SUMMARY
Bromodomain-containing protein 4 (Brd4) is an epigenetic reader and transcriptional regulator recently identified as a cancer therapeutic target for acute myeloid leukemia, multiple myeloma, and Burkitt's lymphoma. Although chromatin targeting is a crucial function of Brd4, there is little understanding of how bromodomains that bind acetylated histones are regulated, nor how the gene-specific activity of Brd4 is determined. Via interaction screen and domain mapping, we identified p53 as a functional partner of Brd4. Interestingly, Brd4 association with p53 is modulated by casein kinase II (CK2)-mediated phosphorylation of a conserved acidic region in Brd4 that selectively contacts either a juxtaposed bromodomain or an adjacent basic region to dictate the ability of Brd4 binding to chromatin and also the recruitment of p53 to regulated promoters. The unmasking of bromodomains and activator recruitment, concurrently triggered by the CK2 phospho switch, provide an intriguing mechanism for gene-specific targeting by a universal epigenetic reader.
INTRODUCTION
Bromodomain (Brd) is an acetyl-lysine (Kac)-binding module of ~110 amino acids found in many transcriptional regulators and chromatin-modifying enzymes. In humans, there are 46 proteins containing a total of 61 Brds classified into eight families, where many Brd structures have been determined (Filippakopoulos et al., 2012). In general, a Kac-containing peptide derived from histones or nonhistone proteins fits into a hydrophobic pocket formed by two α-helix-connecting loops, including the long ZA loop linking αZ and αA, and the short BC loop joining αB and αC. The specificity and affinity of Brd-Kac recognition is determined by amino acids and modifications flanking the Kac peptide and also by spatially oriented residues surrounding the hydrophobic pocket unique to each Brd domain. This configuration underlies recent identification of two anti-cancer therapeutic compounds, JQ1 and I-BET, shown to be effective against acute myeloid leukemia, multiple myeloma, and Burkitt's lymphoma (Dawson et al., 2011; Delmore et al., 2011; Mertz et al., 2011; Zuber et al., 2011) by blocking the chromatin binding activity of a specific Brd family (BET) that harbors two bromodomains (BD1 and BD2) and an extraterminal (ET) domain via competitive binding to Kac-binding pockets of the BET family proteins, including Brd2, Brd3, Brd4, and Brdt (Wu and Chiang, 2007).
Brd4 was originally identified as a mitotic chromosome-binding protein that remains associated with acetylated chromatin throughout the entire cell cycle (Dey et al., 2003) and thus provides epigenetic memory (gene bookmarking) for post-mitotic G1 gene transcription (Zhao et al., 2011). The chromatin-binding activity of Brd4 is noted for preserving acetylated chromatin status, maintaining high-order chromatin structure, and, when anchored by some viruses, for episomal genome segregation (Wu and Chiang, 2007). A direct role of Brd4 in transcription is evident by its association with positive transcription elongation factor b (P-TEFb), general transcription cofactor Mediator, gene-specific proinflammatory factor NFkB, and virus-encoded transcriptional regulators (Chiang, 2009). Deregulation of Brd4 is clinically linked to NUT midline carcinoma (French, 2012), breast, colon and prostate cancers, and is functionally associated with epithelial-to-mesenchymal transition, stem cell-like conversion (Alsarraj et al., 2011), and primary stress responses (Hargreaves et al., 2009; Zippo et al., 2009). While the biological significance of Brd4 has been increasingly recognized, how it controls these diverse processes and how the “universal” chromatin-binding activity of Brd4 is conduced to “gene-specific” targeting are important unresolved issues for our general understanding of the chromatin-decoding processes by epigenetic readers.
Since Brd4 has a short residence time on chromatin as seen with many other chromatin-binding factors that show stable yet dynamic association with chromatin (Phair et al., 2004), we hypothesized that Brd4 gene-specific targeting is jointly conferred by an adjacent sequence-specific DNA-binding protein that likewise binds transiently to its target sequence in vivo. This cooperative binding, triggered by three low-affinity (i.e., Brd4-acetylated histone tail, activator-DNA, and Brd4-activator) interactions, leads to a “commitment” step for gene-specific regulation (Wu and Chiang, 2007). Here we describe a candidate protein-protein interaction screen showing that Brd4 associates directly with many transcription factors and chromatin-modifying enzymes, including p53, YY1, AP2, c-Jun, c-Myc/Max, C/EBPα, C/EBPβ, Acf1, and G9a. Investigation of Brd4-p53 interactions unravels a phospho switch mechanism for Brd4 binding to chromatin and recruitment of p53 to its regulated promoters. This study not only uncovers a molecular mechanism for gene-specific targeting by a universal epigenetic reader, but also identifies Brd4 as a previously unreported regulator for p53 target gene transcription.
RESULTS
Candidate Screen of Brd4-Interacting Proteins
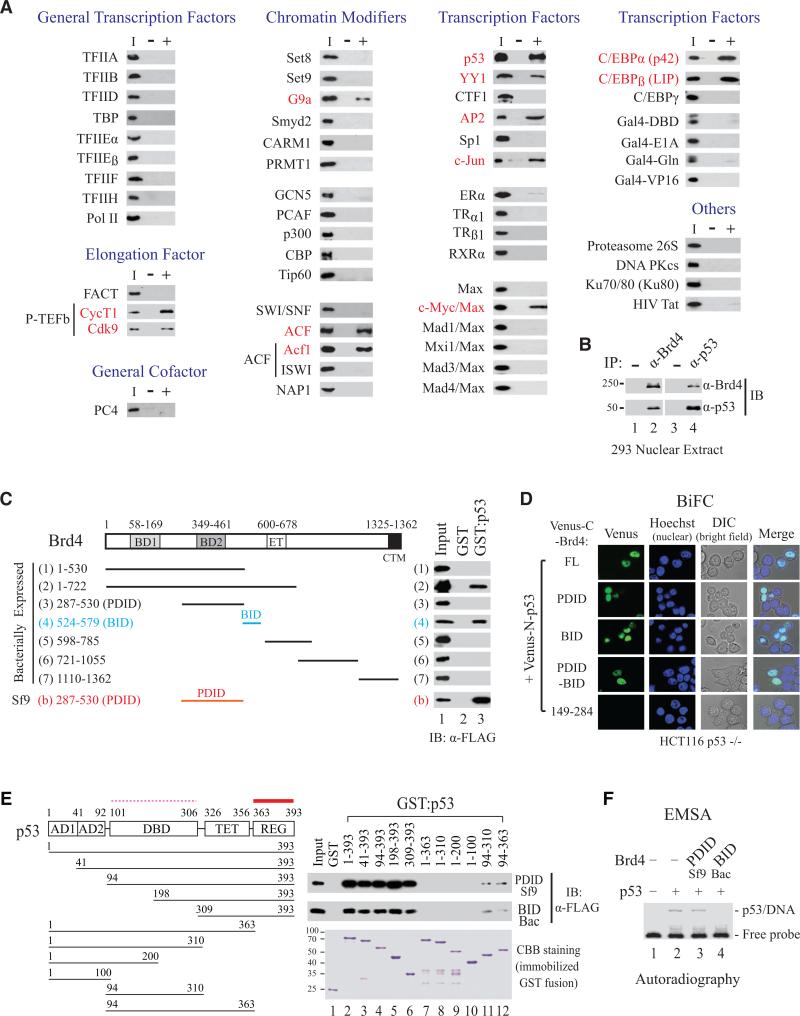
To define the role of Brd4 in chromatin targeting and transcriptional regulation, we performed an unbiased candidate screen to identify cellular proteins interacting directly with Brd4. Using purified proteins for individual incubation with recombinant FLAG-tagged human Brd4 (f:Brd4) and followed by immunoprecipitation (IP) with anti-Brd4 antibody and immunoblotting (IB) using antibodies recognizing tested proteins, we found that none of the human RNA polymerase II (Pol II) general transcription components (TFIIA, TFIIB, TFIID, TBP, TFIIEα, TFIIEβ, TFIIF, TFIIH, and Pol II), elongation cofactor FACT, and general cofactor PC4 interacted directly with Brd4, except for P-TEFb (Figure 1A, left column). A panel of purified histone methyltransferases (Set8, Set9, G9a, Smyd2, CARM1, and PRMT1), histone acetyltransferases (GCN5, PCAF, p300, CBP, and Tip60), chromatin-remodeling enzymes SWI/SNF and ACF (heterodimer of Acf1 and ISWI), and histone chaperone NAP-1 was then screened. Only G9a and ACF (via its Acf1 subunit) interacted directly with Brd4 (Figure 1A, second column). Further survey of sequence-specific DNA-binding proteins identified p53, YY1, AP2, c-Jun, c-Myc/Max heterodimer, C/EBPα, and C/EBPβ also interact directly with Brd4 (Figure 1A, third and fourth columns). The finding that Brd4 contacts particular chromatin-modifying enzymes (e.g., ACF, but not SWI/SNF) and select transcription factors (e.g., c-Myc/Max, but not other Max family heterodimers; and C/EBPα/β, but not C/EBPγ) indicates that Brd4 function is tailored by sequence-specific transcription factors and unique chromatin-modifying enzymes, and that gene-specific targeting is an event jointly determined by Brd4 and its interacting partners.
Figure 1. Brd4 Interacts with Select Chromatin Modifiers and Transcription Factors.
(A) Candidate screen of Brd4-interacting proteins by solution pulldown with purified protein (I, input) incubated with (+) or without (-) f:Brd4. IP with α-Brd4 antibody, followed by IB with anti-test protein antibody.
(B) Brd4 interacts with p53 in 293 nuclear extract.
(C) BID and PDID interact directly and independently with GST:p53, not GST. Proteins purified from bacteria or Sf9 as indicated.
(D) p53 interacts with Brd4 FL, BID and PDID in vivo by BiFC live-cell imaging. Venus-N-p53 and Venus-C-Brd4 containing FL, PDID, BID, PDID-BID, or aa 149-284 were co-expressed in HCT116 p53 -/- cells and imaged by confocal microscopy.
(E) f:PDID (purified from Sf9) and f:BID (purified from bacteria) interact strongly with REG (thick solid line) but weakly with DBD (thin dashed line) of p53. GST pulldown was performed by incubating f:PDID or f:BID with GST or each fusion. Bound f:PDID and f:BID detected by α-FLAG antibody with GST derivatives visualized by CBB staining.
(F) p53 DNA-binding activity inhibited by BID but not PDID. EMSA performed by incubating f:p53, with or without f:PDID or f:BID, with 32P-labeled DNA containing human p21 p53-binding site.
Two New Conserved Regions in Brd4 (i.e., BID and PDID) Directly Interact with p53
To explore the functional significance, we then focused on characterization of Brd4-p53 interaction. Using nuclear extracts from HEK293 cells, we found that endogenous Brd4 associates with endogenous p53 as shown by reciprocal IP and IB detection (Figure 1B). p53-interacting regions in Brd4 were mapped by solution pull-down assay using immobilized GST:p53 or GST (as control) with various recombinant f:Brd4 deletion fragments purified from bacteria. This mapping identified a region spanning amino acids 524-579 of Brd4 interacting directly with p53 (Figure 1C, fragments 1-7). This region was named BID, representing a basic residue-enriched interaction domain conserved in BET proteins (Figure S1A). Intriguingly, amino acids 287-530 of Brd4, when purified from insect Sf9 cells but not from bacteria, showed an even stronger interaction with p53 (Figure 1C, fragments 3 vs. b). This region in Brd4, enriched in acidic residues and containing seven putative CK2 phosphorylation sites, was named PDID for a phosphorylation-dependent interaction domain also conserved among BET proteins (Figure S1B).
To show that BID and PDID could independently interact with p53 in cells, we transfected a series of plasmids expressing GST-tagged Brd4 fragments spanning the entire protein individually into 293 cells. Fragments containing BID or PDID could interact efficiently with p53, and PDID-containing fragments (C and D) again exhibited a stronger interaction with p53 than BID-containing fragments (E, F, and G; Figure S1C). Using bimolecular fluorescence complementation (BiFC) assay linking Brd4 and p53 to nonfluorescent C- and N-terminal fragments of Venus (Nagai et al., 2002) respectively for live-cell imaging in p53-null HCT116 cells, we could directly visualize reconstituted Venus fluorescence mediated by Brd4-p53 interaction (Figure 1D, first row). In vivo association between p53 and PDID, BID, and PDID-BID, but not the non-interacting N-terminal 149-284 fragment of Brd4, was also observed in Venus-fusion-transfected HCT116 p53-/- cells (Figure 1D and Figure S1D), confirming that Brd4 contains two p53-interacting regions and that each domain (BID or PDID) can interact independently and directly with p53 in vitro and in vivo.
PDID and BID Both Interact Strongly with REG but Weakly with DBD of p53 with Differential Effects on p53 Binding to DNA
Reciprocal mapping of p53 regions interacting with PDID and BID was conducted by incubating a series of immobilized N- and C-terminal deletions of GST:p53 individually with Sf9-expressed PDID or bacterially expressed BID, followed by IB detection of bound PDID or BID. Removal of N-terminal 308 amino acids did not diminish p53 interaction with PDID or BID (Figure 1E, lanes 1-6), whereas deletion of the last 30 amino acids (364-393) completely impaired p53 binding to PDID or BID (lanes 7-10). Interestingly, removal of N-terminal 93 amino acids containing two activation domains unmasked p53 DNA-binding domain for interaction with PDID and BID, even though the interaction was significantly weaker than that mediated by the C-terminal regulatory domain (Figure 1E, lanes 7 vs. 12, and 8 vs. 11).
Since PDID and BID interaction with p53 might regulate p53 binding to DNA, we performed electrophoretic mobility shift assay (EMSA) by incubating 32P-labelled DNA containing a p53-binding site from human p21 gene with purified p53, with or without PDID or BID. While p53 retained its DNA-binding activity in the presence of PDID, it failed to bind DNA when BID was present (Figure 1F). This EMSA analysis indicates that BID and PDID differentially regulate p53 DNA-binding activity, likely via select contact with amino acids in the C-terminal regulatory or DNA-binding domain of p53 to sequester (BID-p53 interaction) or support (PDID-p53 interaction) p53 target gene transcription.
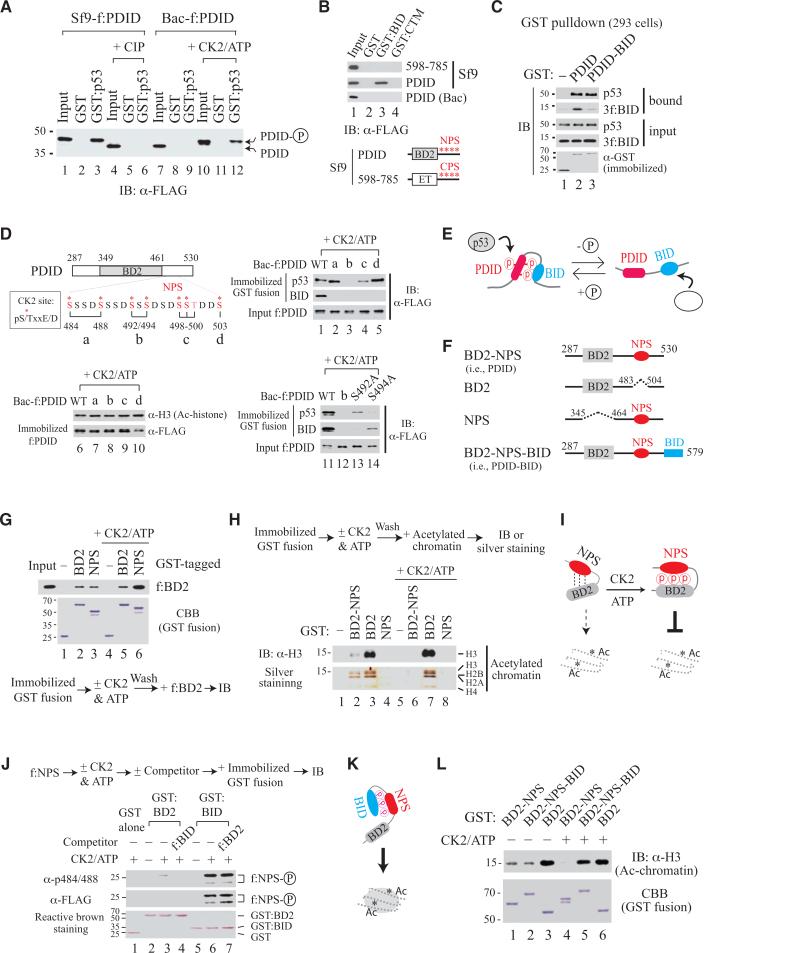
PDID Mediates Intermolecular Interaction with p53 and Intramolecular Contact with BID, Both in a Phosphorylation-Dependent Manner
Since PDID contains seven putative CK2 phosphorylation sites, phosphorylation might be necessary for PDID interaction with p53. Indeed, removal of the phosphate group from f:PDID purified from Sf9 cells (Sf9-f:PDID) by calf intestinal alkaline phosphatase (CIP) treatment not only induced a faster mobility shift but also abolished its interaction with p53, whereas phosphorylation of Bac-f:PDID with CK2 slowed protein migration and restored p53-PDID interaction (Figure 2A). Since BID is enriched in positively charged residues, particularly Lys (K), it might form intramolecular contact with PDID, likely in a phosphorylation-dependent manner as well. This possibility was tested by incubating f:Brd4 fragments purified from Sf9 cells with immobilized GST or GST-fusion containing either BID or CTM of Brd4 as a control. Only Sf9-f:PDID, but not Bac-f:PDID, interacts with immobilized GST:BID (Figure 2B, 2nd and 3rd rows; also Figure S2A). The phosphorylation-dependent interaction between PDID and BID was further verified by reverse phosphorylation treating Sf9-f:PDID with CIP and Bac-f:PDID with CK2 (Figure S2B). It should be mentioned that Brd4 has two clusters of putative CK2 phosphorylation sites. The N-terminal cluster of phosphorylation sites (NPS) is located downstream of BD2 in PDID, whereas the C-terminal cluster of phosphorylation sites (CPS) is situated at the C-terminal side of the ET domain (Figure S2C). Dephosphorylation of either cluster, but not the other parts of Brd4, by CIP treatment of Sf9-purified f:Brd4 fragments induced dramatic mobility shifts during gel migration (Figure S2D, fragments b and c). Failure of an Sf9-purified CPS-containing fragment (amino acids 598-785) to interact with GST:BID (Figure 2B) indicates that both charge and sequence context are important for phosphorylated PDID interaction with BID.
Figure 2. Phosphorylation-Induced Intramolecular Contact Switch in Brd4 Modulates PDID Interaction with p53 and Bromodomain Access to Acetylated Chromatin.
(A) GST pulldown with f:PDID purified from Sf9 or bacteria (Bac) with (+) or without CIP or CK2 treatment.
(B) GST pulldown with NPS- or CPS-containing fragment purified from Sf9 or Bac.
(C) GST pulldown in 293 transfected with GST-tagged plasmid without (for endogenous p53 detection) or with 3f:BID co-expression (for 3f:BID detection).
(D) GST pulldown with CK2-treated WT or mutant f:PDID purified from bacteria. Alanine-substituted S/T is indicated in red for mutants a, b, c, and d.
(E) Model for p53 interaction with BID or PDID.
(F) Drawing of protein fragments with domains retained (solid line) or deleted (dashed line).
(G and H) GST pulldown with GST-fusions untreated or treated (+) with CK2 and ATP, prior to incubation with f:BD2 (G) or acetylated chromatin (H).
(I) Model for phosphorylation-enhanced NPS-BD2 contact further inhibiting chromatin binding.
(J) GST pulldown with GST:BD2 or GST:BID incubated with f:NPS (100 ng), with (+) or without (-) prior CK2 phosphorylation, in the absence (-) or presence (+) of f:BID or f:BD2 competitor (250 ng).
(K) Model for unmasking BD2 binding to acetylated chromatin via NPS-BID contact.
(L) GST pulldown with GST-fusions treated (+) or untreated (-) with CK2 prior to incubation with acetylated chromatin.
Since phosphorylated PDID interacted with BID and p53, we tested whether the intramolecular interaction formed between PDID and BID, as seen in eukaryotic cells, would exclude intermolecular interaction between p53 and phosphorylated PDID. We then co-expressed GST, GST:PDID, and GST:PDID-BID separately with 3xFLAG-tagged BID (3f:BID) in 293 cells and found that GST:PDID interacted with 3f:BID and endogenous p53, respectively, while GST:PDID-BID interacted with endogenous p53, but not 3f:BID (Figure 2C). Thus, intramolecular interaction between PDID and BID (i.e., PDID-BID) in the cell excludes phosphorylated PDID from contacting exogenous 3f:BID (competing for the same binding surface) without compromising its interaction with p53. The data also suggests that BID and p53 might contact different surfaces on phosphorylated PDID. Indeed, GST pulldown using CK2-phosphorylated Bac-f:PDID containing alanine substitutions at different serine residues incubated with immobilized p53 or BID showed that replacement of both S492 and S494 with alanine (i.e., mutant b) severely impairs p53 interaction with CK2-phosphorylated PDID, whereas all phosphoserine residues contribute to intramolecular interaction with BID (Figure 2D, lanes 1-5) and all mutants retain active conformation for binding to acetylated histone H3 (lanes 6-10). Interestingly, single site mutation introduced at S492 or S494 showed that p53 mainly contacts phosphorylated S494, while BID prefers phosphorylated S492 (Figure 2D, lanes 11-14), confirming that intramolecular and intermolecular interaction between BID-PDID and p53-PDID occurs at the same time via differential contact with various phosphoserine residues (Figure 2E).
Phosphorylation at NPS Regulates BD2 Binding to Acetylated Chromatin
Given the importance of phosphoserine (pS) residues to mediate PDID interaction with p53 and BID, we successfully raised a rabbit antibody (α-pS484/488) against diphosphorylated S484 and S488 that detects S484/S488-unaltered wild-type f:PDID and mutants b-e, but not a and f containing alanine-substituted S484 and S488, transiently expressed in human C-33A cervical cancer cells (Figure S3A). Protein degradation occurred in some, but not all, phosphorylation-defective mutants (Figure S3A, lanes 6-8, asterisk), indicating that NPS might contact BD2 depending on extent of phosphorylation to confer protein stability and perhaps regulate BD2 binding to acetylated chromatin. We then selectively deleted either NPS or BD2 from PDID (Figure 2F) and performed GST pulldown by incubating NPS-deleted f:BD2 with immobilized GST:BD2 or GST:NPS purified from bacteria with or without subsequent CK2 treatment. Indeed, f:BD2 interacted with immobilized GST:NPS and GST:BD2 as well, and the interaction between f:BD2 and GST:NPS, but not between f:BD2 and GST:BD2, was significantly enhanced by CK2-mediated phosphorylation (Figure 2G). Thus, NPS contacts BD2 in a phosphorylation-regulated manner, and BD2 in human Brd4 could form a homodimer. We then examined whether BD1 and BD2 in Brd4 could form a heterodimer that might provide molecular insight into Brd4-regulated p53 target gene transcription in vivo (see below). Indeed, interaction was detected between BD1 and BD2, and exogenous BD2 no longer interacted with NPS in BD2-NPS once intramolecular contact between NPS and BD2 was favorably established (Figure S3B).
To determine whether BD2 binding to acetylated chromatin was regulated by intramolecular contact with NPS in a phosphorylation-dependent manner, we performed GST pulldown by incubating acetylated chromatin, typically containing 8-10 nucleosomes prepared from HeLa cells (Figure S3C and S3D), with immobilized GST fusion containing BD2, NPS, or BD2-NPS. Remarkably, BD2 binding to acetylated chromatin, visualized by IB with anti-histone H3 antibody or by silver staining of bound nucleosomal core histones, was diminished by the presence of intramolecular NPS and abolished when phosphorylation was introduced to BD2-NPS by CK2 (Figure 2H, lanes 2 and 3 vs. 6 and 7). Thus, NPS modulates BD2 binding to acetylated chromatin in a phosphorylation-regulated manner (Figure 2I).
Since phosphorylated NPS can contact BD2 upstream or BID downstream, it is important to determine which intramolecular interaction would be dominant, as seen in native Brd4. Using GST pulldown, we found that CK2-phosphorylated NPS interacts with BID stronger than with BD2 (Figure 2J, lanes 3 vs. 6) and that its interaction with BD2 was displaced easily by adding BID as a competitor, but not vice versa (Figure 2J, lanes 3 vs. 4, and 6 vs. 7; see Figure S3E for protein input). Thus, phosphorylated NPS preferentially contacts BID, leading to unmasking of BD2 for binding to acetylated chromatin (Figure 2K). Phosphorylation-induced conformational changes of BD2-NPS-BID and BD2-NPS fragments were monitored by endoproteinase Glu-C digestion, revealing differential sensitivity and cleavage patterns between phosphorylated and unphosphorylated forms (Figure S3F and S3G). Consistent with this hypothesis, inhibition of BD2 binding to acetylated chromatin by phosphorylated NPS (Figure 2L, lanes 4 vs. 6) was relieved by the presence of BID (Figure 2L, lanes 4 vs. 5) and, without CK2 phosphorylation, BID failed to release NPS-inhibited BD2 binding to acetylated chromatin (Figure 2L, lanes 1-2 vs. 4-5), highlighting the existence of a phospho switch mechanism to regulate the chromatin-binding activity of BD2 and likely of full-length Brd4.
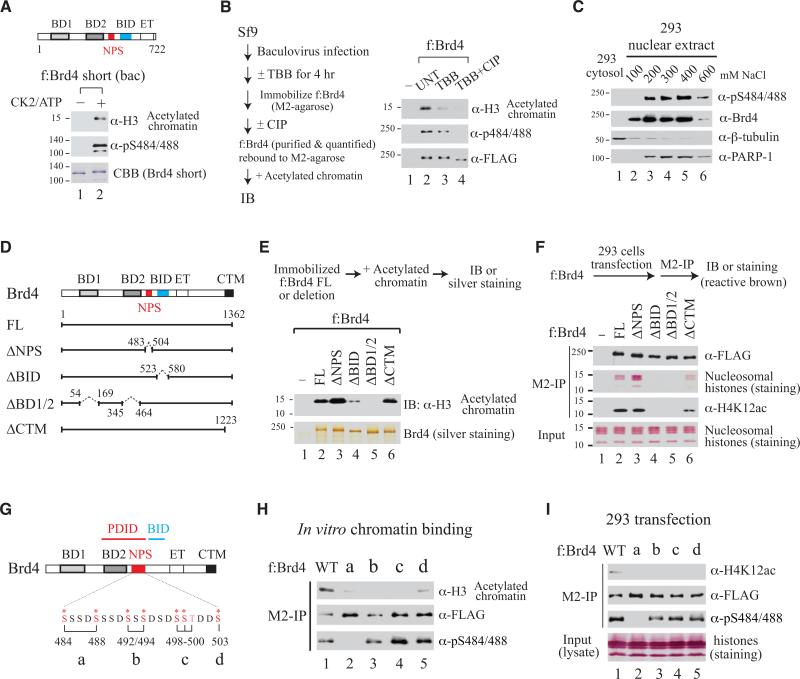
Brd4 Binding to Acetylated Chromatin Requires CK2-Mediated Phosphorylation and Is Regulated by an Intramolecular Contact Switch from NPS-BD2 to NPS-BID
Since intramolecular contacts switch from NPS-BD2 to NPS-BID upon CK2-mediated phosphorylation of NPS, we reasoned that BD2 would become exposed when NPS is phosphorylated. Presumably only phosphorylated forms of naturally existing Brd4, including the full-length protein (aa 1-1362) and a short (S) isoform spanning aa 1-722 (Wu and Chiang, 2007), can bind acetylated chromatin. Thus, we compared the acetylated chromatin-binding activity of unphosphorylated and phosphorylated f:Brd4(S), purified from bacteria and rebound to anti-FLAG M2-agarose beads with or without subsequent CK2 treatment. As predicted, only CK2-phosphorylated f:Brd4(S) exhibited chromatin-binding activity (Figure 3A). Differentially phosphorylated full-length f:Brd4, purified from Sf9 cells treated or untreated with a CK2-specific inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB; Sarno et al., 2001) with half of bead-retained f:Brd4 additionally dephosphorylated by CIP treatment, also showed efficient Brd4 binding to acetylated chromatin depending on extent of CK2-mediated phosphorylation (Figure 3B). Cell fractionation studies showed unphosphorylated Brd4 resides in the nucleoplasm (i.e., 100 mM salt-prepared 293 nuclear extract), whereas all chromatin-bound Brd4 proteins (eluted stepwise from chromatin by 200 to 600 mM NaCl) were phosphorylated, further illustrating that only phosphorylated Brd4 binds chromatin, even in a cellular environment (Figure 3C).
Figure 3. Phosphorylated Brd4 Binding to Acetylated Chromatin Regulated by Intramolecular Contact Switch from NPS-BD2 to NPS-BID.
(A) Short form (S) of f:Brd4 purified from bacteria binds acetylated chromatin after CK2/ATP treatment.
(B) Untreated (UNT) FL f:Brd4 purified from Sf9 loses chromatin-binding activity after dephosphorylation.
(C) Unphosphorylated Brd4 present in nucleoplasm (100 mM salt elution) and phosphorylated Brd4 bound to chromatin eluted at different salt concentrations (200-600 mM) from 293 nuclear extract.
(D) Drawing of Brd4 domain-specific deletion mutants.
(E and F) Chromatin-binding performed with f:Brd4 proteins purified from Sf9 (E) or transiently expressed in 293 cells (F).
(G) Drawing of alanine-substituted mutations (in red) in full-length Brd4.
(H and I) Chromatin-binding performed with mutants constructed in FL f:Brd4 purified from Sf9 (H) or transiently expressed in 293 cells (I).
To define the chromatin-binding activity of phosphorylated Brd4 regulated by intramolecular contact between NPS and BD2, we generated domain-specific deletions that remove NPS (ΔNPS), BID (ΔBID), both BD1 and BD2 (ΔBD1/2), or CTM (ΔCTM) in the context of full-length (FL) Brd4 (Figure 3D). Recombinant FLAG-tagged FL and ΔCTM, purified from Sf9, bound acetylated chromatin to comparable levels (Figure 3E, lanes 2 and 6). While ΔBD1/2 lost chromatin-binding activity as expected, enhanced and diminished chromatin binding were seen with ΔNPS and ΔBID, respectively (Figure 3E, lanes 2-4), consistent with the idea that removal of inhibitory NPS fully exposes BD2 for binding to acetylated chromatin and, conversely, deletion of BID enhances phosphorylated NPS contact with BD2, further masking the chromatin-binding activity of BD2.
To demonstrate the intramolecular regulation of Brd4 chromatin-binding activity in vivo, we transfected each f:Brd4 expression plasmid into 293 cells, performed M2-IP followed by IB detection, and monitored the extent of acetylated chromatin association with Brd4 mutants. The amount of acetylated chromatin pulled down by each Brd4 protein reflected the strength of interaction between acetylated chromatin and individual mutants (Figure 3F, 2nd row). Nucleosomal histones associated with f:Brd4 are derived from chromatin, not from free histones, since inclusion of ethidium bromide that disrupts protein-DNA contact completely abolishes acetylation and methylation marks typically found in cellular chromatin (Figure S4A). Enhanced ΔNPS binding to acetylated chromatin correlates with enrichment of certain, but not all, monitored epigenetic marks (Figure S4B), indicating that effective Brd4 recruitment to cellular chromatin may require specific combinations of acetylated and methylated marks on nucleosomal histones that are unique to different Brd4-regulated genes. When amino acid substitutions that disrupt phosphorylated NPS-BID interaction were introduced in NPS in full-length Brd4 (Figure 3G), all phosphosite mutants showed significantly reduced or abolished binding to acetylated chromatin in vitro (Figure 3H, with purified proteins) and in vivo (Figure 3I, in transfected cells). Thus, phosphorylation-triggered intramolecular contact switch indeed regulates Brd4 binding to acetylated chromatin.
Unmasking BD2 Binding to Chromatin and Association with p53 Are Both Necessary for Brd4-Regulated p21 Gene Transcription
Our finding that phosphorylation of NPS not only unmasks the chromatin-binding activity of BD2 but is also required for p53 interaction suggests gene-specific targeting by a universal epigenetic reader, Brd4, can be accomplished by opening its chromatin-binding domain and activator-recruiting region at the same time. To explore this, we examined whether Brd4 is involved in p53-regulated p21 gene transcription by conducting loss-of-function analysis in 293 cells. Indeed, the RNA level of p21, but not of a p53-unresponsive gene (RPL13A), was significantly reduced upon diminished endogenous Brd4 protein by independent siRNA knockdown targeting different Brd4 regions (Figure 4A). Mapping the region for Brd4 and p53 recruitment to p21 gene by chromatin immunoprecipitation (ChIP) showed Brd4 co-occupied with p53 at -2.3 kb, which contains a distal p53-binding site (Figure 4B). Knockdown of Brd4 or p53 by siRNA reduced occupancy of Brd4 and p53 at -2.3 kb (Figure 4C and 4D). Occupancy of Brd4 did not correlate with acetylation status of nucleosomes (Figure S4C), but rather chromatin association of p53. The mutual dependence of p53 and Brd4 occupancy at p21 gene was also observed in wild-type (p53 +/+) HCT116 but not its isogenic p53-null (p53 -/-) cells, under both unstressed (Figure 4E) and doxorubicin (Dox)-induced DNA-damaging conditions (Figure 4F), correlating with changes of p21 RNA levels in Brd4-knockdown HCT116 p53 +/+ and p53 -/- cells, with or without Dox treatment (Figure 4G). We conclude that p21 is an authentic p53 target gene co-regulated by Brd4.
Figure 4. Brd4-Regulated p21 Gene Expression Is p53-Dependent.
(A) Knockdown of Brd4 in 293 by two separate siRNAs reduces Brd4 protein (bottom) and RNA derived from p21 but not RPL13A (bar graph).
(B) Brd4 co-occupies with p53 at -2.3 kb of p21 gene in 293 detected by ChIP with primer pairs as indicated. Two known p53-binding sites (p53BS) are marked.
(C and D) Occupancy of Brd4 and p53 at p21 -2.3 kb in 293 is reduced upon knockdown of Brd4 (C) or p53 (D). Reduced p53 protein level in p53 knockdown is shown.
(E and F) Co-occupancy of p53 and Brd4 at p21 -2.3 kb detectable in p53 +/+ but not p53 -/-HCT116 cells (E) is further enhanced upon Dox (0.5 μM, 6 hr) treatment (F).
(G) Dox-induced p21 RNA level seen in HCT116 p53 +/+, not p53 -/-, cells diminished upon Brd4 knockdown. Error bars, SD (n=2-3) in (A-G).
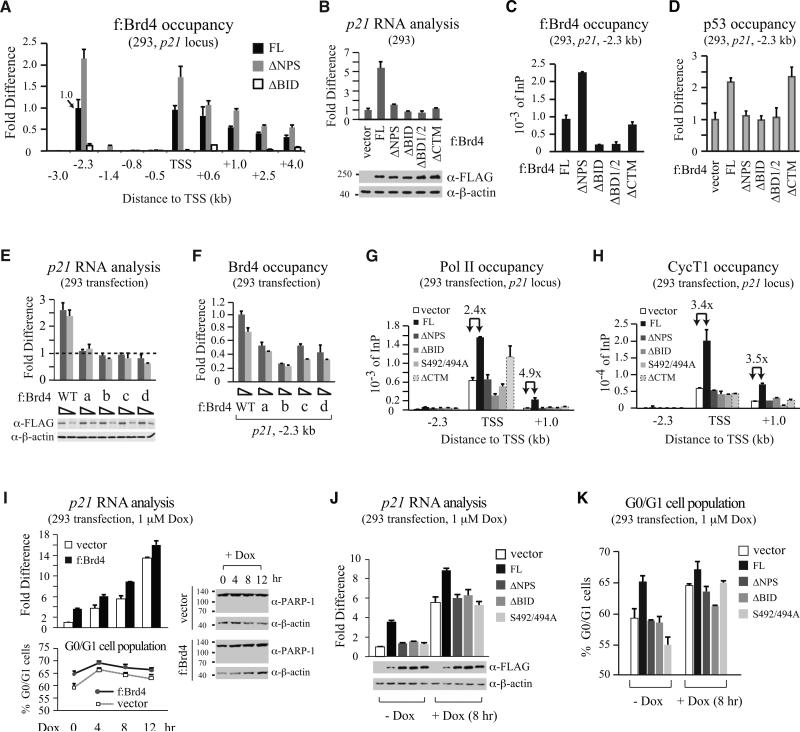
Transient transfection of f:Brd4 FL, ΔNPS, or ΔBID was then performed in 293 cells to examine whether concurrent opening of BD2 chromatin-binding activity and recruitment of p53 dictate Brd4 targeting to endogenous p21 gene. In addition to -2.3 kb detected for endogenous Brd4 binding in unstressed cells, exogenously expressed FL f:Brd4 was also found in the coding region with gradually reduced occupancy toward the 3’ end (Figure 5A, black bars), implicating active transcription driven by exogenous Brd4 occurring at both initiation and elongation steps. Interestingly, ΔNPS showed enhanced recruitment to -2.3 kb and coding regions when compared to FL (Figure 5A, grey vs. black bars), consistent with removal of NPS unmasking BD2 for enhanced binding to chromatin. As predicted, ΔBID exhibited little if any chromatin binding, as deletion of BID reinforces NPS-BD2 contact, further blocking BD2 access to chromatin.
Figure 5. Unmasking BD2 Binding to Chromatin and Recruitment of p53 Are Both Necessary for Brd4-Regulated p21 Gene Transcription.
(A) ChIP of p21 gene occupancy by FL f:Brd4, ΔNPS or ΔBID transiently expressed in 293. FL occupancy at -2.3 kb specified as 1.0.
(B) Measurement of p21 RNA level changes in 293 (bar graph) transiently expressing different f:Brd4 proteins (bottom).
(C and D) ChIP of p21 -2.3 kb bound by FL or domain-deleted f:Brd4 transiently expressed in 293 (C) or by endogenous p53 (D).
(E) Measurement of p21 RNA level changes in 293 (bar graph) transiently expressing WT or NPS serine mutants (bottom) constructed in FL f:Brd4.
(F) ChIP of p21 -2.3 kb bound by WT or NPS serine mutants.
(G and H) Occupancy of Pol II (G) and CycT1 (H) at -2.3 kb, TSS, and +1.0 kb of p21 gene in 293 transiently expressing f:Brd4 FL, domain deletion, or S492/494A mutant.
(I) Measurement of p21 RNA level (top) and G0/G1 population (bottom) in Dox-treated 293 transiently expressing f:Brd4 or vector. Cleavage of PARP-1, monitored by IB, is shown.
(J and K) Measurement of p21 RNA level (J) and G0/G1 population (K) in 293 transiently expressing different f:Brd4 proteins (J, bottom) with or without Dox treatment (1 μM, 8 hr). Error bars, SD (n=2-3) in (A-K).
To determine whether intramolecular contact switch in Brd4 and p53 recruitment are both necessary for active gene transcription, we expressed different f:Brd4 deletions in 293 cells and analyzed p21 RNA levels (Figure 5B) and recruitment of exogenous f:Brd4 (Figure 5C) and endogenous p53 (Figure 5D) to -2.3 kb. While FL f:Brd4 binding to -2.3 kb (Figure 5C, 1st bar) increased p21 RNA due to enhanced p53 recruitment (Figure 5B and 5D, compare 1st two bars), none of the domain-deleted proteins could stimulate p21 RNA (Figure 5B). Failure to recruit p53 by ΔNPS, which bound better than FL to -2.3 kb, indicates that phosphorylated NPS is crucial for recruiting p53 to its chromatin target site in vivo. ΔBID lost chromatin-binding activity as seen with ΔBD1/2, resulting in unproductive recruitment of p53 to p21 gene. The defect of ΔCTM in enhancing p21 RNA level, albeit it binds chromatin and recruits p53 as efficiently as FL, highlights the importance of Brd4-mediated P-TEFb elongation control in p21 gene transcription.
Phosphosite-substituted Brd4 mutants that reduce BD2 binding to acetylated chromatin (see Figure 3H and 3I) also failed to enhance p21 RNA (Figure 5E), correlating with reduced occupancy of these mutants at -2.3 kb (Figure 5F). Since enhanced recruitment of f:Brd4 was observed at -2.3 kb, TSS, and downstream region (Figure 5A), we analyzed whether recruitment of Pol II and P-TEFb to these three regions is regulated by Brd4 depending on its molecular configuration. Enhanced recruitment of Pol II and P-TEFb (monitored by CycT1) by Brd4 was seen at the TSS and coding region, not -2.3 kb, indicating that Brd4 facilitated Pol II and P-TEFb entry to enhance p21 gene transcription (Figure 5G and 5H, black vs. white bars). Transcription-defective Brd4 mutants, including ΔNPS, ΔBID, and S492/494A (i.e., mutant b), were unable to enhance Pol II and P-TEFb recruitment except for ΔCTM, which brought Pol II to TSS but failed to sustain transcription due to its inability to recruit P-TEFb to the promoter region (Figure 5G and 5H). The importance of CTM in recruiting active P-TEFb was further illustrated by displacement of an inhibitory P-TEFb protein (HEXIM1) from the promoter and coding region by wild-type Brd4 but not ΔCTM (Figure S4D). The Brd4-dependent, but CTM-independent, removal of HEXIM1 from -2.3 kb (Figure S4D) indicates P-TEFb-independent recruitment of HEXIM1 occurs at the upstream region, consistent with the finding that HEXIM1 interacts with the C-terminal regulatory region of p53 independent of its association with P-TEFb and 7SK RNA complex (Lew et al., 2012). Thus, Brd4-p53 interaction may also facilitate p53 target gene transcription by displacing HEXIM1 from p53-binding sites via competition for the same binding region in p53.
To address the biological significance of p53/Brd4-coregulated p21 gene transcription, we analyzed G0/G1 cell population and PARP-1 cleavage upon exogenous Brd4 expression and Dox treatment in 293 cells. Brd4 enhanced p21 RNA in unstressed and Dox-treated 293 cells, correlating with induced G0/G1 cell cycle arrest without triggering apoptotic caspase cleavage of PARP-1 (Figure 5I). All transcription-defective Brd4 mutants (ΔNPS, ΔBID, and S492/494A) failed to enhance p21 RNA levels upon Dox treatment (Figure 5J) and did not increase G0/G1 population with or without Dox treatment (Figure 5K), suggesting that Brd4-enhanced p21 RNA contributes to prolonged G0/G1 cell cycle arrest.
Intramolecular Contact Switch Is Crucial for Brd4-Regulated c-Myc and c-Fos Gene Transcription
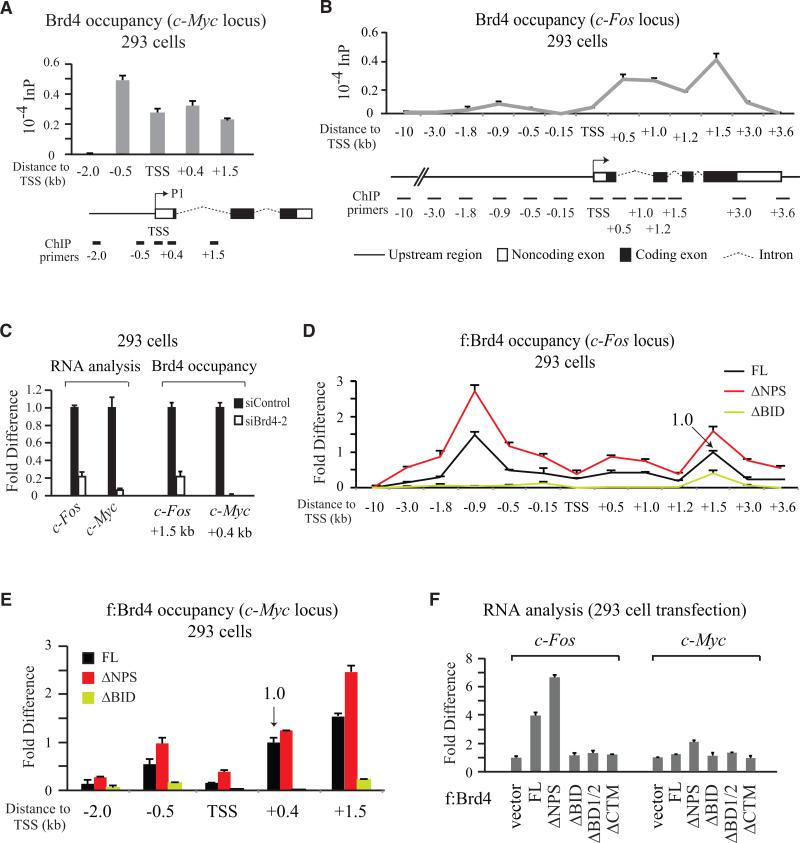
To define whether the intramolecular contact switch is a general mechanism for Brd4-regulated transcription, we further analyzed Brd4 recruitment to c-Myc and c-Fos genes not containing obvious p53-binding sites. The -0.5 kb (known as the P0 promoter) and +1.5 kb (i.e., the P3 promoter) regions, relative to the c-Myc P1 TSS (Levens, 2010), have been shown to associate with Brd4 (Delmore et al., 2011), and the TSS and +0.4 kb regions are positive for P-TEFb recruitment (Yang et al., 2008). Endogenous Brd4 in 293 cells was detected by ChIP at these four regions, but not at -2.0 kb used as negative control (Figure 6A). Brd4 occupancy in c-Fos gene, analyzed by extensive ChIP with primer pairs spanning -10 kb to +3.6 kb, distributed primarily in the coding region peaking at +1.5 kb with a separate smaller peak detectable at -0.9 kb (Figure 6B). Unlike p21 gene showing distinct upstream Brd4 occupancy (Figure 4B), immediate stress response genes, such as c-Fos and c-Myc, showed endogenous Brd4 association predominantly in the coding region. Knockdown of Brd4 significantly diminished c-Fos and c-Myc RNA levels, correlating with reduced Brd4 occupancy at c-Fos +1.5 kb and c-Myc +0.4 kb (Figure 6C).
Figure 6. Brd4-Regulated c-Myc and c-Fos Gene Transcription in 293 Cells Also Modulated by Intramolecular Contact Switch in Brd4.
(A and B) ChIP of endogenous Brd4 occupancy on c-Myc (A) and c-Fos (B) genes.
(C) Reduction of c-Fos and c-Myc RNA correlates with decreased Brd4 occupancy on c-Fos and c-Myc genes upon Brd4 knockdown.
(D) ChIP of c-Fos gene bound by FL f:Brd4, ΔNPS or ΔBID transiently expressed in 293. FL occupancy at +1.5 kb specified as 1.0.
(E) ChIP of c-Myc gene at five different regions bound by FL or domain-deleted f:Brd4 transiently expressed in 293. FL occupancy at +0.4 kb specified as 1.0.
(F) Measurement of c-Fos and c-Myc RNA level changes in 293 transiently expressing different f:Brd4 proteins. Error bars, SD (n=2-3) in (A-F).
The binding profile of exogenous f:Brd4, however, showed a specifically enriched upstream (-0.9 kb) occupancy on c-Fos gene, with ΔNPS and ΔBID displaying enhanced and decreased binding to both -0.9 and +1.5 kb regions (Figure 6D), respectively, similar to that observed in p21 gene (Figure 5A). These results indicate that: 1) overexpressed Brd4 drives initiation of transcription via upstream occupancy in addition to modulating transcription elongation as observed with endogenous Brd4; and 2) intramolecular interaction mediated by the NPS-BD2 and NPS-BID contact switch had a significant effect on Brd4-regulated c-Fos gene transcription. Enhanced chromatin binding exhibited by ΔNPS and the loss of chromatin association seen with ΔBID were likewise reflected at Brd4-binding sites in c-Myc gene (Figure 6E). The efficiency of Brd4 binding to c-Fos gene correlates with c-Fos transcript levels in f:Brd4 domain deletion-transfected cells (Figure 6F, left panel). That c-Myc RNA level was slightly elevated in 293 cells expressing ΔNPS, but not FL f:Brd4 (Figure 6F, right panel), indicates that additional cellular factors besides Brd4 targeting to chromatin are necessary for c-Myc gene transcription.
Inhibition of CK2-Mediated NPS Phosphorylation Reduces Brd4 Chromatin Occupancy and Target Gene Transcription
To demonstrate the phospho switch at NPS regulates Brd4 target gene transcription in vivo, we first identified CK2 as the main kinase phosphorylating NPS by analyzing 30 recombinant kinases for their ability to phosphorylate PDID in vitro (Figure S5A). Involvement of endogenous CK2 in NPS phosphorylation was illustrated by using C-33A lysates that provide cellular kinases phosphorylating wild-type PDID but not its f mutant containing alanine substitutions at all seven consensus CK2 phosphorylation sites (Figure S5B). Inclusion of CK2 inhibitor TBB abolished PDID phosphorylation in the in vitro kinase assay (Figure S5C). When TBB was combined with siRNA knockdown of CK2 in living cells, phosphorylation of NPS in Brd4 was almost completely abolished (Figure S5D).
CK2 activity is elevated in most cancer cells and thus in vivo elucidation of CK2-mediated function requires identification of an appropriate cell system with diminishable CK2 activity that does not trigger a global response and significant cell death. Upon screening ~20 TBB-treated cell lines for Brd4 phosphorylation (Figure S6A), we found that Ker-CT cells, which are human foreskin keratinocytes immortalized by retrovirus-transduced Cdk4 and human telomerase (Ramirez et al., 2003), showed reduced Brd4 phosphorylation (Figure S6B) when treated with TBB (50 μM, no change of Brd4 protein level unlike that seen with 100 μM; Figure S6C) for 6 hr without triggering apoptotic PARP-1 cleavage often observed with prolonged (e.g., 18 hr) TBB treatment (Figure S6D).
p53-regulated p21, HDM2, PUMA, and 14-3-3σ RNA levels were diminished in 6-hr TBB-treated Ker-CT cells (Figure 7A), correlating with reduced Brd4 occupancy on p53-binding sites (Figure 7B) and reduced Brd4 phosphorylation without altering Brd4, CK2 and p53 protein levels as well as CK2-phosphorylated p53 at serine 392 (Figure 7C). Since CK2 is not the only kinase phosphorylating p53 S392 (Claudio et al., 2006), reduced phosphorylation of Brd4 but not p53 likely reflects differential substrate sensitivity of CK2 in the cell. In vitro interaction assay with purified Brd4 and p53, modified by p300 acetylation or CK2 phosphorylation, showed that unmodified and p300-acetylated p53, but not CK2-phosphorylated p53, interact with Brd4 (Figure 7D), indicating that reduced RNA and Brd4 occupancy seen with p21, HDM2, PUMA, and 14-3-3σ genes upon TBB treatment is attributed to reduced CK2 phosphorylation of Brd4, not p53. Similar analysis of other Brd4 targets, such as c-Fos and c-Myc (Figure 6) and CCND1, PIM2, and DCPS whose transcripts were decreased in Brd4-knockdown C-33A cells (Rahman et al., 2011), also showed diminished RNA (Figure 7E) with reduced Brd4 occupancy when known Brd4-binding sites were analyzed (Figure 7F).
Figure 7. Inhibition of CK2-Mediated NPS Phosphorylation Reduces Brd4 Chromatin Occupancy and Target Gene Transcription in Ker-CT Cells.
(A and B) Reduction of p21, HDM2, PUMA, and 14-3-3σ RNA (A) and Brd4 occupancy at p53-binding sites (B) in Ker-CT treated with TBB (vs. DMSO).
(C) IB of phosphorylated Brd4, S392-phosphorylated p53 (following IP with α-p53 DO-1 antibody), and total Brd4, CK2α, and p53 protein levels in Ker-CT treated with DMSO (-) or TBB (+).
(D) CK2-phosphorylated p53, but not p300-acetylated p53, fails to interact with Brd4. Purified p53 was mock-treated or modified by p300 acetylation or CK2 phosphorylation prior to incubation with Brd4, followed by IP with α-Brd4 and IB with various α-p53 antibodies (lanes 1-3). The specificity of antibodies against acetyl-K373/382 and phosphorylated S392 of p53, prepared from untreated (-) or p300- or CK2-modified p53 (+), was shown on the right (lanes 4-7).
(E) Reduction of c-Fos, c-Myc, CCND1, PIM2, and DCPS, but not RPL13A, RNA levels in TBB-treated Ker-CT (vs. DMSO treatment).
(F) ChIP of endogenous Brd4 occupancy at c-Fos and c-Myc genes in DMSO- and TBB-treated Ker-CT. Error bars, SD (n=2-6) in (A,B,E,F).
(G) Model for CK2-mediated phosphorylation of Brd4 leading to intramolecular contact switch, allowing BD2/BD1 binding to acetylated (Ac) chromatin and recruitment of p53 for gene-specific targeting and activation of transcription.
We conclude that CK2 plays an important role in Brd4-regulated gene transcription in vivo, supporting intramolecular contact switch-regulated unmasking of Brd4 chromatin binding and concurrent recruitment of p53 for gene-specific targeting (Figure 7G).
DISCUSSION
CK2-phosphorylated Brd4 is an active chromatin binder and p53 recruiter. The identification of evolutionarily conserved NPS and BID suggests that regulation of bromodomain binding to acetylated chromatin and activator recruitment is likely a common mechanism employed by BET family proteins. Relating to this, yeast homologs of Brd4, Bdf1 and Bdf2, have been shown to be phosphorylated by CK2 at two regions comparable to NPS and CPS reported here, and deletion of either region in Bdf1 results in yeast inviability (Sawa et al., 2004). Clearly, phosphorylation of NPS is essential for the function of Brd4 and its yeast homologs in eukaryotic cells.
Intramolecular Contact Regulates Bromodomain Accessibility
Without CK2 phosphorylation, NPS already enriched in acidic residues could potentially contact a positively charged surface on BD2 (Figure S7A), located approximately 90° spatially to the Kac-binding pocket, to control bromodomain-histone interaction via allosteric regulation. Since the structures of BD1 and BD2 are very similar (with root mean square deviation between 81 Cα atoms within the two domains being 0.7 Å), a comparable charge cluster existing on BD1 (Figure S7B and S7C) suggests that NPS could potentially contact BD1 as well. That BD2 could interact with BD1 (Figure S3B) and that ΔBID lost chromatin binding to a similar extent as observed with ΔBD1/2 in vitro (Figure 3E) and in vivo (Figures 3F and 5C) indicates that NPS masking of BD2 might also occur on BD1 either through BD2-BD1 interaction or direct NPS contact with BD1. Consistent with our biochemical characterization of BD2-BD1 interaction in human Brd4, deletion of either BD1 or BD2 in mouse Brd4 is sufficient to abolish its mitotic chromosome-binding activity in living cells (Dey et al., 2003). Presumably, while active in binding acetylated chromatin as an independent module, BD1 and BD2 in the native protein could bind cooperatively to multiple Kac sites residing on nucleosomal histones and increase overall binding affinity and specificity of Brd4 for its target chromatin. Whether BD2-BD1 and BD2-BD2 interactions functionally modulate intramolecular and intermolecular contact switches of Brd4 during its interaction with chromatin and p53 tetramer that is the active form in transcription and chromatin binding (Wu and Chiang, 2009), and how Brd-Brd crosstalk is regulated jointly or individually by (un)phosphorylated NPS, are important mechanistic questions. In this context, it is worth mentioning that the chromatin-binding activity of Brd4 is best reflected on the analysis of chromatin, not histones, as seen with NPS masking of BD2 binding to acetylated chromatin (e.g., Figures 2L and 3F) but not to acetylated histones (Figure 2D).
Brd4 Partner Association Regulates Protein Function and Stability
Although combinatorial regulation by functional association between interacting partners is a common theme in eukaryotic transcription control, Brd4 may confer other functions, e.g., to help stabilize its partner proteins on or off chromatin and indirectly regulate transcription by modulating protein stability (Lee and Chiang, 2009). Although regulation of c-Myc gene transcription appears to be the primary function of Brd4 targeted by recently identified cancer therapeutic compounds, other mechanisms as revealed by direct association between Brd4 and c-Myc/Max may also control c-Myc-regulated gene transcription and likely modulate c-Myc protein stability as well. These intriguing possibilities regarding the functional implication of each partner interaction (Figure 1A) will need to be experimentally validated in the future as reported here for p53.
Brd4-Regulated Gene Transcription
Recognition of appropriate gene targets in natural chromatin is an essential activity of Brd4 in transcription commitment (Wu et al., 2006). Differential contact with combinations of acetylated lysines in nucleosomal histones dictates the transition of Brd4 from chromatin targeting to initiation of transcription and to elongation (Chiang, 2009), which requires an exchange of Brd4-interacting partners at distinct steps of the chromatin transcription process. It is interesting to note that many histone marks are detected in Brd4-associated chromatin (Figure S4B), suggesting that functional dictation of Brd4-regulated transcription extends beyond the classically defined Brd-Kac interaction and may reflect Brd4 association with diverse and yet-to-be characterized factors modulating chromatin dynamics and transcription. Clearly, multiple control mechanisms are used by Brd4 in transcriptional regulation, which is consistent with live-cell imaging showing that Brd4 association at an induced locus during post-mitotic gene reactivation occurs prior to Pol II and P-TEFb entry, whereas concurrent Brd4 and P-TEFb recruitment to the same locus in interphase takes place only after Pol II entry (Zhao et al., 2011). Although stress-induced signaling tends to induce P-TEFb release from inactive complexes containing 7SK RNA and HEXIM1, knockdown of Brd4 in our studies does not appear to alter the amount of P-TEFb components (e.g., Cdk9) associated with HEXIM1 (Figure S7D), indicating that changes of Brd4-regulated RNA levels are likely caused by direct transcriptional control, rather than indirect modulation of P-TEFb levels in the cell.
Brd4 as a Target in Cancer Therapeutics
Our finding that phosphorylation of Brd4 by CK2 is necessary for Brd4 binding to chromatin and for Brd4-regulated gene transcription, including c-Myc, suggests that one of the major targets of elevated CK2 observed in various types of cancer is to maintain Brd4 chromatin-binding activity in order to sustain high levels of c-Myc gene expression in cancer cells. This possibility is supported by earlier animal studies showing overexpression of a catalytic subunit of CK2 and c-Myc transgenes resulting in neonatal leukemia (Seldin and Leder, 1995). Perhaps combined therapies using lower concentrations of JQ1/I-BET and TBB-derived compounds may reduce off-target effects and significantly enhance the effectiveness of cancer therapeutics.
EXPERIMENTAL PROCEDURES
Plasmid Construction and Protein Purification
Procedures for constructing protein expression plasmids (Table S1) and protein purification were performed as described (Thomas and Chiang, 2005; Wang et al., 2011) and detailed in Supplemental Data.
Co-Immunoprecipitation (IP), GST Pulldown and Candidate Interaction Screen
Protein-protein interaction was examined in vitro and in vivo using purified proteins, nuclear extracts, or in transfected cells typically by IP and immunoblotting detection with anti-epitope or anti-protein antibodies as described in Supplemental Data.
Bimolecular Fluorescence Complementation (BiFC) Assay
BiFC detection of p53-Brd4 interaction in HCT116 p53 -/- cells is described in Supplemental Data.
Electrophoretic Mobility Shift Assay (EMSA)
Detection of p53 binding to DNA by EMSA was performed as described (Wu and Chiang, 2009) with modifications detailed in Supplemental Data.
Kinase Screen and phosphorylation/dephosphorylation Assay
30 recombinant kinases were screened to identify PDID-phosphorylating enzymes with phosphorylation and dephosphorylation assays detailed in Supplemental Data.
Endoproteinase Glu-C Digestion
Phosphorylation-induced conformational changes in BD2-NPS and BD2-NPS-BID were monitored by Glu-C digestion as described in Supplemental Data.
Chromatin and Histone Binding Assay
Binding to acetylated chromatin and core histones by Brd4 proteins purified from bacteria or Sf9, or transiently expressed in 293 cells, was detected by reactive brown staining (for membrane after transfer), silver staining (for wet gels), or IB with antibodies recognizing unmodified or site-specifically modified histone H3 or H4 as detailed in Supplemental Data.
Cell Fractionation by Sequential Salt Extraction
Detection of phospho-Brd4 in chromatin following cell fractionation was described in Supplemental Data.
Cell Cycle and RNA Analysis in Transfected and siRNA-Knockdown Cells
Cell cycle profiles were analyzed by flow cytometry. Endogenous RNA was measured by RT-qPCR using gene-specific primer pairs (Table S2) following transfection with expression plasmids or siRNA as detailed in Supplemental Data.
Kinase Inhibitor Assay
Pharmacological inhibitors against CK2 (TBB), PI3K (LY294002), and AKT (1L6-hydroxymethyl-chiro-inositol-2-(R)-2-O-methyl-3-O-octadecyl-sn-glycerocarbonate) were added individually to C-33A lysate or medium (for cell screen with TBB) to monitor the phosphorylation status of Brd4 as described in Supplemental Data.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was performed (Wu et al., 2006) using primer sequences for various gene loci listed in Table S2 with sources of antibodies and procedures detailed in Supplemental Data.
Electrostatic Potential Analysis of Protein Domains
Electrostatic potentials in BD1 and BD2 structures were calculated using the program ABPS with structural figures drawn using PyMOL.
Supplementary Material
Highlights.
Brd4 interacts directly with multiple DNA-binding proteins and chromatin-modifiers
Brd4 is a newly identified transcription coactivator for p53 tumor suppressor protein
CK2-phosphorylated Brd4 is an active chromatin binder and p53 recruiter
Mechanism for converting a universal epigenetic reader into a gene-specific regulator
ACKNOWLEDGMENTS
We thank David Boothman for providing MCF7, MDA-MB-231, HCC1143, HCC1806, and HCC1934 cells; Ezra Burstein for SR cells; David Chen for DNA-PKcs and Ku70/80 proteins, and α-Ku80 and α-DNA-PKcs antibodies; Ivan D'Orso for purified P-TEFb; Bob Eisenman for pBS0/1Myc, pVZmad, and pVZp21Max; Ron Evans for pGEX2T-hRXRα. Or Gozani for pcDNA3-SET8; Richard Hanson for pRY42; Lily Huang for HEL cells; Robert Kingston for HeLa f:INI1 cells; Thomas Kodadek for 26S proteasome and α-p24 antibody; Mong-Hong Lee for Venus-N-p53 and Venus-C-AurB; Jangwon Park for pCN-GST-Myc and pCN-3xFLAG; Danny Reinberg for pcDNA3-SET9 and baculovirus expressing f:Spt16 and 6His:SSRP1; Bob Roeder for pET-10hismTRα1 and pET-10hishTRβ1; Pier Paolo Scaglioni for NB4 cells; Nima Sharifi for LNCaP cells; Jerry Shay and Kimberly Batten for Ker-CT cells and the culture condition; Michael Stallcup for pSG5-HA:CARM1, pSG5-HA:PRMT1 and pSG5-HA:G9A; Philip Tucker for pcDNA3-Smyd2-Myc; Bert Vogelstein for HCT116 p53 +/+ and p53 -/- cells; and Qiang Zhou for anti-HEXIM1 antibody. We are also grateful to Alison Chiang for constructing pST39-6His:c-Myc/F:Max, pST39-6His:Mad1/F:Max, pST39-6His:Mxi1/F:Max, pST39-6His:Mad3/F:Max, and pST39-6His:Mad4/F:Max; to Celeste Greer for generating pF:hSet8-11d, pF:hSet9-11d, pF:mG9a(587-1267)-11d, pF:mSmyd2-11d, pF:rPRMT1-11d, pF:mCARM1-11d, and pVL-F:mCARM1; and to Ching Lee for making pCN-3F:BID and pCN-GST:Brd4 derivatives. We also thank Bethany Janowski, Julio Morales, Kejin Zhou and Ming-Ming Zhou for discussion, as well as David Boothman, Alison Chiang, William Chiang, David Corey, Carole Mendelson, and Jangwon Park for comments on the manuscript. This work was supported in part by NIH grants (CA103867 and CA124760) and CPRIT grants (RP110471 and RP120340) and is Report CSCN # 067 from Simmons Comprehensive Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL DATA
Supplemental data includes seven figures, two tables, Experimental Procedures, and References.
REFERENCES
- Alsarraj J, Walker RC, Webster JD, Geiger TR, Crawford NP, Simpson RM, Ozato K, Hunter KW. Deletion of the proline-rich region of the murine metastasis susceptibility gene Brd4 promotes epithelial-to-mesenchymal transition- and stem cell-like conversion. Cancer Res. 2011;71:3121–3131. doi: 10.1158/0008-5472.CAN-10-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C-M. Brd4 engagement from chromatin targeting to transcriptional regulation: selective contact with acetylated histone H3 and H4. F1000 Biol. Rep. 2009;1:98. doi: 10.3410/B1-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio PP, Cui J, Ghafouri M, Mariano C, White MK, Safak M, Sheffield JB, Giordano A, Khalili K, Amini S, Sawaya BE. Cdk9 phosphorylates p53 on serine 392 independently of CKII. J. Cell. Physiol. 2006;208:602–612. doi: 10.1002/jcp.20698. [DOI] [PubMed] [Google Scholar]
- Dawson MA, Prinjha RK, Dittman A, Giotopoulos G, Bantscheff M, Chan WI, Robson SC, Chung CW, Hopf C, Savitski MM, Huthmacher C, Gudgin E, Lugo D, Beinke S, Chapman TD, Roberts EJ, Soden PE, Auger KR, Mirguet O, Doehner K, Delwel R, Burnett AK, Jeffrey P, Drewes G, Lee K, Huntly BJ, Kouzarides T. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, Chesi M, Schinzel AC, McKeown MR, Heffernan TP, Vakoc CR, Bergsagel PL, Ghobrial IM, Richardson PG, Young RA, Hahn WC, Anderson KC, Kung AL, Bradner JE, Mitsiades CS. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Chitsaz F, Abbasi A, Miteli T, Ozato K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. USA. 2003;100:8758–8763. doi: 10.1073/pnas.1433065100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P, Picaud S, Mangos M, Keates T, Lambert JP, Barsyte-Lovejoy D, Felletar I, Volkmer R, Müller S, Pawson T, Gingras AC, Arrowsmith CH, Knapp S. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149:214–231. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French CA. Pathogenesis of NUT midline carcinoma. Annu. Rev. Pathol. 2012;7:247–265. doi: 10.1146/annurev-pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Horng T, Medzhitov R. Control of inducible gene expression by signal-dependent transcriptional elongation. Cell. 2009;138:129–145. doi: 10.1016/j.cell.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A-Y, Chiang C-M. Chromatin adaptor Brd4 modulates E2 transcription activity and protein stability. J. Biol. Chem. 2009;284:2778–2786. doi: 10.1074/jbc.M805835200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens D. You don't muck with MYC. Genes Cancer. 2010;1:547–554. doi: 10.1177/1947601910377492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew QJ, Chia YL, Chu KL, Lam YT, Gurumurthy M, Xu S, Lam KP, Cheong N, Chao SH. Identification of HEXIM1 as a positive regulator of p53. J. Biol. Chem. 2012;287:36443–36454. doi: 10.1074/jbc.M112.374157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, Bergeron L, Sims RJ., III Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc. Natl. Acad. Sci. USA. 2011;108:16669–16674. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Phair RD, Scaffidi P, Elbi C, Vecerová J, Dey A, Ozato K, Brown DT, Hager G, Bustin M, Misteli T. Global nature of dynamic protein-chromatin interactions in vivo: three-dimensional genome scanning and dynamic interaction networks of chromatin proteins. Mol. Cell. Biol. 2004;24:6393–6402. doi: 10.1128/MCB.24.14.6393-6402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Sowa ME, Ottinger M, Smith JA, Shi Y, Harper JW, Howley PM. The Brd4 extraterminal domain confers transcription activation independent of pTEFb by recruiting multiple proteins, including NSD3. Mol. Cell. Biol. 2011;31:2641–2652. doi: 10.1128/MCB.01341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez RD, Herbert BS, Vaughan MB, Zou Y, Gandia K, Morales CP, Wright WE, Shay JW. Bypass of telomere-dependent replicative senescence (M1) upon overexpression of Cdk4 in normal human epithelial cells. Oncogene. 2003;22:433–444. doi: 10.1038/sj.onc.1206046. [DOI] [PubMed] [Google Scholar]
- Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 ('casein kinase-2'). FEBS Lett. 2001;496:44–48. doi: 10.1016/s0014-5793(01)02404-8. [DOI] [PubMed] [Google Scholar]
- Sawa C, Nedea E, Krogan N, Wada T, Handa H, Greenblatt J, Buratowski S. Bromodomain factor 1 (Bdf1) is phosphorylated by protein kinase CK2. Mol. Cell. Biol. 2004;24:4734–4742. doi: 10.1128/MCB.24.11.4734-4742.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin DC, Leder P. Casein kinase II alpha transgene-induced murine lymphoma: relation to theileriosis in cattle. Science. 1995;267:894–897. doi: 10.1126/science.7846532. [DOI] [PubMed] [Google Scholar]
- Thomas MC, Chiang C-M. E6 oncoprotein represses p53-dependent gene activation via inhibition of protein acetylation independently of inducing p53 degradation. Mol. Cell. 2005;17:251–264. doi: 10.1016/j.molcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Wang W-M, Wu S-Y, Lee A-Y, Chiang C-M. Binding site specificity and factor redundancy in activator protein-1-driven human papillomavirus chromatin-dependent transcription. J. Biol. Chem. 2011;286:40974–40986. doi: 10.1074/jbc.M111.290874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-Y, Chiang C-M. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J. Biol. Chem. 2007;282:13141–13145. doi: 10.1074/jbc.R700001200. [DOI] [PubMed] [Google Scholar]
- Wu S-Y, Chiang C-M. Crosstalk between sumoylation and acetylation regulates p53-dependent chromatin transcription and DNA binding. EMBO J. 2009;28:1246–1259. doi: 10.1038/emboj.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-Y, Lee A-Y, Hou SY, Kemper JK, Erdjument-Bromage H, Tempst P, Chiang C-M. Brd4 links chromatin targeting to HPV transcriptional silencing. Genes Dev. 2006;20:2383–2396. doi: 10.1101/gad.1448206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, He N, Zhou Q. Brd4 recruits P-TEFb to chromosomes at late mitosis to promote G1 gene expression and cell cycle progression. Mol. Cell. Biol. 2008;28:967–976. doi: 10.1128/MCB.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Nakamura T, Fu Y, Lazar Z, Spector DL. Gene bookmarking accelerates the kinetics of post-mitotic transcriptional re-activation. Nat. Cell Biol. 2011;13:1295–1304. doi: 10.1038/ncb2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippo A, Serafini R, Rocchigiani M, Pennacchini S, Krepelova A, Oliviero S. Histone crosstalk between H3S10ph and H4K16ac generates a histone code that mediates transcription elongation. Cell. 2009;138:1122–1136. doi: 10.1016/j.cell.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, Taylor MJ, Johns C, Chicas A, Mulloy JC, Kogan SC, Brown P, Valent P, Bradner JE, Lowe SW, Vakoc CR. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.