Abstract
Expansion of medical marijuana use in the US and the recently successful decriminalization of recreational marijuana in two States elevates interest in the specific cognitive effects of Δ9tetrahydrocannabinol (Δ9THC), the major psychoactive constituent of marijuana. Controlled laboratory studies in nonhuman primates provide mixed evidence for specific effects of Δ9THC in learning and memory tasks, with a suggestion that frontal-mediated tasks may be most sensitive. In this study, adult male rhesus monkeys were trained on tasks which assess reversal learning, extradimensional attentional shift learning and spatial delayed-response. Subjects were challenged with 0.1–0.5 mg/kg Δ9THC, i.m., in randomized order and evaluated on the behavioral measures. Peak plasma levels of Δ9THC were observed 30 min after 0.2 mg/kg (69 ±29 ng/ml) and 60 min after 0.5 mg/kg (121 ±23 ng/ml) was administered and behavioral effects on a bimanual motor task persisted for up to 2 hrs after injection. An increase in errors-to-criterion (ETC) associated with reversal learning was further increased by Δ9THC in a dose-dependent manner. The increase in ETC associated with extradimensional shifts was not affected by Δ9-THC. Spatial delayed-response performance was impaired by Δ9THC in a retention-interval dependent manner. Overall the pattern of results suggest a more profound effect of Δ9THC on tasks mediated by orbitofrontal (reversal learning) versus dorsolateral (extradimensional shifts) prefrontal mechanisms.
Keywords: cannabis, marijuana, Macaca mulatta, extradimensional shift, reversal learning
1. Introduction
Cannabis has been the most commonly abused illicit drug in the United States across recent decades and within many generational cohorts (Johnston et al. 2011a; Johnston et al. 2011b). Understanding the cognitive effects of the primary psychoactive constituent of cannabis (delta9-tetrahydrocannabinol or Δ9THC) are increasingly important with recent successful efforts to decriminalize recreational cannabis in two US States (Healy 2012). Polysubstance-using human populations exhibit changes in executive function (Verdejo-Garcia et al. 2010), but less is known about how the constituent components of those drug combinations (e.g., cannabis) alter executive function. It is clear, however, that cannabis-associated alterations to executive functions (see (Crean et al. 2011a) for review) can have clear and detrimental effects on personal well-being and work-place competence for some individuals (Crean et al. 2011b).
The macaque monkey is an ideal laboratory species for investigating cognitive effects of Δ9THC due to a wide behavioral capability and a distribution of the CB1 receptor that matches that of the human brain (Eggan and Lewis 2007). Although acute Δ9THC exposure has been demonstrated to disrupt behavior in a variety of nonhuman primate species (Aigner 1988; Brady and Balster 1980; Branch et al. 1980; Ferraro and Grilly 1974; Galbicka et al. 1980; Hienz et al. 1992; Nakamura-Palacios et al. 2000; Pieper 1976; Schulze et al. 1988; Schulze et al. 1989; Stark and Dews 1980; Winsauer et al. 2011), most have been equivocal with respect to memory- or learning-specific effects, as opposed to generally sedative effects, of Δ9THC. As has been briefly reviewed (Taffe 2012b), this may be due to the deployment of tasks which focus on regions other than the frontal cortex. In cases where task-specific, trial-difficulty dependent effects of Δ9THC have been identified, the memory tasks have frequently involved spatial working memory (Rupniak et al. 1991; Verrico et al. 2012). A prior study from this laboratory (Taffe 2012b) found that THC impairs monkeys’ performance of the Self-Ordered Spatial Search task from the Cambridge Neuropsychological Test Automated Battery (CANTAB) which is thought to index spatial working memory and depends on intact frontal cortical function (Collins et al. 1998).
THC interferes with reversal learning but not original acquisition in an odor discrimination task in rats (Sokolic et al. 2011). CB1 receptor null mice display normal learning a Morris water maze task, but perseverate at the previously-correct location after platform has been moved (Varvel and Lichtman 2002). Another study found that THC impairs rats’ reversal learning but not extra-dimensional shifts on a rodent version of the Intra-Dimensional/Extra-Dimensional Attentional Set Shift Task (ID/ED) procedure (Egerton et al. 2005). Chronic daily Δ9THC has been shown to impair reversal, but not acquisition, of a color discrimination in stumptail macaque monkeys (Gluck et al. 1973).
The ID/ED task of the nonhuman primate version of the Cambridge Neuropsychological Test Automated Battery (CANTAB) incorporates a series of two-alternative trial-and-error discrimination learning problems. It has been shown that performance of Extra-dimensional shifts in this task depends on intact function in dorsolateral frontal regions in marmoset monkeys whereas reversal learning depends more on the orbitofrontal cortex (Dias et al. 1996a; 1997). We have also recently developed a spatial delayed-response task variant with the CANTAB which was sensitive to chronic alcohol consumption in peri-adolescent monkeys (Crean et al. 2011c). The experiments reported here employed these tasks to determine the relative influence of acute Δ9THC on performance of complex tasks which depend on intact frontal function.
Our prior studies demonstrated close overlap of Δ9THC doses that disrupted cognitive function and those which induced profound hypothermia (Taffe 2012a; b). This is unlike the rat in which doses which cause hypothermia are higher than those which disrupt cognitive function. In those previous studies, the effects on cognition in the monkeys were observed within about 20–30 min of injection whereas hypothermia developed over the second and third hours after treatment. The study reported here also sought to detail the time course of behavioral effects using repeated assessment with a measure of bimanual motor skill and with evaluation of plasma Δ9THC levels up to 2 hrs after intramuscular injection.
2. Materials and Methods
2.1 Animals
Eleven male rhesus monkeys (Macaca mulatta) were used in these experiments. Animals were of a single birth year and ranged from 5.5–10 years of age during the study. Daily chow (LabDiet® 5038, PMI Nutrition International, Richmond, IN, USA; 3.22 kcal of metabolizable energy (ME) per gram or Teklad® 15% Monkey Diet #8714, Harlan Laboratories, Madison, WI, USA; 3.00 kcal of ME per gram) allocations were supplemented with fruit or vegetables seven days per week and water was available ad libitum in the home cage. Animals were food restricted with reference to our prior studies to maintain adequate body condition scores and stable behavioral responding; see (Taffe 2004a; b). In some cases this was accomplished with a fixed daily post-testing feeding amount and in other cases this required adjusting the chow on a daily basis to account for intake of the chow-based reinforcer pellets. Overall this amounted to daily chow allocations which ranged from 90–240 grams. Animals on this study had previously been immobilized with ketamine (5–20 mg/kg) no less than semiannually for purposes of routine care and some experimental procedures. Animals also had various acute exposures to challenge drugs in additional studies, including THC for a thermoregulatory study (Taffe 2012a). The United States National Institutes of Health guidelines for laboratory animal care (Clark et al. 1996) were followed and all protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
2.2 Behavioral Testing
For behavioral testing, a touch-sensitive computer monitor was placed in front of the animal, unrestrained in a cage. All subjects had been trained to reach out of the cage to touch the location on the screen at which visual stimuli were presented to obtain a food pellet reward. The computer test battery consisted of three variants of the behavioral tasks included in the non-human primate CAmbridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition, Cambridge, UK). General descriptions of the individual tasks and the procedural details have been previously reported (Crean et al. 2011c; Taffe et al. 2004; Weed et al. 1999; Wright et al. 2012a). The Reversal Learning and Extra-dimensional Shift Tasks are task variants that have been recently described (Wright et al. 2012a).
2.2.1 Reversal Learning and Extra-dimensional Shift Tasks
These tasks consisted of a series of two-alternative, visual pattern discrimination tasks from the CANTAB Intra-dimensional/Extra-dimensional Attentional Shift Task (Dias et al. 1996b; Weed et al. 1999; Wright et al. 2012a). In brief, the task presented two “line” stimuli and two “shape” stimuli and the animal was required to determine by trial and error which stimulus was associated with reward on given stage. Each line stimulus was superimposed over one of the shape stimuli (hence a Compound Discrimination (CD) in which only one stimulus dimension was relevant to reward), with the pairings randomized from trial to trial. Likewise, the side on which the shape stimulus appeared was randomly assigned from trial to trial. On a given trial, correct selections resulted in screen blank, a pellet delivery and presentation of the subsequent trial after 5 sec. Incorrect selections resulted in no pellet delivery and a 9 sec blank screen prior to the next trial. Novel stimulus exemplars were used for each of the stages run on a given day and were randomized across animals within a given treatment condition. The criterion for successfully passing a stage was 12 out of 15 responses correct. The primary dependent measure in this task was the number of errors made prior meeting the criterion for completion (Errors-to-criterion; ETC).
For the Reversal Learning task, the “shape” dimension of the Compound Discrimination was always the relevant dimension. Animals were presented with 4 total stages in which there was an initial novel Compound Discrimination and then a reversal of that discrimination (i.e., the same stimuli were used by the previously non-rewarded shape was now rewarded). After completion of the reversal, the animals received a second discrimination using novel stimulus exemplars, followed by a reversal of that discrimination. For the Extra-dimensional Attentional Shift task, the monkeys completed an initial CD stage, a novel CD stage (termed the Intra-dimensional Shift stage) and then an Extra-dimensional Shift stage featuring new stimulus exemplars in which one of the line elements was now the reinforced stimulus. After successful completion of the EDS stage, a reversal stage was run in which the same stimuli were used and the previously un-reinforced line element was the rewarded option.
2.2.2 Spatial Delayed-Response Task
This task used a modification of the CANTAB visuo-spatial Paired Associates Learning task (e.g., (Taffe et al. 2002; 2004) designed to evaluate the decay of short-term spatial working memory. Animals were being trained on the standard vsPAL (which does not incorporate a variable retention interval) and this spatial delayed-response probe was assessed 3 months after the initiation of training. This task is conducted by parametrically increasing the interval between the response to the sample stimulus and the presentation of the choice stimuli. This task variant used one stimulus-location pairing for the sample and then probed 4 locations during the choice phase, constituting 1-stimulus, 4 location trials in the usual vsPAL nomenclature; chance responding was therefore 25% correct. Initial validation of the task was conducted in the present study to replicate our initial report with this novel task (Crean et al. 2011c) and to confirm that performance was systematically reduced with increasing retention intervals; for this the conditions included the standard 1 sec interval and then 5, 10, 20 and 30 second delays. Animals were returned to their ongoing vsPAL training (i.e., with standard 1 sec retention intervals) after this initial validation probe. About a month later, the effect of THC on the performance of this task was determined. The delays evaluated for the THC challenges consisted of 1, 3, 5 and 10 seconds due to an initial concern that drug effects would interact with the retention interval to suppress accuracy and lead to insufficient responding for interpretation. Monkeys received 10 trials of 1-stim (4-loc) with each of the delays being evaluated over sequential sessions in a randomized order for each animal.
2.2.3 Bimanual Motor Skill Task (BMS)
A transparent polycarbonate board (10 cm wide × 25 cm high × 2.75 cm thick) drilled with 15 holes (spaced 13 mm apart in a 3 horizontal × 5 vertical array) was filled with raisins and mounted perpendicular to the door of the transport cage. Subjects acquire a technique wherein they push the raisin out of the hole with one finger before retrieving it with the opposite hand, thus entailing bimanual dexterity. The time elapsed to retrieve all 15 raisins was recorded.
2.3 Drug Challenges
Monkeys were administered acute doses (0.1–0.5 mg/kg, IM) of Δ9THC prior to behavioral testing with active drug challenges being conducted no more than twice per week at 3–4 day intervals. The first Bimanual Motor Skill assessment was 60 min after injection, the Reversal Learning, Extra-dimensional Shift and Spatial Delayed Response tasks were started 20 min after injection. Treatment order was pseudo-randomized across subjects for a given task and the studies were completed within several consecutive weeks. The studies were conducted with substantial intervals between tasks such that animals were approximately 5.5 to 7 years of age for the Reversal Learning, Extra-Dimensional Shift and Bimanual Motor Skill Task studies, 9 years of age for the Spatial Delayed-Response task and 10–10.5 years of age for the plasma blood THC concentration study. The CB1 receptor antagonist SR141716 (Rimonabant) was co-injected (0.1 mg/kg, i.m.) in the Bimanual Task to determine receptor specificity. For injection, Δ9THC and SR141716 were suspended in a vehicle of absolute ethanol, Emulphor and saline in a 1:1:18 ratio. The Δ9THC was provided by the U.S. National Institute on Drug Abuse.
Eight of the subjects had received acute, subtoxic doses of 3,4-methylenedioxymethamphetamine (MDMA) 4 months prior to the Reversal/Extradimensional Shift studies. These studies were conducted across an interval of 6 months with interspersed acute doses of compounds for telemetric and behavioral studies which included THC (0.1–0.5 mg/kg i.m.), MDMA (1.78–2.4 mg/kg, i.m. and/or p.o.), Caffeine (5–10 mg/kg p.o.), SCH23390 (0.032–0.056 mg/kg, i.m.), raclopride (0.032–0.056 mg/kg, i.m.), 8-OH-DPAT (0.056–0.1 mg/kg, i.m.). MDMA was administered no more frequently than once per week and total active drug challenges did not exceed twice per week at 3–4 day intervals. There was an eighteen month interval without any other experimental drug challenges that preceded the Spatial Delayed-Response study.
2.4 Plasma Sampling
Monkeys were treated with Δ9THC (0.2 or 0.5 mg/kg, i.m.) in their home cage and then immobilized with 10 mg/kg ketamine (i.m.) 20 or 80 mins after treatment with Δ9THC. The 0.5 mg/kg dose was chosen because in pilot studies for our ongoing work (Taffe 2012a; b), a subset of the populations were still behaviorally responsive after this dose and it therefore reflects the outside range.
Once sedated, the monkeys were transferred to the procedure room where blood samples were drawn. Monkeys were boosted with an additional 5 mg/kg dose of ketamine if sedation waned during the course of the 30-minute blood draw session. Blood samples, which were drawn at 30 and 60 mins or 90 and 120 mins after Δ9THC administration, were then centrifuged at 2200g for 5 mins. After centrifugation, 500 microliters of plasma was transferred from each sample to siliconized eppendorf tubes containing 25 microliters of a 1 micromolar cannabidiol solution (in methanol) and refrigerated at −20C. Cannabidiol was used as an internal standard to control for possible drug degradation while samples were in storage. Each monkey was treated with both doses of Δ9THC over the course of 4 weeks, with the treatment order balanced across subjects. The study was conducted following prior administration of THC (0.1–0.5 mg/kg i.m.) and 4-methylmethcathinone (1.78–2.4 mg/kg, i.m; see (Wright et al. 2012b) in the 2 month interval proceeding, with washout intervals ranging from 1 week to 1 month. Inspection of the individual plasma data revealed no apparent effects of the pre-study drug challenges/washout interval, nor of treatment order within the plasma sampling study.
After thawing, 50 microliters of plasma from each monkey was transferred to a siliconized eppendorf tube containing 10 nanograms of (−)Δ9THC-D3, an internal standard. Subsequently, 500 microliters of an ethyl acetate/acetonitrile mixture was added and the tube was vortexed briefly. The tubes were then centrifuged in a cold room (4C) for 10 minutes at 3000 g. The organic top layers were transferred to labeled culture tubes and dried in a vacuum centrifuge at room temperature (~22C). After drying, the tubes were sealed with parafilm and refrigerated at −20C until analyzed. Prior to analysis, samples were thawed and reconstituted with 50 microliters of methanol.
Samples were analyzed by liquid chromatography with triple-quadrupole mass spectrometry (LC-QqQ MS). During analysis, a 6 pt curve was run from 200 femtograms/microliter to 500 picograms/microliter. The 200 femtograms/microliter standard was at the level of detection. All standards were run at the beginning of the analysis, in the middle, and at the end. Individual standards were analyzed every 10 samples to check calibration.
2.5 Data Analysis
Analysis of the behavioral data employed randomized block analysis of variance (ANOVA) with a consistent within-subjects factor of drug treatment condition. Analysis of the BMS data employed an additional repeated measures factor of time post-injection. The Reversal Learning data included two additional repeated measures factors for discrimination vs reversal stages and for the first and second pairings within a session and the Extra-dimensional task included a second repeated measures factor for stage. The Spatial Delayed-Response data were analyzed with a second repeated measures factor of retention interval. Post-hoc analyses of any significant main effects in the multi-factor ANOVAs were conducted using the Fisher LSD test including all pairwise comparisons. The criterion for significance in all tests was p< 0.05. Analyses were conducted with GB-STATv7.0; Dynamic Microsystems, Silver Spring MD
3. Results
3.1 Reversal Learning Task
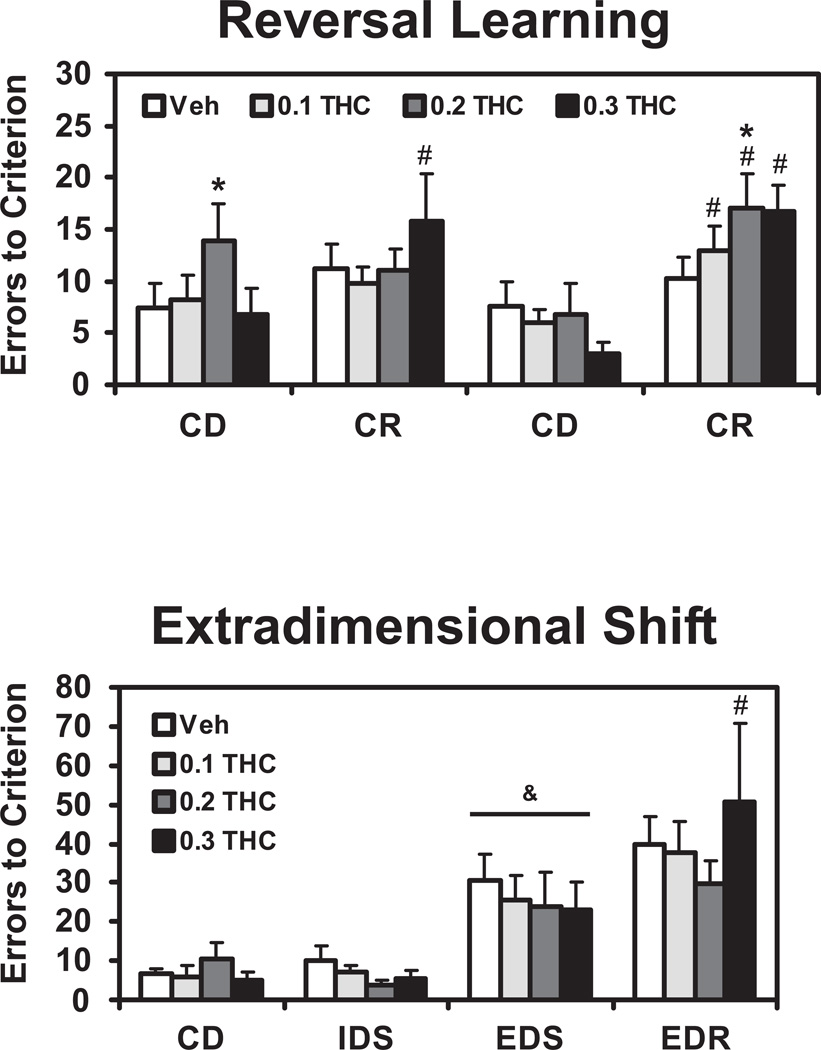
The monkeys (N=10) made more errors-to-criterion (ETC) in reversal learning compared with learning the original novel discriminations (Figure 1). There was a significant main effect of stage type (discrimination versus reversal) in the ANOVA [F1,9 = 73.91; p < 0.0001] and an interaction of this factor with the block factor (first vs second discrimination/reversal pair) in the session [F1,9 = 8.23; p < 0.05]. The post hoc test confirmed significant differences between the second discrimination and reversal pairs (0.1, 0.2 and 0.3 mg/kg Δ9THC) and between the first discrimination and reversal pairs after 0.3 mg/kg Δ9THC only. In addition, more ETC were made on the first discrimination stage and the second reversal stage following 0.2 mg/kg Δ9THC compared with the respective vehicle condition stages.
Figure 1.
The mean errors-to-criterion are presented for Reversal Learning (upper panel; N=10) and Extradimensional Shift (lower panel; N=7) tasks. A significant difference from the vehicle condition within stage is indicated by *, between IDS and EDS stages by & and a difference between a reversal and the prior discrimination by #. Error bars indicate SEM. Task/stage abbreviations: Compound Discrimination, CD; Compound Reversal, CR; Intra-dimensional Shift, IDS; Extra-dimensional Shift, EDS; Extra-dimensional Reversal; EDR.
3.2 Extra-Dimensional Shift Task
Monkeys (N=7) performance in this task depended on the component of the task as was confirmed by a significant main effect of stage in the ANOVA [F3,18=26.9; p<0.001]. The post hoc test likewise confirmed that animals made significantly more errors before reaching criterion in the EDS stages compared with the IDS stages under every treatment condition, but there were no differences between treatment conditions in the EDS stage confirmed. Post-hoc analysis also confirmed that more ETC were made in the EDR compared with EDS stage after 0.3 mg/kg THC but not under any other treatment condition.
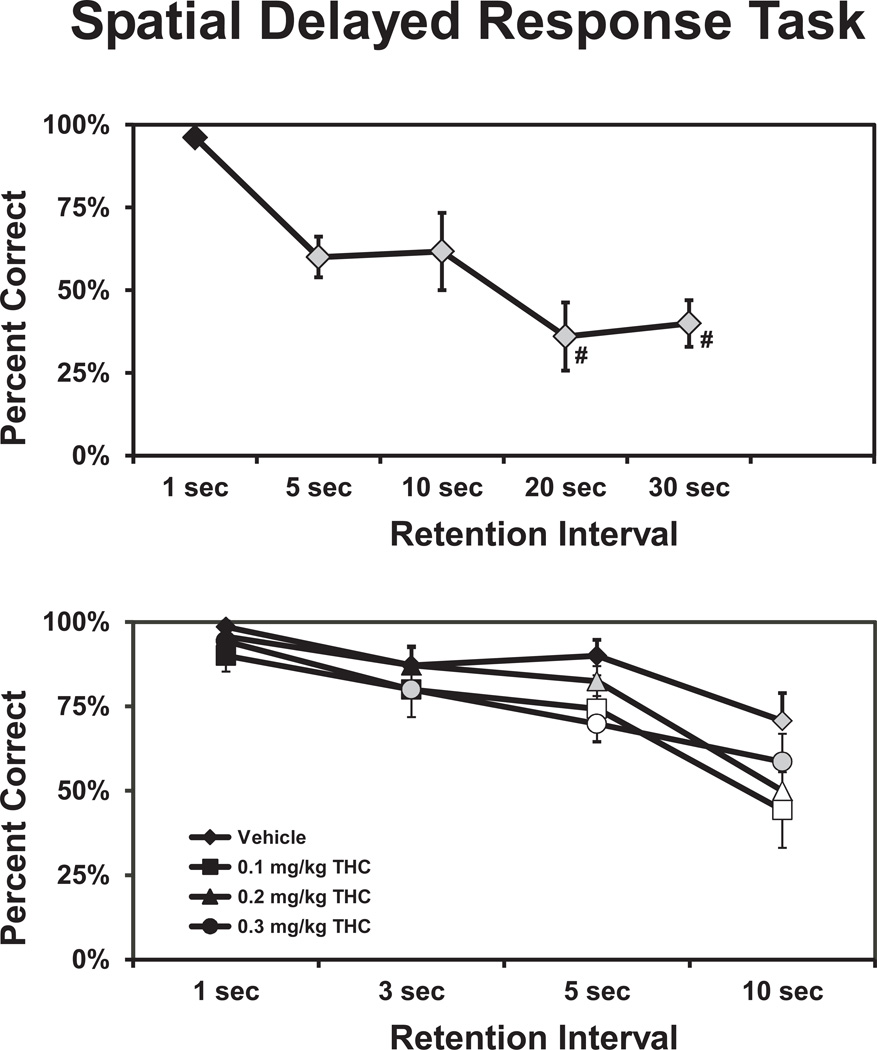
3.3 Spatial Delayed-Response
The monkeys (N=7) accuracy in the spatial delayed response task was detrimentally affected by Δ9THC in a manner that interacted with retention interval as depicted in Figure 2. The ANOVA confirmed a main effect of drug treatment condition (F3,18=7.5; p<0.002) as well as trial retention interval (F3,18=31.5; p<0.0001). Post hoc analysis of the behavior confirmed that significant decreases in the percent correct were obtained relative to the standard, 1-sec retention interval after the administration of vehicle (10 sec delay trials), 0.1 mg/kg (5, 10 sec), 0.2 mg/kg (5, 10 sec) and 0.3 mg/kg (3, 5, 10 sec) Δ9THC. Furthermore, performance on the 10 sec delay trials was significantly lower than for the 3-sec (all treatment conditions) and 5-sec (all conditions except 0.3 mg/kg Δ9THC) trials. The post hoc test further confirmed that THC impaired performance relative to the vehicle condition for retention intervals of 5 sec (0.1, 0.3 mg/kg doses) and 10 sec (0.1, 0.2 mg/kg doses); the performance of the 1 and 3 sec trials was not reliably different from the vehicle condition under any Δ9THC dosing condition. These data show that short-term memory for spatial location is specifically impaired by Δ9THC since other task demands (perception, attention, motor responses, etc) are similar for all trial types.
Figure 2.
The mean (N=7; ±SEM) trial completion accuracy on the Spatial Delayed Response task is given under baseline conditions (upper panel) and following drug challenge (lower panel). A significant difference from the standard 1-sec retention interval (within treatment) is depicted by shaded symbols and from both the 1-sec trials within treatment and the vehicle condition for a given retention interval by open symbols. A difference from the 5-sec and 10-sec trials is depicted by # on the upper panel.
3.4 Bimanual Motor Skill Tasks
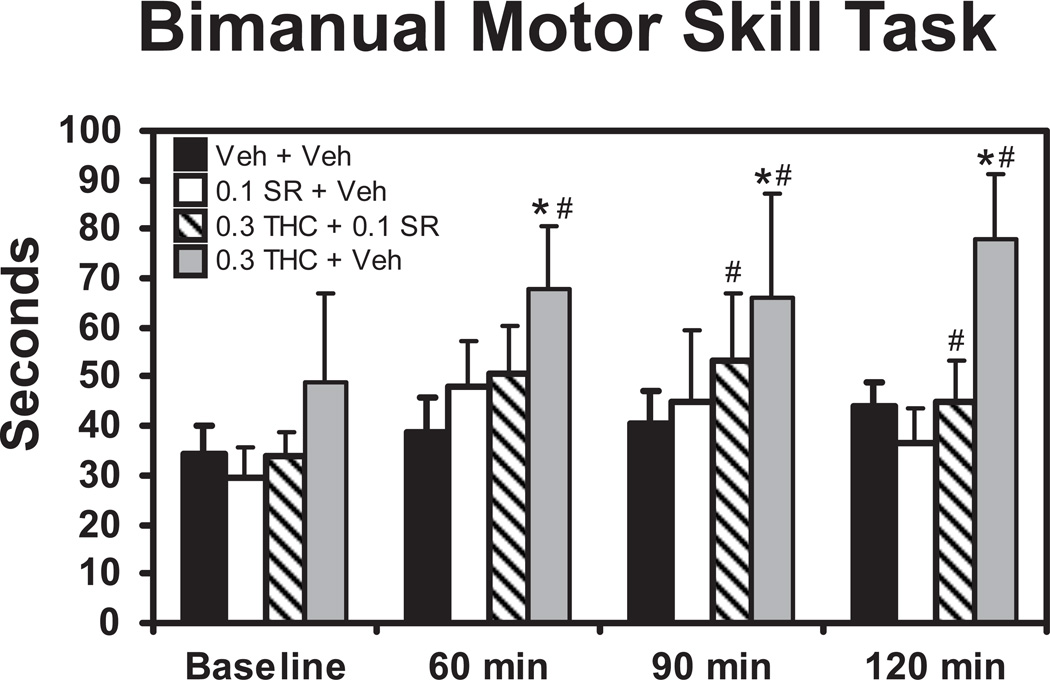
Animals (N=8) were assessed on the Bimanual Motor Skill task immediately prior to injection and then repeatedly at 60, 90 and 120 min post-injection (Figure 3). These assessments were done in combination with a telemetry study previously reported (Taffe 2012a) and the conditions included injection with vehicle, 0.3 mg/kg Δ9THC, 0.1 mg/kg SR141716 and the combination of 0.1 mg/kg SR141716 and 0.3 mg/kg Δ9THC. The ANOVA confirmed that raisin retrieval speed was altered by treatment condition [F3,21 = 4.80; p < 0.05] and time post-injection [F3,21 = 6.70; p < 0.005]. The post-hoc analysis further confirmed that this was because 0.3 mg/kg Δ9THC significantly slowed retrieval relative to the baseline observation when administered alone (60–120 min) or in combination with SR (90–120 min); no effect of time post-injection were confirmed for the other treatment conditions. Similarly, retrieval speed was slower after the administration of 0.3 mg/kg Δ9THC compared with similar times post-injection in the vehicle (60–90 min), 0.1 SR (90–120) and 0.1 SR + 0.3 Δ9THC (120 min) treatment conditions.
Figure 3.
The mean (N=8; ±SEM) latency to retrieve 15 raisins in the Bimanual Motor Skill task is depicted for the four treatment conditions. A significant difference from the baseline is depicted with the # and a difference from the vehicle condition at a given timepoint by *. SR = SR141716 (Rimonabant).
3.5 Plasma
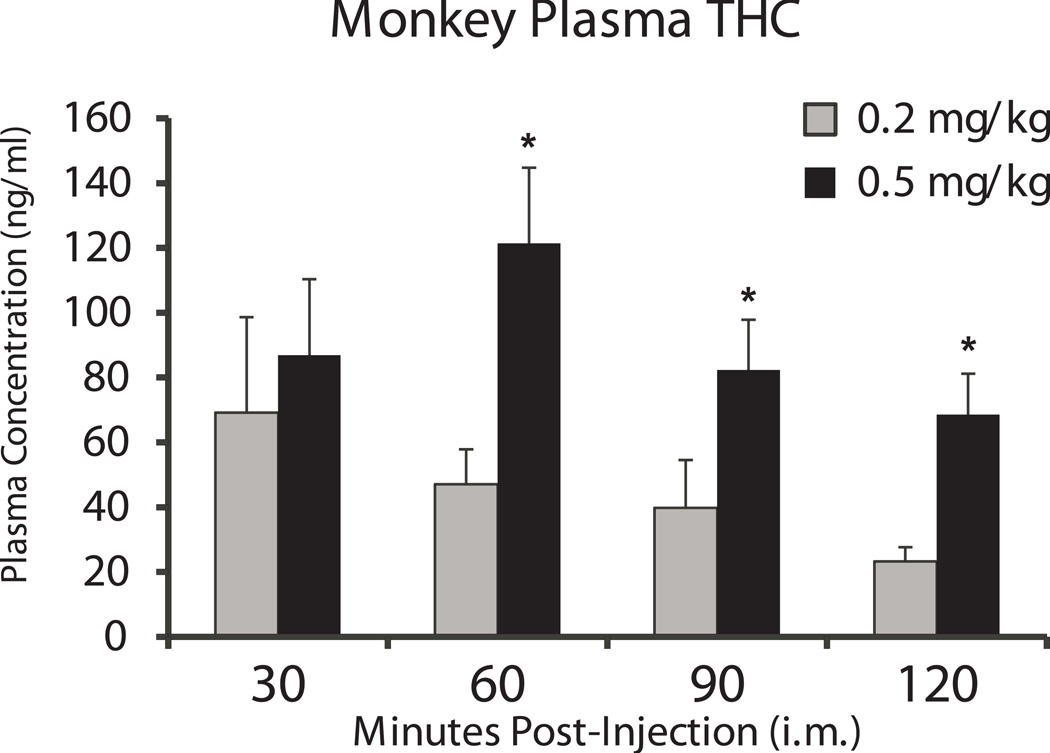
The mean (N=10) peak concentration of 69.3 ng/ml (SEM= 29.3) after 0.2 mg/kg Δ9THC was achieved in the first 30 minutes as is depicted in Figure 4. Mean peak plasma levels (121.3; SEM=23.4 ng/ml) of Δ9THC observed after the 0.5 mg/kg dose were reached at the 60 min time point. In both cases a gradual linear reduction in plasma levels was observed after the peak. The statistical analysis confirmed a main effect of dose [F1,10=12.29; p<0.01] and a trend for an effect of time post-injection [f3,30=2.6; p=0.07]. The post-hoc analysis confirmed that plasma levels differed between the dose conditions from 60–120 min post-injection.
Figure 4.
Mean (N=10; SEM) blood levels of Δ9THC following injection with 0.2 mg/kg or 0.5 mg/kg THC. Statistically significant differences between the two doses are indicated with *.
4. Discussion
This study distinguished the effects of Δ9-tetrahydrocannabinol (Δ9THC) on tasks which depend on intact function of distinct frontal cortical mechanism. Using the same set of tasks, Dias and colleagues (1997) reported that marmosets with orbitofrontal lesions were spared on extra-dimensional shifts (EDS) but were impaired on reversal learning and, conversely, marmosets with lateral frontal lesions were impaired on EDS but spared in reversal learning (Dias et al. 1997). The present data show no effect of acute Δ9THC on EDS but a dose-dependent effect on reversal learning. Consequently it is inferred that the effects of THC were more profound on orbitofrontal function than on dorsolateral prefrontal cortical function. Expression of the CB1 receptor is relatively high throughout frontal cortex but may be slightly higher in dorsolateral prefrontal compared with orbitofrontal regions of the macaque brain, although this difference only amounted to 7 versus 8 on a 10 point scale (Eggan and Lewis 2007).
The relatively modest effect of Δ9THC on spatial working memory, as documented in the scientific literature, is consistent with the effects on the reversal and extra-dimensional shift tasks observed here. Although evidence suggests that dorsolateral regions are particularly important for spatial delayed-response performance in macaque monkeys, it is clear that orbitofrontal regions are involved as well (Inoue et al. 2004; Mao et al. 1999; Oscar-Berman 1975). Electrophysiological investigations suggest that orbitofrontal regions are more associated with the evaluation of reward expectancy versus the spatial information processing (Ichihara-Takeda and Funahashi 2006; 2007; 2008; Tremblay and Schultz 1999) and are more oriented to stimulus-reward associations than to response-reward associations (Rudebeck et al. 2008). These findings are relevant because both reversal and EDS tasks utilized in the present experiments feature changes in reward association but have minimal spatial component. Verrico and colleagues found peri-pubescent monkeys were more impaired by intravenous THC on a spatial delayed-response task than they were on a pattern delayed match-to-sample task (Verrico et al. 2012). Similarly, monkeys were impaired by THC on tasks of spatial working memory and visuo-spatial associative learning and memory in a dose- and task-difficulty dependent manner (Taffe 2012b). Together these results suggest that the retention of information across time is perhaps less affected by THC than is the original storage of that information. The present reversal and extra-dimensional shift results are consistent with that prior study was well. The Self-Ordered Spatial Search task of working memory and the visuo-spatial Paired Associate Learning task both require the flexible alteration of responses based on ongoing within-trial contingencies. A previously correct response becomes incorrect on a response-by-response or trial-by-trial basis, matching the demands of the reversal learning task in the present study.
Interpretation of regional selectivity of behavioral findings must be tempered by the recognition that broader circuitry is likely involved. This is particularly important given that expression of the CB1 receptor is high in hippocampal regions (Eggan and Lewis 2007), including the parahippocampal and perirhinal areas associated with object-in-scene memory (Buffalo et al. 2006; Malkova and Mishkin 2003). A classic paper found that medial temporal lesions in macaque monkeys impaired concurrent discrimination learning and spatial delayed-response in addition to the better-remembered effects on delayed non-match to sample (Zola-Morgan and Squire 1985). Conversely, functional imaging data show delayed match-to-sample activates dorsolateral prefrontal regions (Hampson et al. 2009) that are better known for involvement in spatial delayed-response performance in monkeys (Ichihara-Takeda and Funahashi 2007; Mao et al. 1999). As noted above, monkeys are impaired by THC on a task which requires associating a specific pattern with a given spatial location in a dose- and task-difficulty dependent manner (Taffe 2012b). The Spatial Delayed-Response task in this study may possibly engage temporal mechanisms which were affected by THC. Finally, there was a hint of a biphasic dose-effect of THC on the initial discrimination learning since errors in the first CD stage were significantly higher than vehicle after 0.2 mg/kg THC for the Reversal study and numerically higher than vehicle in the Extradimensional shift study. This effect would have the tendency to obscure reversal effects at that dose since if the initial discrimination is not as well-established, reversing the association may be easier (Wright et al. 2010). The present data also confirm that the 0.02–0.5 mg/kg range injected intramuscularly produced blood levels of Δ9THC in the monkeys comparable to what would be expected in humans smoking marijuana. Humans typically achieve peak plasma Δ9THC levels between 60 – 200 ng/ml after smoking MJ (Azorlosa et al. 1995; Azorlosa et al. 1992; Cone and Huestis 1993; Gorelick et al. 2006; Huang et al. 2001). Marijuana smoke inhalation in monkeys produced 59 ng/ml (Slikker et al. 1991) while 0.03 mg/kg i.v. produced 140 ng/ml peak plasma levels (Schulze et al. 1988) and 0.3 mg/kg. i.v. produced 1419 ng/ml peak plasma three minutes after dosing (Bailey et al. 1987). Most rodent studies have employed considerably higher 10 – 20 mg/kg doses (Ali et al. 1989; Biswas et al. 1975; Breivogel et al. 1999; Corchero et al. 2004; Landfield et al. 1988; Romero et al. 1998; Rubino et al. 2000; Sagredo et al. 2006; Wu et al. 2008), though few data are available regarding the plasma Δ9THC levels attained.
The outcome of the repeated Bimanual Motor Skill testing for up to 2 hours after injection was consistent with the plasma study and confirmed that Δ9THC effects on behavior were reasonably consistent across the testing interval. Test sessions in this study, and a prior investigation (Taffe 2012b), were completed within approximately 60–90 min of initiation of the task. This experiment further supported the conclusion that the effects of Δ9THC on behavior were mediated by the CB1 receptor since the SR141716/Rimonabant antagonist reversed the slowing of raisin retrieval. This latter result is consistent with a similar reversal of Δ9THC-induced hypothermia by Rimonabant (Taffe 2012a); together these results confirm the specificity of both behavioral and thermoregulatory effects of Δ9THC in monkeys despite a different time-course for effects to develop.
In summary, this study used refined and validated memory procedures to assess the cognitive effects of the primary psychoactive constituent of marijuana in a controlled nonhuman primate model. The data verify some aspects of complex cognition are impaired by acute intoxication with Δ9THC including reversal learning and short-term spatial working memory. Other aspects of similarly complex function, such as discrimination learning and extra-dimensional attentional shift may be spared. These selective effects reinforce understanding that effects of this drug on cognition are not an all-or-none phenomenon.
Medical marijuana, and recreational legalization in two US States, will increase use.
There is need to delineate specific cognitive effects of Δ9tetrahydrocannabinol (THC).
This study examined executive function tasks which rely upon the prefrontal cortex.
THC impaired reversal learning without affecting extradimensional shifts of attention.
THC affects functions mediated by orbitofrontal over dorsolateral prefrontal regions.
Acknowledgements
The authors are grateful to the other principal investigators of the Scripps Center for Cannabis Addiction Neurobiology, Drs. Barbara J. Mason, and Susan F. Tapert for many helpful discussions as well as to Rene Croce for technical contributions. This work was supported by US Public Health Service / National Institutes of Health grants DA018418 and DA024194; those bodies had no further role in the design, conduct or analysis of the study. Dr. Wright was supported by a training grant T32 AA007456. The authors do not have any conflicts of interest to declare for this work. This is publication #21672 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: The behavioral studies were conceptualized by MAT and MJW and run by SAV who provided ongoing trouble shooting, experimental refinement and initial data analysis. LHP consulted extensively on the pharmacokinetic experiment and THC analysis. Final data analysis, figure preparation and initial manuscript drafting was conducted by MAT with significant input from MJW. All authors approved the manuscript prior to submission. The authors declare no conflicts of interest that pertain to this study.
REFERENCES
- Aigner TG. Delta-9-tetrahydrocannabinol impairs visual recognition memory but not discrimination learning in rhesus monkeys. Psychopharmacology. 1988;95:507–511. doi: 10.1007/BF00172964. [DOI] [PubMed] [Google Scholar]
- Ali SF, Newport GD, Scallet AC, Gee KW, Paule MG, Brown RM, Slikker W., Jr Effects of chronic delta-9-tetrahydrocannabinol (THC) administration on neurotransmitter concentrations and receptor binding in the rat brain. Neurotoxicology. 1989;10:491–500. [PubMed] [Google Scholar]
- Azorlosa JL, Greenwald MK, Stitzer ML. Marijuana smoking: effects of varying puff volume and breathhold duration. J Pharmacol Exp Ther. 1995;272:560–569. [PubMed] [Google Scholar]
- Azorlosa JL, Heishman SJ, Stitzer ML, Mahaffey JM. Marijuana smoking: effect of varying delta 9-tetrahydrocannabinol content and number of puffs. J Pharmacol Exp Ther. 1992;261:114–122. [PubMed] [Google Scholar]
- Bailey JR, Cunny HC, Paule MG, Slikker W., Jr Fetal disposition of delta 9-tetrahydrocannabinol (THC) during late pregnancy in the rhesus monkey. Toxicol Appl Pharmacol. 1987;90:315–321. doi: 10.1016/0041-008x(87)90338-3. [DOI] [PubMed] [Google Scholar]
- Biswas B, Deb G, Ghosh JJ. Changes in rat adrenal medulla following delta9-tetrahydrocannabinol treatment. A histochemical study. Acta endocrinologica. 1975;80:329–338. doi: 10.1530/acta.0.0800329. [DOI] [PubMed] [Google Scholar]
- Brady KT, Balster RL. The effects of delta 9-tetrahydrocannabinol alone and in combination with cannabidiol on fixed-interval performance in rhesus monkeys. Psychopharmacology. 1980;72:21–26. doi: 10.1007/BF00433803. [DOI] [PubMed] [Google Scholar]
- Branch MN, Dearing ME, Lee DM. Acute and chronic effects of delta 9-tetrahydrocannabinol on complex behavior of squirrel monkeys. Psychopharmacology. 1980;71:247–256. doi: 10.1007/BF00433059. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Bellgowan PS, Martin A. Distinct roles for medial temporal lobe structures in memory for objects and their locations. Learning & memory (Cold Spring Harbor, NY. 2006;13:638–643. doi: 10.1101/lm.251906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JD, Baldwin RL, Bayne KA, Brown MJ, Gebhart GF, Gonder JC, Gwathmey JK, Keeling ME, Kohn DF, Robb JW, Smith OA, Steggarda J-AD, Vandenbergh JG, White WJ, Williams-Blangero S, VandeBerg JL. Guide for the Care and Use of Laboratory Animals. Washington D.C.: Institute of Laboratory Animal Resources, National Research Council; 1996. p. 125. [Google Scholar]
- Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW. Perseveration and strategy in a novel spatial self-ordered sequencing task for nonhuman primates: effects of excitotoxic lesions and dopamine depletions of the prefrontal cortex. J Cogn Neurosci. 1998;10:332–354. doi: 10.1162/089892998562771. [DOI] [PubMed] [Google Scholar]
- Cone EJ, Huestis MA. Relating blood concentrations of tetrahydrocannabinol and metabolites to pharmacologic effects and time of marijuana usage. Ther Drug Monit. 1993;15:527–532. doi: 10.1097/00007691-199312000-00013. [DOI] [PubMed] [Google Scholar]
- Corchero J, Oliva JM, Garcia-Lecumberri C, Martin S, Ambrosio E, Manzanares J. Repeated administration with Delta9-tetrahydrocannabinol regulates mu-opioid receptor density in the rat brain. J Psychopharmacol. 2004;18:54–58. doi: 10.1177/0269881104040237. [DOI] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. Journal of addiction medicine. 2011a;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Tapert SF, Minassian A, Macdonald K, Crane NA, Mason BJ. Effects of chronic, heavy cannabis use on executive functions. Journal of addiction medicine. 2011b;5:9–15. doi: 10.1097/ADM.0b013e31820cdd57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Vandewater SA, Katner SN, Huitron-Resendiz S, Taffe MA. Chronic alcohol consumption impairs visuo-spatial associative memory in periadolescent rhesus monkeys. Drug Alcohol Depend. 2011c;114:31–40. doi: 10.1016/j.drugalcdep.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996a;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996b;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from "on-line" processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Brett RR, Pratt JA. Acute delta9-tetrahydrocannabinol-induced deficits in reversal learning: neural correlates of affective inflexibility. Neuropsychopharmacology. 2005;30:1895–1905. doi: 10.1038/sj.npp.1300715. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical Distribution of the Cannabinoid CB1 Receptor in the Primate Neocortex: A Regional and Laminar Analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Ferraro DP, Grilly DM. Effects of chronic exposure to delta9-tetrahydrocannabinol on delayed matching-to-sample in chimpanzees. Psychopharmacologia. 1974;37:127–138. doi: 10.1007/BF00437419. [DOI] [PubMed] [Google Scholar]
- Galbicka G, Lee DM, Branch MN. Schedule-dependent tolerance to behavioral effects of delta 9-tetrahydrocannabinol when reinforcement frequencies are matched. Pharmacol Biochem Behav. 1980;12:85–91. doi: 10.1016/0091-3057(80)90420-7. [DOI] [PubMed] [Google Scholar]
- Gluck JP, Ferraro DP, Marriott RG. Retardation of discrimination reversal by delta9-tetrahydrocannabinol in monkeys. Pharmacol Biochem Behav. 1973;1:605–608. doi: 10.1016/0091-3057(73)90022-1. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Huestis MA. The cannabinoid CB1 receptor antagonist rimonabant attenuates the hypotensive effect of smoked marijuana in male smokers. American heart journal. 2006;151:754 e1–754 e5. doi: 10.1016/j.ahj.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Espana RA, Rogers GA, Porrino LJ, Deadwyler SA. Mechanisms underlying cognitive enhancement and reversal of cognitive deficits in nonhuman primates by the ampakine CX717. Psychopharmacology (Berl) 2009;202:355–369. doi: 10.1007/s00213-008-1360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy J. Voters Ease Marijuana Laws in 2 States, but Legal Questions Remain New York Times. New York, New York: The New York Times Company; 2012. [Google Scholar]
- Hienz RD, Turkkan JS, Spear DJ, Sannerud CA, Kaminski BJ, Allen RP. General activity in baboons measured with a computerized, lightweight piezoelectric motion sensor: effects of drugs. Pharmacol Biochem Behav. 1992;42:497–507. doi: 10.1016/0091-3057(92)90145-6. [DOI] [PubMed] [Google Scholar]
- Huang W, Moody DE, Andrenyak DM, Smith EK, Foltz RL, Huestis MA, Newton JF. Simultaneous determination of delta9-tetrahydrocannabinol and 11-nor-9-carboxy-delta9-tetrahydrocannabinol in human plasma by solid-phase extraction and gas chromatography-negative ion chemical ionization-mass spectrometry. J Anal Toxicol. 2001;25:531–537. doi: 10.1093/jat/25.7.531. [DOI] [PubMed] [Google Scholar]
- Ichihara-Takeda S, Funahashi S. Reward-period activity in primate dorsolateral prefrontal and orbitofrontal neurons is affected by reward schedules. J Cogn Neurosci. 2006;18:212–226. doi: 10.1162/089892906775783679. [DOI] [PubMed] [Google Scholar]
- Ichihara-Takeda S, Funahashi S. Activity of primate orbitofrontal and dorsolateral prefrontal neurons: task-related activity during an oculomotor delayed-response task. Exp Brain Res. 2007;181:409–425. doi: 10.1007/s00221-007-0941-0. [DOI] [PubMed] [Google Scholar]
- Ichihara-Takeda S, Funahashi S. Activity of primate orbitofrontal and dorsolateral prefrontal neurons: effect of reward schedule on task-related activity. J Cogn Neurosci. 2008;20:563–579. doi: 10.1162/jocn.2008.20047. [DOI] [PubMed] [Google Scholar]
- Inoue M, Mikami A, Ando I, Tsukada H. Functional brain mapping of the macaque related to spatial working memory as revealed by PET. Cereb Cortex. 2004;14:106–119. doi: 10.1093/cercor/bhg109. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Scheulenberg JE. Monitoring the Future national survey results on drug use, 1975–2010. Volume I. Ann Arbor: Secondary school students University of Michigan; 2011a. p. 734. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. College students and adults ages 19–45. Volume II. Ann Arbor: University of Michigan; 2011b. Monitoring the Future national survey results on drug use, 1975–2010; p. 312. [Google Scholar]
- Landfield PW, Cadwallader LB, Vinsant S. Quantitative changes in hippocampal structure following long-term exposure to delta 9-tetrahydrocannabinol: possible mediation by glucocorticoid systems. Brain Res. 1988;443:47–62. doi: 10.1016/0006-8993(88)91597-1. [DOI] [PubMed] [Google Scholar]
- Malkova L, Mishkin M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J Neurosci. 2003;23:1956–1965. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao ZM, Arnsten AF, Li BM. Local infusion of an alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol Psychiatry. 1999;46:1259–1265. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- Nakamura-Palacios EM, Winsauer PJ, Moerschbaecher JM. Effects of the cannabinoid ligand SR 141716A alone or in combination with delta9-tetrahydrocannabinol or scopolamine on learning in squirrel monkeys. Behav Pharmacol. 2000;11:377–386. doi: 10.1097/00008877-200008000-00003. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. The effects of dorsolateral-frontal and ventrolateral-orbitofrontal lesions on spatial discrimination learning and delayed response in two modalities. Neuropsychologia. 1975;13:237–246. doi: 10.1016/0028-3932(75)90033-0. [DOI] [PubMed] [Google Scholar]
- Pieper WA. Great apes and rhesus monkeys as subjects for psychopharmacological studies of stimulants and depressants. Fed Proc. 1976;35:2254–2257. [PubMed] [Google Scholar]
- Romero J, Berrendero F, Manzanares J, Perez A, Corchero J, Fuentes JA, Fernandez-Ruiz JJ, Ramos JA. Time-course of the cannabinoid receptor down-regulation in the adult rat brain caused by repeated exposure to delta9-tetrahydrocannabinol. Synapse. 1998;30:298–308. doi: 10.1002/(SICI)1098-2396(199811)30:3<298::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Massi P, Spinello M, Zagato E, Giagnoni G, Parolaro D. Chronic delta-9-tetrahydrocannabinol treatment increases cAMP levels and cAMP-dependent protein kinase activity in some rat brain regions. Neuropharmacology. 2000;39:1331–1336. doi: 10.1016/s0028-3908(99)00196-3. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, Rushworth MF. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28:13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupniak NM, Samson NA, Steventon MJ, Iversen SD. Induction of cognitive impairment by scopolamine and noncholinergic agents in rhesus monkeys. Life Sci. 1991;48:893–899. doi: 10.1016/0024-3205(91)90036-b. [DOI] [PubMed] [Google Scholar]
- Sagredo O, Ramos JA, Fernandez-Ruiz J, Rodriguez ML, de Miguel R. Chronic Delta(9)-tetrahydrocannabinol administration affects serotonin levels in the rat frontal cortex. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:313–317. doi: 10.1007/s00210-005-0026-1. [DOI] [PubMed] [Google Scholar]
- Schulze GE, McMillan DE, Bailey JR, Scallet A, Ali SF, Slikker W, Jr, Paule MG. Acute effects of delta-9-tetrahydrocannabinol in rhesus monkeys as measured by performance in a battery of complex operant tests. J Pharmacol Exp Ther. 1988;245:178–186. [PubMed] [Google Scholar]
- Schulze GE, McMillan DE, Bailey JR, Scallet AC, Ali SF, Slikker W, Jr, Paule MG. Acute effects of marijuana smoke on complex operant behavior in rhesus monkeys. Life Sci. 1989;45:465–475. doi: 10.1016/0024-3205(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Paule MG, Ali SF, Scallet AC, Bailey JR. Chronic marijuana smoke exposure in the rhesus monkeyIPlasma cannabinoid and blood carboxyhemoglobin concentrations and clinical chemistry parameters. Fundam Appl Toxicol. 1991;17:321–334. doi: 10.1016/0272-0590(91)90222-p. [DOI] [PubMed] [Google Scholar]
- Sokolic L, Long LE, Hunt GE, Arnold JC, McGregor IS. Disruptive effects of the prototypical cannabinoid Delta-tetrahydrocannabinol and the fatty acid amide inhibitor URB-597 on go/no-go auditory discrimination performance and olfactory reversal learning in rats. Behav Pharmacol. 2011;22:191–202. doi: 10.1097/FBP.0b013e328345c82b. [DOI] [PubMed] [Google Scholar]
- Stark P, Dews PB. Cannabinoids. I. Behavioral effects. J Pharmacol Exp Ther. 1980;214:124–130. [PubMed] [Google Scholar]
- Taffe MA. Effects of parametric feeding manipulations on behavioral performance in macaques. Physiol Behav. 2004a;81:59–70. doi: 10.1016/j.physbeh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Erratum: "Effects of parametric feeding manipulations on behavioral performance in macaques". Physiol Behav. 2004b;82:589. doi: 10.1016/j.physbeh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Delta9-Tetrahydrocannabinol attenuates MDMA-induced hyperthermia in rhesus monkeys. Neuroscience. 2012a;201:125–133. doi: 10.1016/j.neuroscience.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA. Delta(9) tetrahydrocannabinol impairs visuo-spatial associative learning and spatial working memory in rhesus macaques. J Psychopharmacol. 2012b;26:1299–1306. doi: 10.1177/0269881112443743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Differential muscarinic and NMDA contributions to visuo-spatial paired-associate learning in rhesus monkeys. Psychopharmacology. 2002;160:253–262. doi: 10.1007/s00213-001-0954-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Modeling a task that is sensitive to dementia of the Alzheimer's type: individual differences in acquisition of a visuo-spatial paired-associate learning task in rhesus monkeys. Behavioural brain research. 2004;149:123–133. doi: 10.1016/s0166-4328(03)00214-6. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Del Mar Sanchez-Fernandez M, Alonso-Maroto LM, Fernandez-Calderon F, Perales JC, Lozano O, Perez-Garcia M. Impulsivity and executive functions in polysubstance-using rave attenders. Psychopharmacology (Berl) 2010;210:377–392. doi: 10.1007/s00213-010-1833-8. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Liu S, Bitler EJ, Gu H, Sampson AR, Bradberry CW, Lewis DA. Delay- and dose-dependent effects of Delta(9)-tetrahydrocannabinol administration on spatial and object working memory tasks in adolescent rhesus monkeys. Neuropsychopharmacology. 2012;37:1357–1366. doi: 10.1038/npp.2011.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LH. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res Cogn Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Molina PE, Amedee AM, Filipeanu CM, McGoey RR, Troxclair DA, Walker EM, Birke LL, Stouwe CV, Howard JM, Leonard ST, Moerschbaecher JM, Lewis PB. Tolerance to chronic delta-9-tetrahydrocannabinol (Delta-THC) in rhesus macaques infected with simian immunodeficiency virus. Exp Clin Psychopharmacol. 2011;19:154–172. doi: 10.1037/a0023000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Jr, Glavis-Bloom C, Taffe MA. Acute ethanol reduces reversal cost in discrimination learning by reducing perseverance in adolescent rhesus macaques. Alcohol Clin Exp Res. 2012a doi: 10.1111/acer.12050. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Taffe MA, Disorders CNA. Effects of Ethanol on Reversal Learning and Extra-Dimensional Attentional Shift in Rhesus Macaques. Alcoholism-Clinical and Experimental Research. 2010;34:30a–30a. [Google Scholar]
- Wright MJ, Vandewater SA, Angrish D, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) and d-methamphetamine improve visuospatial associative memory, but not spatial working memory, in rhesus macaques. Brit J Pharmacol. 2012b;167:1342–1352. doi: 10.1111/j.1476-5381.2012.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YH, Lin KL, Chen SC, Chang YZ. Integration of GC/EI-MS and GC/NCI-MS for simultaneous quantitative determination of opiates, amphetamines, MDMA, ketamine, and metabolites in human hair. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;870:192–202. doi: 10.1016/j.jchromb.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behav Neurosci. 1985;99:22–34. doi: 10.1037//0735-7044.99.1.22. [DOI] [PubMed] [Google Scholar]