Abstract
In a microarray analysis of human retinal pigment epithelial cells (HRPE) treated with TGF-β, in addition to the alteration of a number of known extracellular matrix (ECM)-related genes regulated by TGF-β, we found a significant increase in the expression of Kallmann Syndrome (KAL)-1 gene, that codes for the protein anosmin-1. Enhanced expression of KAL-1 by TGF-β was validated by real-time PCR analysis. In in vitro experiments, TGF-β receptor inhibitor abolished TGF-β-induced expression of KAL-1. Immunofluorescence staining showed increased presence of anosmin-1 in TGF-β treated HRPE cells, with distinct localization at the intercellular junctions. Treatment of HRPE cells with TGF-β enhanced secretion of anosmin-1 and the release of anosmin-1 was further augmented by heparin sulfate. Enhanced secretion of anosmin-1 in the presence of TGF-β and heparin was also observed in other ocular cells such as corneal epithelial and corneal fibroblast cultures. The role of anosmin-1, a protein with adhesion functions, in retinal structure, function and pathology has not been known and remains to be investigated.
Keywords: Anosmin, KAL-1, TGF-β, adhesion proteins, retinal pigment epithelium, retina, cornea
Introduction
Retinal pigment epithelium (RPE) consists of a single layer of epithelial cells strategically located between choroids and neurosensory retina. Extracellular matrix (ECM) components act as cementing substances and play critical roles in the maintenance of adhesion of neurosensory retina to RPE [1, 2]. ECM proteins also play an important role in the integrity of RPE basement membrane complex known as Bruch’s membrane that is critical in preserving the barrier between choroid and retina. Aberrant production and secretion of ECM by RPE would lead to retinal disorders like retinal detachments, proliferative vitreoretinopathy and macular degeneration, the diseases that may lead to loss of visual function [1, 2].
Transforming growth factor-beta (TGF-β) is a multipotent cytokine that regulates cell proliferation, differentiation and synthesis of ECM like collagens, fibronectin, heparin and chondroitin sulfate proteoglycans [3]. Using conditional gene knock-out mice, we have recently shown that retinal specific TGF-β signaling deficiency leads to retinal detachments [4]. Loss of TGF-β actions due to non-functional TGF-β receptor 1 resulted in significantly diminished chondroitin sulfate proteoglycan in the subretinal space causing retinal detachments [4]. In addition, elevated expression of TGF-β in the vitreous, retina and RPE has been reported in retinal fibrosis, retinal detachments and choroidal neovascularization [5, 6].
Our previous studies have showed that TGF-β is a potent inducer of vascular endothelial growth factor, platelet derived growth factors [7, 8, 9]. To further elucidate the genes altered in response to TGF-β in human RPE cells, we employed microarray analysis using Affymetrix GeneChip. One of the genes that demonstrated a significant upregulation following treatment with TGF-β was an extracellular adhesion protein, anosmin-1 (the gene is called Kallmann Syndrome-1 or KAL-1). In this study, we further investigated the expression of anosmin-1 in epithelial and fibroblast cells derived from human retina and cornea. Though we have previously shown that anosmin-1 is a cancer-regulated protein [10], the expression and role of anosmin-1 in the pathophysiology of retinal and other ocular disorders have not been known.
Materials & Methods
Cell Cultures
Human retinal pigment epithelial (HRPE) cell cultures were prepared from donor eyes as reported earlier [7, 8, 9]. Positive staining for cytokeratin confirmed the epithelial nature of HRPE. Human corneal epithelial cell line (HCE-T) was obtained from RIKEN Cell Bank. Human corneal fibroblasts (HCRF) were prepared from donor eyes or corneal buttons as reported earlier [11].
Anosmin polyclonal antibody
Polyclonal antibodies to anosmin-1 were developed in rabbits by using a peptide with the amino acid sequence “CSHLKHRHPHHYKPSPERY” conjugated to KLH [10]. The affinity purified antibody was prepared by coupling the peptide on to a SulfoLink affinity matrix (Pierce) and performing the affinity chromatography with the polyclonal antibody [10].
Analysis of global gene expression profile in HRPE cells by Microarray
Confluent cultures of HRPE were treated with TGF-β1(10 ng/ml) for 8 hr in serum free medium before preparing total RNA. Reverse transcription, cRNA synthesis, hybridization, washing, staining with streptavidin-phycoerythrin (SAPE, Molecular Probes) and amplification with biotinylated anti-streptavidin antibody were performed as per Affymetrix protocols. GeneChip Human Genome U133 Plus 2.0 array (Affymetrix, Santa Clara, CA) was used for hybridization. Affymetrix GeneChip Operating software (GCOS) was used for absolute expression analysis. Normalization, filtering and cluster analysis of the data were performed with GeneSpring software (Silicon Genetica/Agilant, CA)
Real-Time RT-PCR analysis of KAL-1 mRNA expression
Total RNA prepared from cells was used for the relative quantitation of the levels of KAL-1 mRNA. To test the specificity of TGF-β, HRPE cells grown to confluence were treated with TGF-β R1 kinase inhibitor (Calbiochem, La Jolla, CA) 30 minutes prior to TGF-β treatment. Taqman master mix reagents and assays-on-demand gene expression products and FAM labeled KAL-1(Applied Biosystems, CA) were used according to the manufacturer’s instructions. Relative quantification method was used for the expression of KAL-1 gene in TGF-β treated samples in comparison to control samples. KAL-1 mRNA was quantified in relation to GAPDH.
Detection of Anosmin-1 by indirect immunofluorescence
HRPE cultures were grown to near confluence in 8 well chamber slides and treated with TGF-β for 24 hr. Cultures were fixed with acetone:methanol mixture (1:1) for 10 min and preincubated for 30 min in PBS containing 10% normal goat serum for blocking non-specific binding. Then cells were incubated with preimmune rabbit serum, polyclonal anti-anosmin-1 rabbit serum or affinity purified rabbit anosmin-1 antibody prepared in PBS containing 2% goat serum for 1 hr. Cells were washed and further incubated for 1 hr in the presence of goat anti-rabbit IgG conjugated to FITC prepared in PBS containing 2% goat serum. The slides were washed with PBS, mounted (VectashieldAQ, Vector Labs, Burlingame, CA) and observed for fluorescence.
Western blot analysis of secreted Anosmin (KAL-1)
HRPE, HCE-T and HCRF cell cultures were grown to confluence in 100 mm dishes. Cultures were left in serum free medium overnight and then treated with TGF-β1(10 ng/ml) for 24 hr in serum free medium. Two hours before harvesting the culture supernatant fluids, heparin sulfate was added to a final concentration of 50 µg/ml. Culture supernatants were concentrated by 50 fold by using centrifugal filter devices (Centriprep, Millipore, Bedford, MA). Concentrated culture supernatants were separated by PAGE, transferred to nitrocellulose membranes and incubated with affinity purified anosmin antibodies. After incubating with HRP conjugated secondary antibodies, blots were developed by using chemiluminiscence substrate.
Results and Discussion
Microarray analysis of global gene expression profile of primary HRPE cell lines treated with TGF-β showed two fold up or down regulation of 2200 gene targets (data not shown). Several genes coding for proteins related to the ECM were upregulated following TGF-β treatment (Table 1). Since most of the ECM genes are known to be upregulated by TGF-β, we focussed further studies on KAL-1 gene. There was a 3.5 fold increase in the expression of KAL-1 gene in the cells treated with TGF-β (Table 1). The expression of KAL-1 in ocular tissues, its regulation by cytokines and growth factors, and its possible role in normal and pathological conditions in the ocular tissues are not known.
Table 1. Gene expression changes in HRPE cells treated with TGF-β.
HRPE cultures were grown to confluence were treated with TGF-β1(10 ng/ml) for 8h in serum free medium. Total RNA prepared was used for microarray analysis using Affymetrix GeneChip as described in Materials and Methods section.
| Affymetrix ID | Fold Change | Gene name |
|---|---|---|
| 215646_s_at | 17.83 | chondroitin sulfate proteoglycan 2 (versican) |
| 202310_s_at | 15.6 | collagen, type I, alpha 1 |
| 204619_s_at | 14.98 | chondroitin sulfate proteoglycan 2 (versican) |
| 202311_s_at | 14.54 | collagen, type I, alpha 1 |
| 213640_s_at | 14.51 | lysyl oxidase |
| 214701_s_at | 13.42 | fibronectin 1 |
| 201107_s_at | 13.07 | thrombospondin 1 |
| 205828_at | 12.6 | matrix metalloproteinase 3 (stromelysin 1, progelatinase) |
| 226535_at | 12.27 | integrin, beta 6 |
| 221731_x_at | 11.68 | chondroitin sulfate proteoglycan 2 (versican) |
| 226847_at | 11.54 | follistatin |
| 204298_s_at | 10.46 | lysyl oxidase |
| 204620_s_at | 10.32 | chondroitin sulfate proteoglycan 2 (versican) |
| 211571_s_at | 9.588 | chondroitin sulfate proteoglycan 2 (versican) |
| 201645_at | 9.249 | tenascin C (hexabrachion) |
| 215446_s_at | 8.532 | Human lysyl oxidase (LOX) gene, exon 7. |
| 204475_at | 7.224 | matrix metalloproteinase 1 (interstitial collagenase) |
| 214602_at | 6.707 | collagen, type IV, alpha 4 |
| 212865_s_at | 6.676 | collagen, type XIV, alpha 1 (undulin) |
| 214702_at | 6.637 | fibronectin 1 |
| 201109_s_at | 6.37 | thrombospondin 1 |
| 201108_s_at | 5.155 | thrombospondin 1 |
| 216893_s_at | 4.939 | collagen, type IV, alpha 3 (Goodpasture antigen) |
| 221729_at | 4.462 | collagen, type V, alpha 2 |
| 208083_s_at | 4.368 | integrin, beta 6 |
| 222073_at | 4.327 | collagen, type IV, alpha 3 (Goodpasture antigen) |
| 211980_at | 4.317 | collagen, type IV, alpha 1 |
| 211981_at | 3.998 | collagen, type IV, alpha 1 |
| 214641_at | 3.903 | collagen, type IV, alpha 3 (Goodpasture antigen) |
| 216898_s_at | 3.778 | collagen, type IV, alpha 3 (Goodpasture antigen) |
| 219697_at | 3.506 | heparan sulfate (glucosamine) 3-O-sulfotransferase 2 |
| 205206_at | 3.461 | Kallmann syndrome 1 sequence |
| 202620_s_at | 3.087 | procollagen-lysine, 2-oxoglutarate 5-dioxygenase (lysine hydroxylase) 2 |
| 201069_at | 2.845 | matrix metalloproteinase 2 (gelatinase A, 72kDa gelatinase, 72kDa type IV collagenase) |
| 211966_at | 2.299 | collagen, type IV, alpha 2 |
| 212464_s_at | 2.199 | fibronectin 1 |
| 201656_at | 0.485 | integrin, alpha 6 |
| 219909_at | 0.481 | matrix metalloproteinase 28 |
| 239273_s_at | 0.302 | matrix metalloproteinase 28 |
| 239272_at | 0.221 | matrix metalloproteinase 28 |
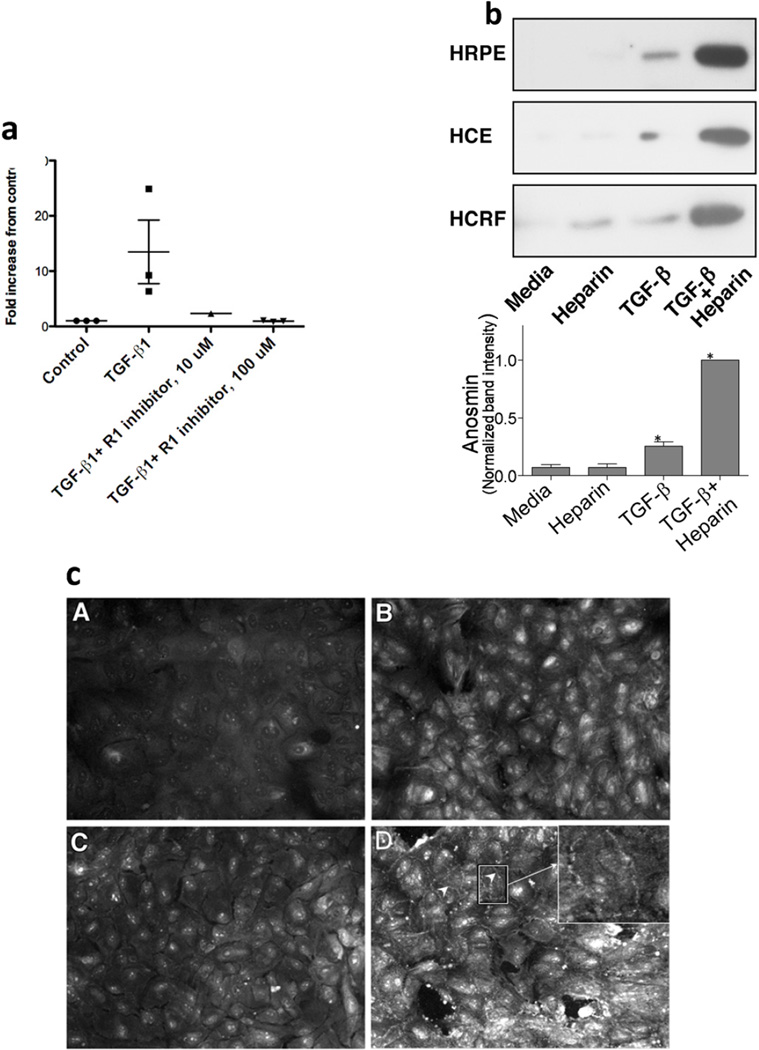
To validate the microarray results of TGF-β enhanced expression of anosmin-1, we performed RT-PCR analysis of total RNA isolated from HRPE cells treated with TGF-β and found that TGF-β treatment significantly enhanced the expression of KAL-1 (Fig.1a) while we did not observe any significant effects by cytokines such as IFN-γ, TNF-α, IL1-β and platelet derived growth factor (data not shown). We used TGF-β R1 kinase inhibitor, a small molecule inhibitor of TGF-β, to test TGF-β specificity in KAL-1 induction (Fig 1a). HRPE cultures were preincubated with the inhibitor for 30 m before adding TGF-β. Total RNA prepared after 8 h was used for the analysis of KAL-1 mRNA. The inhibitor completely abolished TGF-β induced expression of KAL-1 at both concentrations tested (Fig.1a).
Fig. 1.
a. Real-time PCR analysis of KAL-1 gene expression in HRPE cells treated with TGF-β1. HRPE cultures were treated with TGF-β1 (10 ng/ml) in the absence or presence of TGF-β receptor 1 inhibitors for 8 h. Total RNA prepared was used for Real time PCR analysis as described in the methods.
b. Immunoblot analysis of anosmin (KAL-1) secreted by cell cultures. Cultures grown to confluence were treated without or with TGF-β (10 ng/ml) for 24 h in serum free media. Two hr before harvesting, heparin was added to give 50 ug/ml concentration. Culture supernatants collected were concentrated 50 fold and used for immunoblot analysis as described in the methods section. HRPE, Human retinal pigment epithelial cells; HCE, Human corneal epithelial cells; HCRF, Human corneal fibroblast cells. In repeated experiments the molecular size of anosmin appeared around 75kD. Lower panel: Anosmin-1 level in the culture supernatant was quantified by Western blot, in four separate experiments using HRPE cells. Band intensities were normalized to the intensity of the sample with highest anosmin-1 level and expressed as mean+standard error. *=p<0.05 when compared to the controls.
c. Immunofluorescence detection of Anosmin (KAL-1) in HRPE cells. HRPE cultures grown to confluence in 8 well glass slides were treated with TGF-β for 24 hr, fixed, incubated with pre-immune serum or rabbit polyclonal antibody followed by FITC conjugated secondary antibody. A. Control cells incubated with pre-immune serum; B. Control cells incubated with anosmin-1 antibody; C. TGF-β treated cells incubated with pre-immune serum; D. TGF-β treated cells incubated with Anosmin antibody (arrow heads indicate intercellular staining)
As seen in Fig 1b, when HRPE cells were treated with TGF-β, anosmin-1 protein expression was also markedly elevated. In addition, TGF-β treatment resulted in enhanced the expression of anosmin-1 protein in human corneal epithelial (HCE), corneal fibroblast (HCRF) (Fig.1b) and in human choroidal fibroblast (not shown) cells. Intense bands were observed on the protein blots of TGF-β treated culture supernatant to which heparin sulfate was added prior to termination of the culture (Fig.1b), demonstrating heparin-mediated disruption of the cell surface bound anosmin-1 [12].
In order to further confirm anosmin-1 expression, HRPE cultures grown to confluence were treated with TGF-β1 for 24 h in serum free medium and formalin-fixed cells were incubated with either preimmune serum or antibodies to anosmin as described in the methods section. Untreated and TGF-β treated HRPE cells treated with pre-immune serum showed only background fluorescence staining (Fig.1c, panel A). Untreated cells incubated with anosmin antibodies showed faint staining that is diffused and appears to be in the cytoplasm and cell surface (Fig.1c, panel B). In contrast, TGF-β treated cells exhibited intense staining both intracellular and cell surface. In some projections where cell-cell contacts were maintained after fixing, intercellular staining along the borders of the cells is clearly evident (Fig. 1c, panel D).
Anosmin gene (KAL-1) spans 210 kb with 14 exons and encodes a 680 amino acid extracellular matrix protein with a signal peptide and six potential N-glycosylation sites [12,13]. Anosmin has whey acidic protein (WAP) motif and four contiguous fibronectin type III repeats which are reported to be associated with serine protease activity and cell adhesion molecules. Loss of function due to mutations results in Kallmann syndrome, an X linked autosomal disease, characterized by hypogonadism and anosmia [14]. These symptoms are reported to be due to defects in the migration of neuronal precursors of both olfactory and gonadotrophin-releasing hormone cells during embryonic development [14]. Anosmin-1 may also play a role in cancer and inflammation [10,15]. Though the role of anosmin-1 in the olfactory tract extracellular matrix has been strongly implicated in facilitating migration of neurons in embryogenesis, its role in other tissues or in adult-hood is not known. Our finding indicates that TGF-β stimulates the production of anosmin-1; however the mechanism of TGF-b dependent expression of anosmin-1 remains to be investigated.
The results obtained in this study demonstrate that KAL-1 gene expression is enhanced by TGF-β in HRPE and corneal cell lines. The expression of KLA-1 gene in photoreceptors and other neuroretinal cells have not been evaluated in the present studies. However, further studies are needed to elucidate the specific function of anosmin-1 in ocular tissues and its role in diseases such as macular degeneration and diabetic retinopathy where extracellular matrix and TGF-β are known to play a profound role.
Highlights.
*Anosmin-1 is an extracellular matrix protein important in the migration of olfactory neurons in embryogenesis.
*Our experiments demonstrate that TGF-beta upregulates anosmin-1 in human ocular tissues.
*This finding has significance in diseases such as macular degeneration where TGF-beta plays a critical role.
Acknowledgements
This work was supported in part by an HSF-GEF scholar award from UAB (RR) and intramural research support from NEI, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship and disclosures
The authors have no conflict of interests.
References
- 1.Marmor MF, Wolfensberger TG. The retinal pigment epithelium: Function and disease. New York: Oxford University Press; 1998. [Google Scholar]
- 2.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 3.Feng X-H, Derynck R. Specificity and versatility in TGF-β signaling through smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 4.Honjo Y, Nagineni CN, Larsson J, Nandula SR, Hooks JJ, Chan CC, Karlsson S, Kulkarni AB. Neuron-specific TGF-beta signaling deficiency results in retinal detachment and cataracts in mice. Biochem Biophys Res Commun. 2007;352:418–422. doi: 10.1016/j.bbrc.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiedemann P. Growth factors in retinal diseases: proliferative vitreoretinopathy, proliferative diabetic retinopathy, and retinal degeneration. Surv Ophthalmol. 1992;36:373–384. doi: 10.1016/0039-6257(92)90115-a. [DOI] [PubMed] [Google Scholar]
- 6.Frank RN. Growth factors in age-related macular degeneration: pathogenic and therapeutic implications. Opthalmol Res. 1997;29:341–353. doi: 10.1159/000268032. [DOI] [PubMed] [Google Scholar]
- 7.Nagineni CN, Samuel W, Nagineni S, Pardhasaradhi K, Wiggert B, Detrick B, Hooks JJ. Transforming growth factor-beta induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: involvement of mitogen-activated protein kinases. J Cell Physiol. 2003;197:453–462. doi: 10.1002/jcp.10378. [DOI] [PubMed] [Google Scholar]
- 8.Nagineni CN, Kutty V, Detrick B, Hooks JJ. Expression of PDGF and their receptors in human retinal pigment epithelial cells and fibroblasts: regulation by TGF-beta. J Cell Physiol. 2005;203:35–43. doi: 10.1002/jcp.20213. [DOI] [PubMed] [Google Scholar]
- 9.Nagineni CN, Cherukuri KS, Kutty V, Detrick B, Hooks JJ. Interferon-gamma differentially regulates TGF-beta1 and TGF-beta2 expression in human retinal pigment epithelial cells through JAK-STAT pathway. J Cell Physiol. 2007;210:192–200. doi: 10.1002/jcp.20839. [DOI] [PubMed] [Google Scholar]
- 10.Jian B, Nagineni CN, Meleth S, Grizzle W, Bland K, Chaudry I, Raju R. Anosmin-1 involved in neuronal cell migration is hypoxia inducible and cancer regulated. Cell Cycle. 2009;8:3770–3776. doi: 10.4161/cc.8.22.10066. [DOI] [PubMed] [Google Scholar]
- 11.Kommineni VK, Nagineni CN, William A, Detrick B, Hooks JJ. IFN-gamma acts as antiangiogenic cytokine in the human cornea by regulating the expression of VEGF-A and sVEGF-R1. Biochem Biophys Res Commun. 2008;374:479–484. doi: 10.1016/j.bbrc.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soussi-Yanicostas N, Hardelin JP, Arroyo-Jimenez MM, Ardouin O, Legouis R, Levilliers J, Traincard F, Betton JM, Cabanie L, Petit C. Initial characterization of anosmin-1, a putative extracellular matrix protein synthesized by definite neuronal cell populations in the central nervous system. J Cell Sci. 1996;109(Pt 7):1749–1757. doi: 10.1242/jcs.109.7.1749. [DOI] [PubMed] [Google Scholar]
- 13.Franco B, Guioli S, Pragliola A, Incerti B, Bardoni B, Tonlorenzi R, Carrozzo R, Maestrini E, Pieretti M, Taillon-Miller P, et al. A gene deleted in Kallmann's syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991;353:529–536. doi: 10.1038/353529a0. [DOI] [PubMed] [Google Scholar]
- 14.Hu y, Bouloux P-M. X-linked GnRH deficiency: Role of KAL-1 mutations in GnRH deficiency. Mol Cell Endocrinol. 2011;346:13–20. doi: 10.1016/j.mce.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Raju R, Dalakas MC. Gene expression profile in the muscles of patients with inflammatory myopathies: effect of therapy with IVIg and biological validation of clinically relevant genes. Brain. 2005;128(Pt8):1887–1896. doi: 10.1093/brain/awh518. [DOI] [PubMed] [Google Scholar]