Abstract
Treatment of cells with carbonyl cyanide m-chlorophenylhydrazone (CCCP), a mitochondrial proton gradient uncoupler, can result in mitochondrial damage and autophagy activation, which in turn eliminates the injured mitochondria in a Parkin-dependent way. How CCCP mobilizes the autophagy machinery is not fully understood. By analyzing a key autophagy step, LC3 lipidation, we examined the roles of two kinase complexes typically involved in the initiation and nucleation phases of autophagy, namely the ULK kinase complex (UKC) and the Beclin 1/Atg14 complex. We found that CCCP-induced LC3 lipidation could be independent of Beclin 1 and Atg14. In addition, deletion or knockdown of the UKC component FIP200 or Atg13 only led to a partial reduction in LC3 lipidation, indicating that UKC could be also dispensable for this step during CCCP treatment. In contrast, Atg9, which is important for transporting vesicles to early autophagosomal structure, was required for CCCP-induced LC3 lipidation. Taken together, these data suggest that CCCP-induced autophagy and mitophagy depends more critically on Atg9 vesicles than on UKC and Beclin 1/Atg14 complex.
Keywords: autophagy, CCCP, Atg9, Beclin 1, FIP200, Atg13, Atg14, mitophagy
Introduction
Macroautophagy (hereafter referred to as autophagy) represents an evolutionarily conserved self-degradation process, in which cellular constituents are sequestered into double-membraned autophagosomes and delivered to the lysosome for hydrolytic digestion [1; 2]. The autophagy machinery is controlled by autophagy-associated (Atg) proteins. More than 30 Atg genes have so far been characterized in yeast [1; 2; 3]. The mammalian homologues of most Atg proteins have been identified. Among them, the mammalian ULK kinase complex (UKC) is composed of ULK1/ULK2, Atg13, focal adhesion kinase family interactional protein of 200 kD (FIP200) and Atg101, which can be regulated by metabolic signals through the mammalian target of rapamycin (mTOR) [4; 5]. The autophagy-specific class Ⅲ phosphatidylinositol 3-kinase complex (PI-3KCIII), or the Beclin 1/Atg14 complex, has been identified as a Beclin 1-Atg14- Vps34-Vps15 complex [6; 7]. The hierarchical relationship of Atg proteins has been well established in canonical autophagy, such as that stimulated by nutrient deprivation [8], in which the above two kinase complexes are required for the initiation and nucleation of autophagosomes. Beclin 1 complex plays an essential role in bridging the upstream UKC to the downstream Atg12-Atg5-Atg16 complex [7; 9], and the Atg8/microtube-associated protein light chain 3 (LC3) conjugation system [10]. Finally, vesicle transportation by Atg9-mediated process is critical in the assembly of pre-autophagosomal structure [11].
Carbonyl cyanide m-chlorophenylhydrazone (CCCP) is known as an uncoupling agent, increasing the proton permeability across the mitochondrial inner membranes and thus depolarizing the mitochondria. CCCP has been used extensively in recent years to study mitochondrial damage and to induce autophagic degradation of damaged mitochondria (i.e. mitophagy) [12]. Previous studies demonstrated that CCCP can induce autophagy via a ROSmediated mechanism [13], and anti-oxidants, such as N-acetyl cysteine, can inhibit autophagy induced by CCCP. CCCP-induced autophagy and LC3 lipidation required the Atg7 and Atg5- mediated conjugation system [13]. In addition, the depolarized mitochondria were targeted by structures containing ULK1, Atg14 or Atg9 [14]. However, the contribution of these canonical Atg proteins to CCCP-induced autophagy remained unknown. In this study we used genetically manipulated cells to define the role of UKC, Beclin 1/Atg14 complex and Atg9 using LC3 lipidation as the parameter. We found that the importance of these molecules varies in CCCPinduced autophagy.
Materials and Methods
Reagents and Antibodies
The following primary antibodies were used: anti-FIP200 (Gene Tex, Irvine, CA); anti-Atg13 and anti-β-actin (Sigma-Aldrich, St. Louis MO); anti-LC3 (MBL International, Woburn, MA); anti-Beclin 1 (Santa Cruz Biotechnology, Dallas, TX); anti-Atg14 (Cell Signaling Technology, Danvers, MA); anti-Atg9 (Abgent, San Diego, CA); and peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA). The Atg13 and Atg14 shRNA were purchased from Santa Cruz Biotechnology (catalogue # sc- 97013) and Cell Signaling Technology (catalogue #6286), respectively.
Cell Culture and Fluorescence Microscopy
All cells were maintained in DMEM with 10% FBS and the standard supplements. Mouse embryonic fibroblasts (MEF), and human cervical cancer cell line HeLa stably expressing GFP-LC3 were constructed as previously described [13; 15]. FIP200-deficient and Atg9A-deficient MEFs, and the glioblastoma cell line U251 with constitutive knock-down of Beclin 1 had been described [16; 17; 18]. Some experiments were conducted with a prior infection of the cells with an adenoviral vector encoding GFP-LC3. For gene knock-down experiments, Atg13 or Atg14 siRNA (100 nM) was transfected into 1x106 cells using Lipofectamine2000 (Invitrogen) for 48 h. To induce autophagy, cells were treated with CCCP (20 µM) for 6 h, or cultured in Earle’s balanced salt solution (EBSS) in the presence or absence of chloroquine (CQ, 10 µM) (Sigma) for 4 h. Images were acquired using an inverted epifluorescence microscope (Nikon Eclipse TE 2000). GFP-LC3 punctation were quantified and calculated as the average number of puncta per cell.
Immunoblotting Assay
Cells (5×105 per well) were seeded into 6-well plates for indicated treatments, washed in PBS and lysed in RIPA buffer with protease/phosphatase inhibitors (Sigma). Thirty micrograms of protein was separated by SDS-PAGE and then transferred to PVDF membranes. The membranes were probed with the indicated antibodies and developed with SuperSignal West Pico chemiluminescent substrate (Pierce). Images were taken using a Kodak Image 4000MM with the companion Software (Carestream Health).
Statistical analysis
Each experiment was performed with replicates for at least three times. Images were acquired from multiple randomly selected fields for each group. At least 100 or more cells per condition were analyzed for quantification. Data were presented as mean±S.D, which were subjected to One-Way ANOVA with post-hoc analysis using the GraphPad software. P<0.05 was considered significant.
Results and Discussion
CCCP is able to initiate autophagy in FIP200 or Atg13-deficient cells
A well-defined example of autophagy is the one induced by starvation, in which mTOR is inhibited, which in turn allows the activation of UKC to initiate autophagy [19]. We had previously shown that CCCP can inhibit mTOR via reactive oxygen species [13]. We thus investigated whether CCCPinduced autophagy would depend on UKC.
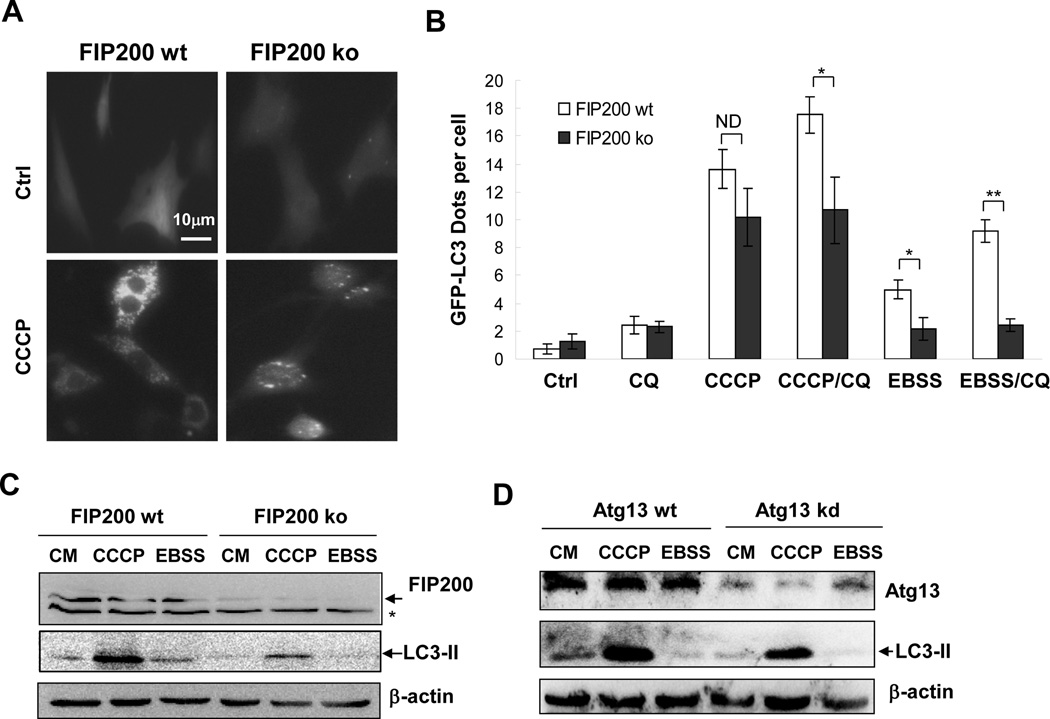
We assessed the autophagy process by measuring LC3 lipidation, a well-defined parameter for autophagy, using both fluorescence microscopy and immunoblot assay. MEFs deficient in FIP200, a key component of UKC were unable to mount autophagy response to starvation [16]. Indeed, starvation of cells by incubating them in EBSS induced an upregulation of GFP-LC3 puncta (Figure 1A–B) and LC3-II (Figure 1C) in wild type MEFs but not in FIP200-deficient MEF. Addition of a lysosome inhibitor, such as chloroquine (CQ), blocked the degradation of GFP-LC3, thus further elevating the number of GFL-LC3 dots in starved wild type cells, but again not in starved FIP200-deficient cells. The data confirmed the dependence of starvationinduced autophagy on FIP200. As previously shown [13] CCCP induced both GFP-LC3 puncta and LC3-II in wild type MEFs, which was dependent on Atg5 and Atg7. The level of LC3 lipidation was more significant than that induced by starvation. Strikingly, LC3 lipidation remained prominent, although reduced, in FIP200-deficient cells, suggesting that CCCP, unlike starvation, could still promote LC3 lipidation in the absence of FIP200.
Figure 1. CCCP induces LC3 lipidation in cells with deficient FIP200 or Atg13 expression.
(A–C). Wild type (wt) and FIP200-deficient (ko) MEFs stably expressing GFP-LC3 were treated with CCCP or EBSS in the presence or absence of CQ. Fluorescent images were taken (A) for quantification of GFP-LC3 punctation (B). Cell lysates were analyzed by immunoblotting assay with indicated antibodies (C). The asterisk indicates a nonspecific band. (D). HeLa cells with or without Atg13 knocked down by a specific siRNA were treated with CCCP with or without CQ. Cell lysates were prepared for immunoblotting assay with indicated antibodies (A). *: P<0.05; **: P<0.01; ND: no statistical difference.
We then explored the importance of another key component of UKC, Atg13, in CCCP-induced autophagy. Immunoblotting analysis confirmed the reduction of Atg13 protein level following the transfection of a specific siRNA into HeLa cells (Figure 1D). However, the level of LC3-II was only slightly decreased in the knockdown cells after CCCP treatment (Figure 1D). Atg13 knockdown also resulted in no significant change in GFP-LC3 dots upon CCCP challenge (data not shown). These observations suggested that CCCP could employ a FIP200/Atg13- independent pathway to activate autophagy in addition to the FIP200/Atg13-dependent mechanism.
CCCP can induce LC3 lipidation in the absence of functional Beclin 1/Atg14 complex
In starvation-induced autophagy the Beclin 1/Atg14 complex acts downstream of UKC and plays important roles in the nucleation of autophagosomal membrane [8]. To determine the role of Beclin-1/Atg14 complex in CCCP-induced autophagy, we first analyzed a glioblastoma cell line (U251), which stably expressed a Beclin-1-specific shRNA construct [18]. Immunoblotting assay confirmed Beclin 1 knock-down, which was accompanied by a decrease of Atg14 expression (Figure 2A). The Beclin 1 complex components stabilize each other so that a reduction in one component can result in disintegration of the whole complex and the reduction in other components [6; 7]. As anticipated starvation- triggered elevation in GFP-LC3 punctation and LC3-II were observed in wild type, but not in Beclin 1 knockdown U251 cells (Fig. 2A–B).
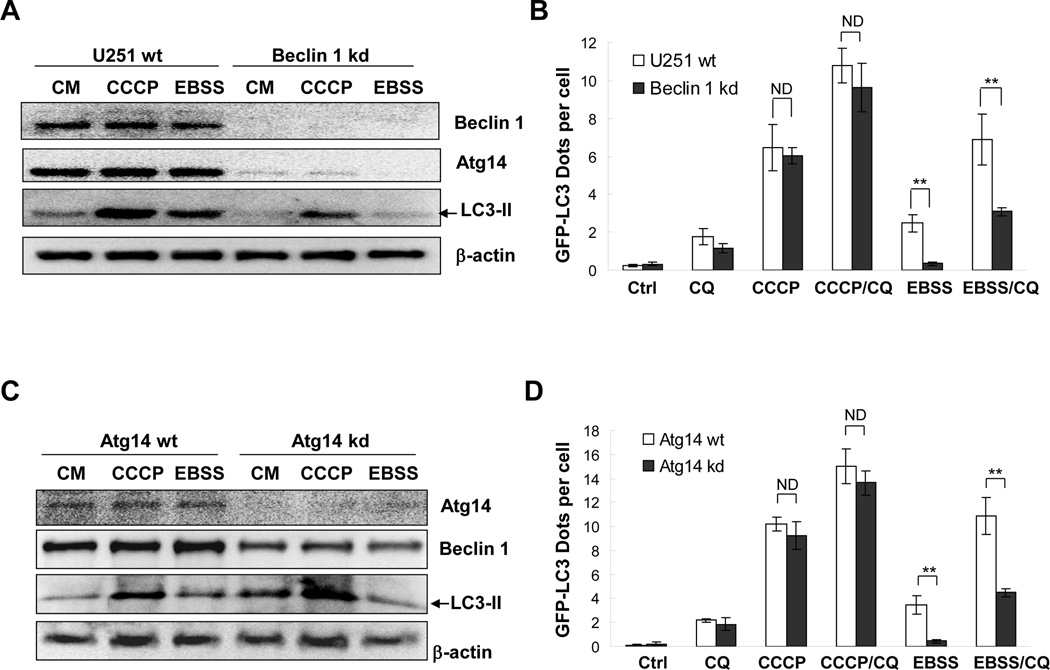
Figure 2. CCCP induces LC3 lipidation in cells with compromised Beclin 1/Atg14 complex.
(A–B). Wild type (wt) U251 cells and U251 cells with constitutive Beclin 1 knockdown (kd) were infected with Ad-GFP-LC3 overnight and then treated with CCCP or EBSS in the presence or absence of CQ. Cell lysates were analyzed by immunoblotting assays with indicated antibodies (A). Fluorescent images were taken for quantification of GFP-LC3 punctation (B). (C–D). HeLa cells expressing GFP-LC3 were transfected with Atg14-specific siRNAs for 48 hours and then treated with CCCP or EBSS in the presence or absence of CQ. Cell lysates were analyzed by immunoblotting assay with indicated antibodies (C). Fluorescent images were taken for quantification of GFP-LC3 punctation (D). *: P<0.05; **: P<0.01; ND: no statistical difference.
Following the treatment with CCCP there was no difference in GFP-LC3 punctation between Beclin 1-wild type and -knockdown cells (Figure 2A–B). Both types of cells had significantly increased GFP-LC3 punctation following CCCP treatment. Consistently, the level of lipidated LC3-II also remained high in the absence of Beclin 1(Figure 2A). These findings suggested that there were Beclin-1-independent mechanisms for CCCP-induced LC3 lipidation.
To confirm the finding with the Beclin-1 knockdown cells we inhibited the expression of Atg14 in HeLa cells by transient transfection of a specific siRNA. Immunoblotting analysis indicated that the siRNA successfully diminished Atg14 expression and promoted a concomitant degradation of Beclin 1 (Figure 2C). We observed that Atg14 knockdown significantly suppressed starvation-induced GFP-LC3 punctation and LC3 lipidation (Figure 2C–D). In contrast, Atg14 knockdown resulted in no changes in GFP-LC3 punctation or LC3-II level after CCCP treatment (Figure 2C–D). Consistent with the above studies, we had also found that 3-MA, a class III PI-3K inhibitor, did not affect CCCP-induced LC3 lipidation (data not shown). Taken together, these experiments suggested that the Beclin 1/Atg14 complex was indispensable for starvation-induced autophagy, but dispensable for CCCP-initiated autophagy.
The Beclin 1-independent autophagy had been frequently seen in cases where ROS and/or mitochondrial damage are involved [20; 21; 22; 23], which is also the case in CCCP-induced autophagy. Indeed, CCCP-induced autophagy can be effectively suppressed by antioxidants [13]. ROS-triggered Beclin-1 independent autophagy had been associated with ERK and JNK [21; 24]. We found that ERK and JNK could be activated by CCCP and suppression of these kinases partially inhibited CCCP-induced autophagy (data not shown), suggesting that MAP kinases could play an important role in the signaling of CCCP-induced autophagy.
Atg9 contributes to CCCP-induced autophagy and mitophagy
Atg9 is the only membrane protein involved in autophagy and it shuttles between different compartments especially between the Golgi apparatus and endosomes [11; 25]. It is speculated to be a membrane carrier that contributes to autophagosome expansion. We thus would like to determine whether CCCPinduced autophagy would dependent on Atg9 by comparing the wild type and Atg9A-deficient MEFs [17].
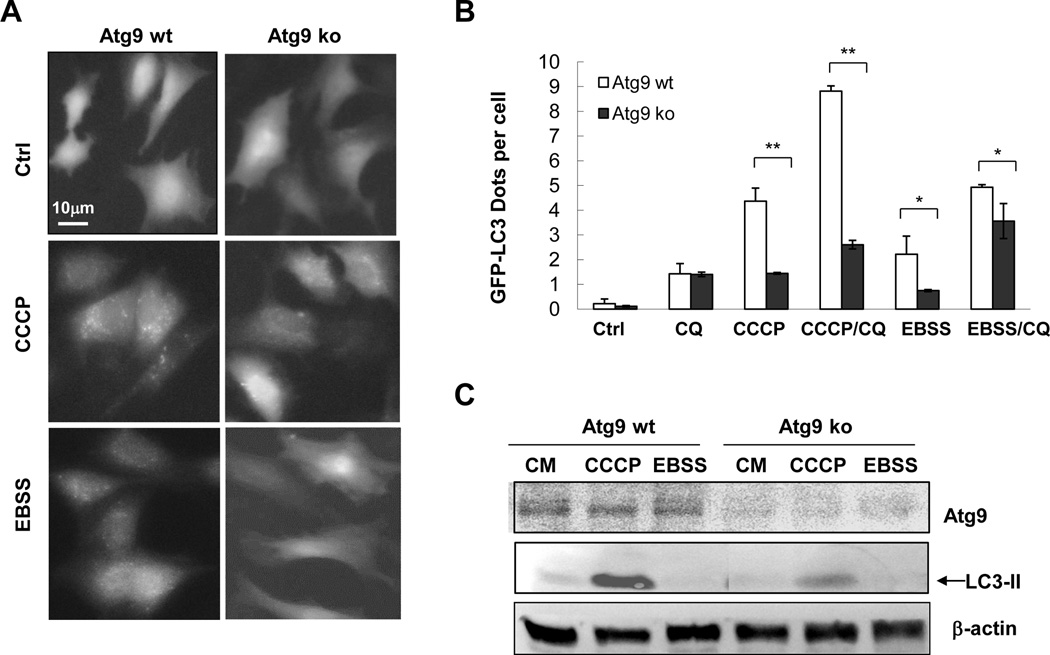
We found that consistent with previous reports [17; 26] loss of Atg9 significantly decreased EBSS-induced GFP-LC3 punctation (Figure 3A–B). Notably, Atg9 deletion also resulted in significantly reduced levels of GFP-LC3 puncta and LC3-II level following CCCP treatment (Figure 3A–C), indicating that Atg9 was important for autophagosome biogenesis induced by CCCP.
Figure 3. CCCP-induced LC3 lipidation is largely abrogated in Atg9-deficient cells.
Wild type (wt) and Atg9-deficient (ko) MEFs expressing GFP-LC3 were stimulated with CCCP or cultured in EBSS in the presence or absence of CQ. Fluorescent images were taken (A) for GFP-LC3 puncta quantification (B). Cell lysates were prepared for immunoblotting assay with the indicated antibodies (C). *: P<0.05; **: P<0.01.
Although Atg9 trafficking can be regulated by ULK1, Atg13 and PI-3 kinase activity [25; 27; 28] a recent study found that following CCCP treatment Atg9 and ULK1/Atg14 were independently recruited to depolarized mitochondria [14]. In addition, LC3 is also found to colocalize with the damaged mitochondria [12; 13]. Consistently we found that deletion of Atg9 reduced the colocalization of LC3 with the fragmented mitochondria following CCCP treatment (data not shown). It is thus possible that LC3 lipidation during CCCP-triggered mitophagy could be independently regulated by Atg9 and UKC/Beclin 1/Atg14, and that Atg9 may mediate a more crucial process since UKC could be dispensable (Figure 1).
To summarize, in the work presented here we demonstrate that CCCP-induced LC3 lipidation is more dependent on Atg9 than on UKC and Beclin 1 complex, suggesting that CCCP can utilize initiating mechanisms other than that of ULK and Beclin 1 complexes. Autophagy activation in the absence of ULK1 and/or ULK2 had been reported [29; 30]. Notable, a kinaseinactivated ULK1 could inhibit starvation-induced autophagy via an Atg13-indpenent mechanism [28], supporting the notion that other autophagy proteins could be involved in parallel to the classical UKC to activate autophagy.
Though not well defined a major role of UKC in autophagy would have to be related to the activation of the Beclin 1/Atg14-directed class III PI3-kinase. Thus it may not be surprising that Beclin 1 and Atg14 could be also dispensable for CCCP-induced LC3 lipidation when the UKC was dispensable. Interestingly, Beclin 1-independent autophagy has been well reported in several other studies [20; 31; 32] and has been considered as a form of non-canonical autophagy [33].
How does the Beclin-1-indepenednet pathway leading to the engagement of the conjugation system is still an open question and could vary in different scenarios. There are potentially many factors that could affect the Atg7-Atg5-LC3conjugation system, such as those affecting the level of phosphatidylinositol-3 phosphate, the extent of enzymatic reactions, the selection of the membrane source, and the processing/deconjugation of LC3 by Atg4. It is therefore important to sort through these different regulatory steps to determine whether disturbance at one of them could offer the compensation to the loss of Becclin-1 complex components. It is also possible that LC3 lipidation initiated by the mechanisms independent of UKC and/or Beclin 1/Atg14 may not be ultimately associated with productive autophagosomes and/or efficient autophagy process. Future works would have to focus more on the physiological significance of the UKC and Beclin 1/Atg14-independent LC3 lipidation and autophagy. In addition, careful dissections of the noncanonical pathway will likely lead to the revelation of new molecular information for autophagy regulation under different contexts.
Highlight.
CCCP-induced LC3 lipidation can be independent of initiation/nucleation molecules.
Atg9-mediated trafficking is critically required for CCCP-induced LC3 lipidation.
CCCP-induced mitophagy may thus be more dependent on Atg9-positive vesicles.
Acknowledgement
We thank Dr. William A. Maltese (University of Toledo Health Science Campus, Toledo, OH) for U251 cells expressing Beclin 1-specific shRNA, Dr. Jun-Lin Guan (University of Michigan, Ann Arbor, MI) for FIP200-deficient MEF, and Dr. Shizuo Akira (Osaka University, Japan) for Atg9-deficient MEF. This research was supported by a grant from the National Institutes of Health (CA111456) and funding from Department of Pathology and Laboratory Medicine, Indiana University School of Medicine to XMY.
Abbreviations
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- CQ
chloroquine
- EBSS
Earle’s balanced salt solution
- LC3
microtube-associated protein light chain 3
- MEFs
mouse embryonic fibroblasts
- UKC
ULK kinase complex.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mehrpour M, Esclatine A, Beau I, Codogno P. Overview of macroautophagy regulation in mammalian cells. Cell Res. 2010;20:748–762. doi: 10.1038/cr.2010.82. [DOI] [PubMed] [Google Scholar]
- 2.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki K, Ohsumi Y. Molecular machinery of autophagosome formation in yeast, Saccharomyces cerevisiae. FEBS Lett. 2007;581:2156–2161. doi: 10.1016/j.febslet.2007.01.096. [DOI] [PubMed] [Google Scholar]
- 4.Ganley IG, Lam du H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, Mizushima N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol. 2008;181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell. 2008;19:5360–5372. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT, Heintz N, Yue Z. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol. 2009;11:468–476. doi: 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itakura E, Mizushima N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy. 2010;6:764–776. doi: 10.4161/auto.6.6.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weidberg H, Shpilka T, Shvets E, Abada A, Shimron F, Elazar Z. LC3 and GATE-16 N termini mediate membrane fusion processes required for autophagosome biogenesis. Dev Cell. 2011;20:444–454. doi: 10.1016/j.devcel.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto H, Kakuta S, Watanabe TM, Kitamura A, Sekito T, Kondo-Kakuta C, Ichikawa R, Kinjo M, Ohsumi Y. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. The Journal of cell biology. 2012;198:219–233. doi: 10.1083/jcb.201202061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB, Dorn GW, 2nd, Yin XM. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem. 2010;285:27879–27890. doi: 10.1074/jbc.M110.119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itakura E, Kishi-Itakura C, Koyama-Honda I, Mizushima N. Structures containing Atg9A and the ULK1 complex independently target depolarized mitochondria at initial stages of Parkin-mediated mitophagy. Journal of cell science. 2012;125:1488–1499. doi: 10.1242/jcs.094110. [DOI] [PubMed] [Google Scholar]
- 15.Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, Stolz DB, Shao ZM, Yin XM. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007;282:4702–4710. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- 16.Gan B, Peng X, Nagy T, Alcaraz A, Gu H, Guan JL. Role of FIP200 in cardiac and liver development and its regulation of TNFalpha and TSC-mTOR signaling pathways. J Cell Biol. 2006;175:121–133. doi: 10.1083/jcb.200604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saitoh T, Fujita N, Hayashi T, Takahara K, Satoh T, Lee H, Matsunaga K, Kageyama S, Omori H, Noda T, Yamamoto N, Kawai T, Ishii K, Takeuchi O, Yoshimori T, Akira S. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci U S A. 2009;106:20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;119:259–270. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- 19.Chan EY. Regulation and function of uncoordinated-51 like kinase proteins. Antioxidants & redox signaling. 2012;17:775–785. doi: 10.1089/ars.2011.4396. [DOI] [PubMed] [Google Scholar]
- 20.Chu CT, Zhu J, Dagda R. Beclin 1-independent pathway of damage-induced mitophagy and autophagic stress: implications for neurodegeneration and cell death. Autophagy. 2007;3:663–666. doi: 10.4161/auto.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong CH, Iskandar KB, Yadav SK, Hirpara JL, Loh T, Pervaiz S. Simultaneous induction of non-canonical autophagy and apoptosis in cancer cells by ROS-dependent ERK and JNK activation. PLoS One. 2010;5:e9996. doi: 10.1371/journal.pone.0009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo G, Kim SK, Byun YJ, Oh E, Jeong SW, Chae GT, Lee SB. Hydrogen peroxide induces Beclin 1-independent autophagic cell death by suppressing the mTOR pathway via promoting the ubiquitination and degradation of Rheb in GSH-depleted RAW 264.7 cells. Free Radic Res. 2011;45:389–399. doi: 10.3109/10715762.2010.535530. [DOI] [PubMed] [Google Scholar]
- 23.Smith DM, Patel S, Raffoul F, Haller E, Mills GB, Nanjundan M. Arsenic trioxide induces a beclin-1-independent autophagic pathway via modulation of SnoN/SkiL expression in ovarian carcinoma cells. Cell Death Differ. 2010;17:1867–1881. doi: 10.1038/cdd.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4- phenylpyridinium-induced cell death. Am J Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young AR, Chan EY, Hu XW, Kochl R, Crawshaw SG, High S, Hailey DW, Lippincott-Schwartz J, Tooze SA. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006;119:3888–3900. doi: 10.1242/jcs.03172. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Li M, Chen D, Gao W, Guan JL, Komatsu M, Yin XM. Autophagy induced by calcium phosphate precipitates involves endoplasmic reticulum membranes in autophagosome biogenesis. PloS one. 2012;7:e52347. doi: 10.1371/journal.pone.0052347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reggiori F, Tucker KA, Stromhaug PE, Klionsky DJ. The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Dev Cell. 2004;6:79–90. doi: 10.1016/s1534-5807(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 28.Chan EY, Longatti A, McKnight NC, Tooze SA. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol Cell Biol. 2009;29:157–171. doi: 10.1128/MCB.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheong H, Lindsten T, Wu J, Lu C, Thompson CB. Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc Natl Acad Sci U S A. 2011;108:11121–11126. doi: 10.1073/pnas.1107969108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alers S, Loffler AS, Paasch F, Dieterle AM, Keppeler H, Lauber K, Campbell DG, Fehrenbacher B, Schaller M, Wesselborg S, Stork B. Atg13 and FIP200 act independently of Ulk1 and Ulk2 in autophagy induction. Autophagy. 2011;7:1423–1433. doi: 10.4161/auto.7.12.18027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R. Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 2008;15:1318–1329. doi: 10.1038/cdd.2008.51. [DOI] [PubMed] [Google Scholar]
- 32.Tian S, Lin J, Jun Zhou J, Wang X, Li Y, Ren X, Yu W, Zhong W, Xiao J, Sheng F, Chen Y, Jin C, Li S, Zheng Z, Xia B. Beclin 1-independent autophagy induced by a Bcl-XL/Bcl-2 targeting compound, Z18. Autophagy. 2010;6:1032–1041. doi: 10.4161/auto.6.8.13336. [DOI] [PubMed] [Google Scholar]
- 33.Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat Rev Mol Cell Biol. 2011;13:7–12. doi: 10.1038/nrm3249. [DOI] [PubMed] [Google Scholar]