Abstract
Great effort has been made toward defining and characterizing the pre-ictal state. Many studies have pursued the idea that there are recognizable electrographic (EEG-based) features that occur before overt clinical seizure activity. However, development of reliable EEG-based seizure detection and prediction algorithms has been difficult. In this review, we discuss the concepts of seizure detection vs. prediction and the pre-ictal “clinical milieu” and “EEG milieu”. We proceed to discuss novel concepts of seizure detection based on the pre-ictal “physiological milieu”; in particular, we indicate some early evidence for the hypothesis that pre-ictal cell swelling/extracellular space constriction can be detected with novel optical methods. Development and validation of optical seizure detection technology could provide an entirely new translational approach for the many patients with intractable epilepsy.
Keywords: pre-seizure state, electroencephalography, optics, hippocampus, seizure, detection, prediction
Introduction: clinical importance of seizure detection/prediction
Epilepsy, affecting approximately 2% of the population [1], comprises a group of disorders of the brain characterized by the periodic and unpredictable occurrence of seizures. As seizures are intermittent in patients with epilepsy, prediction or early detection of seizures before they occur would be revolutionary in both warning patients and also developing “closed-loop” seizure detection and termination paradigms. Indeed, the NIH Curing Epilepsy 2000 and Curing Epilepsy 2007 conferences have designated the creation of an effective closed-loop seizure detection/termination device as a critical benchmark in epilepsy research. The most important application would be to detect a focal seizure before it can generalize or spread through the brain, and couple this detection to any of a variety of seizure termination methods.
Seizure prediction and early detection methods based on electroencephalographic and clinical features have been extensively reviewed [2-5], and are discussed briefly below. Herein we present other novel approaches to identify the pre-ictal state, with a focus on physiologic examinations of the pre-ictal cellular milieu, and in particular optical imaging prior to electrographic seizure onset.
Prediction vs. detection
Although there is a large literature concerning the pre-ictal or early ictal state, the terminology is not uniform. The terms prediction and detection are often used interchangeably but actually describe different brain states. Seizure “prediction” implies that an impending seizure is predicted prior to some measure of ictal manifestation, while seizure “detection” suggests that the seizure is being identified at the earliest ictal manifestation. Certainly this distinction is not always clear, for example some changes in intracranial EEG may represent a true “pre-ictal” state or may be more accurately considered to be the earliest EEG manifestation of the seizure.
We propose a conceptual system that will translate into different intervention strategies. Seizure “prediction” takes place during the truly pre-ictal period, at a time when either the clinical, electrographic and/or physiological milieu represents an “at risk” state where the likelihood of progression to seizure is high. Interventions at this phase would be considered pre-emptive. In contrast, seizure “detection” takes place during the earliest physiological detection of an ictal state. This may be well before either a clinical or EEG manifestation is evident. Interventions at this phase would be abortive.
“Clinical milieu” of the pre-ictal state
The search for a clinical pre-ictal state has largely focused on constellations of symptoms and/or the presence of precipitants that indicate a high likelihood of impending seizure. Many patients report awareness of an impending seizure hours or days prior to the event. However, the challenges implicit in a rigorous investigation of this phenomenon are many, including the fact that these combinations of symptoms and precipitants may be specific for individuals, and the reality that this type of data is subject to recall bias. Thus the optimal study might include a large cohort, with subjects having frequent seizures and data collection consisting of time stamped prospective data collected for long periods of time. There have been an increasing number of studies focused on this area, although no published study meets those criteria.
To date, the most widely studied feature of a clinical pre-ictal “milieu” is the presence of prodromal or premonitory symptoms. Both questionnaire studies and prospective diary studies have identified symptoms such as irritable mood, headache, dizziness, visual changes and concentration difficulties, to name a few, as being reliably prodromal [6-11]. However, not all studies confirm this finding [12], including a recent electronic diary study [13], and in fact this topic was recently debated at a seizure prediction workshop [14].
Precipitating factors are likely essential features of the clinical pre-ictal milieu, by contributing to the creation of a time period where the risk of a seizure is increased. Such precipitants as derived from both questionnaire and prospective studies appear to reliably include increased stress, decreased sleep, and menstrual status [11, 15-20]. It is likely that the reported ability of a subset of patients to self-report their seizures [21, 22] is related to awareness of precipitants and prodromal features.
While intriguing, clinical investigations of the clinical pre-ictal milieu have not resulted yet in prediction with adequate sensitivity and specificity for a pre-emptive pharmacologic trial. However, the importance of studying this area is clear: if the period of time preceding a seizure is characterized by specific prodromal or predictive symptoms, identifying this “at risk” period clinically may enhance other methodologies for identifying the pre-ictal state, and suggest clinical interventions.
“EEG milieu” of the pre-ictal state
The concept of the pre-ictal state has existed since the early 1970s [23]. To date, seizure prediction and early detection algorithms have been solely based on analysis of either surface or intracranial electroencephalography (EEG). More recent studies have used a variety of computational algorithms to attempt to define characteristics of the pre-ictal state with EEG modalities [2, 24-26].
Recent efforts in optimizing EEG-based seizure detection have generally followed two distinct strategies: improving seizure detection algorithms vs. improving EEG spatial and temporal resolution. Seizure detection algorithms aim to recognize specific patterns that occur in the EEG that represent impending or early seizure activity, and produce a “trigger” that alerts the clinician to the seizure. Using various mathematical methods, investigators have evaluated EEG trends that occur prior to seizures in the attempt to predict seizure onset [3, 24, 26, 27]. However, despite over a decade of research on this topic, no seizure prediction/detection algorithm has been proven prospectively to perform better than chance [3, 4, 26].
The second strategy, methodology development, is largely based on improved spatial and temporal EEG sampling through engineering advancements and new monitoring/analysis protocols [4]. Increased spatial sampling has been obtained with recent advances in electrode design, which has led to the discovery of “microseizures” detectable by individual microelectrodes [28-31]. Increased temporal sampling has led to the finding that high-frequency oscillations (HFOs) have a close relationship with the seizure onset zone [28, 31-34].
It is likely that further technological advancements in EEG and analysis algorithms will continue to yield important clues toward the physiology of seizure generation and propagation in the brain. However, current EEG modalities still have several drawbacks. First, most clinical EEG systems have low spatial resolution, too low to detect “microseizures” generated in localized cortical regions requiring invasive electrodes. Second, the temporal resolution of most clinical EEG systems is too low to detect HFOs. Third, for high spatial and temporal resolution EEG data, analysis algorithms and automated signal processing techniques are still in development. Fourth, current paradigms require offline, retrospective analysis to achieve reliable prediction [27, 35]. Initial human trials with implanted recording electrodes had inconsistent detection rates and high rates of false positive triggering events [36, 37].
We would like to suggest that a more important conceptual problem is that the synchronized neuronal discharges which EEG measures are the end result of a preceding physiologic cascade, making early seizure detection or seizure prediction difficult with EEG modalities. While great efforts are underway, there are currently no real-time methods of EEG analysis capable of reliably and reproducibly predicting seizure onset [4, 38].
“Physiological milieu” of the pre-ictal state
Since patients ultimately desire a seizure prediction device with high sensitivity and specificity [39], is there any approach distinct from EEG that may give us clues to the pre-ictal state? Our hypothesis is that physiological changes that occur during the preictal state may lead us to novel approaches to early seizure detection and prediction.
A wealth of research in animal models has led to many insights regarding the neurochemical, histological, and behavioral characteristics of acquired epilepsy (epileptogenesis) [2]. However, most of these studies are not directly relevant to the exact physiological changes occurring in the brain just prior to a seizure.
Several studies have directly addressed physiological changes occurring in the brain immediately prior to seizure activity. In 1989, Traynelis and Dingledine performed a series of in vitro studies using the high-K+ model in the CA1 region of rat hippocampal slices. Among other findings, they noted that “a slight (approximately 2%) but significant increase in electrical resistance gradually occurred over the 20 s immediately preceding seizure generation. The observed increase in tissue resistance suggests extracellular space is decreased during these events” [40]. These results suggested for the first time that brain extracellular space (ECS) may be decreased just prior to seizure activity. In a separate group of studies, Dudek's group found that reduction of ECS size with hypoosmolar treatment powerfully induced epileptiform bursting in rat hippocampal slices, an effect that was reversed by mannitol [41].
Does pre-ictal ECS constriction occur in vivo? Early pioneering studies of ECS parameters using the iontophoretic TMA+ method demonstrated significant constriction of the ECS during seizure activity in vivo [42-44], but the temporal onset of ECS constriction relative to seizure onset remained unclear. To address this question, Binder et al. developed a novel technique termed cortical fluorescence recovery after photobleaching (cFRAP) to determine real-time extracellular space diffusion parameters in vivo and applied this technique in the PTZ model of generalized seizure [45]. The cFRAP technique involves in vivo loading of fluorescein-dextrans into the extracellular space (ECS) of intact brain, followed by laser-induced spot photobleaching and measurement of fluorescence recovery using confocal optics (Figure 1). Like cytotoxic edema produced by water intoxication, seizure activity slowed diffusion and created dead-space microdomains in which free diffusion was prevented. Critically, slowed ECS diffusion preceded electroencephalographic seizure activity (Figure 1, bottom). These experiments established proof-of-principle for the idea of seizure detection by detection of water movement/ECS constriction prior to seizure onset [45].
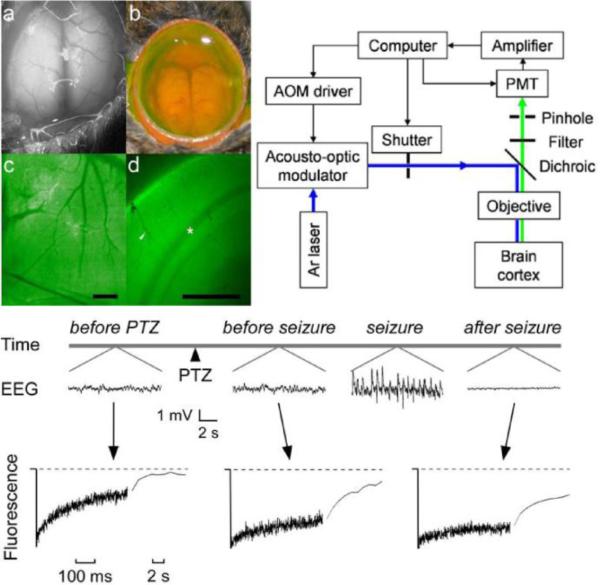
Figure 1. In vivo cortical surface photobleaching detects pre-ictal ECS constriction.
Top left. In vivo loading of brain ECS by fluorescein-dextrans. a. Brain surface exposure following craniectomy showing cortical blood vessels and intact dura. b. Transdural loading of brain ECS with ACSF solution of fluorescein-dextran. c. Fluorescence image of cortical surface after dye loading (scale bar, 1 mm). d. Coronal 300 μm brain slice obtained ex vivo following loading demonstrates fluorescence loading of cortex (scale bar, 1 mm). Arrowhead, cortical blood vessel; asterisk, white matter. Top right. Photobleaching apparatus. A laser beam is modulated by an acousto-optic modulator and directed onto the surface of the cortex using a dichroic mirror and objective lens. Emitted fluorescence is focused through a pinhole and detected by a gated photomultiplier (PMT). Bottom. Upper traces: Electroencephalographic recordings before and after intraperitoneal injection of PTZ (100 mg/kg). Lower traces: Fluorescence recovery curves for 70-kD fluorescein-dextran before PTZ administration, after PTZ but prior to electroencephalographic seizure activity, and following seizure activity. Note the change in fluorescence recovery signal prior to EEG seizure onset, suggesting ECS constriction prior to seizure onset and providing the potential for seizure detection. Modified from [45].
Similar to Traynelis and Dingledine's 1989 measurements of tissue electrical resistance before seizure activity in vitro, Willoughby's group has performed studies in vivo with electrical impedance monitoring combined with EEG [46, 47]. In these studies [46, 47], animals were treated with picrotoxin, kainic acid or fluorocitrate. Epileptiform discharges were preceded by small increases in electrical tissue impedance. Interestingly, increases in baseline impedance were correlated with increases in power of non-ictal high-frequency EEG activity. This may suggest that pre-ictal HFOs may be associated with detectable tissue impedance and ECS changes.
The finding of pre-ictal ECS constriction leads naturally to the question of what is the mechanism of ECS constriction before and during seizure activity. It is noteworthy that McBain, Traynelis, and Dingledine used the identical hippocampal slice preparation and the high-K+ model to demonstrate marked (30%) ictal reductions in ECS volume fraction in the hippocampus [48]. Presumably, ECS constriction is related to cell swelling. However, the cell type responsible for swelling in these early studies is unclear. Recently, it has become clear that astrocytes but not neurons express aquaporin water channels that allow rapid facilitated water transport and swelling in response to osmotic gradients [49, 50]. In addition, astrocytes but not neurons have been shown to swell after osmotic stimulation [51]. It remains unresolved whether astrocytes vs. neurons swell before and during seizure activity. However, it is clear that astrocytes but not neurons express aquaporin-4 water channels abundantly in the hippocampus [52]. Using two-photon imaging, Tian et al. demonstrated consistent pre-ictal calcium signaling in cortical astrocytes [53], suggesting that astrocytes may be activated during the pre-ictal period and responsible for pre-ictal swelling and ECS constriction.
New optical approaches to seizure detection
The above findings suggesting pre-ictal cell swelling and ECS constriction together with our experience in sensitive optical detection of cellular swelling [54] lead us to our new unifying hypothesis that pre-ictal ECS constriction can be detected with optical methods.
The optical properties of brain tissue during neuronal activity have been under investigation for decades [55, 56]. Changes in the intrinsic optical signal (IOS), a measure of cortical diffuse reflectance, have been observed during focal cortical seizures in animal models [57] and during intraoperative mapping in patients with neocortical epilepsy [58]. IOS changes are believed to result primarily from increased light absorption by hemoglobin during increased tissue perfusion due to neurovascular coupling. As such, these absorption-derived optical changes typically occur at or slightly after the onset of electrographic seizure, limiting the potential of this modality in seizure prediction.
Our work has focused on a different approach—optical detection of physiologic changes that occur prior to EEG seizure onset. Seizures are associated with depolarization of neurons and glial cells and concomitant ion flux and water movement from the extracellular (ECS) to the intracellular space (ICS). This depolarization of small neuronal populations putatively occurs prior to recruitment of enough neurons to generate a true “clinical” seizure detectable by standard EEG.
The importance of cell swelling (and reduction of the extracellular space) to the generation of seizure activity in vitro has been appreciated for some time [40, 59] but not well studied in vivo. We hypothesize that during the pre-ictal period, hyperactivity induces glial (in particular astrocyte) activation [53] and swelling resulting from ion and water fluxes during uncontrolled neuronal firing, which leads to the observed pre-seizure ECS constriction [45]. ECS constriction is predicted to decrease optical back-scattering of near-infrared light back to the surface of the brain [60]. This decreased backscattering thus manifests itself as an optical dip from the baseline. This suggests the possibility that a correctly configured optical apparatus could indeed provide reliable detection in the pre-ictal period as ECS constriction should alter photon propagation.
We hypothesized that quantitative measurements of brain optical scattering coefficient, an optical property largely ignored in previous IOS studies, would provide a correlate to the ECS constriction and associated glial swelling preceding seizure onset.
Recently, we have employed neurophotonic technology including spatial frequency domain imaging (SFDI), near-infrared (NIR) fiberoptic probes, and optical coherence tomography (OCT) to interrogate brain tissue in vivo prior to and during seizure activity. Our 850 nm NIR dual-fiberoptic probe system demonstrated a reduction in NIR reflectance during early cell swelling and cerebral edema in the water intoxication model [54]. Spatial frequency domain imaging (SFDI) is capable of generating quantitative absorption and scattering maps of tissue while simultaneously imaging across multiple wavelengths of light (Figure 2) [61]. Our optical results in the PTZ model of acute cortical seizure activity with SFDI indeed indicate that a decrease in NIR optical scattering occurs several seconds to minutes prior to electrographic seizure onset (Figure 2). Scattering returns to baseline levels after seizure termination. We obtained similar results with fiberoptic NIR probes (data not shown). Interestingly, fiberoptic NIR probes can be stereotactically implanted in deep brain structures important for epilepsy (e.g. the hippocampus), potentially providing a solution to optical seizure detection in deep brain structures.
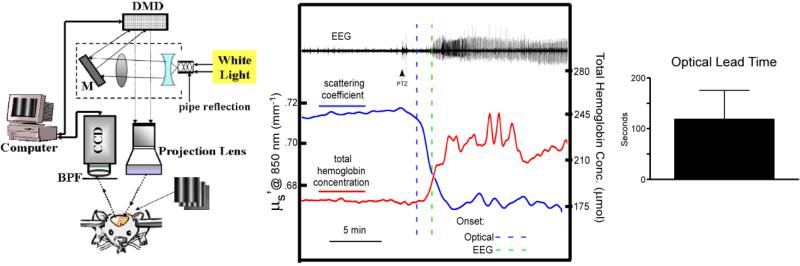
Figure 2. Spatial frequency domain imaging (SFDI) detects pre-ictal reduction in optical scattering.
Left: SFDI system. BPF, bandpass filter. CCD, charge-cooled device. DMD, digital micromirror device. M, mirror. Middle: Optical scattering coefficient (blue) and simultaneous EEG demonstrates a reduction in optical scattering coefficient (at 850 nm) (dashed blue line, 2 S.D. reduction in scattering coefficient) following convulsant administration (PTZ) but prior to electrographic seizure onset (dashed green line, 2 S.D. increase in EEG power). SFDI-derived total hemoglobin concentration (red) demonstrates cortical hyperperfusion following seizure onset. Right: optical lead time defined as time at optical “trigger” (2 S.D. change in optical scattering from baseline) to time of EEG seizure onset (analyzed blindly and by power analysis of EEG epochs). Mean optical lead time was 118 sec (n=5). Courtesy of Owen et al. (unpublished data).
OCT can be thought of as an optical analog of ultrasound imaging, in which the intensity and time delay of reflected light is used to generate cross-sectional images of tissue microstructure with micron-level resolution [62]. Optical scattering and reflectivity coefficients can be extracted from OCT images accurately and with high spatiotemporal resolution [63, 64]. These findings make OCT attractive for development as a real-time structural and functional brain imaging modality for use in neuroscience. While OCT has already been employed clinically in retinal and vascular imaging and diagnosis [65, 66], it remains to be developed and applied for neurological diagnosis. Our working hypothesis is that OCT, an imaging modality sensitive to NIR scattering changes, will be able to detect, with high temporal and spatial resolution, the changes in NIR scattering that occur prior to seizure activity. Recently, we have developed the ability of our OCT imaging system to reliably image the adult mouse cerebral cortex. In preliminary experiments, we have prepared anesthetized mice with atraumatic cranial windows as previously described [45, 54] and also with thinned-skull preparations (Figure 3) [67] and performed in vivo OCT imaging [68]. Preliminary data from these experiments indicate that our OCT imaging system demonstrates a stable optical baseline and a reduction in OCT-derived scattering intensity of NIR light following PTZ injection but prior to clinical seizure onset (Figure 4). These results provide proof-of-principle for optical detection of the pre-ictal state on a clinically relevant timescale. One important advantage of OCT is its noninvasive nature relative to approaches requiring implanted fiberoptics. Clearly, further studies with careful simultaneous electrophysiological and optical recordings are necessary to further delineate the spatiotemporal dynamics of pre-ictal optical signal changes and validate these optical signal changes as reliable biomarkers of seizures.
Figure 3. Spectral domain OCT setup for in vivo OCT imaging of mouse brain.
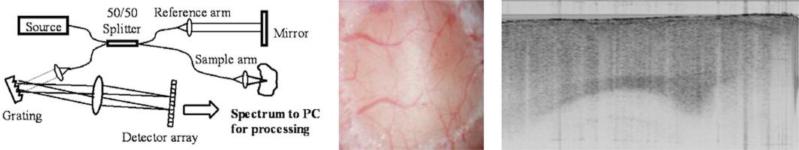
Left: schematic of SD-OCT setup. The light source, super luminescent diodes centered at 1310 nm, is split between the sample and reference arm. The reflected light from these arms recombines in the detector arm and the resulting spectrum is imaged onto the detector array. A Fourier transform of the spectrum generates the depth profile information. Middle: OCT imaging of mouse cortex can be done through a thinned-skull preparation and does not require craniectomy. Right: sample sagittal OCT image of mouse cortex (image dimension: 3×2 mm). The entire depth of the cerebral cortex and some subcortical structures lie within the optical interrogation volume and can be observed serially for reflectance intensity changes in vivo. Courtesy of Eberle et al. (unpublished data).
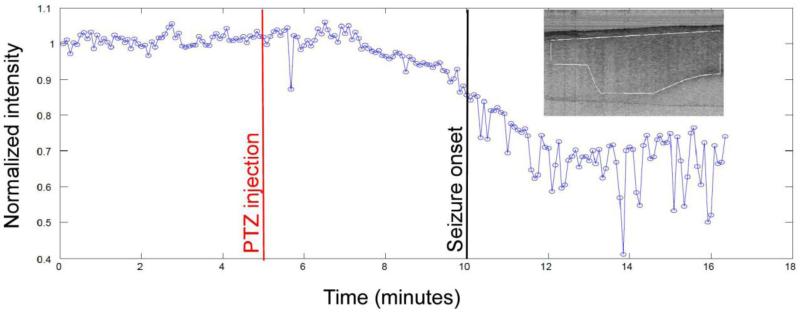
Figure 4. Reduction in OCT scattering signal prior to seizure onset.
Stable baseline OCT images were obtained for 5 minutes prior to administration of PTZ (red line). Inset shows region of interest (ROI) in brain cortex OCT image in which near-infrared (NIR) backscatter intensity was analyzed at every time point (every 5 seconds or 0.2 Hz) indicated by the blue circles. Approximately 10% reduction in intensity was observed prior to generalized seizure onset at 10 minutes (black line). These data provide proof-of-principle for real-time OCT-based seizure detection with excellent optical stability, sensitivity and temporal resolution. Courtesy of Eberle et al. (unpublished data).
Summary and conclusions
Detection of impending seizures prior to clinical manifestation would be groundbreaking in the treatment of epilepsy. Based on previous results demonstrating pre-ictal constriction of the extracellular space (ECS), we hypothesized that optical detection systems configured to detect changes in scattering of near-infrared (NIR) light can be developed for early seizure detection. Early results using fiberoptic NIR probes, SFDI, and OCT appear consistent with this hypothesis. Future directions include: (1) comparison of optical changes with new higher temporal resolution EEG modalities that detect high-frequency oscillations or “HFOs” in the seizure onset zone [31, 32, 34, 69]; (2) test the spatial resolution of OCT to determine seizure onset location in focal seizure models; and (3) develop a real-time predictive algorithm for early warning of seizure onset based on pre-ictal NIR scattering changes. A predictive algorithm would incorporate online interaction with the optical system, instantaneous computational analysis of optical reflectance and scattering, and real-time EEG analysis to alert of optical changes indicative of impending seizure.
Ultimately, a real-time seizure detector could in principle be integrated into existing closed-loop methods for seizure termination. Optical detection of seizure activity could be coupled to focal drug injection, deep brain stimulation, cortical cooling, or other seizure termination methods [70-74] to provide “closed-loop” control of seizures. Recently described optical methods of seizure termination could provide an all-optical solution [75, 76]. In summary, development and validation of optical seizure detection technology could provide an entirely new translational technology for the many patients with intractable epilepsy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.England MJ, Liverman CT, Schultz AM, Strawbridge LM. Committee on the Public Health Dimension of the Epilepsies: Institute of Medicine. The National Academies Press; Washington, DC: 2012. Epilepsy Across the Spectrum: Promoting Health and Understanding. [PubMed] [Google Scholar]
- 2.Dudek FE, Staley KJ. Seizure probability in animal models of acquired epilepsy: a perspective on the concept of the preictal state. Epilepsy Res. 2011;97:324–31. doi: 10.1016/j.eplepsyres.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Mormann F, Andrzejak RG, Elger CE, Lehnertz K. Seizure prediction: the long and winding road. Brain. 2007;130:314–33. doi: 10.1093/brain/awl241. [DOI] [PubMed] [Google Scholar]
- 4.Stacey W, Le Van Quyen M, Mormann F, Schulze-Bonhage A. What is the present-day EEG evidence for a preictal state? Epilepsy Res. 2011;97:243–51. doi: 10.1016/j.eplepsyres.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Haut SR, Lipton RB. Predicting seizures: a behavioral approach. Neurol Clin. 2009;27:925–40. doi: 10.1016/j.ncl.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Hughes J, Devinsky O, Feldmann E, Bromfield E. Premonitory symptoms in epilepsy. Seizure. 1993;2:201–3. doi: 10.1016/s1059-1311(05)80128-1. [DOI] [PubMed] [Google Scholar]
- 7.Rajna P, Clemens B, Csibri E, Dobos E, Geregely A, Gottschal M, György I, Horváth A, Horváth F, Mezöfi L, Velkey I, Veres J, Wagner E. Hungarian multicentre epidemiologic study of the warning and initial symptoms (prodrome, aura) of epileptic seizures. Seizure. 1997;6:361–8. doi: 10.1016/s1059-1311(97)80035-0. [DOI] [PubMed] [Google Scholar]
- 8.Schulze-Bonhage A, Kurth C, Carius A, Steinhoff BJ, Mayer T. Seizure anticipation by patients with focal and generalized epilepsy: a multicentre assessment of premonitory symptoms. Epilepsy Res. 2006;70:83–8. doi: 10.1016/j.eplepsyres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Scaramelli A, Braga P, Avellanal A, Bogacz A, Camejo C, Rega I, Messano T, Arciere B. Prodromal symptoms in epileptic patients: clinical characterization of the preictal phase. Seizure. 2009;18:246–50. doi: 10.1016/j.seizure.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Pinikahana J, Dono J. The lived experience of initial symptoms of and factors triggering epileptic seizures. Epilepsy Behav. 2009;15:513–20. doi: 10.1016/j.yebeh.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Haut SR, Hall CB, Borkowski T, Tennen H, Lipton RB. Clinical features of the pre-ictal state: mood changes and premonitory symptoms. Epilepsy Behav. 2012;23:415–21. doi: 10.1016/j.yebeh.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Maiwald T, Blumberg J, Timmer J, Schulze-Bonhage A. Are prodromes preictal events? A prospective PDA-based study. Epilepsy Behav. 2011;21:184–8. doi: 10.1016/j.yebeh.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Taylor DC. Whatever happened to the “epileptic prodrome”? Epilepsy Behav. 2007;11:251–2. doi: 10.1016/j.yebeh.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Schulze-Bonhage A, Haut S. Premonitory features and seizure self-prediction: artifact or real? Epilepsy Res. 2011;97:231–5. doi: 10.1016/j.eplepsyres.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Neugebauer R, Paik M, Hauser WA, Nadel E, Leppik I, Susser M. Stressful life events and seizure frequency in patients with epilepsy. Epilepsia. 1994;35:336–43. doi: 10.1111/j.1528-1157.1994.tb02441.x. [DOI] [PubMed] [Google Scholar]
- 16.Swinkels WA, Engelsman M, Kasteleijn-Nolst Trenite DG, Baal MG, de Haan GJ, Oosting J. Influence of an evacuation in February 1995 in The Netherlands on the seizure frequency in patients with epilepsy: a controlled study. Epilepsia. 1998;39:1203–7. doi: 10.1111/j.1528-1157.1998.tb01312.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakken KO, Solaas MH, Kjeldsen MJ, Friis ML, Pellock JM, Corey LA. Which seizure-precipitating factors do patients with epilepsy most frequently report? Epilepsy Behav. 2005;6:85–9. doi: 10.1016/j.yebeh.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Badawy RA, Curatolo JM, Newton M, Berkovic SF, Macdonell RA. Sleep deprivation increases cortical excitability in epilepsy: syndrome-specific effects. Neurology. 2006;67:1018–22. doi: 10.1212/01.wnl.0000237392.64230.f7. [DOI] [PubMed] [Google Scholar]
- 19.Herzog AG, Fowler KM, Sperling MR, Liporace JD, Kalayjian LA, Heck CN, Krauss GL, Dworetzky BA, Pennell PB. Variation of seizure frequency with ovulatory status of menstrual cycles. Epilepsia. 2011;52:1843–8. doi: 10.1111/j.1528-1167.2011.03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haut SR, Hall CB, Masur J, Lipton RB. Seizure occurrence: precipitants and prediction. Neurology. 2007;69:1905–10. doi: 10.1212/01.wnl.0000278112.48285.84. [DOI] [PubMed] [Google Scholar]
- 21.Haut SR, Hall CB, LeValley AJ, Lipton RB. Can patients with epilepsy predict their seizures? Neurology. 2007;68:262–6. doi: 10.1212/01.wnl.0000252352.26421.13. [DOI] [PubMed] [Google Scholar]
- 22.DuBois JM, Boylan LS, Shiyko M, Barr WB, Devinsky O. Seizure prediction and recall. Epilepsy Behav. 2010;18:106–9. doi: 10.1016/j.yebeh.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viglione SS, Walsh GO. Proceedings: Epileptic seizure prediction. Electroencephalogr Clin Neurophysiol. 1975;39:435–6. [PubMed] [Google Scholar]
- 24.Litt B, Echauz J. Prediction of epileptic seizures. Lancet Neurology. 2003;1:22–30. doi: 10.1016/s1474-4422(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 25.Jiruska P, Csicsvari J, Powell AD, Fox JE, Chang WC, Vreugdenhil M, Li X, Palus M, Bujan AF, Dearden RW, Jefferys JG. High-frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J Neurosci. 2010;30:5690–701. doi: 10.1523/JNEUROSCI.0535-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mormann F, Kreuz T, Rieke C, Andrzejak RG, Kraskov A, David P, Elger CE, Lehnertz K. On the predictability of epileptic seizures. Clin Neurophysiol. 2005;116:569–87. doi: 10.1016/j.clinph.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Lehnertz K, Mormann F, Osterhage H, Muller A, Prusseit J, Chernihovskyi A, Staniek M, Krug D, Bialonski S, Elger CE. State-of-the-art of seizure prediction. J Clin Neurophysiol. 2007;24:147–53. doi: 10.1097/WNP.0b013e3180336f16. [DOI] [PubMed] [Google Scholar]
- 28.Schevon CA, Trevelyan AJ, Schroeder CE, Goodman RR, McKhann G, Jr., Emerson RG. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain. 2009;132:3047–59. doi: 10.1093/brain/awp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schevon CA, Cappell J, Emerson R, Isler J, Grieve P, Goodman R, McKhann G, Jr., Weiner H, Doyle W, Kuzniecky R, Devinsky O, Gilliam F. Cortical abnormalities in epilepsy revealed by local EEG synchrony. NeuroImage. 2007;35:140–8. doi: 10.1016/j.neuroimage.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stead M, Bower M, Brinkmann BH, Lee K, Marsh WR, Meyer FB, Litt B, Van Gompel J, Worrell GA. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010;133:2789–97. doi: 10.1093/brain/awq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–37. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engel J, Jr., Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 33.Bragin A, Mody I, Wilson CL, Engel J., Jr. Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–21. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- 35.van Putten MJ. Nearest neighbor phase synchronization as a measure to detect seizure activity from scalp EEG recordings. J Clin Neurophysiol. 2003;20:320–5. doi: 10.1097/00004691-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Navakatikyan MA, Colditz PB, Burke CJ, Inder TE, Richmond J, Williams CE. Seizure detection algorithm for neonates based on wave-sequence analysis. Clin Neurophysiol. 2006;117:1190–203. doi: 10.1016/j.clinph.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 37.van Putten MJ, Kind T, Visser F, Lagerburg V. Detecting temporal lobe seizures from scalp EEG recordings: a comparison of various features. Clin Neurophysiol. 2005;116:2480–9. doi: 10.1016/j.clinph.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 38.Andrzejak RG, Chicharro D, Elger CE, Mormann F. Seizure prediction: any better than chance? Clin Neurophysiol. 2009;120:1465–78. doi: 10.1016/j.clinph.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Schulze-Bonhage A, Sales F, Wagner K, Teotonio R, Carius A, Schelle A, Ihle M. Views of patients with epilepsy on seizure prediction devices. Epilepsy Behav. 2010;18:388–96. doi: 10.1016/j.yebeh.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Traynelis SF, Dingledine R. Role of extracellular space in hyperosmotic suppression of potassium-induced electrographic seizures. J Neurophysiol. 1989;61:927–38. doi: 10.1152/jn.1989.61.5.927. [DOI] [PubMed] [Google Scholar]
- 41.Roper SN, Obenaus A, Dudek FE. Osmolality and nonsynaptic epileptiform bursts in rat CA1 and dentate gyrus. Ann Neurol. 1992;31:81–5. doi: 10.1002/ana.410310115. [DOI] [PubMed] [Google Scholar]
- 42.Heinemann U, Dietzel I. Extracellular potassium concentration in chronic alumina cream foci of cats. J Neurophysiol. 1984;52:421–434. doi: 10.1152/jn.1984.52.3.421. [DOI] [PubMed] [Google Scholar]
- 43.Dietzel I, Heinemann U. Dynamic variations of the brain cell microenvironment in relation to neuronal hyperactivity. Ann N Y Acad Sci. 1986;481:72–86. doi: 10.1111/j.1749-6632.1986.tb27140.x. [DOI] [PubMed] [Google Scholar]
- 44.Lux HD, Heinemann U, Dietzel I. Ionic changes and alterations in the size of the extracellular space during epileptic activity. Adv Neurol. 1986;44:619–39. [PubMed] [Google Scholar]
- 45.Binder DK, Papadopoulos MC, Haggie PM, Verkman AS. In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J Neurosci. 2004;24:8049–56. doi: 10.1523/JNEUROSCI.2294-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broberg M, Pope KJ, Lewis T, Olsson T, Nilsson M, Willoughby JO. Cell swelling precedes seizures induced by inhibition of astrocytic metabolism. Epilepsy Res. 2008;80:132–41. doi: 10.1016/j.eplepsyres.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Olsson T, Broberg M, Pope KJ, Wallace A, Mackenzie L, Blomstrand F, Nilsson M, Willoughby JO. Cell swelling, seizures and spreading depression: an impedance study. Neuroscience. 2006;140:505–15. doi: 10.1016/j.neuroscience.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 48.McBain CJ, Traynelis SF, Dingledine R. Regional variation of extracellular space in the hippocampus. Science. 1990;249:674–7. doi: 10.1126/science.2382142. [DOI] [PubMed] [Google Scholar]
- 49.Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta. 2006;1758:1085–93. doi: 10.1016/j.bbamem.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Binder DK, Nagelhus EA, Ottersen OP. Aquaporin-4 and epilepsy. Glia. 2012;60:1203–14. doi: 10.1002/glia.22317. [DOI] [PubMed] [Google Scholar]
- 51.Andrew RD, Labron MW, Boehnke SE, Carnduff L, Kirov SA. Physiological evidence that pyramidal neurons lack functional water channels. Cereb Cortex. 2007;17:787–802. doi: 10.1093/cercor/bhk032. [DOI] [PubMed] [Google Scholar]
- 52.Hsu MS, Seldin M, Lee DJ, Seifert G, Steinhauser C, Binder DK. Laminar-specific and developmental expression of aquaporin-4 in the mouse hippocampus. Neuroscience. 2011;178:21–32. doi: 10.1016/j.neuroscience.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–81. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gill AS, Rajneesh KF, Owen CM, Yeh J, Hsu M, Binder DK. Early optical detection of cerebral edema in vivo. J Neurosurg. 2011;114:470–477. doi: 10.3171/2010.2.JNS091017. [DOI] [PubMed] [Google Scholar]
- 55.Grinvald A, Hildesheim R. VSDI: a new era in functional imaging of cortical dynamics. Nat Rev Neurosci. 2004;5:874–85. doi: 10.1038/nrn1536. [DOI] [PubMed] [Google Scholar]
- 56.Chen-Bee CH, Polley DB, Brett-Green B, Prakash N, Kwon MC, Frostig RD. Visualizing and quantifying evoked cortical activity assessed with intrinsic signal imaging. J Neurosci Methods. 2000;97:157–73. doi: 10.1016/s0165-0270(00)00180-1. [DOI] [PubMed] [Google Scholar]
- 57.Bahar S, Suh M, Zhao M, Schwartz TH. Intrinsic optical signal imaging of neocortical seizures: the ‘epileptic dip’. Neuroreport. 2006;17:499–503. doi: 10.1097/01.wnr.0000209010.78599.f5. [DOI] [PubMed] [Google Scholar]
- 58.Haglund M, Hochman D. Optical imaging of epileptiform activity in human neocortex. Epilepsia. 2004;45(Suppl 4):43–7. doi: 10.1111/j.0013-9580.2004.04010.x. [DOI] [PubMed] [Google Scholar]
- 59.Dudek FE, Obenaus A, Tasker JG. Osmolality-induced changes in extracellular volume alter epileptiform bursts independent of chemical synapses in the rat: importance of non-synaptic mechanisms in hippocampal epileptogenesis. Neurosci Lett. 1990;120:267–70. doi: 10.1016/0304-3940(90)90056-f. [DOI] [PubMed] [Google Scholar]
- 60.Buchheim K, Wessel O, Siegmund H, Schuchmann S, Meierkord H. Processes and components participating in the generation of intrinsic optical signal changes in vitro. Eur J Neurosci. 2005;22:125–32. doi: 10.1111/j.1460-9568.2005.04203.x. [DOI] [PubMed] [Google Scholar]
- 61.Weber JR, Cuccia DJ, Johnson WR, Bearman GH, Durkin AJ, Hsu M, Lin A, Binder DK, Wilson D, Tromberg BJ. Multispectral imaging of tissue absorption and scattering using spatial frequency domain imaging and a computed-tomography imaging spectrometer. J Biomed Opt. 2011;16:011015. doi: 10.1117/1.3528628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knuttel A, Boehlau-Godau M. Spatially confined and temporally resolved refractive index and scattering evaluation in human skin performed with optical coherence tomography. J Biomed Opt. 2000;5:83–92. doi: 10.1117/1.429972. [DOI] [PubMed] [Google Scholar]
- 64.Schmitt JM, Knuttel A, Bonner RF. Measurement of optical properties of biological tissues by low-coherence reflectometry. Applied Optics. 1993;32:6032–6042. doi: 10.1364/AO.32.006032. [DOI] [PubMed] [Google Scholar]
- 65.Farooq MU, Khasnis A, Majid A, Kassab MY. The role of optical coherence tomography in vascular medicine. Vasc Med. 2009;14:63–71. doi: 10.1177/1358863X08095153. [DOI] [PubMed] [Google Scholar]
- 66.Galetta KM, Calabresi PA, Frohman EM, Balcer LJ. Optical coherence tomography (OCT): imaging the visual pathway as a model for neurodegeneration. Neurotherapeutics. 2011;8:117–32. doi: 10.1007/s13311-010-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szu JI, Eberle MM, Reynolds CL, Hsu MS, Park BH, Binder DK. Thinned-skull cortical window technique for in vivo optical coherence tomography imaging. JoVE. 2012 doi: 10.3791/50053. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park BH, Pierce MC, Cense B, Yun SH, Mujat M, Tearney GJ, Bouma BE, de Boer JF. Real-time fiber-based multi-functional spectral-domain optical coherence tomography at 1.3 mu m. Optics Express. 2005;13:3931–3944. doi: 10.1364/opex.13.003931. [DOI] [PubMed] [Google Scholar]
- 69.Blanco JA, Stead M, Krieger A, Viventi J, Marsh WR, Lee KH, Worrell GA, Litt B. Unsupervised classification of high-frequency oscillations in human neocortical epilepsy and control patients. J Neurophysiol. 2010;104:2900–12. doi: 10.1152/jn.01082.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Franaszczuk PJ, Kudela P, Bergey GK. External excitatory stimuli can terminate bursting in neural network models. Epilepsy Res. 2003;53:65–80. doi: 10.1016/s0920-1211(02)00248-6. [DOI] [PubMed] [Google Scholar]
- 71.Ludvig N, Kuzniecky RI, Baptiste SL, John JE, von Gizycki H, Doyle WK, Devinsky O. Epidural pentobarbital delivery can prevent locally induced neocortical seizures in rats: the prospect of transmeningeal pharmacotherapy for intractable focal epilepsy. Epilepsia. 2006;47:1792–802. doi: 10.1111/j.1528-1167.2006.00642.x. [DOI] [PubMed] [Google Scholar]
- 72.Yang X, Duffy D, Morley R, Rothman S. Neocortical seizure termination by focal cooling: temperature dependence and automated seizure detection. Epilepsia. 2002;43:240–5. doi: 10.1046/j.1528-1157.2002.33301.x. [DOI] [PubMed] [Google Scholar]
- 73.Yang XF, Chang JH, Rothman SM. Long-lasting anticonvulsant effect of focal cooling on experimental neocortical seizures. Epilepsia. 2003;44:1500–5. doi: 10.1111/j.0013-9580.2003.23003.x. [DOI] [PubMed] [Google Scholar]
- 74.Stacey WC, Litt B. Technology insight: neuroengineering and epilepsy-designing devices for seizure control. Nat Clin Pract Neurol. 2008;4:190–201. doi: 10.1038/ncpneuro0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rothman SM, Perry G, Yang XF, Hyrc K, Schmidt BF. Optical suppression of seizure-like activity with an LED. Epilepsy Res. 2007;74:201–9. doi: 10.1016/j.eplepsyres.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–9. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]