Abstract
BACKGROUND
Methadone maintenance for heroin dependence reduces illicit drug use, crime, HIV risk, and death. Typical dosages have increased over the past few years, based on strong experimental and clinical evidence that dosages under 60 mg/day are inadequate and that dosages closer to 100 mg/day produce better outcomes. However, there is little experimental evidence for the benefits of exceeding 100 mg/day, or for individualizing methadone dosages. We sought to provide such evidence.
METHODS
We combined individualized methadone dosages over 100 mg/day with voucher-based cocaine-targeted contingency management (CM) in 58 heroin- and cocaine-dependent outpatients. Participants were randomly assigned to receive a fixed dose increase from 70 mg/day to 100 mg/day, or to be eligible for further dose increases (up to 190 mg/day, based on withdrawal symptoms, craving, and continued heroin use). All dosing was double-blind. The main outcome measure was simultaneous abstinence from heroin and cocaine.
RESULTS
We stopped the study early due to slow accrual. Cocaine-targeted CM worked as expected to reduce cocaine use. Polydrug use (effect-size h = .30) and heroin craving (effect-size d = .87) were significantly greater in the flexible/high-dose condition than in the fixed-dose condition, with no trend toward lower heroin use in the flexible/high-dose participants.
CONCLUSIONS
Under double-blind conditions, dosages of methadone over 100 mg/day, even when prescribed based on specific signs and symptoms, were not better than 100 mg/day. This counterintuitive finding requires replication, but supports the need for additional controlled studies of high-dose methadone.
Keywords: methadone maintenance, methadone dose, individualized dosing, flexible dosing, polydrug dependence, contingency management
1. INTRODUCTION
Methadone maintenance is an effective treatment for opioid dependence, particularly when given with other psychosocial services (Ball et al., 1988; Barthwell et al., 1989; Gerstein and Lewin, 1990; McLellan et al., 1993; Caplehorn et al., 1994; Goldstein and Herrera, 1995; Bell et al., 1997). Nevertheless, some methadone patients continue to abuse heroin during treatment, even with extensive psychosocial services (McLellan et al., 1993).
One longstanding issue in methadone maintenance is appropriate dosing. Historically, methadone dosage practices have sometimes been ideologically driven (Williams, 1970; Maddux et al., 1991). The average methadone dose in community clinics has increased over the past few decades, with the proportion of patients receiving doses <80 mg decreasing from 94% in 1988 to 56% in 2005 (Maddux et al., 1991; D'Aunno and Vaughn, 1992; D'Aunno et al., 1999; D'Aunno and Pollack, 2002; Pollack and D'Aunno, 2008), and prior outside approval of doses over 100 mg/day is no longer required (Rettig and Yarmolinsky, 1995). The trend toward higher doses has been supported by clinical trials and by retrospective analyses of outcome in clinical populations (Caplehorn and Bell, 1991; Strain et al., 1993; Maremmani et al., 1994; Hartel et al., 1995; Ling et al., 1996; Schottenfeld et al., 1997; Strain et al., 1999; Farré et al., 2002). A Cochrane review of randomized trials found that higher doses of methadone (60 to 100 mg/day) were more effective than lower doses (1 to 39 mg/day) in reducing heroin use (Faggiano et al., 2003). Some addictions specialists have advocated for doses above 100 mg (Maremmani et al., 2003; Fareed et al., 2009), particularly in special populations (McCarthy et al., 2005). One criticism of nearly all recent major trials, including our previous studies, is that they used fixed doses (Strain et al., 1993; Ling et al., 1996; Schottenfeld et al., 1997; Preston et al., 2000) rather than the more flexible, individualized approach to dosing now endorsed by Center for Substance Abuse Treatment (CSAT, 2005) and currently used in most community clinics.
A complication in clinical practice is that many methadone patients abuse nonopiate drugs such as cocaine. Cocaine abuse during methadone maintenance indicates poor prognosis, both in terms of treatment dropout (Greenfield et al., 1996; Simpson et al., 1997; Kidorf et al., 1998) and heavy concurrent use of heroin (Hartel et al., 1995). In many cases, the relationship between cocaine abuse and heroin abuse appears causal: 36% of cocaine-abusing methadone patients report using heroin to modify the effects of cocaine, either by co-injection or to lessen dysphoria as cocaine effects dissipate (Kidorf and Stitzer, 1993). All these data suggest that methadone programs seeking to reduce treatment dropout and heroin abuse should put a high priority on reducing cocaine abuse.
One of the most effective treatments for cocaine abuse is contingency management (CM), in which desired behaviors are externally reinforced (Prendergast et al., 2006; Dutra et al., 2008). We previously showed that CM targeted at opiate abstinence, along with a fixed increase in daily methadone dose from 50 mg to 70 mg, each significantly increased opiate abstinence (Preston et al., 2000).
In a more recent CM study (Epstein et al., 2009), we administered higher doses of methadone (70 or 100 mg/day) combined with CM to promote abstinence from both heroin and cocaine. We used a novel contingency that reinforced abstinence from either drug while doubly reinforcing simultaneous abstinence from both. Each intervention was effective in specific ways: cocaine-targeted CM reduced cocaine use, and the methadone dose increase reduced heroin use. However, achievement of polydrug abstinence was difficult for most patients. This finding suggested that for CM to promote simultaneous abstinence from cocaine and heroin, a relatively high dose of methadone is necessary. Therefore, we decided to investigate an approach in which CM was targeted exclusively toward cocaine use, while methadone was deployed against heroin use more effectively.
The aim of this study was to evaluate the efficacy and safety of individualized methadone doses over 100 mg/day (based on each patient's opioid use, craving, and withdrawal symptoms and on avoidance of side effects such as constipation and sedation) combined with voucher-based cocaine-targeted CM to reduce opioid and cocaine use. We hypothesized that the individualized dose increases would increase abstinence from heroin and that the addition of CM would result in greater simultaneous opioid and cocaine abstinence, and higher treatment retention, when compared to methadone doses fixed at 100 mg and a control condition for CM. As described below, surprisingly, these hypotheses were not supported. One limitation of the study is a partial failure of randomization (discussed below), but that limitation does not seem to account for the unexpected results.
2. METHOD
2.1 Participants
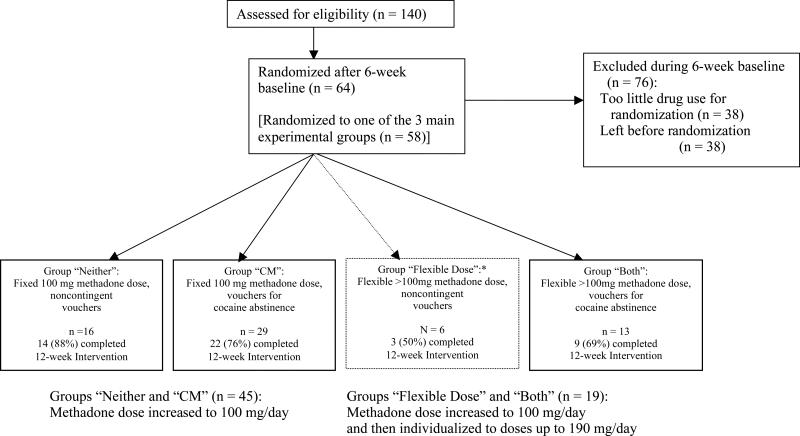
Participants were selected from 140 outpatients admitted for methadone maintenance at a research clinic in Baltimore, MD. Screening included medical, psychiatric, and drug-use histories, physical examination, standard laboratory tests, and a battery of assessment instruments, including the Addiction Severity Index (ASI) (McLellan et al., 1985) and the Diagnostic Interview Schedule (DIS-IV; Robins et al., 1995). Eligibility criteria for initial enrollment were: age 18-65, cocaine and opiate use (by self-report and urine screen), and physical dependence on opiates. Exclusion criteria were: current psychotic, bipolar, or major depressive disorders; current physical dependence on alcohol or sedatives (because our outpatient clinic did not have the resources to meet the medical needs that would arise during detoxification); unstable serious medical illness; estimated IQ below 80, per the Shipley Institute of Living Scale (Zachary, 1986); and conditions precluding urine collection. Eligibility for randomization to a group was based on subsequent heroin and cocaine use during a six-week baseline (see below). DSM-IV diagnoses of heroin or cocaine dependence were not required. Of 140 patients enrolled, 38 failed to meet continued-drug-use criteria for randomization, and 38 dropped out before being randomized. Six were assigned to a group whose only purpose was to maintain the blind (details below); the remaining 58 were randomized to one of the three experimental groups (Figure 1).
Figure 1.
Flowchart of participants1 progress through the study. *Group 3Flexible Dose2 (indicated by dotted-line box) existed solely to maintain the blind and was not part of the data analysis specified a priori. Likelihood of allocation to that group was deliberately set low. The main pairwise comparisons of interest were group 3Both2 versus groups 3Neither2 and 3CM2; those three groups were intended to be equal in size The disproportionately large N in group 3CM2 reflects an error in the input to the randomization algorithm for the last 12 participants randomized (see Methods section).
This study was approved by the Institutional Review Board of the NIDA Intramural Research Program; each participant gave written informed consent.
2.2. Standard treatment
All participants received daily methadone and weekly individual counseling for up to 40 weeks, of which the last 10 weeks were a scheduled dose taper (before and during which, our clinic staff helped participants transfer to community methadone clinics). In weekly individual-counseling sessions, counselors completed a semistructured psychosocial assessment and treatment plan for each participant. Reduction of substance use was the primary goal. Methadone HCl (Mallinckrodt, Inc., St. Louis, MO) was administered orally in a fixed volume of 95 ml of cherry-flavored solution throughout the study. Dose was stabilized at 70 mg/day within seven days.
2.3. Urine and breath toxicology
Mondays, Wednesdays, and Fridays, urine specimens were collected under observation. Testing was conducted with an Enzyme Multiplied Immunoassay Technique (EMIT; Syva Corp., Palo Alto, California) system that provided qualitative results for cocaine (benzoylecgonine equivalents; BZE), opiates (morphine), marijuana, and benzodiazepines (oxazepam). Cutoffs were 300 ng/ml for cocaine, opiates, and benzodiazepines, and 50 ng/ml for marijuana. Breath alcohol was determined with an Alco-Sensor III (Intoximeters, Inc., St. Louis, MO). Use of alcohol, benzodiazepines, and nonheroin opiates was rarely detected or reported; use of cannabis was detected in approximately 19% of urine screens.
2.4. Other measures of treatment response
Once a week, in clinic, participants completed questionnaires in which they rated on a scale of 0 to 4 how much they had “wanted” heroin or cocaine in the past week, with the response anchors “not at all,” “a little,” “moderately,” “quite a bit,” and “extremely.” On the same occasions, with the same 0-4 response anchors, participants rated 24 symptoms of opiate withdrawal (nausea, runny nose, etc.) in the last two days. The 24 opiate-withdrawal items were summed to generate a score between 0 and 96. Cronbach alphas, computed separately for each week, ranged from .93 to .95.
Every two weeks, during the experimental phase of the study, participants completed questionnaires on which they rated their degree of constipation and sedation, the two main side effects expected from high doses of methadone. In addition, every two weeks, a staff member rated the participants’ objective signs of opiate withdrawal, using the Clinical Opiate Withdrawal Scale (COWS; Wesson and Ling, 2003). A study physician (JPS or KAP) reviewed participants’ progress at least every two weeks to determine whether dose adjustments were appropriate. Actual dose increases were based on randomization group assignment and were double-blind, with neither the physician nor participant knowing whether they actually occurred.
2.5. Study timeline and groups
Baseline began upon enrollment and continued until the participant had provided 18 urine specimens (at least 6 weeks). Participants were then eligible for randomization if they had tolerated 70 mg/day and tested positive for heroin and cocaine at least four times each (not necessarily on the same days) with at least one positive urine occurring in the last 2 weeks of Baseline. Participants not meeting these criteria (n = 38) were excluded from randomization but were permitted to remain in treatment for the rest of the study; their data are not reported here. Participants were not told about the randomization criteria.
For the 16-week Intervention, participants were randomized to one of three main experimental groups or a fourth group (described below). For the 8-week Maintenance, standard treatment was resumed. Thereafter, participants were encouraged to transfer to community treatment programs; those who did not were offered a 10-week methadone taper.
We used a 2 × 2 design, with each participant assigned to one of two methadone-dose conditions and one of two CM conditions (Figure 1). Assignment was unequal to maximize power for the two pairwise comparisons of interest (Woods et al., 1998; Dumville et al., 2006). We hypothesized that group “Both” (flexible/!100 mg dose, cocaine-contingent vouchers) would have greater sustained simultaneous abstinence from heroin and cocaine than group “Neither” (fixed/100 mg dose, noncontingent vouchers) or group “CM” (fixed/100 mg dose, cocaine-contingent vouchers). Group “Flexible” (flexible/>100 mg dose, noncontingent vouchers) existed solely to maintain the blind.
When we began enrollment, toward the end of 2004, we intended to collect data from 55 participants in each of the three main experimental groups. We stopped enrolling participants in 2010, before reaching our sample-size target, partly due to slow enrollment and reduced randomization eligibility due to not meeting continued-drug-use criteria and partly because we believed that real-world clinical practice had largely resolved our experimental question about flexible/high-dose methadone. Thus, we expected that we would see at least a trend toward results in the hypothesized direction.
When we broke the blind, we noted that group “CM” was larger than the other two main groups. This was due to an undetected change in the inputs to our urn-randomization algorithm. The algorithm, which had been programmed into our in-house medical-records system to maintain the double blinding of dose conditions, stratified group assignments by sex, race, and frequency of opiate- and cocaine-positive urine specimens during baseline. The algorithm had automated access to the baseline urine data, which came from an external laboratory. Before the last 12 participants were randomized, the external laboratory changed the format of its data so that the necessary fields were empty. The randomization algorithm assigned all 12 of those participants to group “CM.”
2.6. CM conditions
In all of the CM conditions, participants could receive vouchers exchangeable for goods and services in the community consistent with treatment goals (not for cash). The maximum total value was $1418.
2.6.1. Noncontingent (Groups “Neither” and “Flexible”)
In this control condition, each participant received vouchers independent of individual urine results on a schedule designed to simulate the frequency of voucher earnings of participants in the CM condition. This ranged from 20% to 100% of urine-collection days. Participants were told that they would receive vouchers on a “completely unpredictable schedule.”
2.6.2. Cocaine contingency (Groups “CM” and “Both”)
Participants received a voucher immediately upon provision of each cocaine-negative urine. The value of the vouchers began at $4.00 and increased by $2.00 for each consecutive cocaine-negative urine, to a maximum of $30 per voucher. Upon provision of a cocaine-positive urine sample or failure to provide a urine sample, the voucher was withheld, and the value of the next earned voucher was reset to $4.00. An additional voucher worth $10.00 was given for every three consecutive cocaine-negative urine samples.
2.7. Methadone-Dose Conditions
2.7.1. Dose increase to 100 mg/day, then fixed at that dose (Groups “Neither” and “CM”)
Participants assigned to this condition received dose increases to a target of 100 mg/day over the first 4 days of Intervention and remained at that dose throughout the Intervention.
2.7.2. Dose increase to 100 mg/day, followed by individualized dose adjustments (Groups “Flexible” and “Both”)
Participants assigned to this condition received dose increases to a target of 100 mg/day over the first 4 days of Intervention, and were thereafter eligible to receive increases to a maximum of 190 mg/day, based on withdrawal symptoms, adverse effects, and opiate-positive urines. Each dose increase was 15 mg/day, given at intervals of at least two weeks. If the participant showed signs of opioid intoxication or other adverse effects, the dose increase was suspended, but could resume if signs diminished.
Dose changes did not require breaking of the blind. When a participant met the requirement for a dose increase, the clinic physician wrote a pharmacy order. If the participant was in a flexible-dose group, the in-house pharmacist increased the methadone dose; if not, the pharmacist left the dose unchanged. For dose decreases, the pharmacist completed all physician orders.
2.8. Data analysis
The primary outcome measure was urine drug screens simultaneously negative for opiates and cocaine. Analyses in this report focus on the Intervention phase. The “Flexible Dose” group not receiving cocaine-targeted CM was not included in the statistical analyses because it existed only to maintain the blind and was not part of the a priori plan for analysis. Only six participants were randomized to that group. For all analyses, the alpha level was p ≤ .05 (two-tailed), with trends noted at p ≤ .10. Analysis was on an intent-to-treat basis. Degrees of freedom differ across analyses, reflecting inclusion of different numbers of covariates.
Intake measures were analyzed by ANOVA (for continuous variables), Pearson ! 2 (for categorical variables), or Fisher's exact test (for categorical variables with expected cell sizes under 5).
Study retention was analyzed with a log-rank test (SAS Lifetest procedure) of time until provision of the final urine sample; participants who left before the final week of the intervention phase were classified as “dropouts” in statistical analyses (see next paragraph).
Urine results from Intervention were analyzed by repeated-measures logistic regression (SAS Proc Glimmix). In three sets of analyses, the dependent variables were opiate-negative urines, cocaine-negative urines, and (the primary outcome measure) urines simultaneously negative for opiates and cocaine. We treated Group as a three-level variable and made Tukey-adjusted pairwise planned comparisons between groups. Thus, each model contained the following predictors: Group (CM, Both, Neither), a control term for baseline drug use (percentage of urine specimens negative for the drug or drugs being analyzed), and a control term for the nonrandomness of the missing data (coded 1 for participants who were intervention-phase “dropouts,” 0 for those who were not; Hedeker and Gibbons, 1997). To avoid convergence problems, no parameter estimates were made for Day (which would have had 48 levels, corresponding to the 48 urines screens done across 16 weeks). A first-order autoregressive error structure was used.
Longest duration of simultaneous abstinence from opiates and cocaine, based on urine data, was analyzed by an ANCOVA in which the categorical predictor was Group (CM, Both, Neither) and the continuous covariate was each participant's baseline percentage of urines simultaneously negative for opiates and cocaine. The ANCOVA was followed by Tukey-adjusted pairwise comparisons between groups.
Withdrawal symptoms and drug craving were analyzed by repeated-measures linear regression (SAS Proc Mixed), using models similar to the Glimmix models for urine results. These models converged when parameter estimates were made for Week (17 levels) and Group × Week interaction, so those terms were included. Each model also included a control term for each participant's baseline mean on the response being analyzed. Tukey-adjusted pairwise planned comparisons were made between groups.
COWS, sedation ratings, and constipation ratings were also analyzed by repeated-measures linear regression (SAS Proc Mixed). These models converged when parameter estimates were made for Week (8 levels) and Group × Week interaction, so those terms were included. However, the measures were administered during the intervention phase only, so no control term for baseline ratings could be included.
Effect sizes for differences between percentages are expressed in terms of Cohen's h; h values of .2, .5, and .8 denote small, medium, and large effect sizes, corresponding roughly to R2 values of 1%, 5%, and 14% (Cohen, 1988). Effect sizes for differences between means are expressed in terms of Cohen's d; d values of .2, .5, and .8 denote small, medium, and large, corresponding roughly to R2 values of 1%, 6%, and 14%. The values used to calculate effect sizes were the covariate-adjusted values.
3. RESULTS
3.1. Participant Characteristics
Demographic characteristics (shown in Table 1) did not differ significantly among the three main experimental groups, nor between the randomized participants and those who dropped out before randomization or who did not meet criteria for randomization (data not shown).
Table 1.
Participant Characteristics
| Total N | 58 participants |
|---|---|
| Women | 22% |
| African American | 47% |
| Age | 36.8 years (SD = 8.0, range 22-52) |
| Education | 11.8 years of (SD = 1.7, range 7-16), |
| Estimated IQ | 95.1 (SD = 7.7, range 81-110) |
| Employment | |
| Unemployed | 28% |
| Employed part time | 25% |
| Employed full time | 47% |
| Marital status | |
| Never married | 71% |
| Separated or divorced | 20% |
| Married | 9% |
| Drug History | |
| Cocaine | |
| Lifetime use | 6.5 years (SD = 6.1, range 0-24); |
| Use in past 30 days | 11.3 days (SD = 7.7, range 2-30). |
| Route | smoked 59%, injected intravenously 33%, insufflated 8%. |
| Heroin | |
| Lifetime use | 10.7 years (SD = 7.7, range 1-35) |
| Use in past 30 days | 28.7 days (SD = 4.5, range 3-30) |
| Route | injected intravenously 64%, insufflated 36% |
| Urine-detected drug use during 6-week study baseline | |
| Urines testing opiate negative (mean %) | 23% (SD 23, range 0-78%) |
| Urines testing cocaine negative (mean %) | 16% (SD 19, range 0-72%) |
| Urines testing both opiate and cocaine negative (mean %) | 6% (SD 11, range 0-44%) |
3.2. Retention
The mean total number of weeks in the study (maximum 22, for Baseline plus Intervention) was 19.9 (SD 4.3, range 7.3 to 22) for group “CM,” 18.9 (SD 5.1, range 9.6 to 22) for group “Both,” and 21.1 (SD 3.6, range 7.6 to 22) for group “Neither.” Survival analysis showed no significant difference across groups (log-rank chi-square = 1.49, df = 2, n.s.).
3.3. Methadone dose given
The maximum daily dose actually given in group “Both” averaged 139.2 mg/day (SD = 30.9, median = 115, range = 100 to 190). All but one participant in that group received a maximum dose greater than 100 mg/day. The participant who did not was incarcerated during the study before qualifying for increases over 100 mg/day.
3.4. Treatment outcome - Opiate and cocaine use
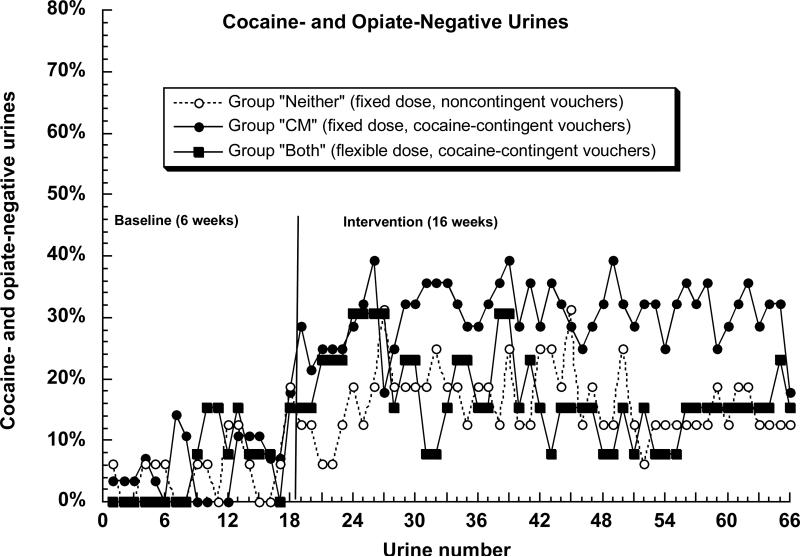
The primary outcome measure, urine specimens simultaneously negative for opiates and cocaine, is shown in Figure 2. Controlling for the six-week baseline and for dropout, there was a significant difference among groups during the 16-week intervention, F(2, 51) = 9.60, p = .0003. However, the nature of the differences was not as predicted: abstinence was greater in group “CM” than in group “Both” (Tukey p = .018; effect-size h = 0.30) and did not differ between groups “Both” and “Neither” (Tukey p = .70; h = 0.08).
Figure 2.
Percentage of participants simultaneously abstinent from cocaine and opiates on 66 successive urine test days in the Baseline phase (18 specimens) and Intervention phase (48 specimens) in the three experimental groups. In the figure, missing specimens (including those due to dropout) are assumed positive. The statistical analyses (SAS Proc Glimmix) treated missing specimens as missing. Contrary to our expectation, group “Both” was less likely to achieve abstinence than group “CM” (see Results section for statistics).
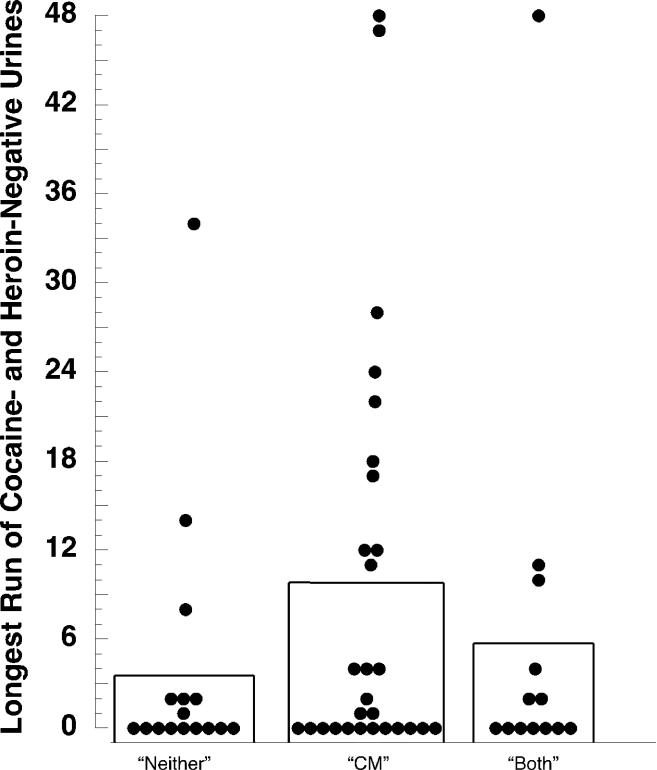
Figure 3 shows another measure of simultaneous abstinence from opiates and cocaine, longest duration of abstinence. An ANCOVA controlling for baseline abstinence showed no significant difference among groups, F(2,53) = 1.09, p = .34 (no pairwise comparisons significant).
Figure 3.
Longest duration of simultaneous abstinence from cocaine and opiates during the Intervention phase (48 specimens) in the three experimental groups. The bars show group means; the filled circles show values for individual participants. Contrary to our expectation, group “Both” was no more likely to achieve sustained abstinence than the other two groups (see Results section for statistics). The possible influence of “outlier” values is discussed in the online supplement.
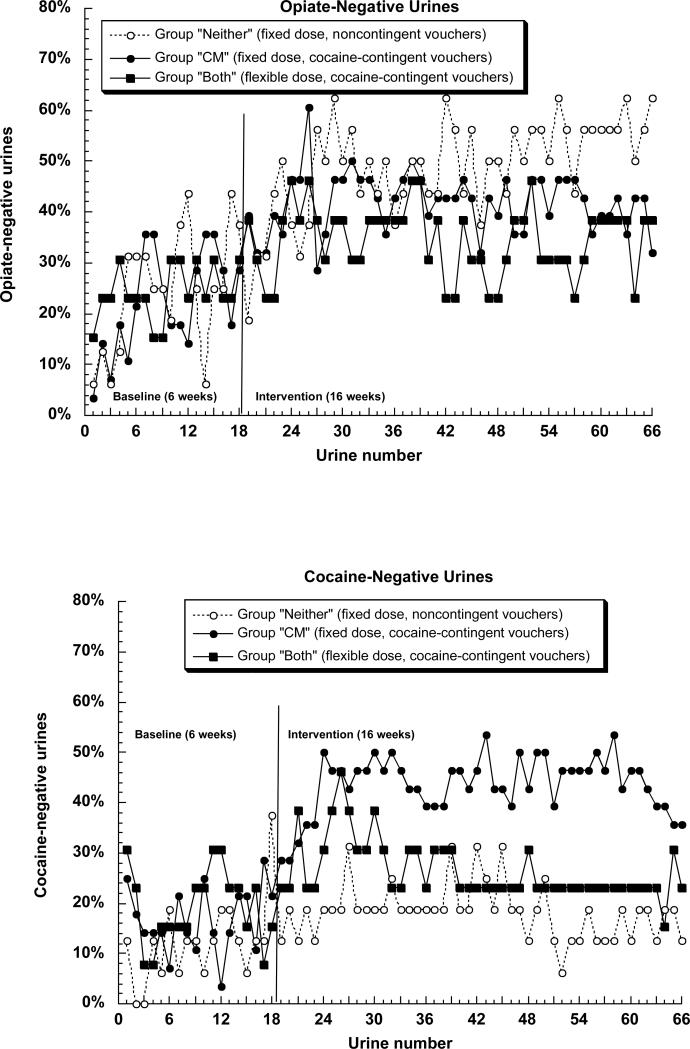
Figures 4a and 4b show urine specimens negative for opiates and cocaine, respectively. For opiates (Figure 4a), the difference among groups was only marginally significant, F(2,51) = 2.68, p = .078. Again, this reflected greater abstinence in group “CM” than in group “Both” (Tukey p = .076, h = 0.28) and no significant difference between groups “Both” and “Neither” (Tukey p = .13; h = 27, with the absolute values favoring group “Neither”). For cocaine, the difference among groups was significant, F(2,51) = 21.61, p < .0001, and again, this reflected greater abstinence in group “CM” then in group “Both” (Tukey p = .0003; h = .49) but no difference between groups “Both” and “Neither” (Tukey p = .60; h = 09).
Figures 4a and 4b.
Percentage of participants abstinent from (A) opiates or (B) cocaine on 66 successive urine test days. Details are the same as those for figure 2.
3.5. Treatment outcome - Withdrawal
3.5.1. COWS and Withdrawal symptoms
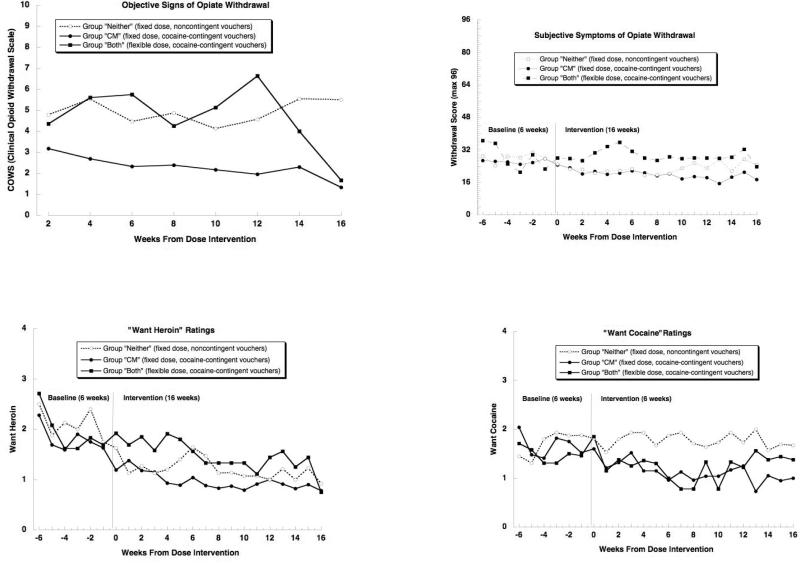
Objective signs of opiate withdrawal, as reflected in COWS scores (Figure 5, upper left panel), differed among groups, F(2,52) = 12.35, p < .0001. Contrary to our hypothesis, this reflected lower COWS scores in group “CM” than in group “Neither” (Tukey p < .0001; effect-size d = 1.39) or Group “Both” (Tukey p = .0039; d = 1.18), and no difference between groups “Both” and “Neither” (Tukey p = .93; d = 0.14).
Figure 5.
a. Objective signs of opiate withdrawal as assessed on the Clinical Opioid Withdrawal Scale (COWS) during the 16-week Intervention phase. Contrary to our expectation, group “Both” showed more signs of withdrawal than group “CM” (see Results section for statistics).
Figure 5b. Subjective symptoms of opiate withdrawal as rated on questionnaires during the 16-week Intervention phase. Contrary to our expectation, group “Both” tended to report more symptoms of withdrawal than group “CM” (see Results section for statistics).
Figures 5c and 5d. Participant ratings of “wanting” for (A) heroin and (b) cocaine during the 6-week Baseline phase and the 16-week Intervention phase. Contrary to our expectation, heroin wanting was higher in group “Both” than in group “CM” (see Results section for statistics).
Subjective symptoms of opiate withdrawal (Figure 5, upper right panel) also differed among groups at trend level, F(2,54) = 2.95, p = .061. The pattern was similar to what was seen with objective signs on the COWS, except that group “Neither” seemed to have fewer symptoms relative to its frequency of objective signs. The salient and unpredicted findings were that withdrawal symptoms were greater in group “Both” than in group “CM” (Tukey p = .048; d = 0.82) and did not differ between groups “Both” and “Neither” (Tukey p = .26; d = 0.60; the absolute difference favored group “Neither”).
3.5.2. Craving (“Want heroin” and “want cocaine”)
Weekly ratings of “Want Heroin” and “Want Cocaine” (Figure 5, lower panels) differed among groups, F(2,54) = 3.61, p = .034 (heroin), F(2,54) = 5.08, p = .009 (cocaine). In both cases, ratings were lowest in group “CM.” For “Want Heroin,” ratings were significantly lower in group “CM” than in group “Both” (Tukey p = .034; d = 0.87) and also tended to be lower in group “Neither” than in group “Both” (Tukey p = .073; d = 0.85). For “Want Cocaine,” ratings were significantly lower in group “CM” than in group “Neither” (Tukey p = .003; d = 0.96) and also tended to be lower in group “CM” than in group “Both” (Tukey p = .092; d = 0.58), and did not differ between groups “Both” and “Neither” (Tukey p = .38; d = 0.34; the absolute difference favored group “Both”).
3.6 Methadone adverse effects
Weekly reports of sedation did not differ among groups; the frequency of such reports was generally around 10% per week, and 38% of participants reported sedation on at least one assessment (weekly data are shown in Figure S11). Weekly reports of constipation did differ among groups, F(2,52) = 3.98, p = .025. However, the difference was not related to the methadone dose: constipation was reported by 59% of participants in group “CM,” 88% in group “Neither,” and 69% in group “Both” (weekly data are shown in Figure S22; in Tukey pairwise comparisons, the only significant difference was “CM” vs. “Neither,” Tukey p = .053).
4. DISCUSSION
We examined whether simultaneous abstinence from heroin and cocaine could be achieved by combining individualized (flexible) high-dose (>100 mg) methadone and voucher-based CM in heroin- and cocaine-dependent individuals. We ended the study before reaching our planned sample size, partly due to slow accrual, and partly because we had begun to suspect that a clinical trial comparing flexible high-dose methadone to fixed lower-dose methadone was becoming like a clinical trial of whether parachutes protect against the effects of free fall (Smith and Pell, 2003). Therefore, we were surprised to find that our flexible/high-dose methadone participants were less likely to achieve simultaneous abstinence from heroin and cocaine than participants receiving a lower, fixed dose, and that they did not even show a greater likelihood of abstinence from heroin alone. Equally surprising, our flexible/high-dose methadone participants reported more opiate-withdrawal symptoms and more heroin “wanting” than participants receiving a lower, fixed dose. Our study had several limitations, which we discuss below, but even in light of those limitations, our unexpected results merit attention.
One possibility, which we suggest only tentatively, is that higher doses can paradoxically worsen outcome. This possibility has been discussed in the context of long-term opioid treatment for chronic pain: “more is not necessarily better,” concluded one review (Doleys et al., 2006). Issues raised in that context included opioid-induced hyperalgesia (White, 2004). We know of no studies suggesting analogous phenomena in addiction treatment, and although there are survey studies that fail to show the expected positive relationship between methadone dose and heroin abstinence (e.g., Blaney and Craig, 1999), interpretation of these studies is obviously impeded by their nonrandomized nature: presumably, the patients who receive the highest methadone dosages in clinical practice are those who are hardest to treat. Randomized, double-blind trials have shown that higher methadone doses are better within a dose range of 30 to 100 mg/day, but ours is one of very few such trials to have tested doses over 100 mg/day. We hesitate to conclude on the basis of one study that “more can be worse.” A more prudent conclusion is that (unlike parachutes) higher methadone dosages need more experimental evaluation.
At the same time, one of the core methods of experimental pharmacology, double-blind dosing, may cause problems that could explain our unexpected results. In clinical practice, treatment retention is better in methadone programs in which patients are informed of their dosages; this finding persists after statistical control for other program differences (Condelli and Dunteman, 1993). Prior studies showing expected dose-response effects of methadone have been double-blinded, but ours was unusual in that we were testing a flexible-dose intervention in which dosages could change fairly frequently. Our participants may have found these changes aversive under double-blind conditions, possibly more aversive than receiving a lower, fixed dose under double-blind conditions (though it should be noted that both groups were blinded, and the fixed-dose group had unmet expectations of possible dose increases). If so, then fixed and individualized methadone dosing may not be amenable to comparison using the standard tools of a clinical trial.
Another possibility is that our implementation of dose individualization was insufficiently sensitive to our participants’ needs. We think this is unlikely. Dose individualization was done by two physicians (JS from 2004 to 2007; KAP from 2007 to 2010). One physician based dose-change decisions on questionnaire and face-to-face interview data gathered by research assistants every two weeks or more, meeting with participants only when the data seemed ambiguous. The other physician used the same data but also met with each participant when a dose change seemed indicated, to discuss the participant's needs in a more collaborative way. We found no trend toward any difference between physicians in the numbers of dose changes ordered per patient-week.
The effects of cocaine-targeted CM in this study were as expected, increasing our confidence that the study cannot be written off as a “failed trial” due to some idiosyncrasy of the sample.
Nonetheless, our study has two clear limitations. One was the partial failure of urn randomization; however, this does not explain our unexpected results (see Supplementary Material.3) The more salient limitation is that we stopped the trial early, before achieving the targeted sample size. Trials stopped early can produce false-positive findings (Bassler et al., 2008), and we cannot rule out the possibility that we would have had different results if we had completed our trial. Therefore, we do not suggest that our findings be immediately acted upon in clinical settings. We find it both counterintuitive and unlikely that a fixed, lower dose could be superior to an individualized higher dose. However, we think our findings call for additional controlled studies to bolster or qualify the assumptions that underlie recent trends in methadone maintenance.
Supplementary Material
Acknowledgments
We wish to thank the NIDA IRP Archway Clinic staff for data collection.
Role of the funding source: This research was supported by the Intramural Research Program of the NIH National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors: Drs. Schmittner, Preston, and Epstein designed the study and wrote the protocol. Dr. Schmittner, Dr. Phillips, and Mr. Reamer interviewed participants and reviewed information for dose change decisions and managed data collection. Dr. Epstein undertook the statistical analysis, and Dr. Kennedy wrote the first draft of the manuscript. All authors contributed to and approved the final manuscript.
Conflict of Interest: All authors declare that they have no conflict of interests.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
REFERENCES
- Ball JC, Lange WR, Myers CP, Friedman SR. Reducing the risk of AIDS through methadone maintenance treatment. J. Health Soc. Behav. 1988;29:214–226. [PubMed] [Google Scholar]
- Barthwell A, Senay E, Marks R, White R. Patients successfully maintained with methadone escaped human immunodeficiency virus infection. Arch. Gen. Psychiatry. 1989;46:957–958. doi: 10.1001/archpsyc.1989.01810100099020. [DOI] [PubMed] [Google Scholar]
- Bassler D, Montori VM, Briel M, Glasziou P, Guyatt G. Early stopping of randomized clinical trials for overt efficacy is problematic. J. Clin. Epidemiol. 2008;61:241–246. doi: 10.1016/j.jclinepi.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Bell J, Mattick R, Hay A, Chan J, Hall W. Methadone maintenance and drug-related crime. J. Subst. Abuse. 1997;9:15–25. doi: 10.1016/s0899-3289(97)90003-1. [DOI] [PubMed] [Google Scholar]
- Blaney T, Craig RJ. Methadone maintenance: does dose determine differences in outcome? J. Subst. Abuse Treat. 1999;16:221–228. doi: 10.1016/s0740-5472(98)00031-2. [DOI] [PubMed] [Google Scholar]
- Caplehorn J, Bell J. Methadone dosage and retention of patients in maintenance treatment. Med. J. Australia. 1991;154:195–199. [PubMed] [Google Scholar]
- Caplehorn J, Dalton M, Cluff M, Petrenas A. Retention in methadone maintenance and heroin addicts’ risk of death. Addiction. 1994;89:203–207. doi: 10.1111/j.1360-0443.1994.tb00879.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Condelli WS, Dunteman GH. Exposure to methadone programs and heroin use. Am. J. Drug Alcohol Ab. 1993;19:65–78. doi: 10.3109/00952999309002666. [DOI] [PubMed] [Google Scholar]
- CSAT . Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs: Treatment Improvement Protocol (TIP) Series 43. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2005. [PubMed] [Google Scholar]
- D'Aunno T, Vaughn TE. Variations in methadone treatment practices. JAMA. 1992;267:253–258. [PubMed] [Google Scholar]
- D'Aunno T, Folz-Murphy N, Lin X. Changes in methadone treatment practices: results from a panel study, 1988-1995. Am. J. Drug Alcohol Ab. 1999;25:681–699. doi: 10.1081/ada-100101886. [DOI] [PubMed] [Google Scholar]
- D'Aunno T, Pollack HA. Changes in methadone treatment practices: results from a national panel study, 1988-2000. JAMA. 2002;288:850–856. doi: 10.1001/jama.288.7.850. [DOI] [PubMed] [Google Scholar]
- Doleys DM, Brown JL, Ness T. Multidimensional outcomes analysis of intrathecal, oral opioid, and behavioral-functional restoration therapy for failed back durgery syndrome: a retrospective study with 4 years’ follow-up. Neuromodulation. 2006;9:270–283. doi: 10.1111/j.1525-1403.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- Dumville JC, Hahn S, Miles JN, Torgerson DJ. The use of unequal randomisation ratios in clinical trials: a review. Contemp. Clin. Trials. 2006;27:1–12. doi: 10.1016/j.cct.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am. J. Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Schmittner J, Umbricht A, Schroeder JR, Moolchan ET, Preston KL. Promoting abstinence from cocaine and heroin with a methadone dose increase and a novel contingency. Drug Alcohol Depend. 2009;101:92–100. doi: 10.1016/j.drugalcdep.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggiano F, Vigna-Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst. Rev. 2003:CD002208. doi: 10.1002/14651858.CD002208. [DOI] [PubMed] [Google Scholar]
- Fareed A, Casarella J, Roberts M, Sleboda M, Amar R, Vayalapalli S, Drexler K. High dose versus moderate dose methadone maintenance: is there a better outcome? J. Addict. Dis. 2009;28:399–405. doi: 10.1080/10550880903183042. [DOI] [PubMed] [Google Scholar]
- Farré M, Mas A, Torrens M, Moreno V, Cami J. Retention rate and illicit opioid use during methadone maintenance interventions: a meta-analysis. Drug Alcohol Depend. 2002;65:283–290. doi: 10.1016/s0376-8716(01)00171-5. [DOI] [PubMed] [Google Scholar]
- Gerstein D, Lewin L. Treating Drug Problems. New Engl. J. Med. 1990;323:844–848. doi: 10.1056/NEJM199009203231230. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Herrera J. Heroin addicts and methadone treatment in Albuquerque—a 22-year follow-up. Drug Alcohol Depend. 1995;40:139–150. doi: 10.1016/0376-8716(95)01205-2. [DOI] [PubMed] [Google Scholar]
- Greenfield L, Brady JV, Besteman KJ, De Smet A. Patient retention in mobile and fixed-site methadone maintenance treatment. Drug Alcohol Depend. 1996;42:125–131. doi: 10.1016/0376-8716(96)01273-2. [DOI] [PubMed] [Google Scholar]
- Hartel DM, Schoenbaum EE, Selwyn PA, Kline J, Davenny K, Klein RS, Friedland GH. Heroin use during methadone maintenance treatment: the importance of methadone dose and cocaine use. Am. J. Public Health. 1995;85:83–88. doi: 10.2105/ajph.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol. Methods. 1997;2:64–78. [Google Scholar]
- Kidorf M, Stitzer M. Descriptive analysis of cocaine use of methadone patients. Drug Alcohol Depend. 1993;32:267–275. doi: 10.1016/0376-8716(93)90091-4. [DOI] [PubMed] [Google Scholar]
- Kidorf M, Brooner RK, King VL, Stoller KB, Wertz J. Predictive validity of cocaine, sedative, and alcohol dependence diagnoses. J. Consult. Clin. Psych. 1998;66:168–173. doi: 10.1037//0022-006x.66.1.168. [DOI] [PubMed] [Google Scholar]
- Ling W, Wesson DR, Charuvastra C, Klett CJ. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Arch. Gen. Psychiatry. 1996;53:401–407. doi: 10.1001/archpsyc.1996.01830050035005. [DOI] [PubMed] [Google Scholar]
- Maddux JF, Esquivel M, Vogtsberger KN, Desmond DP. Methadone dose and urine morphine. J. Subst. Abuse Treat. 1991;8:195–201. doi: 10.1016/0740-5472(91)90039-d. [DOI] [PubMed] [Google Scholar]
- Maremmani I, Nardini R, Zolesi O, Castrogiovanni P. Methadone dosages and therapeutic compliance during a methadone maintenance program. Drug Alcohol Depend. 1994;34:163–166. doi: 10.1016/0376-8716(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Maremmani I, Pacini M, Lubrano S, Lovrecic M. When “enough” is still not “enough”: effectiveness of high-dose methadone in the treatment of heroin addiction. Heroin Addict. Rel. Cl. 2003;5:17–32. [Google Scholar]
- McCarthy JJ, Leamon MH, Parr MS, Anania B. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am. J. Obstet. Gynecol. 2005;193:606–610. doi: 10.1016/j.ajog.2005.03.072. [DOI] [PubMed] [Google Scholar]
- McLellan A, Arndt I, Metzger D, Woody G, O'Brien P. The effects of psychological services in substance abuse treatment. JAMA. 1993;269:1953–1959. [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola JS, Griffith J, Evans F, Barr HL, O'Brien CP. New data from the Addiction Severity Index: reliability and validity in three centers. J. Nerv. Ment. Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Pollack HA, D'Aunno T. Dosage patterns in methadone treatment: results from a national survey, 1988-2005. Health Serv. Res. 2008;43:2143–2163. doi: 10.1111/j.1475-6773.2008.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Epstein DH. Methadone dose increase and abstinence reinforcement for treatment of continued heroin use during methadone maintenance. Arch. Gen. Psychiatr. 2000;57:395–404. doi: 10.1001/archpsyc.57.4.395. [DOI] [PubMed] [Google Scholar]
- Rettig RA, Yarmolinsky A. Federal regulation of methadone treatment. In: Rettig RA, Yarmolinsky A, editors. Federal Regulation of Methadone Treatment. National Academy Press; Washington: 1995. pp. 120–150. [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM., III . The Diagnostic Interview Schedule, version IV. Washington University; St. Louis, MO: 1995. [Google Scholar]
- Schottenfeld RS, Pakes JR, Oliveto A, Zeidonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch. Gen. Psychiatry. 1997;54:713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Broome KM, Hiller ML, Knight K, Rowanszal GA. Program diversity and treatment retention rates in the Drug Abuse Treatment Outcome Study (DATOS). Psychol. Addict. Behav. 1997;11:279–293. [Google Scholar]
- Smith GC, Pell JP. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ. 2003;327:1459–1461. doi: 10.1136/bmj.327.7429.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain E, Bigelow G, Liebson I, Stitzer M. Moderate- vs high-dose methadone in the treatment of opioid dependence. JAMA. 1999;281:1000–1005. doi: 10.1001/jama.281.11.1000. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Methadone dose and treatment outcome. Drug Alcohol Depend. 1993;33:105–117. doi: 10.1016/0376-8716(93)90052-r. [DOI] [PubMed] [Google Scholar]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J. Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- White JM. Pleasure into pain: the consequences of long-term opioid use. Addict. Behav. 2004;29:1311–1324. doi: 10.1016/j.addbeh.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Williams HR. Low and high methadone maintenance in the out-patient treatment of the hard core heroin adict. Int. J. Addict. 1970;5:439–447. doi: 10.3109/10826087009057011. [DOI] [PubMed] [Google Scholar]
- Woods SW, Sholomskas DE, Shear MK, Gorman JM, Barlow DH, Goddard AW, Cohen J. Efficient allocation of patients to treatment cells in clinical trials with more than two treatment conditions. Am. J. Psychiatry. 1998;155:1446–1448. doi: 10.1176/ajp.155.10.1446. [DOI] [PubMed] [Google Scholar]
- Zachary R. Shipley Institute of Living Scale. Revised Manual. Western Psychological Services; Los Angeles: 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.