in fat body. Among three paralytic peptide binding proteins (PPBP-1, -2, and -3), PPBP-1 had a slight increase in fat body while PPBP-3 expression was up-regulated more than two-fold in hemocytes. PPBP-3 mRNA level was higher than those of PPBP-1 and -2.
3.7. Autophagy
Autophagy governs the lysosome-dependent turnover of proteins or organelles and plays key roles in other cellular processes as well as human diseases (Shintani and Klionsky, 2004). Among nine different autophagy-related (Atg) molecules found are two ubiquitin-like proteins (Atg8 and Atg12), E1-like Atg5, Cys proteinase Atg4, and Atg4-like proteins (Table S4). These proteins are implicated in the process of macroautophagy (Geng and Klionsky, 2008). Except for up-regulated Atg8, all the other members are either expressed at low levels or down-regulated in fat body.
3.8. AMPs
We previously found 25 unique AMPs encoded by 61 highly up-regulated contigs (Zhang et al., 2011). Despite near complete coverage in that study, we identified other antimicrobial molecules, namely M. sexta lysozyme-like protein 1 (LLP1), four WAP-domain proteins, and attacin-3 through -6 (Table S5). LLP1 is slightly up-regulated in fat body. Two of the WAP-containing proteins showed increased expression in fat body, despite these genes are poorly expressed. Attacins are highly expressed in fat body except for attacin-6, which is highly expressed only in hemocytes. All other attacins are also highly up-regulated in hemocytes with the exception of attacin-5, which is expressed only in the infected fat body. A closer look at the multiple sequence alignment of attacin-coding contigs revealed that the attacin family of AMPs comprise six members as opposed to two reported previously in M. sexta (Table S5, Fig. 4). There is a cluster of two attacin genes in the B. mori genome, closely similar to MsAttacin-2. Similar gene duplications gave rise to 2–3 attacin genes in other lepidopteran insects. In M. sexta, a different gene expansion yielded five other genes (MsAttacin-1, -3, -4, -5, and -6) in a lineage-specific way. A monophylatic group of four D. melanogaster attacin genes as well as T. castaneum attacin-1, -2 and -3 was probably generated in a similar way.
Fig. 4. Phylogenetic relationships among insect attacins.
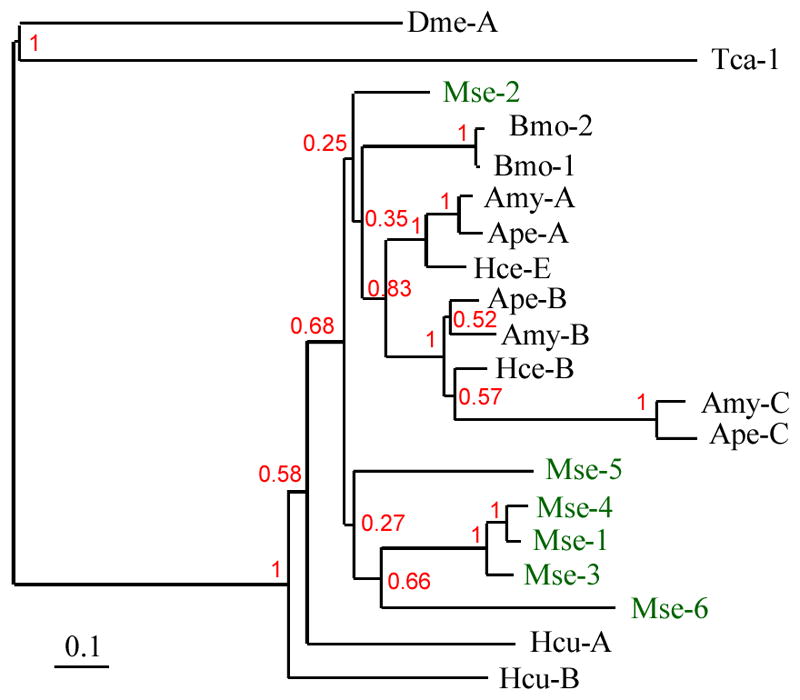
Amino acid sequences of M. sexta attacin-1 through -6 (Mse-1, 2, 3, 4, 5, 6, green); Antheraea mylitta attacin-A, B, C (Amy-A, B, C), Antheraea pernyi attacin-A, B, C (Ape-A, B, C), B. mori attacin-1 and 2 (Bmo-1, 2), D. melanogaster attacin-A (Dme-A), Hyalophora cecropia attacin-B and E (Hce-B, E), Hyphantria cunea attacin-A and B (Hcu-A, B), T. castaneum attacin-1 (Tca-1) are aligned using MUSCLE 3.7 at www.phylogeny.fr. The phylogenetic tree was constructed using JTT substitution matrix in ProtDist/FastDist+ neighbor with a bootstrap replicates of 1000. The bootstrap values (%) are indicated at nodes. Correct assembling of Mse-5 (17350 + 13563) and -6 (15744 + 16576 + 15159) from multiple contigs is confirmed by the draft genome sequence (data not shown).
3.9. Others
This category comprises other genes involved in signaling, hemocyte proliferation and development, reactive molecular species synthesis and regulation, and gene silencing (Table S6). Focal adhesion kinase mediates signals from integrin receptors to MAPK pathway and, hence, plays a central role in regulating cellular immunity (Sieg et al., 1999). It has characteristic functional domains, such as FERM-M, PTK, and Focal-AT. The ligand Serrate and its receptor Notch mediate signal transduction to control hematopoiesis (Williams, 2007). MsSerrate and MsNotch are among the most highly expressed after AMPs. MsSerrate mRNA level reduced in fat body while MsNotch transcripts in hemocytes remained unchanged after the challenge. A transcription factor known as Brahma in D. melanogaster may also control hematopoiesis (Remillieux-Leschelle et al., 2002), and MsBrahma shows no change in expression.
Reactive oxygen species (ROS) play a part in cytotoxic defense against microbes via activating AMPs or enhancing melanogenesis (Lavine and Strand, 2002). Nitric oxide synthase (NOS) generates nitric oxide (Nappi et al., 2000). However, the expression of MsNOS is low in both the tissues even after the induction. Thioredoxin peroxidases and peroxiredoxins regulate amount of ROS, especially after an oxidative burst in the case of an infection, to maintain cellular homeostasis (Christensen et al., 2005; Nappi and Christensen, 2005). We found three thioredoxin peroxidases and one peroxiredoxin. Thioredoxin POD1 is up-regulated in hemocytes while thioredoxin POD3 and peroxiredoxin in fat body. However, thioredoxin POD2 is slightly down-regulated in fat body.
Homology-based gene silencing is involved in the Drosophila antiviral response (Wang et al., 2006). We identified homologs of Argonaute-1 and Dicer-2 that compose a part of the RNA interference silencing complex (RISC) (Ding et al., 2004).
4. Discussion
This extended study of the quantitative transcriptome data unveiled 95 new immunity-related genes in M. sexta. Along with the 137 reported previously (Jiang et al., 2010; Zhang et al., 2011), the number of such genes summed up to 232. In comparison to the 205, 462 and 184 genes retrieved from B. mori, D. melanogaster and A. mellifera, our studies, not based on annotated genome, revealed a similar number of genes. The deep analysis of fat body and hemocyte transcriptomes did uncover a large portion of the complete set of immunity-related genes that would come from a genome analysis. This is a valuable piece of information for researchers doing similar transcriptome studies in organisms that lack sequenced genomes. The extensiveness and depth of our transcriptome data are further supported by the discovery and analysis of six attacin genes in M. sexta (Fig. 4). Another important aspect of our transcriptomic data is that the inducibility of certain genes reported conformed well to known expression data on each of those genes (Zhang et al., 2011). Along the same line, we found significant similarities between inducibility of genes involved in the Drosophila Toll pathway (De Gregorio et al., 2001), further corroborating the data generated by Zhang et al. (2011) and utilized in this study.
A major goal of this research was not limited to discover sequences similar to the queries; rather it was to identify genes most likely related to immunity. For instance, BLAST search using aPKC as a query revealed 34 contigs at a commonly used cutoff E-value of 10−5, but there is only one ortholog (contigs 5708 and 74333) in M. sexta. Twenty-eight of the hits were identified because they encode a kinase domain commonly found in genes, which may not be related to immunity. As such, many studies may have yielded inflated lists of homologous genes with limited value in orthology-based function predictions. Contrary to that practice, we took measures to reduce false positives, such as adopting a stringent threshold in the initial BLASTX analysis, searching for domain structures, and placing more weight on experimentally proven ontology in the GO annotation. Our initial BLASTX search (E-value <10−15) against NCBI NR database resulted in 411 hits, dominated by lectins (80), proPO subunits (32), attacins (25), serine proteinases (30), and serine proteinase inhibitors (37). The parallel, local BLASTX analysis using known immunity-related genes from B. mori, A. mellifera and D. melanogaster, along with domain searches and multiple sequence alignments, yielded 379 highly scrutinized contigs. Although the number difference was only 18, the second list overlapped with the first only in 197 cases. Over 50% or 214 of the positives in the first list were incorrect: the use of a stringent threshold did not greatly reduce false positives; it, instead, yielded a lower number of valuable hits. Therefore, we adopted the 2nd list and improved it by merging 379 contigs into 232 groups, each of which represents one or more contigs putatively encoded by a single gene (Tables 1, S1–S6).
Based on the categorization of immune functions, we found genes for signal transduction and modulation account for 54% or 179 of the 232 genes whose products form pathways which crosstalk in multiple steps (Fig. 3). Genes for pathogen recognition and execution account for 16% and 10% of the gene set and, unlike signaling proteins, their products exert similar functions by extensively complementing each other to cope with a broad spectrum of infectious agents. The remaining 20% are involved in other processes, such as cell adhesion and autophagy. While this functional classification provided a good overview of the immune system, general GO analysis at level 2 did not yield clear differences in gene counts in the I-C and F-H comparisons (data not shown). Only after we took mRNA levels into consideration could significant differences be observed in certain categories of CC, MF, and BP at GO level 2 (Fig. 2). Six of the thirty groups are significantly different (p < 0.20) between fat body and hemocytes, whereas four categories are in the I-C comparison. Considering the high level of generalization in GO terms at level 2, we believe p < 0.20 is remarkable, especially when a large percentage of increase or decrease (>50%) is observed. The most dramatic changes occur in the categories of extracellular region (31,038, CC), catalytic activity (48,149, MF), and metabolism (46,433, BP) in fat body after the immune challenge. Highly induced expression of AMPs and other plasma defense proteins is partly responsible for the increase in total mRNA levels of extracellular molecules.
Phagocytosis, nodulation, and encapsulation as a result of pathogen recognition and cell adhesion comprise insect cellular immunity. Except for slightly up-regulated nimrod A, Dscam and Draper, up-regulated leureptin and IML-3, and down-regulated galectin-4, other genes showed no major change at the transcription level (Table S1), suggesting a complex regulation of phagocytosis. A majority of the genes involved in autophagy showed low transcript levels (Table S4). Atg-8, which plays a critical role in autophagy, had a 3.1-fold mRNA level increase in fat body, whereas Atg-3, -4, and -5 transcripts reduced to 1/3 in the same tissue after the immune challenge. Total NRNs of the Atg genes were 4.4-fold higher in fat body than hemocytes. Since active engulfment of microbes occurs in the latter, the regulation of autophagy seems complicated, like phagocytosis, nodulation, and encapsulation. Hemolectin had a 2.5-fold down-regulation of mRNA level in hemocytes. In contrast, the increases in Reeler (IFARN 97.9; IH/CH: 3.0) and proPSP (IF/IH: 2.6) transcripts may enhance nodulation.
Melanogenesis plays a key role in immunity by participating in killing of entrapped microbes and wound healing. In M. sexta, an extracellular network of serine proteinases generates active PO, PSP, and Spätzle by proteolytic processing (Jiang et al., 2010). Many HP-related proteins were up-regulated after the immune challenges, including HP14, HP6, PAPs, and SPH1 (Table S2). Serpin-3, 4, 5, and 6, whose mRNAs became more abundant, negatively regulate some of these HPs that activate proPO. PO catalyzes the key steps for quinone and melanin formation, whereas other enzymes (e.g. Phe and Tyr hydroxylases, Punch, and dopa decarboxylase) also contribute to melanization. Substantial increases in their transcript levels (Table S2) further indicate the enzyme system for melanogenesis is highly coordinated and regulated at that level. In addition to proPAP1 activation, M. sexta HP6 generates active HP8 that processes Spätzle precursor (IF/CF 3.6, IH/CH 4.0) to initiate the Toll pathway (An et al., 2009 and 2010) for AMP induction. The increases in HP6 (IF/CF 1.6) and HP8 (IF/CF 1.9) mRNA levels indicate that reverse transcription-PCR is less quantitative than deep sequencing in detecting less than twofold induction.
Massively parallel pyrosequencing of transcripts from larval fat body and hemocytes allowed us to identify most components of the intracellular signaling pathways and quantify changes in their transcript levels after the immune challenges (Fig. 3). Although Dif, TRAF, PGRP-LE, and Wengen are missing in our contigs, evidence for the existence of Toll, IMD, MAPK-JNK-p38, and JAK-STAT pathways is compelling. We plan to search the genome sequence for these genes and profile their expression in these two tissues in the future. The current data did not show dramatic mRNA level changes, consistent with the fact that many members of the intracellular signal transduction pathways are activated via posttranslational modifications (i.e. phosphorylation and dephosphorylation). However, this does not exclude a possible regulation at the transcription level. In fact, major increases in mRNA levels were observed for Tube (IF/CF: 8.4), Cactus (IF/CF: 10.0), and Relish (IF/CF: 7.0), and the induced production of Cactus and Relish are probably related to their cleavage during immune signaling and the need to be replenished for a secondary response. After excluding these three genes, we calculated the averages and ranges of induction for Toll, IMD, MAPK-JNK-p38 pathways as 1.8 (1.3–2.2), 1.2 (0.3–2.0), and 1.2 (0.8–1.5) in fat body, as well as 1.4 (0.8–2.2), 1.1 (0.2–2.1), and 1.1 (0–1.9) in hemocytes, respectively. These increases, although small, may substantially contribute to the induction of AMP synthesis (Table S5). Interestingly, those values for the antiviral JAK-STAT pathway were 0.6 (0.2–0.9) in fat body and 0.8 (0.5–1.1) in hemocytes, consistent with the fact that we did not use any elicitor to mimic viral infection. It would be interesting to compare effects of virulent and incompatible viruses on transcription of the genes in the antiviral signaling pathway.
Concluding remarks
The next-generation sequencing approach we adopted has yielded a set of 19,020 contigs and corresponding read numbers from control and induced larval fat body and hemocytes of M. sexta. The long average size (923 bp), known immunity-related genes of other insects, and extensive sequence comparisons have facilitated the identification of 232 genes (or 383 contigs), assignment of GO terms and immune processes, and examination of transcriptional regulation of the entire system. The results validated our previous study, uncovered genes (e.g., components of signaling pathways), demonstrated the practicality of genome-independent expression profiling of a complex process, and paved the way for annotation of the immunogenome.
Supplementary Material
Acknowledgments
We thank Drs. Ulrich Melcher and Jack Dill with for their critical comments on the manuscript. This work was supported by National Institutes of Health Grants GM58634 (to H. Jiang). This article was approved for publication by the Director of the Oklahoma Agricultural Experiment Station and supported in part under project OKL02450.
Abbreviations
- CF and CH
control (C) fat body (F) and hemocytes (H) from naïve larvae
- IF and IH
induced (I) fat body and hemocytes from larvae injected with bacteria
- Alk
anaplastic lymphoma kinase
- AMP
antimicrobial peptide
- ANK
ankyrin
- aPKC
atypical protein kinase C
- ARN
adjusted read number
- AtgX
autophagy-related protein X
- BP
biological process
- CC
cellular component
- CRD
carbohydrate recognition domain
- CTL
C-type lectin
- Dscam
Down syndrome cell adhesion molecule
- ECSIT
evolutionarily conserved intermediate in Toll pathway
- EGF
NIM and EMI, epidermal growth factor, nimrod and emilin
- EST
expressed sequence tag
- FN
fibronectin
- GO
gene ontology
- HAIP
hemocyte aggregation inhibitor protein
- Hem
hemipterous
- HP
hemolymph proteinase
- IAP
inhibitor of apoptosis
- Ig
immunoglobulin
- IKK
IκB kinase
- IMD
immune deficiency
- IML
immulectin
- JAK-STAT
Janus kinase-signal transducer and activator of transcription
- JNK
Jun N-terminal kinase
- Jra
Jun-related antigen
- LNF
library normalization factor
- LPS
lipopolysaccharide
- LRR
leucine-rich repeat
- MAPK
mitogen-activated protein kinase
- MASK
multiple ankyrin repeats single KH domain
- MEKK
MAP kinase kinase kinase
- MF
molecular function
- MLK
mixed-linage kinase
- NFκB and IκB
nuclear factor-κB and its inhibitor
- NO and NOS
nitric oxide and its synthase
- NRN
normalized read number
- NTF
nuclear translocation
- ORF
open reading frame
- PAP
proPO-activating proteinase
- PDGF and VEGF
platelet-derived and vascular endothelial growth factors
- PG and PGRP
peptidoglycan and its recognition protein
- PIAS
protein inhibitor of activated STAT
- PO and proPO
phenoloxidase and its precursor
- POSH
plenty of SH3 domains
- PPBP
paralytic peptide-binding protein
- PSP
plasmatocyte spreading peptide
- PVR
PDGF/VEGF receptor
- RA
relative abundance
- RISC
RNA interference silencing complex
- ROS
reactive oxygen species
- SH2/3
src homology 2/3 domain
- SOCS
suppressor of cytokine signaling
- SP and SPH
serine proteinase and its homolog
- SUMO
small ubiquitin-like modifier
- TAK
transforming growth factor β (TGFβ) activated kinase
- TEP
thioester-containing protein
- TIR
Toll/interleukin-1 receptor
- TRAF
tumor necrosis factor (TNF) receptor-associated factor
- UBC
ubiquitin-conjugating domain
- VWD
Von Willebrand disease factor
- WAP
whey acidic protein
- ZnF
zinc finger
- α2M
α2-macrogobulins
- βGRP
β-1, 3-glucanas related protein
Footnotes
To get the CIFH contigs, readers can search the database “CIFH_contigs (March, 2010)” (http://darwin.biochem.okstate.edu/blast/blast.html) and retrieve sequences from a text file named “MsextaCIFHcontigs.fna” (http://entoplp.okstate.edu/profiles/jiang.htm). According to GenBank policy, contigs from more than one tissue or treatment are not allowed to be deposited there. Only CF, IF, CH, and IH contigs can be found in Transcriptome Shotgun Assembly Sequence Database at GenBank.
References
- Abraham J. Ohio University; Ohio: 2007. The role of Drosophila SUMO conjugating enzyme Lesswright in larval hematopoiesis: Effects on Cactus, Dorsal and Dorsal-related immunity factor (Dif) LINK. http://rave.ohiolink.edu/etdc/view?acc_num=ohiou1187275172. [Google Scholar]
- Agaisse H. An adaptive immune response in Drosophila? Cell Host Microbe. 2007;1:91–93. doi: 10.1016/j.chom.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Altincicek B, Vilcinskas A. Identification of immune-related genes from an apterygote insect, the firebrat Thermobia domestica. Insect Biochem Mol Biol. 2007;37:726–731. doi: 10.1016/j.ibmb.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- An C, Jiang H, Kanost MR. Proteolytic activation and function of the cytokine Spatzle in the innate immune response of a lepidopteran insect, Manduca sexta. FEBS J. 2010;277:148–162. doi: 10.1111/j.1742-4658.2009.07465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C, Ishibashi J, Ragan EJ, Jiang H, Kanost MR. Functions of Manduca sexta hemolymph proteinases HP6 and HP8 in two innate immune pathways. J Biol Chem. 2009;284:19716–19726. doi: 10.1074/jbc.M109.007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoun RB, Hetru C, Troxler L, Doucet D, Ferrandon D, Matt N. Analysis of thioester-containing proteins during the innate immune response of Drosophila melanogaster. J Innate Immunity. 2011;3:52–64. doi: 10.1159/000321554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg GH, Zhou R, Perrimon N. Genome-wide RNAi analysis of JAK/STAT signaling components in Drosophila. Genes Dev. 2005;19:1861–1870. doi: 10.1101/gad.1320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar V, Smith M, Courey AJ. Conjugation of Smt3 to dorsal may potentiate the Drosophila immune response. Mol Cell Biol. 2002;22:492. doi: 10.1128/MCB.22.2.492-504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar V, Valentine SA, Courey AJ. A functional interaction between dorsal and components of the Smt3 conjugation machinery. J Biol Chem. 2000;275:4033–4040. doi: 10.1074/jbc.275.6.4033. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Steward R. The Drosophila homolog of NTF-2, the nuclear transport factor-2, is essential for immune response. EMBO Rep. 2002;3:378–383. doi: 10.1093/embo-reports/kvf072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin S, Levashina EA. Thioester-containing proteins and insect immunity. Mol Immunol. 2004;40:903–908. doi: 10.1016/j.molimm.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163–171. doi: 10.1016/j.tcb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Boutros M, Agaisse H, Perrimon N. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev Cell. 2002;3:711–722. doi: 10.1016/s1534-5807(02)00325-8. [DOI] [PubMed] [Google Scholar]
- Brey PT, Hultmark D. Molecular mechanisms of immune responses in insects. Chapman & Hall; London; New York: 1998. [Google Scholar]
- Chapman AD Australian Biodiversity Information, S., Australia. Numbers of living species in Australia and the world. Australian Govt., Dept. of Environ. Heritage; Canberra, ACT: 2006. [Google Scholar]
- Choe KM, Werner T, Stoven S, Hultmark D, Anderson KV. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science. 2002;296:359–362. doi: 10.1126/science.1070216. [DOI] [PubMed] [Google Scholar]
- Christensen BM, Li J, Chen CC, Nappi AJ. Melanization immune responses in mosquito vectors. Trends Parasitol. 2005;21:192–199. doi: 10.1016/j.pt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, Hoa NT, Hoffmann JA, Kanzok SM, Letunic I, Levashina EA, Loukeris TG, Lycett G, Meister S, Michel K, Moita LF, Muller HM, Osta MA, Paskewitz SM, Reichhart JM, Rzhetsky A, Troxler L, Vernick KD, Vlachou D, Volz J, von Mering C, Xu JN, Zheng LB, Bork P, Kafatos FC. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- De Gregorio E, Spellman PT, Rubin GM, Lemaitre B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci USA. 2001;98:12590–12595. doi: 10.1073/pnas.221458698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding SW, Li H, Lu R, Li F, Li WX. RNA silencing: a conserved antiviral immunity of plants and animals. Virus Res. 2004;102:109–115. doi: 10.1016/j.virusres.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, Hoffmann JA, Imler JL. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat Immunol. 2005;6:946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom Y. Induction and regulation of antimicrobial peptides in Drosophila. Dev Comp Immunol. 1999;23:345–358. doi: 10.1016/s0145-305x(99)00016-6. [DOI] [PubMed] [Google Scholar]
- Evans JD, Aronstein K, Chen YP, Hetru C, Imler JL, Jiang H, Kanost M, Thompson GJ, Zou Z, Hultmark D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol Biol. 2006;15:645–656. doi: 10.1111/j.1365-2583.2006.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvarque MO, Williams MJ. Drosophila cellular immunity: a story of migration and adhesion. J Cell Sci. 2011;124:1373–1382. doi: 10.1242/jcs.064592. [DOI] [PubMed] [Google Scholar]
- Foley E, O’Farrell PH. Functional dissection of an innate immune response by a genome-wide RNAi screen. PLoS Biol. 2004;2:E203. doi: 10.1371/journal.pbio.0020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol. 2002;3:663–672. doi: 10.1038/nrm906. [DOI] [PubMed] [Google Scholar]
- Geng JF, Klionsky DJ. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. EMBO Rep. 2008;9:859–864. doi: 10.1038/embor.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardo NM, Altincicek B, Anselme C, Atamian H, Barribeau SM, De Vos M, Duncan EJ, Evans JD, Gabaldon T, Ghanim M, Heddi A, Kaloshian I, Latorre A, Moya A, Nakabachi A, Parker BJ, Perez-Brocal V, Pignatelli M, Rahbe Y, Ramsey JS, Spragg CJ, Tamames J, Tamarit D, Tamborindeguy C, Vincent-Monegat C, Vilcinskas A. Immunity and other defenses in pea aphids, Acyrthosiphon pisum. Genome biology. 2010;11:R21. doi: 10.1186/gb-2010-11-2-r21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie JP, Kanost MR, Trenczek T. Biological mediators of insect immunity. Annu Rev Entomol. 1997;42:611–643. doi: 10.1146/annurev.ento.42.1.611. [DOI] [PubMed] [Google Scholar]
- Gottar M, Gobert V, Michel T, Belvin M, Duyk G, Hoffmann JA, Ferrandon D, Royet J. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature. 2002;416:640–644. doi: 10.1038/nature734. [DOI] [PubMed] [Google Scholar]
- Gotz S, Garcia-Gomez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talon M, Dopazo J, Conesa A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36:3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Han ZQS, Enslen H, Hu XD, Meng XJ, Wu IH, Barrett T, Davis RJ, Ip YT. A conserved p38 mitogen-activated protein kinase pathway regulates Drosophila immunity gene expression. Mol Cell Biol. 1998;18:3527–3539. doi: 10.1128/mcb.18.6.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RAB. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA, Reichhart JM. Drosophila immunity. Trends Cell Biol. 1997;7:309–316. doi: 10.1016/S0962-8924(97)01087-8. [DOI] [PubMed] [Google Scholar]
- Hou XS, Perrimon N. The JAK-STAT pathway in Drosophila. Trends Genet. 1997;13:105–110. doi: 10.1016/s0168-9525(97)01006-8. [DOI] [PubMed] [Google Scholar]
- Huang L, Ohsako S, Tanda S. The lesswright mutation activates Rel-related proteins, leading to overproduction of larval hemocytes in Drosophila melanogaster. Dev Biol. 2005;280:407–420. doi: 10.1016/j.ydbio.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet. 1993;9:178–183. doi: 10.1016/0168-9525(93)90165-e. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Drosophila immunity: paths and patterns. Curr Opin Immunol. 2003;15:12–19. doi: 10.1016/s0952-7915(02)00005-5. [DOI] [PubMed] [Google Scholar]
- Inoue H, Tateno M, Fujimura-Kamada K, Takaesu G, Adachi-Yamada T, Ninomiya-Tsuji J, Irie K, Nishida Y, Matsumoto K. A Drosophila MAPKKK, D-MEKK1, mediates stress responses through activation of p38 MAPK. EMBO J. 2001;20:5421–5430. doi: 10.1093/emboj/20.19.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving P, Troxler L, Heuer TS, Belvin M, Kopczynski C, Reichhart JM, Hoffmann JA, Hetru C. A genome-wide analysis of immune responses in Drosophila. Proc Natl Acad Sci USA. 2001;98:15119–15124. doi: 10.1073/pnas.261573998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IH, Nam HJ, Lee WJ. CLIP-domain serine proteases in Drosophila innate immunity. BMB Rep. 2008;41:102–107. doi: 10.5483/bmbrep.2008.41.2.102. [DOI] [PubMed] [Google Scholar]
- Jiang H. The biochemical basis of antimicrobial responses in. Insect Sci. 2008;15:53–66. [Google Scholar]
- Jiang H, Vilcinskas A, Kanost MR. Immunity in lepidopteran insects. Adv Exp Med Biol. 2010;708:181–204. doi: 10.1007/978-1-4419-8059-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Gu Y, Guo X, Zou Z, Scholz F, Trenczek TE, Kanost MR. Molecular identification of a bevy of serine proteinases in Manduca sexta hemolymph. Insect Biochem Mol Biol. 2005;35:931–943. doi: 10.1016/j.ibmb.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio J, Leinonen A, Ulvila J, Valanne S, Ezekowitz RA, Ramet M. Functional analysis of immune response genes in Drosophila identifies JNK pathway as a regulator of antimicrobial peptide gene expression in S2 cells. Microbes Infect. 2005;7:811–819. doi: 10.1016/j.micinf.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Goldman WE, Mellroth P, Steiner H, Fukase K, Kusumoto S, Harley W, Fox A, Golenbock D, Silverman N. Monomeric and polymeric Gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity. 2004;20:637–649. doi: 10.1016/s1074-7613(04)00104-9. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Yano T, Aggarwal K, Lim JH, Ueda K, Oshima Y, Peach C, Erturk-Hasdemir D, Goldman WE, Oh BH. PGRP-LC and PGRP-LE have essential yet distinct functions in the Drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006;7:715–723. doi: 10.1038/ni1356. [DOI] [PubMed] [Google Scholar]
- Kanost MR. Serine proteinase inhibitors in arthropod immunity. Dev Comp Immunol. 1999;23:291–301. doi: 10.1016/s0145-305x(99)00012-9. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Gorman MG. Phenoloxidases in insect immunity. In: Beckage N, editor. Insect Immunology. Academic Press/Elsevier; San Diego: 2008. pp. 69–96. [Google Scholar]
- Kanost MR, Jiang HB, Wang Y, Yu XQ, Ma CC, Zhu YF. Hemolymph proteinases in immune responses of Manduca sexta. Phylogenetic Perspectives on the Vertebrate Immune System. 2001;484:319–328. doi: 10.1007/978-1-4615-1291-2_32. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Jiang HB, Yu XQ. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol Rev. 2004;198:97–105. doi: 10.1111/j.0105-2896.2004.0121.x. [DOI] [PubMed] [Google Scholar]
- Khush RS, Lemaitre B. Genes that fight infection - what the Drosophila genome says about animal immunity. Trends Genet. 2000;16:442–449. doi: 10.1016/s0168-9525(00)02095-3. [DOI] [PubMed] [Google Scholar]
- Kim T, Kim YJ. Overview of innate immunity in Drosophila. J Biochem Mol Biol. 2005;38:121–127. doi: 10.5483/bmbrep.2005.38.2.121. [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- Kleino A, Valanne S, Ulvila J, Kallio J, Myllymaki H, Enwald H, Stoven S, Poidevin M, Ueda R, Hultmark D, Lemaitre B, Ramet M. Inhibitor of apoptosis 2 and TAK1-binding protein are components of the Drosophila Imd pathway. EMBO J. 2005;24:3423–3434. doi: 10.1038/sj.emboj.7600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnakumar S, Burton D, Rasco J, Chen XY, O’Donnell J. Functional interactions between GTP cyclohydrolase I and tyrosine hydroxylase in Drosophila. J Neurogenet. 2000;14:1–23. doi: 10.3109/01677060009083474. [DOI] [PubMed] [Google Scholar]
- Kurata S, Ariki S, Kawabata S. Recognition of pathogens and activation of immune responses in Drosophila and horseshoe crab innate immunity. Immunobiol. 2006;211:237–249. doi: 10.1016/j.imbio.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochem Mol Biol. 2002;32:1295–1309. doi: 10.1016/s0965-1748(02)00092-9. [DOI] [PubMed] [Google Scholar]
- Lee KZ, Ferrandon D. Negative regulation of immune responses on the fly. EMBO J. 2011;30:988–990. doi: 10.1038/emboj.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- LeMosy EK, Hong CC, Hashimoto C. Signal transduction by a protease cascade. Trends Cell Biol. 1999;9:102–107. doi: 10.1016/s0962-8924(98)01494-9. [DOI] [PubMed] [Google Scholar]
- Mabb AM, Miyamoto S. SUMO and NF-κB ties. Cell Mol Life Sci. 2007;64:1979–1996. doi: 10.1007/s00018-007-7005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmaras VJ, Lampropoulou M. Regulators and signalling in insect haemocyte immunity. Cell Signal. 2009;21:186–195. doi: 10.1016/j.cellsig.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Myhre S, Tveit H, Mollestad T, Laegreid A. Additional gene ontology structure for improved biological reasoning. Bioinformatics. 2006;22:2020–2027. doi: 10.1093/bioinformatics/btl334. [DOI] [PubMed] [Google Scholar]
- Nappi A, Christensen B. Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem Mol Biol. 2005;35:443–459. doi: 10.1016/j.ibmb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Nappi AJ, Vass E, Frey F, Carton Y. Nitric oxide involvement in Drosophila immunity. Nitric Oxide Biol Chem. 2000;4:423–430. doi: 10.1006/niox.2000.0294. [DOI] [PubMed] [Google Scholar]
- Nardi JB, Pilas B, Bee CM, Zhuang S, Garsha K, Kanost MR. Neuroglian-positive plasmatocytes of Manduca sexta and the initiation of hemocyte attachment to foreign surfaces. Dev Comp Immunol. 2006;30:447–462. doi: 10.1016/j.dci.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Pace KE, Baum LG. Insect galectins: roles in immunity and development. Glycoconj J. 2004;19:607–614. doi: 10.1023/B:GLYC.0000014092.86763.2f. [DOI] [PubMed] [Google Scholar]
- Park JM, Brady H, Ruocco MG, Sun H, Williams D, Lee SJ, Kato T, Jr, Richards N, Chan K, Mercurio F, Karin M, Wasserman SA. Targeting of TAK1 by the NF-κB protein Relish regulates the JNK-mediated immune response in Drosophila. Genes Dev. 2004;18:584–594. doi: 10.1101/gad.1168104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauchet Y, Wilkinson P, Vogel H, Nelson DR, Reynolds SE, Heckel DG, ffrench-Constant RH. Pyrosequencing the Manduca sexta larval midgut transcriptome: messages for digestion, detoxification and defence. Insect Mol Biol. 2010;19:61–75. doi: 10.1111/j.1365-2583.2009.00936.x. [DOI] [PubMed] [Google Scholar]
- Petersen UM, Kadalayil L, Rehorn KP, Hoshizaki DK, Reuter R, Engstrom Y. Serpent regulates Drosophila immunity genes in the larval fat body through an essential GATA motif. EMBO J. 1999;18:4013–4022. doi: 10.1093/emboj/18.14.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. Plos Pathog. 2007;3:e26. doi: 10.1371/journal.ppat.0030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevillon E, Silventoinen V, Pillai S, Harte N, Mulder N, Apweiler R, Lopez R. InterProScan: protein domains identifier. Nucleic Acids Res. 2005;33:W116–120. doi: 10.1093/nar/gki442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragab A, Buechling T, Gesellchen V, Spirohn K, Boettcher AL, Boutros M. Drosophila Ras/MAPK signalling regulates innate immune responses in immune and intestinal stem cells. EMBO J. 2011;30:1123–1136. doi: 10.1038/emboj.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramet M, Manfruelli P, Pearson A, Mathey-Prevot B, Ezekowitz RA. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature. 2002a;416:644–648. doi: 10.1038/nature735. [DOI] [PubMed] [Google Scholar]
- Ramet M, Lanot R, Zachary D, Manfruelli P. JNK signaling pathway is required for efficient wound healing in Drosophila. Dev Biol. 2002b;241:145–156. doi: 10.1006/dbio.2001.0502. [DOI] [PubMed] [Google Scholar]
- Remillieux-Leschelle N, Santamaria P, Randsholt NB. Regulation of larval hematopoiesis in Drosophila melanogaster: a role for the multi sex combs gene. Genetics. 2002;162:1259–1274. doi: 10.1093/genetics/162.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolff J, Reynolds SE. Insect infection and immunity: Evolution, ecology, and mechanisms. Oxford University Press; Oxford: 2009. [Google Scholar]
- Sackton TB, Lazzaro BP, Schlenke TA, Evans JD, Hultmark D, Clark AG. Dynamic evolution of the innate immune system in Drosophila. Nat Genet. 2007;39:1461–1468. doi: 10.1038/ng.2007.60. [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ. The innate signaling of dangers and the dangers of innate signaling. Nat Immunol. 2006;7:1237–1242. doi: 10.1038/ni1420. [DOI] [PubMed] [Google Scholar]
- Shintani T, Klionsky DJ. Autophagy in health and disease: A double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- Silverman N, Maniatis T. NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001;15:2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- Silverman N, Zhou R, Erlich RL, Hunter M, Bernstein E, Schneider D, Maniatis T. Immune activation of NF-κB and JNK requires Drosophila TAK1. J Biol Chem. 2003;278:48928–48934. doi: 10.1074/jbc.M304802200. [DOI] [PubMed] [Google Scholar]
- Stoven S, Silverman N, Junell A, Hedengren-Olcott M, Erturk D, Engstrom Y, Maniatis T, Hultmark D. Caspase-mediated processing of the Drosophila NF-κB factor Relish. Proc Natl Acad Sci USA. 2003;100:5991–5996. doi: 10.1073/pnas.1035902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand MR. The insect cellular immune response. Insect Sci. 2008;15:1–14. [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Ishibashi J, Fujita K, Nakajima Y, Sagisaka A, Tomimoto K, Suzuki N, Yoshiyama M, Kaneko Y, Iwasaki T, Sunagawa T, Yamaji K, Asaoka A, Mita K, Yamakawa M. A genome-wide analysis of genes and gene families involved in innate immunity of Bombyx mori. Insect Biochem Mol Biol. 2008;38:1087–1110. doi: 10.1016/j.ibmb.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Valanne S, Wang JH, Ramet M. The Drosophila Toll signaling pathway. J Immunol. 2011;186:649–656. doi: 10.4049/jimmunol.1002302. [DOI] [PubMed] [Google Scholar]
- Vilmos P, Kurucz E. Insect immunity: evolutionary roots of the mammalian innate immune system. Immunol Lett. 1998;62:59–66. doi: 10.1016/s0165-2478(98)00023-6. [DOI] [PubMed] [Google Scholar]
- Vogel H, Altincicek B, Glockner G, Vilcinskas A. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genomics. 2011;12:308. doi: 10.1186/1471-2164-12-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajant H, Scheurich P. Analogies between Drosophila and mammalian TRAF pathways. Prog Mol Subcell Biol. 2004;34:47–72. doi: 10.1007/978-3-642-18670-7_3. [DOI] [PubMed] [Google Scholar]
- Wang XH, Aliyari R, Li WX, Li HW, Kim K, Carthew R, Atkinson P, Ding SW. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jiang H. Reconstitution of a branch of the Manduca sexta prophenoloxidase activation cascade in vitro: snake-like hemolymph proteinase 21 (HP21) cleaved by HP14 activates prophenoloxidase-activating proteinase-2 precursor. Insect Biochem Mol Biol. 2007;37:1015–1025. doi: 10.1016/j.ibmb.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse RM, Kriventseva EV, Meister S, Xi ZY, Alvarez KS, Bartholomay LC, Barillas-Mury C, Bian GW, Blandin S, Christensen BM, Dong YM, Jiang HB, Kanost MR, Koutsos AC, Levashina EA, Li JY, Ligoxygakis P, MacCallum RM, Mayhew GF, Mendes A, Michel K, Osta MA, Paskewitz S, Shin SW, Vlachou D, Wang LH, Wei WQ, Zheng LB, Zou Z, Severson DW, Raikhel AS, Kafatos FC, Dimopoulos G, Zdobnov EM, Christophides GK. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science. 2007;316:1738–1743. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson FL, Puttmann-Holgado R, Thomas F, Lamar DL, Hughes M, Kondo M, Rebel VI, Schmucker D. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science. 2005;309:1874–1878. doi: 10.1126/science.1116887. [DOI] [PubMed] [Google Scholar]
- Weber AN, Tauszig-Delamasure S, Hoffmann JA, Lelievre E, Gascan H, Ray KP, Morse MA, Imler JL, Gay NJ. Binding of the Drosophila cytokine Spatzle to Toll is direct and establishes signaling. Nat Immunol. 2003;4:794–800. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- Williams MJ. Drosophila hemopoiesis and cellular immunity. J Immunol. 2007;178:4711–4716. doi: 10.4049/jimmunol.178.8.4711. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Wiklund ML, Wikman S, Hultmark D. Rac1 signalling in the Drosophila larval cellular immune response. J Cell Sci. 2006;119:2015–2024. doi: 10.1242/jcs.02920. [DOI] [PubMed] [Google Scholar]
- Xu H, Hao W, He D, Xu Y. Smt3 is required for the immune response of silkworm, Bombyx mori. Biochimie. 2010;92:1306–1314. doi: 10.1016/j.biochi.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Zhu YF, Ma C, Fabrick JA, Kanost MR. Pattern recognition proteins in Manduca sexta plasma. Insect Biochem Mol Biol. 2002;32:1287–1293. doi: 10.1016/s0965-1748(02)00091-7. [DOI] [PubMed] [Google Scholar]
- Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz E, Ando I, Hultmark D. A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci USA. 2004;101:14192–14197. doi: 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FJ, Guo HY, Zheng HJ, Zhou T, Zhou YJ, Wang SY, Fang RX, Qian W, Chen XY. Massively parallel pyrosequencing-based transcriptome analyses of small brown planthopper (Laodelphax striatellus), a vector insect transmitting rice stripe virus (RSV) BMC Genomics. 2010;11:303. doi: 10.1186/1471-2164-11-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Gunaratna RT, Zhang X, Najar F, Wang Y, Roe B, Jiang H. Pyrosequencing-based expression profiling and identification of differentially regulated genes from Manduca sexta, a lepidopteran model insect. Insect Biochem Mol Biol. 2011;41:733–746. doi: 10.1016/j.ibmb.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YF, Ragan EJ, Kanost MR. Leureptin: a soluble, extracellular leucine-rich repeat protein from Manduca sexta that binds lipopolysaccharide. Insect Biochem Mol Biol. 2010;40:713–722. doi: 10.1016/j.ibmb.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang SF, Kelo L, Nardi JB, Kanost MR. Neuroglian on hemocyte surfaces is involved in homophilic and heterophilic interactions of the innate immune system of Manduca sexta. Dev Comp Immunol. 2007a;31:1159–1167. doi: 10.1016/j.dci.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Zhuang SF, Kelo LS, Nardi JB, Kanost MR. An integrin-tetraspanin interaction required for cellular innate immune responses of an insect, Manduca sexta. J Biol Chem. 2007b;282:22563–22572. doi: 10.1074/jbc.M700341200. [DOI] [PubMed] [Google Scholar]
- Zhuang SF, Nardi JB, Kanost MR. An integrin, neuroglian, and a tetraspanin modulate adhesion in hemocytes of an insect, Manduca sexta. FASEB J. 2005;19:A1061–A1062. [Google Scholar]
- Zou Z, Evans JD, Lu ZQ, Zhao PC, Williams M, Sumathipala N, Hetru C, Hultmark D, Jiang HB. Comparative genomic analysis of the Tribolium immune system. Genome Biol. 2007;8(8):R177. doi: 10.1186/gb-2007-8-8-r177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Najar F, Wang Y, Roe B, Jiang H. Pyrosequence analysis of expressed sequence tags for Manduca sexta hemolymph proteins involved in immune responses. Insect Biochem Mol Biol. 2008;38:677–682. doi: 10.1016/j.ibmb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.