Abstract
IL-17 is an inflammatory cytokine associated with anti-microbial host defense and pathogenesis of autoimmune diseases. Obesity is considered to be an inflammatory condition, but how cytokines and fat metabolism are interconnected remains poorly understood. Mesenchymal stem cells can differentiate into adipocytes, which serve as depots for stored fat. Despite the pro-inflammatory properties of IL-17, both IL-17- and IL-17RA-deficient mice are overweight. Consistently, IL-17 suppresses maturation of cells with adipogenic potential. However, the mechanism underlying IL-17-mediated inhibition is not defined. In this study, we addressed this question by evaluating the impact of IL-17 on a variety of transcription factors (TFs) that control adipogenesis, using 3T3-L1 cells to model adipocyte differentiation. Surprisingly, IL-17 does not suppress adipogenesis via C/EBPβ and C/EBPδ, TFs often considered to be central regulators of adipogenesis. Rather, IL-17 suppresses expression of several pro-adipogenic TFs, including PPARγ and C/EBPα. Moreover, we found that IL-17 regulates expression of several members of the Krüppel-like family (KLF). Specifically, IL-17 suppresses KLF15, a pro-adipogenic TF, and enhances expression of KLF2 and KLF3, which are anti-adipogenic. Thus, IL-17 suppresses adipogenesis at least in part through the combined effects of TFs that regulate adipocyte differentiation.
Introduction
Interleukin (IL)-17A is the founding member of a unique family of pro-inflammatory cytokines, composed of IL-17A (referred to here as IL-17), IL-17B, IL-17C, IL-17D (SEF), IL-17E (IL-25) and IL-17F [1]. IL-17 and IL-17F are the hallmark cytokines of Th17 cells, a CD4+ T effector population whose discovery in 2005 revolutionized our understanding of T helper cell biology [2]. A number of innate cell types also produce IL-17 and bear marked functional and phenotypic similarity to Th17 cells [3]. IL-17 is centrally involved in tissue inflammation, acting on epithelial, endothelial and mesenchymal cell types to induce expression of proinflammatory chemokines and other factors that induce neutrophil chemotaxis, as well as promoting a highly pro-inflammatory state [4]. Consequently, IL-17A−/− or IL-17R−/− mice are highly susceptible to infection, especially to mucosal pathogens [5].
Conversely, IL-17 and other Th17-derived cytokines promote pathology of autoimmune diseases such as rheumatoid arthritis, psoriasis and lupus. Consistent with its role as a pro-inflammatory cytokine, IL-17 signals cooperatively or synergistically with other inflammatory effectors, particularly TNFα but also IFNγ, LTα, BAFF among others [6–9], which may explain its role in driving autoimmunity. Antibodies to TNFα have long been used in the clinic, and IL-17 and its receptor are now in clinical trials to treat autoimmune disease. Therefore, understanding the broader effects of IL-17 and its interactions with other cytokines such as TNFα beyond the immune system has important clinical implications [10].
In that regard, emerging data indicate that IL-17 regulates non-inflammatory activities, including mesenchymal cell differentiation. In particular, IL-17 was shown to enhance differentiation of mesenchymal stem cells (MSCs) into osteoblasts, and conversely to suppress MSC differentiation to adipocytes [11–13]. Consistently, IL-17A−/− and IL-17R−/− mice are overweight, show defects in glucose metabolism, and experience enhanced bone loss during osteoporosis [14, 15]. However, little is understood about how IL-17 regulates these events at a mechanistic level.
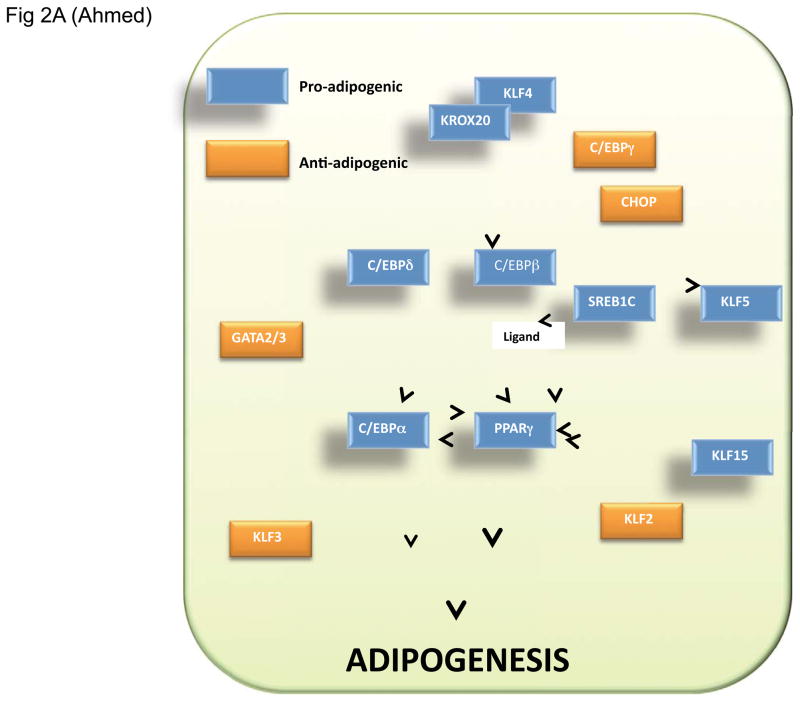
The process of differentiation from a precursor preadipocyte to a fully mature adipocyte follows a precisely ordered and temporally regulated series of gene expression events (see Fig 2A). A number of transcription factors (TFs) regulate adipogenesis, including the peroxisome proliferator-activated receptor (PPAR) [16, 17] and CCAAT/enhancer-binding protein (C/EBP) family proteins [18, 19]. C/EBPδ and C/EBPβ are induced rapidly, followed by expression of C/EBPα and PPARγ. C/EBPα and PPARγ in turn induce programs of gene expression leading to the differentiation of mature adipocytes. Additional TFs further influence this process, including sterol-regulated element binding protein 1c (SREBP1c, also known as ADD1, adipocyte differentiation-1), GATA2 and GATA3, and members of the Krüppel-like factor family (KLFs) [16, 19, 20]. KLF zinc finger proteins play diverse roles in regulation of cell proliferation, differentiation, and development [21, 22]. Several members of the KLF family are implicated in adipogenesis. KLF4, KLF5 and KLF15 are pro-adipogenic, while KLF2 and KLF3 exert negative effects on adipogenesis.
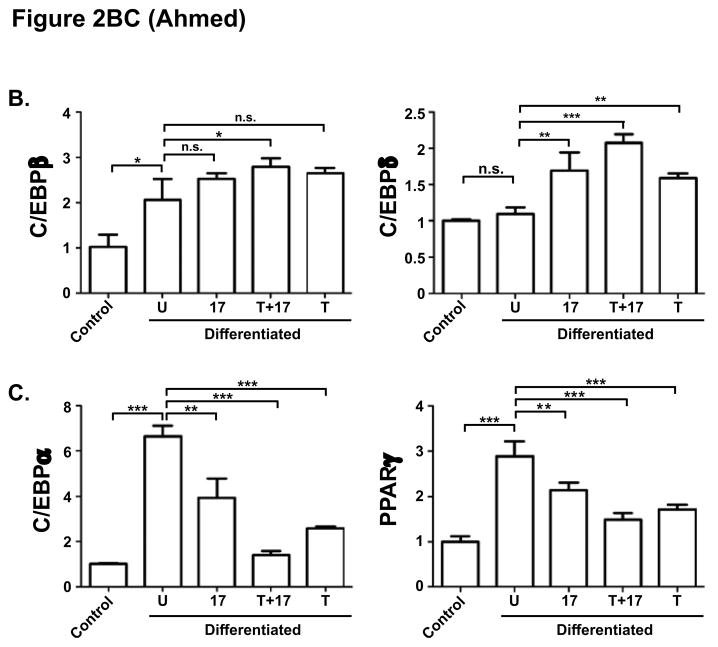
Figure 2. IL-17 suppression of adipogenesis correlates with C/EBPα and PPARγ but not C/EBPβ and C/EBPδ expression.
A. Diagram of TFs that regulate adipocyte differentiation. Pro-adipogenic TFs are indicated in blue, anti-adipogenic TFs are in orange. B–C. Effect of IL-17 on C/EBP and PPARγ expression during adipogenic differentiation. 3T3-L1 cells were cultured alone (“control”) or in adipogenic culture conditions (“Differentiated”) in the presence of IL-17 (200 ng/ml) and/or a suboptimal dose of TNFα (2 ng/ml) for 2 days. Total RNA was extracted and subjected to qRT-PCR in triplicate with primers specific for the indicated genes.
Here, we analyzed expression of a panel of TFs that regulate adipogenesis to ascertain where IL-17 might exert its suppressive effects. We show that IL-17 signaling alters expression of several adipogenic TFs in a manner correlating with suppressed adipogenesis, including C/EBPα, PPARγ and several KLF family members, and surprisingly appears to act downstream of C/EBPβ and C/EBPδ. This is the first evidence for a modulatory role of IL-17A on KLF proteins, and provides new insight into the interconnection between IL-17-driven inflammation and adipocyte development.
Results
IL-17A inhibits adipogenesis downstream of C/EBPβ and C/EBPδ and upstream of PPARγ and C/EBPα
We and others previously reported that IL-17 inhibits adipocyte differentiation of adipogenic cell lines and MSC cultures [12, 14, 15]. The 3T3-L1 cell line can be induced to differentiate into mature adipocytes by a hormonal cocktail that triggers a cascade of transcription factor (TF) expression, considered the gold standard for modeling this process [23]. Here, we first verified the suppressive effect of IL-17 in this system (Fig. 1). Confluent 3T3-L1 cells were grown to confluence and then subjected to an adipogenic cocktail (insulin, dexamethasone, IBMX and ciglitizone) in the presence or absence of IL-17 for 8 days. IL-17 is typically a very modestly-acting cytokine, Therefore, we also included control samples in which IL-17 was included together with TNFα, with which IL-17 exhibits potent synergy [7]. Cells were stained with Oil Red O to visualize fat droplets (Fig. 1A). This effect was quantified by measuring Oil Red O extracted from stained cells by spectroscopy (Fig. 1B), or by measuring triglyceride content 10 days post differentiation (Fig 1C). Similar results were obtained with another adipogenic cell line, ST2 (data not shown) [24]. C/EBPβ and C/EBPδ are mutually reinforcing TFs that are central to adipocyte development (Fig 2A) [25]. Expression of both C/EBPβ and C/EBPδ is positively regulated by IL-17 in several mesenchymal cell types [26, 27]. Thus, we sought to determine whether the effects of IL-17 on C/EBPβ or C/EBPδ might explain how this cytokine inhibits adipogenesis. Expression was assessed by real-time RT-PCR (qPCR) on day 2 following initiation of adipogenesis. 18S rRNA was used as a normalization control for qPCR, as its expression was more uniform during differentiation than the more commonly used housekeeping gene GAPDH (data not shown). As expected, C/EBPβ mRNA was induced significantly in adipogenic conditions compared to undifferentiated controls (Fig 2B). IL-17 and/or TNFα treatment individually did not further enhance C/EBPβ expression, which is consistent with our previous findings that its induction is generally very weak at the mRNA level [26, 28]. There was a mild cooperative enhancement of IL-17 plus TNFα, which we observed previously [29]. C/EBPδ, on the other hand, was not upregulated on day 2 of differentiation in the absence of cytokines, but was induced by IL-17 about ~1.5–2 fold. TNFα also induced C/EBPδ in this setting, and there was a cooperative effect with IL-17 (Fig 2B). These results indicated that the regulation of C/EBPβ and C/EBPδ by IL-17 does not appear to explain its suppressive effect on adipogenesis, since their upregulation by IL-17 would be expected to promote maturation rather than inhibit it.
Figure 1. IL-17 suppresses adipogenesis.
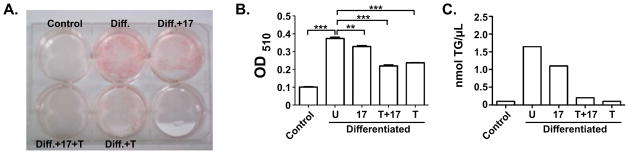
A. 3T3-L1 cells were cultured alone (“control”) or in adipogenic culture conditions (“Diff.”) in the presence of IL-17 (200 ng/ml) and/or a suboptimal dose of TNFα (2 ng/ml) for 10 days. Cells were stained with Oil Red O to visualize fat formation. B. Lysates from the cells described in panel A were solubilized and evaluated by light spectroscopy at OD510. C. Cells were differentiated in duplicate for 10 days with the indicated cytokines and triglyceride content (TG) was assessed.
In addition to C/EBPβ and C/EBPδ, PPARγ and C/EBPα are central to control of adipocyte-specific gene expression [19] (Fig 2A). As expected, both PPARγ and C/EBPα were upregulated on day 2 of differentiation (Fig 2C). IL-17 treatment downregulated PPARγ by 1.3-fold, and C/EBPα by 1.7-fold, in comparison to differentiated adipocytes without cytokine treatment. TNFα and IL-17 cooperatively suppressed C/EBPα in a manner more potent than either cytokine alone, but this was not true for PPARγ (Fig 2C). Collectively, these data suggested that IL-17 acts downstream of C/EBPβ and C/EBPδ but upstream or at the level of PPARγ and C/EBPα.
Pro-adipogenic TFs: IL-17A regulation of Krüppel-like factors correlates with inhibition of adipogenesis
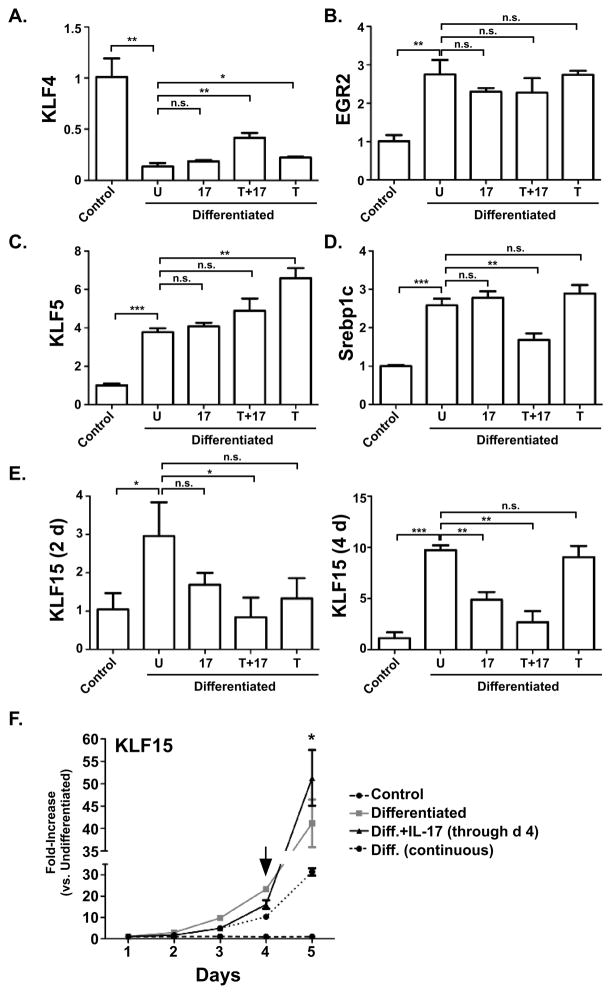
To determine where in the hierarchy of adipogenic TFs IL-17A exerts its suppressive effect, expression of other genes known to control adipocyte differentiation was assessed. We first KLF4, which is pro-adipogenic [30]. At day 2, mRNA expression of KLF4 was strongly suppressed in differentiating cells; although counterintuitive, this observation is consistent with a prior report describing the early kinetics of this TF [30]. There was no discernable effect of IL-17 or TNFα individually on the expression of KLF4. However, TNFα and IL-17 synergistically exhibited a 3-fold increase in KLF4 expression compared to untreated cells (Fig 3A). We also evaluated expression of early growth response gene 2 (EGR2, also known as KROX20), which transactivates C/EBPβ [30]. Results from qPCR analysis confirmed that EGR2 is induced during adipogeneis, but neither IL-17 nor TNFα affected EGR2 expression (Fig 3B).
Figure 3. A–E. Effect of IL-17 on adipogenic TF expression during adipogenic differentiation.
3T3-L1 cells were cultured alone (“control”) or in adipogenic culture conditions (“Differentiated”) in the presence of IL-17 (200 ng/ml) and/or a suboptimal dose of TNFα (2 ng/ml) for 2 days. Total RNA was extracted and subjected to qRT-PCR in triplicate and subjected to qRT-PCR in triplicate with primers specific for the indicated genes. F. KLF15 expression kinetics in differentiation. Expression of KLF15 was assessed by qRT-PCR as bove from 1–5 days of differentiation with or without IL-17. Arrow indicates time of withdrawal of IL-17, as described in the text.
During differentiation, C/EBPβ and C/EBPδ induce KLF5, which acts in concert with C/EBPβ and C/EBPδ to drive expression of PPARγ [31]. As expected, KLF5 was induced during differentiation (Fig 3C). However, IL-17 did not affect KLF5, although TNFα alone enhanced its expression. Like KLF5, sterol regulatory element binding protein 1c (SREBP1c) enhances the adipogenic activity of PPARγ [32, 33]. SREBP1c was induced on day 2 but its expression was not significantly affected by IL-17 stimulation. Notably, however, SREB1c expression was suppressed by a combination of IL-17 and TNFα, and thus might contribute to the combined effects of these cytokines (Fig 3D). Thus, we concluded that, among these positively-acting TFs, only Srebp1 expression correlated with suppression of adipogenesis by IL-17, but only in combination with TNFα.
KLF15 synergizes with C/EBPα to upregulate activity of the PPARγ promoter [34]. KLF15 mRNA was suppressed by IL-17 on day 2 and more potently on day 4 after differentiation (Fig 3E). The inhibitory effect was slightly more pronounced in the presence of TNFα. Thus, the suppressive effect of IL-17 on KLF15 correlates well with its impact on adipogenesis. To determine IL-17 regulation of KLF15 expression in more detail, 3T3-L1 cells were treated with the adipogenic cocktail over an extended time course, and IL-17 treatment was either sustained throughout or removed at day 4. As shown in Fig 3F, continuous treatment of IL-17 during differentiation inhibited KLF15 continuously. However, removal of IL-17 on day 4 (arrow) was associated with an immediate increase of KLF15 expression (Fig 3F). Thus, IL-17 downregulates KLF15 in differentiating adipocytes, and continuous IL-17 signaling is required in order for the inhibitory effect to be sustained.
Anti-adipogenic TFs: IL-17 regulation of Krüppel-like factors correlates with inhibition of adipogenesis
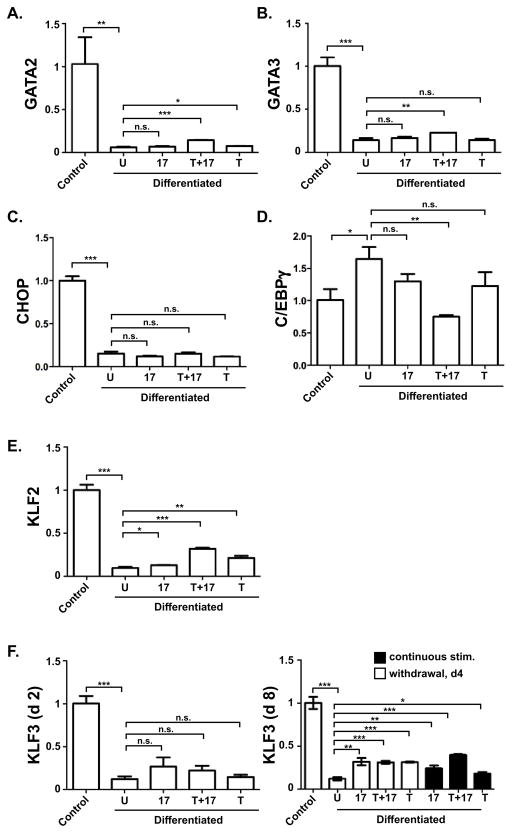
In addition to positively-acting TFs, many factors negatively regulate adipocyte development. GATA2 and GATA3 are specifically expressed in adipocyte precursors where they repress C/EBPα and PPARγ, setting the stage for terminal differentiation [35]. As shown in Fig 4A–B, GATA2 and GATA3 mRNA expression was suppressed strongly at 2 days of differentiation. However, expression was not affected by IL-17 or TNFα. Similarly, expression of the inhibitory factor CHOP was not significantly altered by IL-17A or TNFα (Fig 4C). C/EBPγ negatively regulates transactivation of target genes by forming a heterodimer with C/EBPβ [36]. At day 2 there was a 1.6 fold increase of C/EBPγ mRNA in differentiated 3T3-L1 cells (Fig. 4D). Although IL-17 or TNFα individually had no statistically significant effect on C/EBPγ expression, IL-17 and TNFα together downregulated C/EBPγ by 2.3-fold compared to differentiated cells. This observation is consistent with the observed increased expression of C/EBPβ, but does not explain the IL-17-mediated suppression of adipogenesis. Thus, these anti-adipogenic TFs are unlikely to be the basis for IL-17 regulation of differentiation.
Figure 4. A-F. IL-17 regulation of anti-adipogenic TFs.
3T3-L1 cells were cultured alone (“control”) or in adipogenic culture conditions (“Differentiated”) in the presence of IL-17 (200 ng/ml) and/or a suboptimal dose of TNFα (2 ng/ml) for 2 or 8 days. Total RNA was extracted and subjected to qRT-PCR in triplicate with primers specific for the indicated genes.
KLF2 and KLF3 are also negative regulators of adipogenesis. KLF2 directly inhibits the PPARγ promoter [37], and KLF3 associates with the C/EBPα promoter to block adipocyte differentiation [38]. TNFα individually and IL-17 plus TNFα induced a modest but significant and reproducible increase of KLF2 (Fig 4E). IL-17 and/or TNFα did not induce KLF3 on day 2, but did show increased expression by day 8 (Fig 4F). Taken together, these data indicate that IL-17 affects several KLF family TFs involved in regulating adipogenesis, providing a potential new insight into how this complex process is orchestrated.
IL-17 does not regulate Glucocorticoid Receptor expression or the regulatory enhancer and promoter regions of the KLF15 gene
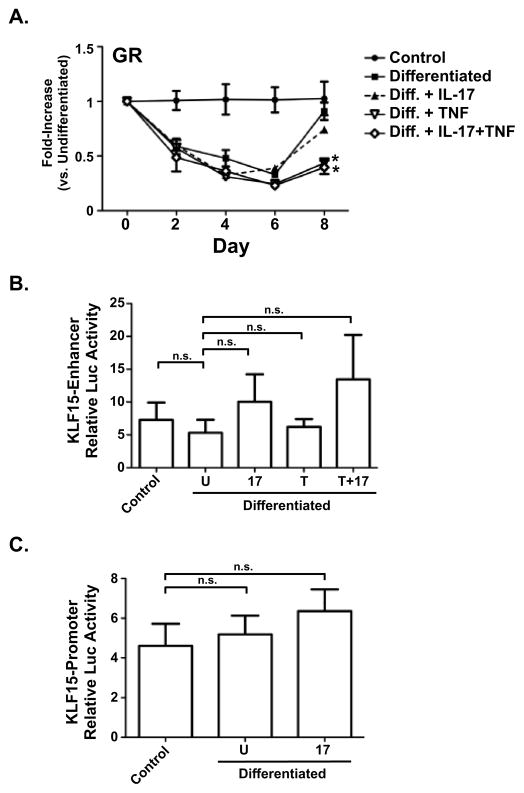
Glucocorticoids are potent regulators of adipose differentiation [39, 40]. Indeed, the synthetic glucocoticoid dexamethasome (Dex) is a key component of the adipogenic-cocktail. Dex exerts its effect through the glucocorticoid receptor (GR), a ligand-activated intracellular TF that binds to conserved glucocorticoid response elements (GREs) in target promoters. A recent report showed that GR directly binds to the intronic enhancer of KLF15 gene, inducing KLF expression and promoting adipogenesis [41]. In order to determine whether IL-17 acts via targeting the GR, 3T3-L1 cells were induced to differentiate with continuous IL-17 stimulation. As shown, IL-17 treatment had no effect on GR mRNA expression (Fig 5A), whereas TNF exerted a significantly suppressive effect as previously reported [42–44]. Therefore, IL-17-dependent effects appear to be independent of GR.
Figure 5. IL-17 regulation of KLF15 and GR. A. Regulation of GR by IL-17 during adipogenic differentiation.
3T3-L1 cells were cultured alone (“control”) or in adipogenic culture conditions (“Differentiated”) in the presence of IL-17 (200 ng/ml) and/or TNFα (2 ng/ml) for the indicated times. Total RNA was extracted and subjected to qRT-PCR. B. IL-17 does not impact the KLF15 intronic enhancer. 3T3-L1 cells were transiently transfected with the reporter construct pGL3-intron-KLF15 enhancer ± IL-17. After 48 h, cell lysates were analyzed for luciferase. C. IL-17 does not regulate the KLF15 proximal promoter. 3T3-L1 cells were transiently transfected with the promoter construct pGL3-promoter-KLF15 with or without IL-17. After 48 h, cell lysates were analyzed for luciferase.
KLF15 is regulated at the transcriptional level by a proximal promoter and an intronic enhancer [41]. The enhancer contains two GRE binding sites, previously shown to be the main regulatory element for KLF15 [41]. To determine whether IL-17 alters activity of the KLF15 DNA regulatory elements, we subcloned the murine KLF15 intronic enhancer into pGL3-luciferase (LUC)-promoter reporter plasmid. The effect of IL-17 on the KLF intergenic enhancer was evaluated. As shown, there was no statistically significant change in luciferase activity in the presence of IL-17 (Fig 5B). We then questioned whether IL-17 might downregulate KLF15 expression via its proximal promoter elements. To that end, we cloned the promoter region conserved between human and mouse (−765 to −1 relative to the transcriptional start site) into the pGL3-Luc-Enhancer vector. This construct was transfected into differentiating 3T3-L1 cells and luciferase reporter activity was assessed on day 4 of differentiation. Again, there was no impact of IL-17 on KLF15- promoter-driven luciferase reporter activity (Fig 5C). These data indicating that IL-17-mediated inhibition of adipogenesis does not act by targeting the KLF intronic enhancer or proximal promoter.
Discussion
Adipose tissue is designed to store lipids but also plays a significant role in integrating endocrine, metabolic, and inflammatory signals [45, 46]. Mature adipocytes synthesize and secrete numerous enzymes, growth factors, cytokines and hormones that are involved in overall energy homeostasis [46]. It is relevant to understand adipocyte differentiation not only to gain insight into the pathogenesis of metabolic diseases, but also for identifying proteins or pathways that serve as targets for pharmological intervention. Many of the factors that influence adipogenesis are also involved in diverse physiological processes including lipid homeostasis and modulation of inflammation. In this regard, recent studies have revealed that the inflammatory cytokine IL-17 functions in obesity and regulation of adipocyte development, with the unexpected finding that IL-17 suppresses adipogenesis [47]. Although this finding was made in several independent labs [12, 14, 15], the underlying mechanism has not been probed in depth. Interestingly a new report indicates that IL-17 increases leptin in differentiation MSCs, which exerts a negative feedback effect in cooperation with IL-17 to suppress adipogenic differentiation [13]. Our study provides the first evidence for IL-17-mediated regulation of key adipogenic transcription factors, including C/EBPα, PPARγ and the recently characterized Krüppel-like factor family.
In evaluating the impact of IL-17 on TFs that control adipogenesis, we were surprised that IL-17 had no discernable inhibitory effect on upstream positive TFs C/EBPβ and C/EBPδ (Fig 2B), which are widely considered to be master regulators of adipocyte transcription. However, our findings in 3T3-L1 cells agree with most reported effects of IL-17 on these TFs, would be consistent with enhancing adipogenesis rather than suppressing it. For example, IL-17 upregulates C/EBPδ [26], and induces phosphorylation of C/EBPβ on the RD2 domain, which is a pro-adipogeneic event [48, 49].
We focused instead on TFs that act downstream of C/EBPβ and C/EBPδ, and found that C/EBPα and PPARγ are regulated in a manner consistent with IL-17-mediated suppression (Fig 2C). C/EBPα and PPARγ induce programs of gene expression leading to the differentiation of mature adipocytes, supporting a scenario in which IL-17 may suppress adipogenesis by regulating C/EBPα and PPARγ [16, 20, 50]. We then evaluated TFs that regulate C/EBPβ and PPARγ, and identified KLF15 as a promising candidate based on its regulation by IL-17 (Fig. 3E–F). The downregulation of KLF15 coincided with the subsequent suppression of PPARγ and C/EBPα that occurs upon IL-17 treatment. Krüppel-like factors (KLFs) have been identified as key components of the transcription network responsible for controlling adipocyte differentiation, adipogenesis and obesity [51]. KLF15, in synergy with C/EBPα, has been shown to upregulate the PPARγ promoter [34], suggesting a model wherein IL-17A-mediated suppression of KLF15 and C/EBPα in turn leads to the reduced expression of PPARγ. In addition to KLF15, IL-17 also exerted a mild regulation of KLF2 and KLF3 (Fig 4E–F), which may further contribute to the suppressive effects of IL-17 on adipogenesis. This is, to our knowledge, the first report demonstrating IL-17-mediated regulation of KLFs, and provides new insight regarding mechanisms underlying the suppressive effect of IL-17 on adipocyte differentiation.
What is the basis for IL-17 regulation of KLF15? Thus far, our attempts to measure promoter activity in differentiating 3T3-L1 cells have not been fruitful due to complexities of such analyses in the context of accumulating fat (data not shown), and so we do not know whether this occurs directly. IL-17 stimulation did not significantly affect the enhancer or promoter regions of the KLF15 gene (Fig 5B–C). Although the glucocorticoid receptor (GR) is reported to control KLF15 [41], we found that IL-17 does not modulate GR expression (Fig 5A). On the contrary, in asthmatic subjects IL-17 is associated with increased GR-β expression in epithelial cells, though this may be indirect [52]. TNF-induced repression of GR function has been reported in various studies, which we also observed (Fig 5A). We conclude that IL-17 mediated suppression of KLF15 gene expression apparently occurs indirectly, and the inhibitory mechanism of adipocyte differentiation by IL-17 may involve a far more intricate pathway.
It will be important to investigate further how circulating IL-17 impacts adipose tissue in vivo. Some clinical studies suggest a potential role for IL-17 in obesity. One report showed that obese women exhibited increased levels of circulating IL-17, which was independent of leptin or other inflammatory cytokines [53]. In a zymosan-induced peritonitis mouse model of obesity, levels of IL-17 are increased in obese mice in response to zymosan [54]. Therefore increased levels of IL-17 may in some way restrain the differentiation of mesenchymal progenitor cells. Intriguingly, a recent study reports that KLF15 expression is decreased in adipose tissue of obese mice [55]. It is plausible to speculate that this decrease of KLF15 expression may be mediated by circulating IL-17 in obese mice. Clearly much more work is needed to understand the effects on IL-17 on adipose tissue regulation in vivo.
Materials and Methods
Cell culture and differentiation
3T3-L1 cells were from American Type Culture Collection (ATCC) (Manassas,VA). Cells were grown to confluence for 2 days in Dulbecco’s modified Eagle’s medium (DMEM) (Cellgro, Manassas, VA) supplemented with FCS (GemCell, West Sacramento, CA) Penn-Strep, non-essential amino acids, pantothenate and biotin (Invitrogen, Carlsbad, CA). Differentiation was induced on Day 0 with DMEM supplemented with 10% FCS (FBS), 0.07 mg/mL insulin (bovine pancreas), 0.4 mg/mL dexamethasone, 0.5mM 3-isobutyl-1-methylxanthine, 0.003 mg/mL ciglitizone (Sigma, St. Louis, MO) with IL-17A (200 ng/mL) and TNF-α (1–2 ng/mL) (Peprotech, Rocky Hill, NJ). Cells were maintained in DMEM containing 10% FBS.
Oil Red O Staining and TGA Assay
Monolayers of differentiated 3T3-L1 cells were washed with PBS and fixed with 10% formalin in PBS for 1 h. After washing with 60% isopropanol, cells were stained with Oil-Red-O (Cayman Chemical, Ann Arbor, MI). Oil-Red-O in isopropanol was diluted with 3/2 volumes of distilled water, filtered and added to the fixed cell monolayers for 1 hour at room temperature. Monolayers were washed with distilled water and visualized by microscopy. Quantitation was carried out by extracting Oil-Red-O-stained triglyceride droplets with 100% isopropanol, and OD was measured at 510 nm. Triglyceride content was assessed in 5μl from duplicate samples 10 days after induction of differentiation with a kit from BioVision (Mountain View, CA), per manufacturer’s instructions.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cells using an RNeasy Mini Kit (Qiagen, Germantown, MD) and reverse transcribed to cDNA using Superscript III Reverse Transcriptase (Invitrogen, Grand Island, NY). Resulting cDNA was then used as a template in quantitative real-time PCR reactions performed using Perfecta SYBR Green FastMix (Quanta Biosciences, Gaithersburg, MD) and various RT2 qPCR Primer Assays specific for adipogenic genes (SABiosciences, Qiagen) in the ABI-7300 fast detection system (Applied Biosystems, Invitrogen). Relative expression (fold-change vs. undifferentiated control) was quantified by the 2−ΔΔCt method. For normalization, mouse 18s rRNA primers were used. The average value of three separate control sample was set to a value of 1 and was used to determine fold-change of both controls and experimental groups. Error bars indicate standard deviations.
Statistical Analysis
All values are mean ± s.d. For statistical analysis, the p value was calculated using ANOVA with a post-hoc Tukey test, and p<0.05 considered significant. *p< 0.05, ** p< 0.005, *** p< 0.0005. Ns., not significant
Plasmids, transfections and luciferase reporter assays
The amplicons for PCR were generated from 3T3-L1 genomic DNA. The KLF15 promoter was generated by PCR (−765 to −1 relative to the transcriptional start site) using specific primers (sense: 5′-CGCGTAGCAGTGTAGGCTAA-3′; antisense 5′-CTCGAGGCCGGCCCGGCTCCGT-3′) and subcloned into the pGL3-Luc-Enhancer vector (Promega, Madison, WI). The KLF15 enhancer was amplified with the sense primer 5′-CGCGTGCACTGACCCAATGGC-3′ and the antisense primer 5′-GATCCAGGACTTCCTGGACCC-3′ and inserted into the pGL3-Luc-Promoter vector (Promega). For luciferase reporter assays, 3T3-L1 cells were plated into 12-well plates and transfections were performed in triplicate on day 1 after adipogenic induction with or without IL-17, using FuGENE-6 (Roche Applied Science, Indianapolis, IN). After 48 h, cells were lysed and luciferase assays were performed using a Dual-Glo Luciferase kit (Promega).
IL-17A suppresses adipogenic differentiation of 3T3-L1 cells.
IL-17 acts downstream of C/EBPβ and C/EPBδ and upstream of C/EBPα and PPARγ
Expression of the anti-adipogenic transcription factor KLF15 is suppressed by IL-17, suggesting a mechanism of inhibition
Acknowledgments
SLG was supported by NIH (AR054389). We thank S. Khader for helpful suggestions.
Abbreviations
- KLF
Krüppel like factor
- IL-
interleukin
- GR
glucocorticoid receptor
- Egr-2
Early growth response gene2 (KROX 20)
- Srebp1c
sterol regulatory element binding protein 1c
- PPARγ
peroxisome proliferator- activated receptor
- GRE
glucocorticoid response element
- TF
transcription factor
- TG
triglyceride
Footnotes
Conflicts of Interest
SLG received travel reimbursements from Amgen and Novartis, and a research grant from Amgen.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–6. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–89. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 4.Onishi R, Gaffen SL. IL-17 and its Target Genes: Mechanisms of IL-17 Function in Disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–11. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–32. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77:388–99. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 8.Onishi R, Park S, Hanel W, Maitra A, Gaffen S. The SEFIR is not enough: An extended region downstream of the Interleukin-17RA SEFIR domain is required for IL-17-dependent signal transduction. J Biol Chem. 2010;285:32751–9. doi: 10.1074/jbc.M110.121418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, Durieu I, Tebib J, Kassai B, Ansieau S, Puisieux A, Eliaou JF, Bonnefoy-Berard N. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–85. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 10.Gaffen SL. Recent advances in the IL-17 cytokine family. Curr Opin Immunol. 2011;23:613–9. doi: 10.1016/j.coi.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H, Kim HJ, Chang EJ, Lee ZH, Hwang SJ, Kim HM, Lee Y, Kim HH. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ. 2009 doi: 10.1038/cdd.2009.74. [DOI] [PubMed] [Google Scholar]
- 12.Shin JH, Shin DW, Noh M. Interleukin-17A inhibits adipocyte differentiation in human mesenchymal stem cells and regulates pro-inflammatory responses in adipocytes. Biochem Pharmacol. 2009;77:1835–44. doi: 10.1016/j.bcp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Noh M. Interleukin-17A increases leptin production in human bone marrow mesenchymal stem cells. Biochem Pharmacol. 2012;83:661–70. doi: 10.1016/j.bcp.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Goswami J, Hernandez-Santos N, Zuniga L, Gaffen S. A bone-protective role for IL-17 receptor signaling in ovariectomy-induced bone loss. Eur J Immunol. 2009;39:2831–2839. doi: 10.1002/eji.200939670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuniga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB, Butcher EC. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947–59. doi: 10.4049/jimmunol.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–56. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 17.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–95. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 18.Freytag SO, Paielli DL, Gilbert JD. Ectopic expression of the CCAAT/enhancer-binding protein alpha promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 1994;8:1654–63. doi: 10.1101/gad.8.14.1654. [DOI] [PubMed] [Google Scholar]
- 19.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–96. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 20.Lin FT, Lane MD. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci U S A. 1994;91:8757–61. doi: 10.1073/pnas.91.19.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dang DT, Pevsner J, Yang VW. The biology of the mammalian Kruppel-like family of transcription factors. Int J Biochem Cell Biol. 2000;32:1103–21. doi: 10.1016/s1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–60. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 23.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 24.Ding J, Nagai K, Woo JT. Insulin-dependent adipogenesis in stromal ST2 cells derived from murine bone marrow. Biosci Biotechnol Biochem. 2003;67:314–21. doi: 10.1271/bbb.67.314. [DOI] [PubMed] [Google Scholar]
- 25.Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–52. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 26.Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-a is mediated by CCAAT/enhancer binding protein family members. J Biol Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 27.Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, Vincenti M, Barnes JL, Chandrasekar B. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am J Physiol Heart Circ Physiol. 2007;293:H3356–65. doi: 10.1152/ajpheart.00928.2007. [DOI] [PubMed] [Google Scholar]
- 28.Maitra A, Shen F, Hanel W, Mossman K, Tocker J, Swart D, Gaffen SL. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci, USA. 2007;104:7506–11. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559–67. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 30.Birsoy K, Chen Z, Friedman J. Transcriptional regulation of adipogenesis by KLF4. Cell Metab. 2008;7:339–47. doi: 10.1016/j.cmet.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, Nishimura G, Maemura K, Yamauchi T, Kubota N, Suzuki R, Kitamura T, Akira S, Kadowaki T, Nagai R. Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005;1:27–39. doi: 10.1016/j.cmet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10:1096–107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- 33.Kim JB, Wright HM, Wright M, Spiegelman BM. ADD1/SREBP1 activates PPARgamma through the production of endogenous ligand. Proc Natl Acad Sci U S A. 1998;95:4333–7. doi: 10.1073/pnas.95.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori T, Sakaue H, Iguchi H, Gomi H, Okada Y, Takashima Y, Nakamura K, Nakamura T, Yamauchi T, Kubota N, Kadowaki T, Matsuki Y, Ogawa W, Hiramatsu R, Kasuga M. Role of Kruppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J Biol Chem. 2005;280:12867–75. doi: 10.1074/jbc.M410515200. [DOI] [PubMed] [Google Scholar]
- 35.Tong Q, Dalgin G, Xu H, Ting CN, Leiden JM, Hotamisligil GS. Function of GATA transcription factors in preadipocyte-adipocyte transition. Science. 2000;290:134–8. doi: 10.1126/science.290.5489.134. [DOI] [PubMed] [Google Scholar]
- 36.Cooper C, Henderson A, Artandi S, Avitahl N, Calame K. Ig/EBP (C/EBP gamma) is a transdominant negative inhibitor of C/EBP family transcriptional activators. Nucleic Acids Res. 1995;23:4371–7. doi: 10.1093/nar/23.21.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee SS, Feinberg MW, Watanabe M, Gray S, Haspel RL, Denkinger DJ, Kawahara R, Hauner H, Jain MK. The Kruppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-gamma expression and adipogenesis. J Biol Chem. 2003;278:2581–4. doi: 10.1074/jbc.M210859200. [DOI] [PubMed] [Google Scholar]
- 38.Sue N, Jack BH, Eaton SA, Pearson RC, Funnell AP, Turner J, Czolij R, Denyer G, Bao S, Molero-Navajas JC, Perkins A, Fujiwara Y, Orkin SH, Bell-Anderson K, Crossley M. Targeted disruption of the basic Kruppel-like factor gene (Klf3) reveals a role in adipogenesis. Mol Cell Biol. 2008;28:3967–78. doi: 10.1128/MCB.01942-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–70. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 40.Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing’s syndrome. Lancet. 2006;367:1605–17. doi: 10.1016/S0140-6736(06)68699-6. [DOI] [PubMed] [Google Scholar]
- 41.Asada M, Rauch A, Shimizu H, Maruyama H, Miyaki S, Shibamori M, Kawasome H, Ishiyama H, Tuckermann J, Asahara H. DNA binding-dependent glucocorticoid receptor activity promotes adipogenesis via Kruppel-like factor 15 gene expression. Lab Invest. 2011;91:203–15. doi: 10.1038/labinvest.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tliba O, Damera G, Banerjee A, Gu S, Baidouri H, Keslacy S, Amrani Y. Cytokines induce an early steroid resistance in airway smooth muscle cells: novel role of interferon regulatory factor-1. Am J Respir Cell Mol Biol. 2008;38:463–72. doi: 10.1165/rcmb.2007-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhandare R, Damera G, Banerjee A, Flammer JR, Keslacy S, Rogatsky I, Panettieri RA, Amrani Y, Tliba O. Glucocorticoid receptor interacting protein-1 restores glucocorticoid responsiveness in steroid-resistant airway structural cells. Am J Respir Cell Mol Biol. 2010;42:9–15. doi: 10.1165/rcmb.2009-0239RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Bogaert T, De Bosscher K, Libert C. Crosstalk between TNF and glucocorticoid receptor signaling pathways. Cytokine Growth Factor Rev. 2010;21:275–86. doi: 10.1016/j.cytogfr.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–73. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 46.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed M, Gaffen SL. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev. 2010;21:449–53. doi: 10.1016/j.cytogfr.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen F, Li N, Gade P, Kalvakolanu DV, Weibley T, Doble B, Woodgett JR, Wood TD, Gaffen SL. IL-17 Receptor Signaling Inhibits C/EBP{beta} by Sequential Phosphorylation of the Regulatory 2 Domain. Sci Signal. 2009;2:ra8. doi: 10.1126/scisignal.2000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang QQ, Gronborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc Natl Acad Sci U S A. 2005;102:9766–71. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–6. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White UA, Stephens JM. Transcriptional factors that promote formation of white adipose tissue. Mol Cell Endocrinol. 2010;318:10–4. doi: 10.1016/j.mce.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vazquez-Tello A, Semlali A, Chakir J, Martin JG, Leung DY, Eidelman DH, Hamid Q. Induction of glucocorticoid receptor-beta expression in epithelial cells of asthmatic airways by T-helper type 17 cytokines. Clin Exp Allergy. 2010;40:1312–22. doi: 10.1111/j.1365-2222.2010.03544.x. [DOI] [PubMed] [Google Scholar]
- 53.Sumarac-Dumanovic M, Stevanovic D, Ljubic A, Jorga J, Simic M, Stamenkovic-Pejkovic D, Starcevic V, Trajkovic V, Micic D. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond) 2009;33:151–6. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- 54.Pini M, Gove ME, Sennello JA, van Baal JW, Chan L, Fantuzzi G. Role and regulation of adipokines during zymosan-induced peritoneal inflammation in mice. Endocrinology. 2008;149:4080–5. doi: 10.1210/en.2008-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagare T, Sakaue H, Matsumoto M, Cao Y, Inagaki K, Sakai M, Takashima Y, Nakamura K, Mori T, Okada Y, Matsuki Y, Watanabe E, Ikeda K, Taguchi R, Kamimura N, Ohta S, Hiramatsu R, Kasuga M. Overexpression of KLF15 transcription factor in adipocytes of mice results in down-regulation of SCD1 protein expression in adipocytes and consequent enhancement of glucose-induced insulin secretion. J Biol Chem. 2011;286:37458–69. doi: 10.1074/jbc.M111.242651. [DOI] [PMC free article] [PubMed] [Google Scholar]