Abstract
Exposure to alcohol during adolescence exerts long-term effects on the adult brain stress circuits, causing many changes that persist into adulthood. Here we examined the consequences of adolescent intermittent ethanol [AIE, administered from postnatal day (PND) 28–42] on the hypothalamic-pituitary-adrenal (HPA) axis-related brain circuitry of rats challenged with an intragastric administration of alcohol in adulthood (PND 70–71). Both male and female adolescent rats were exposed to alcohol vapors, while controls did not receive the drug, to assess whether AIE alters adult alcohol response in a sex-specific manner. We demonstrated that AIE increased PVN Avp mRNA levels during late (PND 42) but not middle (PND 36) adolescence in males. While an alcohol challenge administered to 70–71-day-old rats increased Crf mRNA levels in males and Avp mRNA levels in females, AIE blunted both effects. These results suggest that AIE produced long-lasting changes in the responsiveness of the HPA axis to a subsequent alcohol challenge in a sex-specific manner. Furthermore, AIE altered adrenergic brain stem nuclei involved in stress responses in adulthood, resulting in increased numbers of phenylethanolamine N-methyltransferease (PNMT) neurons in male C2 and female C1 regions. This tended to enhance activation of the male C2 nucleus upon alcohol challenge. Collectively, these results suggest that AIE exerts long-term effects on the ability of the PVN to respond to an alcohol challenge in adulthood, possibly mediated by catecholaminergic input from the brain stem to the PVN.
Keywords: adolescent, alcohol, catecholamines, corticotropin-releasing factor, vasopressin
INTRODUCTION
Alcohol abuse and dependence are significant societal problems whose likelihood is greatly enhanced by starting alcohol consumption during adolescence (Dawson et al., 2008). Thus, determining the long-term impact of adolescent alcohol exposure is critical for developing interventions to treat alcohol-related disorders. One possible locus of persistent drug-induced adaptations is the hypothalamic-pituitary-adrenal (HPA) axis, the system that mediates responses to stressors. As the HPA response has been shown to change between adolescence and adulthood, it is thought that adolescence presents a vulnerable period for shaping the function of the adult HPA axis (Romeo, 2010). Both male and female rats initiating alcohol consumption in adolescence show greater stress-induced increases in alcohol intake in adulthood than similarly alcohol-experienced rats whose alcohol access commenced in adulthood (Siegmund et al., 2005, Fullgrabe et al., 2007). This enhanced susceptibility to stress-induced alcohol intake suggests that adolescent alcohol experience may permanently alter stress responses, with deleterious consequences for alcohol consumption in adulthood. Thus, understanding how adolescent alcohol exposure modifies adult stress responses is of great importance for developing therapies to treat individuals with early onset of alcohol drinking.
The HPA response to stressors is initiated by activation of neurons in the paraventricular nucleus (PVN) of the hypothalamus, triggering the release of corticotropin-releasing factor (CRF) and vasopressin (VP) into the pituitary gland (Herman and Cullinan, 1997). This causes release of adrenocorticotropic hormone (ACTH), which then acts on the adrenal gland to increase corticosterone (cort) production. The HPA axis is activated by an acute alcohol challenge in adult rats (Rivier et al., 1984, Rivier et al., 1990, Ogilvie et al., 1997), while repeated alcohol exposure and dependence lead to a reduction in the magnitude of alcohol-induced HPA activation (Lee and Rivier, 1997, Richardson et al., 2008). The HPA response to alcohol develops during the adolescent period, increasing in magnitude from postnatal day (PND) 28 and reaching a maximal level in adulthood, an effect much more prominent in females than in males (Silveri and Spear, 2004, Willey et al., 2012). Exposure of male rats to repeated alcohol injections during adolescence resulted in both blunted adult baseline cort levels and enhanced cort release following an alcohol challenge in adulthood (Przybycien-Szymanska et al., 2011). Cort responses to acute stressors in adolescents, as compared to adult rats, were significantly prolonged in both sexes (Romeo et al., 2004b, Romeo et al., 2006). However, stress responses in chronically stressed adolescent rats were blunted in males (Romeo et al., 2006, Weintraub et al., 2010) but enhanced in females (Weintraub et al., 2010). Together, these data suggest adolescence as a period of prolonged activity and enhanced plasticity of HPA axis responses that may significantly impact adult HPA axis function, with differential susceptibility of females and males to such long-term adaptations.
Additional studies support persistent effects of adolescent exposure to stressors, including alcohol, on HPA axis activity and anxiety-like behavior. Both isolated housing (Weintraub et al., 2010) and binge-like alcohol drinking (Gilpin et al., 2012) during adolescence reduced baseline anxiety-like behavior in adult male rats, as assessed by time spent on the open arms of the elevated plus maze (EPM). However, female rats did not display any significant changes in EPM anxiety-like behavior following adolescent isolation (Weintraub et al., 2010), but did show reduced anxiety-like behavior in adulthood during estrus if exposed to social stress in adolescence (McCormick et al., 2008). Despite the greater behavioral effect of adolescent isolation in adult males, females, but not males, displayed elevated baseline PVN Avp mRNA, with no changes in baseline Crf mRNA in either sex, as compared to rats housed socially during adolescence (Weintraub et al., 2010). Similarly, adolescent binge drinking did not alter baseline PVN CRF neuron number or activity in males (Allen et al., 2011a), while alcohol injections administered to mimic a binge pattern in adolescence elevated both baseline and alcohol challenge-induced PVN Crf mRNA in adult male rats (Przybycien-Szymanska et al., 2011). These data support long-term effects of adolescent exposure to stressors, including alcohol, on adult stress responses and HPA axis activity, as well as possible sexual dimorphism in these effects. However, the fact that differences in PVN gene and protein expression do not directly align with behavioral effects of adolescent stress/alcohol history in adulthood suggests that long-term modifications in HPA axis activity may not be restricted to the PVN but rather may involve changes in afferent regions regulating PVN activation.
One source of projections that modulate PVN neuronal activity in response to stress is the catecholaminergic neurons in the brain stem, particularly the noradrenergic neurons in the locus coeruleus and regions A1–A2, as well as the adrenergic neurons of regions C1–C3 (Dayas et al., 2001). Previously, we showed that acute alcohol activates these brain stem nuclei in adult male rats (Lee et al., 2011). We have also demonstrated in male rats that adolescent binge drinking attenuated alcohol-induced activation of C3 neurons in adulthood (Allen et al., 2011a), while intermittent exposure to alcohol vapor throughout adolescence increased the number of phenylethanolamine N-methyltransferase (PNMT)-labeled neurons in the C2 region following an acute alcohol challenge in early adulthood [PND 60–61] (Allen et al., 2011b). Unlike these long-term changes resulting from adolescent alcohol experience, prenatal alcohol exposure enhanced the footshock stress responsiveness of C1 neurons in adulthood in females, but not males (Choi et al., 2008). Together, these data indicate that alcohol exposure during various developmental epochs may alter the responsiveness of brain stem catecholaminergic neurons, suggesting these neurons as a possible locus of the long-term effects of adolescent alcohol exposure on adult HPA axis function.
Taken together, these previous studies led to the hypothesis that adolescent intermittent ethanol (AIE) exposure would generate long-lasting changes in the HPA axis sensitivity to alcohol challenge, resulting in a reduced alcohol response in adulthood. We also theorized that the depressive effects of AIE on adult HPA axis function would result from decreased activation of either PVN neurons or the afferent C1–C3 adrenergic neurons upon alcohol challenge. Finally, we proposed that these persistent effects of AIE would differ by sex.
EXPERIMENTAL PROCEDURES
Animals
Male and female Sprague Dawley rats (Harlan, San Diego, CA, USA) were housed 3–4 per standard plastic cage with wood chip bedding, with standard rat chow and water available ad libitum throughout the study. Rats lived in a humidity- and temperature-controlled vivarium under a 12 h light/12 h dark light cycle with lights off at 1800. Each experimental group consisted of 5–7 animals. Experiments were performed during the early portion of the light cycle in the trough of the circadian HPA cycle (Atkinson and Waddell, 1997), to minimize the experimental impact of individual differences. All experiments met the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by The Salk Institute Animal Care and Use Committee.
Intermittent alcohol vapor exposure
Adolescent rats were exposed to alcohol vapor in an air-tight chamber system provided by La Jolla Alcohol Research, Inc. (La Jolla, CA, USA, http://www.ljari.com), as described in detail previously (Lee et al., 2000a, Lee et al., 2000b). Briefly, 3–4 animals per cage were exposed to alcohol vapor daily for 6 h (0700–1300; AIE) or air (Control) for the duration of the 15-day adolescent period (PND 28–42), except where indicated for rats euthanized at an earlier time point. After each exposure, the rats were returned to the housing racks in clean cages. Blood alcohol levels (BALs) were sampled from one rat per cage every 2–3 days and alcohol drip rates adjusted, as necessary, to maintain BALs at approximately 200 mg%. Each rat was sampled twice during the exposure period, with the second sampling performed on the same day for all rats in the cohort (PND 39 for males, PND 41 for females). Experimental control rats were exposed to similar handling and tail nicking to standardize any procedural stress effects across all subjects. This exposure paradigm was used to generate controlled daily cycles of alcohol intoxication and withdrawal during the adolescent period. To analyze the time course of BAL and cort levels in response to vapor exposure during the adolescent period, rats were euthanized by rapid decapitation at the time points specified, relative to the onset of vapor exposure, and trunk blood collected. Control samples were collected in parallel, matched for time and age, but collapsed into a single group for analysis due to similar low basal cort levels in both age groups.
Blood alcohol levels
Blood samples (50–100 μl) were collected from the tails of all animals, including the air-exposed rats to control for any procedural stress effects. BALs were measured in 5 μl samples using an Analox AM 1 analyzer (Analox Instruments Ltd., Lunenburg, MA, USA) (Lee et al., 2000a, Lee et al., 2000b). The precision of this assay is 1–2%, the sensitivity is 0.1 mg/100 ml, and the curve is linear up to 400 mg/100 ml.
Corticosterone assay
Plasma cort levels were measured using a kit optimized for small sample volumes (MP Biomedicals, Inc., Orangeburg, NY, USA), according to manufacturer’s instructions. The detection limit of this assay is 10 ng/ml.
Animal surgery and alcohol injection
Upon reaching PND 62–63, male and female rats were implanted with intragastric (ig) catheters under isoflurane anesthesia (Butler Animal Health Supply, Dublin, OH, USA) [see (Ogilvie et al., 1997) for methods], and were allowed to recover from surgery for 7–8 days before experimentation. Rats were housed individually post-surgery to prevent chewing of external cannulae. Despite individual housing, we did not observe differences in c-fos expression in any region analyzed between ig saline injected experimental control rats and group-housed absolute control rats that did not undergo experimental procedures on the test day, nor did we observe activation of c-fos expression in the PVN in either experimental or absolute controls (data not shown). Estrous cycles were synchronized in female rats on the third day prior to experimentation via subcutaneous injection of 2 μg of a GnRH analog, [D-Trp6-Pro9-Net-GnRH], at 9 am and 2 pm, such that female rats were in diestrus II at the time of experimentation, as previously described by ourselves (Lee et al., 2003, Richardson et al., 2006) and others (Roberts et al., 1998, Cottone et al., 2007, Parylak et al., 2012). On the day of the experiment (PND 70–71), the animals were singly housed in opaque buckets with wood chip bedding in a quiet room with extension cannulae connected such that the animals could be injected without being handled, to prevent procedural stress. Rats were left undisturbed for 2 h to allow hormone levels to return to baseline (Lee and Rivier, 1997, Ogilvie et al., 1997, Rivier and Lee, 2001), then administered 4.5 g/kg alcohol (<20% v/v in water) or an equivalent volume of water via the ig cannula. The alcohol challenge dose corresponds to that previously used in our laboratory (Rivier and Lee, 2001, Lee and Rivier, 2003, Seo and Rivier, 2003). BALs at the time of euthanasia can be found in Table 1. Because of the large volume administered, injections were slowly infused over a 2-min period. Compared to absolute, non-infused controls, ig delivery of this volume of fluid did not induce c-fos expression in any of the brain regions studied (P’s>0.05, absolute control vs. control/vehicle group; data not shown).
Table 1.
Blood alcohol levels at time of brain collection following acute intragastric alcohol administration on PND 70–71
| Sex | Control | AIE |
|---|---|---|
| M | 310.8 ± 27.3 | 332.8 ± 19.8 |
| F | 258.3 ± 11.6 | 293.4 ± 30.7 |
Blood alcohol levels are expressed as mean ± SEM mg alcohol per dl blood.
Immunohistochemistry
Rats were euthanized at the end of the 6-h alcohol vapor session on PND 36 or PND 42, or 2 h after acute ig alcohol challenge on PND 70–71, based on our previous studies (Ogilvie et al., 1998). All animals were deeply anesthetized by an intraperitoneal injection of 35% chloral hydrate (Ogilvie et al., 1997) followed by transcardial perfusion with 0.9% NaCl for 2 min and 4% cold paraformaldehyde (PFA) for 18 min. Brains were placed in 4% PFA, then cryoprotected by overnight incubation in a 10% sucrose/4% PFA solution prior to coronal sectioning by microtome at a thickness of 30 μm. All sections were maintained in an antifreeze solution (50% 0.1 M phosphate buffered saline, 20% glycerol, 30% ethylene glycol) at 20°C until analysis. Every fourth section throughout the rostral-caudal extent of the PVN and brain stem was used for analysis. Each immunohistochemistry (IHC) staining or in situ hybridization reaction was performed using brains obtained from AIE-treated and control animals. Double DAB IHC staining was performed on free-floating sections as described previously (Choi et al., 2008, Allen et al., 2011b), with a rabbit anti-c-fos antibody (1:10,000, Calbiochem, San Diego, CA, USA) stained black and a sheep anti-PNMT antibody (1:7,500, Chemicon/Millipore, Billerica, MA, USA) stained brown. Sections were mounted on gelatin-coated sub slides, dehydrated, and coverslipped using DPX mounting medium (Electron Microscopy Sciences, Hatfield, PA). Negative controls without primary or secondary antibody were included. The black (c-fos) stain indicates activated nuclei whereas the brown PNMT-ir stain shows cytoplasmic signals. Individual and colocalized immuno-labeled cells were counted using a 20X dry objective in 3–6 sections throughout each brain region examined, and the average values per section for each rat were determined by brain region. The data are expressed as both the total number of PNMT-positive cells per section and as the number of Fos-positive cells colocalized with PNMT-positive cell bodies.
In situ hybridization
In situ hybridization was performed according to a previously published protocol that was adapted and described extensively (Lee et al., 2000a, Lee et al., 2000b). Briefly, autoradiographic localization of Crf and Avp mRNA signals was obtained using 35S-labeled cRNA probes, and densitometric analysis was carried out using the same exposure time on brain sections mounted onto slides that were dipped in nuclear emulsion. Autoradiograph signals for Crf or Avp mRNA levels were measured bilaterally for 3 sections containing the PVN. Data were calculated as average autoradioagraphic density per section for each rat.
Imaging
IHC and in situ sections were imaged using a Nikon optical system, the Eclipse E600 microscope (Nikon Instruments Inc., Melville, NY, USA), equipped with a Micro*Color filter (Model RGB-MS-C, CRI Inc., Woburn, MA, USA) and CoolSNAP camera (Photometrics, Tucson, AZ, USA), coupled to a PC. IHC images were obtained using a 20X objective, while autoradiographic images were captured under 100X magnification. Image Pro Plus software (version 4.5.029, Media Cybernetics Inc., Bethesda, MD, USA) was used to obtain the densitometric analyses of the autoradiographic signals. Gray level measurements (optical density) were taken under dark-field illumination of hybridized sections in the PVN.
Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM). Data were analyzed using two-way ANOVA with post-hoc comparisons via the Tukey method. Statistical analyses were performed using Prism (Version 4.0, Graphpad Software Inc., La Jolla, CA, USA) and SigmaPlot (Version 11.0, Systat Software Inc., Chicago, IL, USA). Statistical significance was accepted for P < 0.05.
RESULTS
Effect of AIE on plasma corticosterone levels
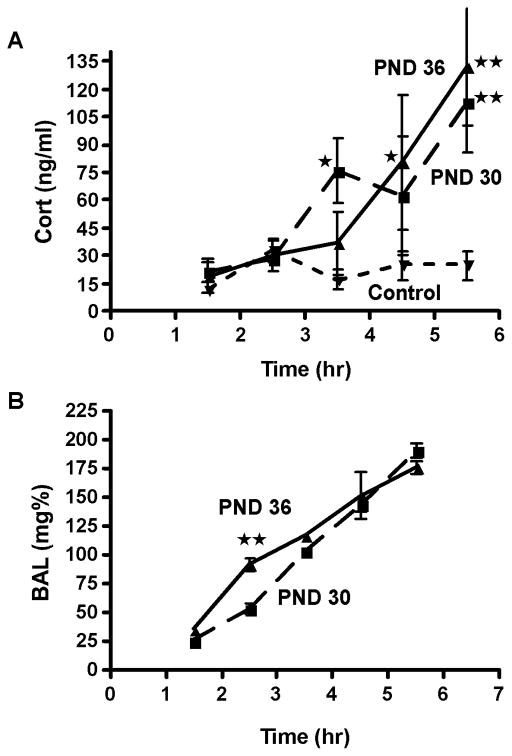
In order to study the effects of adolescent alcohol exposure on the HPA axis, we utilized a model of AIE in which rats were exposed to alcohol vapor or air for 6 h per day from PND 28–42 (Allen et al., 2011b). On PND 30 and 36, plasma cort release and BALs were assessed in adolescent males at hourly intervals beginning 1.5 h after the onset of alcohol vapor exposure, or at the same time points for age-matched controls (Fig. 1). Analysis of plasma cort data by two-way ANOVA with the factors Time (1.5, 2.5, 3.5, 4.5 and 5.5 h post-vapor onset) and AIE Group (Control, AIE PND 30 and AIE PND 36) yielded main effects of Time (F4,55=8.98, P<0.001) and AIE Group (F2,55=10.42, P<0.001). Cort levels were elevated by 3.5 h into vapor exposure on PND 30 and by 4.5 h into vapor exposure on PND 36 vs. alcohol-naïve controls (Fig. 1A). The more rapid increase in plasma cort on PND 30, as compared to PND 36, manifested as an interaction between the factors Time and AIE Group (F8,55=3.21, P<0.01). BALs also increased steadily across the 6-h vapor sessions on PND 30 and 36 (Fig. 1B, main effect of Time, F4,30 = 122.83, P<0.001). Although BALs increased similarly over the 6-h period on both PND 30 and PND 36, an Age x Time Point interaction was observed (F4,24=2.98, P<0.05) due to significantly higher BALs at the 2.5-h time point for PND 36 rats. This difference did not persist and was observed in the group showing delayed, rather than earlier, elevations in cort levels, suggesting that differences in plasma cort levels were independent of BAL discrepancies.
Figure 1.
Time course of elevated corticosterone (cort) and blood alcohol levels (BALs) during adolescent alcohol vapor exposure. Plasma samples were collected by rapid decapitation at 1-h intervals in male rats during a 6-h vapor exposure session or at the same time points in air-exposed controls on PND 30 and PND 36. Samples were analyzed to determine plasma cort levels (A) and BALs (B). Data are represented as mean ± SEM of 4–6 rats per time point. Inverted triangle with short dashed line, Control (collapsed across ages); Square with large dashed line, AIE PND 30; Triangle with solid line, AIE PND36. *, P<0.05, **, P<0.01, AIE vs. Control (A) or PND 30 vs. PND 36 (B).
Effect of AIE on PVN Crf and Avp mRNA levels
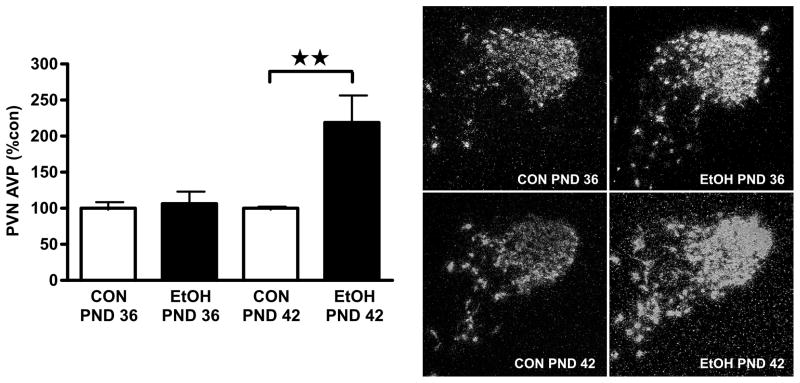
As the observed elevation in cort levels indicated activation of the HPA axis by the daily AIE exposure, we hypothesized that AIE would also increase Crf and/or Avp mRNA levels in the PVN of male rats during the vapor exposure period. To test this, Crf and Avp mRNA levels were assessed in male rats at the end of the 6-h vapor exposure period on PND 36 and 42. As shown in Fig. 2, PVN Avp mRNA levels were significantly increased in AIE male rats at the end of vapor access on PND 42 (mean ± SEM BAL: 271.1 ± 9.1 mg/dl) but not PND 36 (mean ± SEM BAL: 285.8 ± 4.2 mg/dl). Analysis by two-way ANOVA showed a Group x Age interaction (F1,12=6.39, P<0.05), with significantly higher Avp mRNA levels in the PND 42 AIE group as compared to both its respective control and PND 36 AIE (P’s<0.01). PVN Crf mRNA levels of AIE rats were not significantly different from controls at either time point (PND 36: control, 100.0 ± 4.0%; AIE, 85.1 ± 7.5 %, P>0.05; PND 42: control, 100.0 ±16.4 %; AIE, 125.6± 14.2%, P>0.05). Together, these data show that AIE increased PVN Avp but not Crf gene expression at the end of a 6-h vapor exposure session only in late adolescent males.
Figure 2.
Exposure to intermittent alcohol vapor during adolescence increased Avp mRNA in the PVN of male rats on PND 42 but not PND 36. Male rats were exposed to alcohol vapor for 6 h per day from PND 28 and brains collected for analysis at the conclusion of the 6 h vapor exposure period on PND 36 and PND 42. (Left panel) Avp mRNA levels were quantified using optical density (arbitrary units) and normalized to average values of age-matched controls. Data are expressed as mean ± SEM percent control of 4–6 male rats per group. **, P<0.01. (Right panel) Representative dark-field photomicrographs (100X magnification) of PVN Avp mRNA in alcohol vapor- (EtOH) and air-exposed (CON) rats euthanized on PND 36 and PND 42.
Sex differences in AIE effects on the PVN Crf and Avp response to alcohol challenge in adulthood
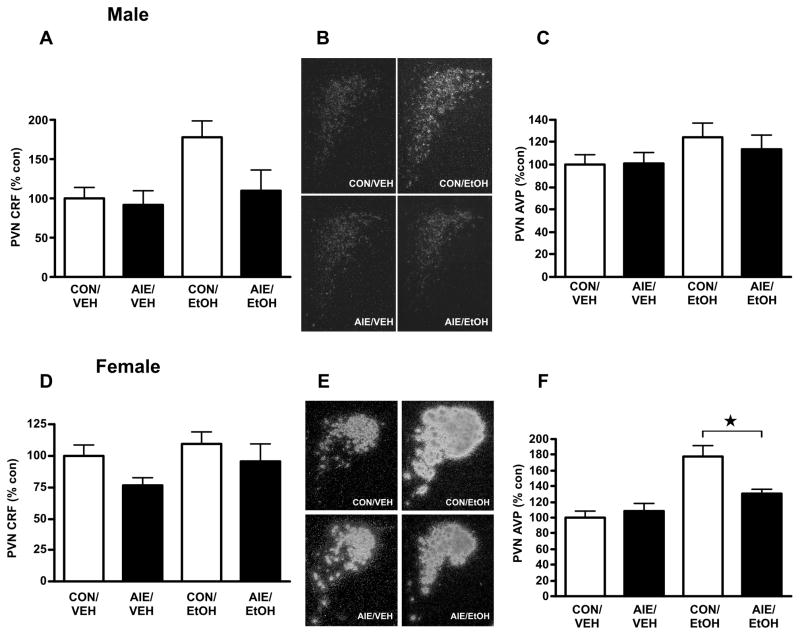
Previously, we reported a dampened PVN Crf response to an alcohol challenge in young adulthood, at PND 60–61, in males exposed to AIE as compared to adolescent air-exposed controls (Allen et al., 2011b). We wanted to determine (1) whether this difference resulted from altered basal PVN gene expression in AIE-experienced adult rats, (2) whether differences in response to alcohol challenge in rats with a history of AIE persisted past early adulthood, and (3) whether males and females responded differentially to AIE. Rats were exposed to air (Control) or AIE (mean ± SEM peak BALs: male PND 39, 270.8 ± 5.6; female PND 41, 196.3 ± 4.4) until PND 42, then challenged on PND 70–71 with ig infusion of 4.5 g/kg alcohol or vehicle. As shown in Figure 3, AIE did not significantly alter basal Crf mRNA levels on PND 70–71 in males (Fig. 3A,B), although alcohol challenge did increase Crf mRNA levels (two-way ANOVA, main effect of Adult Challenge, F1,20=5.12, P<0.05; no significant effect of AIE, F1,20=3.31, P=0.08). We found no interaction between the factors, suggesting that while AIE appeared to reduce the increase in PVN Crf mRNA levels observed following 4.5 g/kg alcohol challenge on PND 70–71, this effect was not significant (F1,20=2.00, P=0.17). In contrast, there was no statistical difference in PVN Avp mRNA levels either at baseline or following alcohol challenge between control and vapor-exposed male rats (F’s>2.68, P’s>0.11; Fig 3C).
Figure 3.
Effects of intermittent alcohol vapor exposure during adolescence on Crf and Avp mRNA expression in the PVN of adult (PND 70–71) male (A–C) and female (D–F) rats following alcohol challenge. Control, air-exposed (CON) and adolescent intermittent ethanol-exposed (AIE) rats were euthanized on PND 70–71 2 h after administration of a 4.5 g/kg ig alcohol challenge (EtOH) or equivalent volume of water (VEH), and in situ hybridization was performed to quantify Crf and Avp mRNA levels. (A) Quantification of CRF mRNA by densitometric analysis of autoradiographic signals (arbitrary units) in male rats. (B) Representative dark-field photomicrographs (100X magnification) of PVN Crf mRNA in male rats. (C) Quantification of Avp mRNA by densitometric analysis of autoradiographic signals (arbitrary units) in male rats. (D) Quantification of Crf mRNA by densitometric analysis of autoradiographic signals (arbitrary units) in female rats. (E) Representative dark-field photomicrographs (100X magnification) of PVN Avp mRNA in female rats. (F) Quantification of Avp mRNA by densitometric analysis of autoradiographic signals (arbitrary units) in female rats. Data in histograms are expressed as mean ± SEM optical density expressed percent control relative to CON/VEH average for the given experiment from 4–6 rats per treatment. * P<0.05.
In contrast to AIE male rats, challenging females with alcohol in adulthood did not significantly modify PVN Crf mRNA levels, regardless of AIE history (F’s<2.73, P’s>0.11, Fig. 3D). Instead, following administration of alcohol on PND 70–71, female rats demonstrated a striking elevation in PVN Avp mRNA levels which was blunted by AIE exposure (Fig. 3E,F). Analysis by two-way ANOVA yielded an interaction between AIE and adult alcohol challenge (F1,16=6.80, P<0.05) due to significant effects of adult alcohol challenge in controls (P<0.001) as well as AIE history in alcohol-challenged rats (P<0.05). Together, these results demonstrated differential effects of AIE on the adult PVN response to alcohol challenge in adult male and female rats.
Effects of AIE on adult alcohol responses in brain stem catecholaminergic neurons
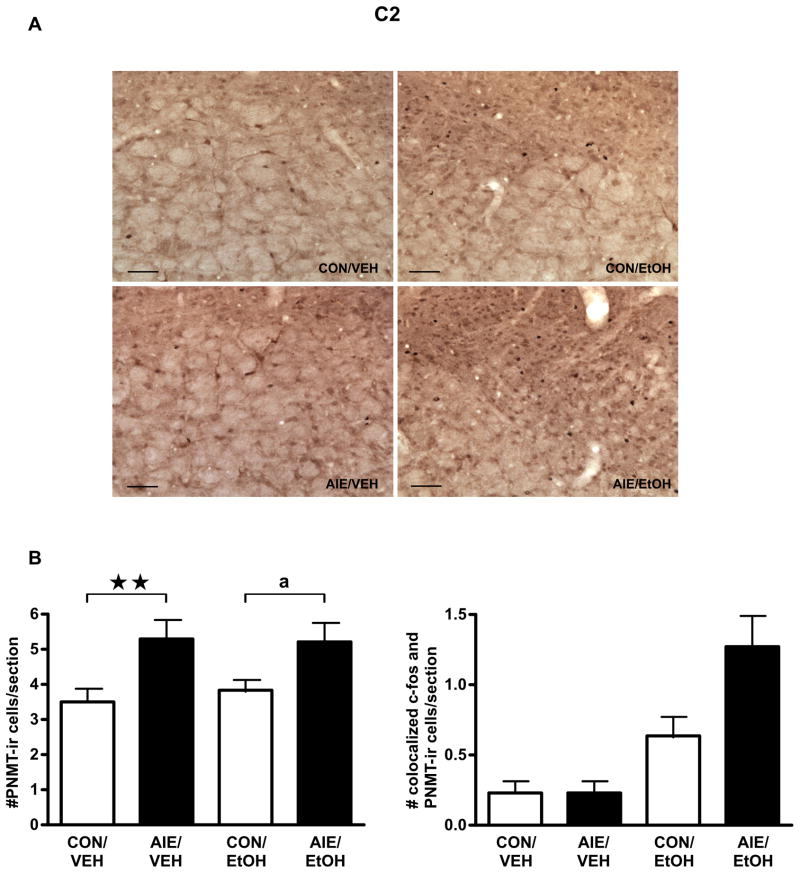
Brain stem catecholaminergic neurons provide a significant input driving the activity of PVN neurons (Dayas et al., 2001). We hypothesized that the sex differences observed in the PVN might result from sex-specific effects of AIE and/or adult alcohol challenge on the medullary C1–C3 nuclei. We performed IHC to examine changes in neurons expressing PNMT, a final enzyme in adrenergic synthesis, following alcohol challenge on PND 70–71 in male and female rats with or without a history of AIE exposure. As shown in Table 2, AIE male rats showed elevated colocalization of c-fos-ir and PNMT-ir cells/section in the C1 medullary region on PND 70–71 (main effect of AIE, F1,16=6.31, P<0.05). Alcohol challenge also increased the number of colocalized neurons (main effect of alcohol challenge, F1,16=46.15, P<0.001), but there was no interaction between the factors (F1,16=0.16, P=0.70) and no change in the total number of PNMT-positive cells (F’s<1.64, P’s>0.21). A similar trend for increase in alcohol challenge-induced c-fos/PNMT colocalization was observed in the C2 region of AIE-exposed male rats (Fig. 4; AIE, F1,16=3.68, P<0.08; alcohol challenge, F1,16=20.05, P<0.001; AIE x alcohol challenge interaction, F1,16=3.68, P<0.08). Unlike C1, AIE significantly increased the number of PNMT-ir cells per section, regardless of adult alcohol challenge, in male rats (Fig. 4; main effect of AIE, F1,16=12.59, P<0.005).
Table 2.
Brain stem region C1 PNMT and c-fos expression in male rats exposed to AIE and challenged with alcohol on PND 70–71
| Group | Treatmenta | Number of PNMT-ir cells per section in C1 | Number of colocalized c-fos-ir and PNMT-ir cells per section in C1 |
|---|---|---|---|
| Male | Control/vehicle ig | 68.6 ± 13.6 | 0.6 ± 0.2 |
| Control/alcohol ig | 78.1 ± 11.8 | 4.3 ± 0.9b | |
| AIE/vehicle ig | 79.2 ± 3.9 | 1.8 ± 0.4c | |
| AIE/alcohol ig | 92.5 ± 6.4 | 6.0 ± 0.6b,c |
Male rats were exposed to alcohol vapor (AIE) or air (Control) from PND 28–42, then injected with alcohol (4.5 g/kg, ig) or vehicle at PND 70–71. Values are mean ± SEM, n=4–6 rats per group.
P<0.001, alcohol challenge vs. vehicle.
P<0.05, AIE vs. control.
Figure 4.
Exposure to intermittent alcohol vapor during adolescence increased the number of colocalized c-fos and PNMT immunoreactive (ir) cells/section and the number of PNMT-ir cells/section in the C2 region of the brain stem of male rats following adult alcohol challenge. Male rats exposed in adolescence to intermittent ethanol (AIE) or air controls (CON) were euthanized 2 h after administration of a 4.5 g/kg ig alcohol challenge (EtOH), or equivalent volume of water (VEH). (A) Double immunohistochemical staining labeled c-fos-containing nuclei black and PNMT-containing cell bodies brown. Representative images were captured using a 20X dry objective (scale bar=100 μm). (B) Quantification of PNMT-positive cell numbers (left) and PNMT-positive cells containing c-fos staining (right) are expressed as mean ± SEM cells/section for 4–6 male rats per treatment. ** P=0.01, a P=0.053.
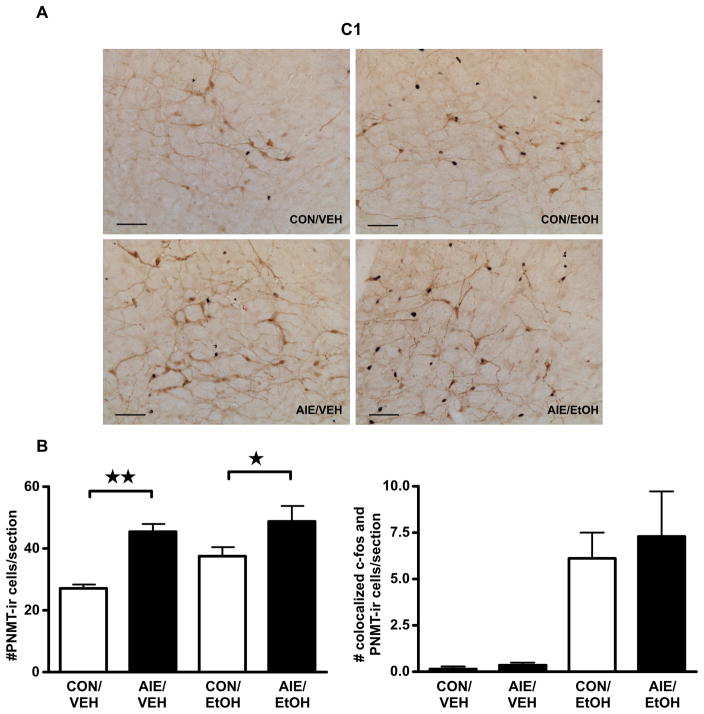
Based on the differential effects of adult alcohol challenge on PVN mRNA expression in male and female rats, we hypothesized disparate effects of AIE on brain stem PNMT neuron activation as well. As predicted, female rats demonstrated a significant AIE-dependent increase in the number of PNMT-ir neurons per section in the C1 region on PND 70–71 (F1,17=17.33, P<0.001), but no AIE-related effect on the alcohol challenge-induced increase in c-fos/PNMT colocalization (Fig. 5; main effect of alcohol challenge, F1,17=22.35, P<0.001; no effect of AIE or interaction, F’s<0.04, P’s>0.84). Although adult alcohol challenge activated C2 PNMT-expressing neurons in the brain stem of female rats (Table 3, F1,17=5.24, P<0.05), no AIE-related significant statistical differences were observed (all F’s<1.59, all P’s>0.22 for AIE and AIE by Alcohol Challenge interaction). No significant differences were observed in the C3 region of either male or female rats, regardless of AIE exposure (Table 4). Together, these data demonstrate that AIE differentially altered medullary brain stem regions in adult male and female rats, both at baseline and following an alcohol challenge, suggesting a central role for catecholamines in modulating the long-term consequences of AIE on the adult HPA axis.
Figure 5.
Exposure to intermittent alcohol vapor during adolescence increased the number of colocalized c-fos and PNMT immunoreactive (ir) cells/section and the number of PNMT-ir cells/section in the C1′ region of the brain stem of female rats following adult alcohol challenge. Female rats exposed in adolescence to intermittent ethanol (AIE) or air controls (CON) were euthanized 2 h after administration of a 4.5 g/kg ig alcohol challenge (EtOH), or equivalent volume of water (VEH). (A) Double immunohistochemical staining labeled c-fos-containing nuclei black and PNMT-containing cell bodies brown. Representative images were captured using a 20X dry objective (scale bar=100 μm). (B) Quantification of PNMT-positive cell numbers (left) and PNMT-positive cells containing c-fos staining (right) are expressed as mean ± SEM cells/section for 4–6 female rats per treatment. * P<0.05.
Table 3.
Brain stem region C2 PNMT and c-fos expression in female rats exposed to AIE and challenged with alcohol on PND 70–71
| Group | Treatmenta | Number of PNMT-ir cells per section in C2 | Number of colocalized c-fos-ir and PNMT-ir cells per section in C2 |
|---|---|---|---|
| Female | Control/vehicle ig | 7.4 ± 0.4 | 0 ± 0 |
| Control/alcohol ig | 8.7 ± 1.2 | 0.4 ± 0.2b | |
| AIE/vehicle ig | 7.7 ± 0.9 | 0.1 ± 0.1 | |
| AIE/alcohol ig | 9.2 ± 1.1 | 1.2 ± 0.6b |
Female rats were exposed to alcohol vapor (AIE) or air (Control) from PND 28–42, then injected with alcohol (4.5 g/kg, ig) or vehicle at PND 70–71. Values are mean ± SEM, n=4–6 rats per group.
P<0.05, alcohol challenge vs. vehicle.
Table 4.
Brain stem region C3 PNMT and c-fos expression in female and male rats exposed to AIE and challenged with alcohol on PND 70–71
| Group | Treatmenta | PNMT-ir cells per section | Number of colocalized c-fos-ir and PNMT-ir cells per section in C3 |
|---|---|---|---|
| Female | Control/vehicle ig | 18.7 ± 2.0 | 0.1 ± 0.1 |
| Control/alcohol ig | 17.8 ± 1.0 | 0.4 ± 0.3 | |
| AIE/vehicle ig | 16.6 ± 1.9 | 0.1 ± 0.1 | |
| AIE/alcohol ig | 18.3 ± 1.8 | 0.7 ± 0.4 | |
| Male | Control/vehicle ig | 34.4 ± 4.1 | 0.3 ± 0.2 |
| Control/alcohol ig | 21.0 ± 2.7 | 0.9 ± 0.5 | |
| AIE/vehicle ig | 24.6 ± 0.7 | 0.4 ± 0.1 | |
| AIE/alcohol ig | 21.8 ± 2.0 | 1.8 ± 0.4 |
Male and female rats were exposed to alcohol vapor (AIE) or air (Control) from PND 28–42, then injected with alcohol ig (4.5 g/kg) or vehicle at PND 70–71. Values are mean ± SEM, n=4–6 rats per group.
DISCUSSION
The purpose of this study was to investigate long-term effects of AIE on the brain stress circuits that alter HPA axis activity, and to determine whether such effects differ by sex. Here we demonstrated that AIE increases Avp gene expression during late (PND 42) but not middle (PND 36) adolescence in the male. Unlike adolescence, alcohol exposure in the 70–71-day-old male altered Crf, but not Avp, mRNA levels. Conversely, adult females did not show significant changes in Crf mRNA levels following alcohol challenge, but rather displayed increased Avp mRNA levels. These effects were blunted by AIE, suggesting that alcohol exposure during adolescence produced long-lasting changes in the responsiveness of the HPA axis to subsequent alcohol challenge in a sex-specific manner. AIE also altered brain stem nuclei involved in stress responses in adulthood, with increased numbers of PNMT-immunoreactive neurons in male C2 and female C1 regions, resulting in enhanced activation of the male C2 nucleus upon alcohol challenge. Together, these data show sex-specific, persistent modifications of stress-responsive nuclei in rats exposed to intermittent alcohol during adolescence.
Modulation of alcohol responsiveness during adolescent maturation
The data herein demonstrate differential effects of alcohol exposure as adolescence progresses, with HPA axis responsiveness changing over adolescence. Specifically, PVN Avp gene expression increased only at PND 42, not PND 36, in males, despite similar BALs in both groups. The elevation in Avp in later adolescence agrees with results from Przbycien-Szymanska et al. (2010) following daily 3 g/kg i.p. alcohol treatments on PND 37–44, although that treatment regimen also generated elevated Crf, which was not seen in the present study. This discrepancy may be due to the more prolonged alcohol exposure utilized herein, both in number of days (beginning at PND 28 vs. PND 37) and in discrete alcohol exposure duration (6 h vapor session vs. single acute injection). Indeed, prolonged alcohol exposure generates dysregulation of the HPA axis in human alcoholics (Heuser et al., 1988, von Bardeleben et al., 1989), and the possibility exists that the greater severity of the 15-day vapor exposure paradigm may cause such an adaptation in the HPA axis. Alternatively, the difference may arise from the earlier onset of alcohol exposure in the periadolescent period. Nonetheless, the results indicate both that repeated alcohol exposure in later adolescence increased Avp and that greater magnitude of adolescent alcohol exposure may trigger an adaptation of the Crf response within the adolescent period.
In line with the change in alcohol vapor effects on Avp mRNA expression as adolescence progresses, developmental stage impacts the behavioral consequences of alcohol exposure. For example, alcohol increased open arm exploration in the elevated plus maze, suggestive of decreased anxiety-like behavior, in early but not late adolescence or adulthood in mice (Hefner and Holmes, 2007). Adolescent rats also failed to show the alcohol withdrawal-induced anxiety observed in adults (Doremus et al., 2003). These results are in line with studies that demonstrated reduced behavioral suppressive effects of alcohol in younger rats, including sedation/intoxication (Silveri and Spear, 2004, Pian et al., 2008), tolerance (Swartzwelder et al., 1998, Varlinskaya and Spear, 2006) and motor impairment (Silveri and Spear, 2004). These data demonstrate blunted sensitivity to the inhibitory and anxiogenic-like effects of alcohol, particularly during early adolescence, in agreement with PVN gene expression changes emerging only later in the adolescent period.
Sex differences in AIE effects on stress circuitry
While both male and female rats received equivalent alcohol treatment during adolescence, alterations in the response to adult alcohol challenge were quite different. Males demonstrated a slight attenuation of Crf responses and nonexistent Avp responses, similar to our previously reported findings in younger males (Allen et al., 2011b), while females showed inhibition of Avp activation by alcohol, without Crf effects. These data contrast directly with previous findings of elevated PVN Crf mRNA after an acute alcohol challenge in adult male rats with a history of adolescent alcohol injections (PND 37–42), but not in adolescent alcohol-naïve controls following the same adult alcohol challenge (Przybycien-Szymanska et al., 2011). The authors also showed reduced PVN Avp mRNA in control rats, but not adolescent alcohol-experienced rats, following adult alcohol challenge, whereas we did not see any significant Avp changes in adult males following acute alcohol challenge, regardless of AIE history. These discrepancies may result from differences in the adolescent alcohol exposure paradigms (PND 28–42 vapor vs. PND 37–44 injections), the routes of adult alcohol exposure (ig vs. intraperitoneal injection), or the time points analyzed (2 h vs. 1 h after adult alcohol challenge). Sex differences in gonadal maturation during the adolescent period, which occurs earlier in females than males, may also impact the sex differences observed in the effects of AIE on adult HPA responses, as differences in stress responses pre- and post-puberty (Viau et al., 2005), as well as variation in stress responses across the estrous cycle (Viau and Meaney, 1991), suggest that gonadal steroids modify HPA axis activity. Alternatively, alcohol exposure in adolescence could disrupt or delay puberty, as adult alcohol exposure reduces gonadal hormone levels (Cicero et al., 1978, Ogilvie and Rivier, 1997). However, removal of female sex hormones via ovariectomy (Romeo et al., 2004b) or augmentation of circulating testosterone to adult levels (Romeo et al., 2004a) in adolescent male rats did not alter HPA responses to stressors during early adolescence, suggesting that adolescent stress responses, including the observed AIE effects, may differ from adult responses via mechanisms independent of pubertal maturation.
Interestingly, rather than mirroring other studies investigating the adult impact of adolescent alcohol (Przybycien-Szymanska et al., 2011), our observations are more directly in line with findings reported following 6 months of alcohol liquid diet consumption in adulthood (Silva et al., 2009). Male rats exposed to 6-month alcohol intake showed significantly blunted numbers of CRF-positive neurons (Silva et al., 2009) and Crf mRNA levels (Silva and Madeira, 2012) in the PVN, with no significant change in VP-positive neurons (Silva et al., 2009), although a slight reduction in Avp mRNA levels was reported (Silva and Madeira, 2012). Conversely, females that consumed alcohol liquid diet for 6 months showed significant reductions in numbers of both CRF- and VP-positive PVN neurons (Silva et al., 2009), as well as decreases in both Crf and Avp mRNA levels (Silva and Madeira, 2012). Importantly, while withdrawal of alcohol access attenuated alcohol-induced changes in males exposed to the drug for 6 months, the inhibitions seen in female PVN were either maintained or enhanced during the withdrawal period (Silva et al., 2009, Silva and Madeira, 2012). Together, these studies suggest an increased sensitivity of the female PVN to permanent changes following prolonged alcohol exposure, and indicate that alcohol experience during the brief adolescent period may be as deleterious to PVN function as months of alcohol intake in adulthood.
In congruence with our previous study in young adult (PND 60–61) male rats (Allen et al., 2011b), AIE increased brain stem adrenergic neuronal PNMT expression and/or activation of PNMT-positive neurons. Specifically, PND 70–71 males showed elevated activation of C1 PNMT neurons and elevated C2 PNMT neuron numbers and alcohol responsiveness according to AIE history, while adult AIE females displayed elevated numbers of PNMT-ir neurons in C1. As these brain stem adrenergic nuclei project to the PVN (Cunningham et al., 1990), they are believed to participate in activation of the PVN; however, the increased activity of the brain stem adrenergic cell groups contrasts with the decreased Crf and Avp mRNA levels observed in AIE PVN following adult alcohol challenge. This discrepancy may be due to circuit alterations generated by AIE, although the brain stem adrenergic nuclei have been suggested to play a role independent of the initial HPA axis activation, as unilateral transection of the ventral noradrenergic bundle did not alter footshock-induced PVN activation (Li et al., 1996).
Adolescence as a period of heightened vulnerability for the HPA axis
During adolescence, many neural circuits undergo maturation, leaving them highly susceptible to insult. Not surprisingly, adolescent alcohol exposure has been shown to generate a variety of long-lasting neuroadaptations, including prefrontal cortical modifications of synaptic structure (Crews et al., 2007) and neurotransmission-related gene expression (Coleman et al., 2011), inhibition of hippocampal neurogenesis in both rats (Crews et al., 2006) and nonhuman primates (Taffe et al., 2010), blunting of the alcohol-responsiveness of tonic inhibitory current in adult hippocampus (Fleming et al., 2012), altered EEG frequencies in the hippocampus and parietal cortex (Slawecki et al., 2001), and reduced numbers of CRF-expressing neurons in the central nucleus of the amygdala (Gilpin et al., 2012). Some changes elicited by adolescent alcohol exposure demonstrated sex-specific patterns, such as reduced glial cell numbers in the medial prefrontal cortex, an effect only observed in males (Koss et al., 2012). Together, these studies highlight the deleterious effects caused by adolescent alcohol exposure in a variety of neural systems, and suggest a role for sex in modifying alcohol’s impact on adolescent brain development.
Differential stress response patterns in adolescent and adult rats, indicative of developmental changes in the stress system, accompany the progression to adulthood. Both male and female rats show prolonged cort responses to acute restraint stress during adolescence as compared to adulthood, independent of gonadal sex steroids (Romeo et al., 2004a, Romeo et al., 2004b). Acute stress exposure also triggers a greater activation of PVN neurons, as measured by c-fos-ir, in adolescent than adult male rats, particularly within cells that express CRF (Romeo et al., 2006), although we observed elevated Avp, not Crf, mRNA levels during adolescent alcohol exposure in male rats. Nonetheless, adolescent alcohol exposure in both males and females resulted in permanent perturbations of the adult HPA axis, particularly among females, who showed a striking inhibition of alcohol-induced PVN Avp mRNA expression. Additionally, the fact that our AIE males and females displayed elevated numbers of PNMT-positive neurons in brain stem areas C2 and C1, respectively, suggests the possibility that AIE modifies adult HPA responsiveness to alcohol challenge via modified connectivity to adrenergic neurons of the rostroventrolateral medulla. Determination of the specific PVN inputs responsible for the sex differences in AIE modulation of PVN responsiveness to alcohol challenge presents an important area for future study.
Implications for susceptibility to adult alcohol dependence following AIE
While the data clearly demonstrate alterations in the alcohol responsiveness of PVN Avp mRNA levels in females and, to a lesser degree, Crf mRNA levels in males following AIE experience, the role of these changes in shaping adult alcohol intake are currently unknown. Several studies have demonstrated elevated alcohol intake in adult rats (Pascual et al., 2009, Maldonado-Devincci et al., 2010, Gilpin et al., 2012) and mice (Moore et al., 2010) with binge-like patterns of adolescent alcohol exposure, suggesting the possibility that the observed changes in the stress circuitry of AIE rats might promote high alcohol intake in adulthood. Interestingly, alcohol-preferring P and HAD male rats showed higher levels of PVN Avp mRNA (Hwang et al., 1998) but not Crf mRNA (Hwang et al., 2004) than their low-drinking NP and LAD counterparts. VP has been demonstrated to support alcohol tolerance in mice via activation of the VP 1B (V1B) receptor (Hoffman, 1994). In line with these results, alcohol-dependent, but not nondependent, male rats were sensitive to suppression of alcohol intake during withdrawal by treatment with a V1B inhibitor (Edwards et al., 2012). Interestingly, during abstinence, human alcoholics displayed prolonged depression of basal circulating VP levels, lasting at least 9 months after cessation of alcohol intake (Doring et al., 2003), possibly due to epigenetic modification of the AVP gene (Hillemacher et al., 2009). The CRF system has also been implicated in human alcohol use disorders, as genetic polymorphisms in the CRF-binding protein, which inhibits CRF interactions with its receptors, have been associated with elevated stress-induced alcohol craving in heavy drinkers (Ray, 2011). Together, these studies suggest possible involvement of both VP and CRF in the maintenance of alcohol use disorders, thus making both systems attractive targets for the development of pharmacotherapies to treat alcohol use disorders, particularly for those patients whose alcohol use begins in adolescence.
HIGHLIGHTS.
Adolescent alcohol exposure alters the adult PVN response to alcohol challenge
Adolescent alcohol increases the number of PNMT neurons in the adult brain stem
Effects of adolescent alcohol on adult PVN and brain stem differ by sex
Acknowledgments
The authors would like to thank Jonathan Tjong, Bryant Chee, Brian Yip, Casey Peto and Debbie Doan for technical assistance. The project described was supported by Award Number AA019973 and training was provided under AA018914, both from the National Institute on Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health.
Footnotes
Authors’ Contributions
MLL, CR and SL conceptualized and designed the research; MLL, CL, SI, JV AND SL performed the experiments and analyzed the data; MLL, CR and SL wrote and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen CD, Lee S, Koob GF, Rivier C. Immediate and prolonged effects of alcohol exposure on the activity of the hypothalamic-pituitary-adrenal axis in adult and adolescent rats. Brain Behav Immun. 2011a;25(Suppl 1):S50–60. doi: 10.1016/j.bbi.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Rivier CL, Lee SY. Adolescent alcohol exposure alters the central brain circuits known to regulate the stress response. Neuroscience. 2011b;182:162–168. doi: 10.1016/j.neuroscience.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138:3842–3848. doi: 10.1210/endo.138.9.5395. [DOI] [PubMed] [Google Scholar]
- Choi IY, Lee S, Rivier C. Novel role of adrenergic neurons in the brain stem in mediating the hypothalamic-pituitary axis hyperactivity caused by prenatal alcohol exposure. Neuroscience. 2008;155:888–901. doi: 10.1016/j.neuroscience.2008.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Bernstein D, Badger TM. Effects of acute alcohol administration on reproductive endocrinology in the male rat. Alcohol Clin Exp Res. 1978;2:249–254. doi: 10.1111/j.1530-0277.1978.tb05807.x. [DOI] [PubMed] [Google Scholar]
- Coleman LG, Jr, He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res. 2011;35:671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. FG 7142 specifically reduces meal size and the rate and regularity of sustained feeding in female rats: evidence that benzodiazepine inverse agonists reduce food palatability. Neuropsychopharmacology. 2007;32:1069–1081. doi: 10.1038/sj.npp.1301229. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1990;292:651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Doring WK, Herzenstiel MN, Krampe H, Jahn H, Pralle L, Sieg S, Wegerle E, Poser W, Ehrenreich H. Persistent alterations of vasopressin and N-terminal proatrial natriuretic peptide plasma levels in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2003;27:849–861. doi: 10.1097/01.ALC.0000065433.17403.DE. [DOI] [PubMed] [Google Scholar]
- Edwards S, Guerrero M, Ghoneim OM, Roberts E, Koob GF. Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addict Biol. 2012;17:76–85. doi: 10.1111/j.1369-1600.2010.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. In the rat, chronic intermittent ethanol exposure during adolescence alters the ethanol sensitivity of tonic inhibition in adulthood. Alcohol Clin Exp Res. 2012;36:279–285. doi: 10.1111/j.1530-0277.2011.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav. 2007;86:320–326. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology (Berl) 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Heuser I, von Bardeleben U, Boll E, Holsboer F. Response of ACTH and cortisol to human corticotropin-releasing hormone after short-term abstention from alcohol abuse. Biol Psychiatry. 1988;24:316–321. doi: 10.1016/0006-3223(88)90200-4. [DOI] [PubMed] [Google Scholar]
- Hillemacher T, Frieling H, Luber K, Yazici A, Muschler MA, Lenz B, Wilhelm J, Kornhuber J, Bleich S. Epigenetic regulation and gene expression of vasopressin and atrial natriuretic peptide in alcohol withdrawal. Psychoneuroendocrinology. 2009;34:555–560. doi: 10.1016/j.psyneuen.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Hoffman PL. Neuroadaptive functions of the neuropeptide arginine vasopressin. Ethanol tolerance. Ann N Y Acad Sci. 1994;739:168–175. doi: 10.1111/j.1749-6632.1994.tb19818.x. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Froehlich JC, Hwang WS, Lumeng L, Li TK. More vasopressin mRNA in the paraventricular hypothalamic nucleus of alcohol-preferring rats and high alcohol-drinking rats selectively bred for high alcohol preference. Alcohol Clin Exp Res. 1998;22:664–669. doi: 10.1111/j.1530-0277.1998.tb04309.x. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Stewart R, Zhang JK, Lumeng L, Li TK. Corticotropin-releasing factor gene expression is down-regulated in the central nucleus of the amygdala of alcohol-preferring rats which exhibit high anxiety: a comparison between rat lines selectively bred for high and low alcohol preference. Brain Res. 2004;1026:143–150. doi: 10.1016/j.brainres.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Koss WA, Sadowski RN, Sherrill LK, Gulley JM, Juraska JM. Effects of ethanol during adolescence on the number of neurons and glia in the medial prefrontal cortex and basolateral amygdala of adult male and female rats. Brain Res. 2012 doi: 10.1016/j.brainres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Blanton CA, Rivier C. Prenatal ethanol exposure alters the responsiveness of the rat hypothalamic-pituitary-adrenal axis to nitric oxide. Alcohol Clin Exp Res. 2003;27:962–969. doi: 10.1097/01.ALC.0000076120.67014.ED. [DOI] [PubMed] [Google Scholar]
- Lee S, Craddock Z, Rivier C. Brain stem catecholamines circuitry: activation by alcohol and role in the hypothalamic-pituitary-adrenal response to this drug. J Neuroendocrinol. 2011;23:531–541. doi: 10.1111/j.1365-2826.2011.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Rivier C. An initial, three-day-long treatment with alcohol induces a long-lasting phenomenon of selective tolerance in the activity of the rat hypothalamic-pituitary-adrenal axis. J Neurosci. 1997;17:8856–8866. doi: 10.1523/JNEUROSCI.17-22-08856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Rivier C. Long-term influence of an initial exposure to alcohol on the rat hypothalamic-pituitary axis. Alcohol Clin Exp Res. 2003;27:1463–1470. doi: 10.1097/01.ALC.0000086065.06203.DD. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Cole M, Smith A, Rivier C. Prolonged exposure to intermittent alcohol vapors blunts hypothalamic responsiveness to immune and non-immune signals. Alcohol Clin Exp Res. 2000a;24:110–122. [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Rivier C. Increased activity of the hypothalamic-pituitary-adrenal axis of rats exposed to alcohol in utero: role of altered pituitary and hypothalamic function. Mol Cell Neurosci. 2000b;16:515–528. doi: 10.1006/mcne.2000.0890. [DOI] [PubMed] [Google Scholar]
- Li HY, Ericsson A, Sawchenko PE. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc Natl Acad Sci U S A. 1996;93:2359–2364. doi: 10.1073/pnas.93.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Alipour KK, Michael LA, Kirstein CL. Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacol Biochem Behav. 2010;96:476–487. doi: 10.1016/j.pbb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melon LC, Boehm SL., 2nd Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcohol Clin Exp Res. 2010;34:734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Rivier C. Effect of three different modes of alcohol administration on the activity of the rat hypothalamic-pituitary-adrenal axis. Alcohol Clin Exp Res. 1997;21:467–476. doi: 10.1111/j.1530-0277.1997.tb03792.x. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Lee S, Rivier C. Divergence in the expression of molecular markers of neuronal activation in the parvocellular paraventricular nucleus of the hypothalamus evoked by alcohol administration via different routes. J Neurosci. 1998;18:4344–4352. doi: 10.1523/JNEUROSCI.18-11-04344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Effect of alcohol on the proestrous surge of luteinizing hormone (LH) and the activation of LH-releasing hormone (LHRH) neurons in the female rat. J Neurosci. 1997;17:2595–2604. doi: 10.1523/JNEUROSCI.17-07-02595.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Cottone P, Sabino V, Rice KC, Zorrilla EP. Effects of CB1 and CRF1 receptor antagonists on binge-like eating in rats with limited access to a sweet fat diet: lack of withdrawal-like responses. Physiol Behav. 2012;107:231–242. doi: 10.1016/j.physbeh.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Differential effects of acute alcohol on EEG and sedative responses in adolescent and adult Wistar rats. Brain Res. 2008;1194:28–36. doi: 10.1016/j.brainres.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Mott NN, Paul CR, Gillespie RA, Pak TR. Binge-pattern alcohol exposure during puberty induces long-term changes in HPA axis reactivity. PLoS One. 2011;6:e18350. doi: 10.1371/journal.pone.0018350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Rao YS, Pak TR. Binge-pattern alcohol exposure during puberty induces sexually dimorphic changes in genes regulating the HPA axis. Am J Physiol Endocrinol Metab. 2010;298:E320–328. doi: 10.1152/ajpendo.00615.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA. Stress-induced and cue-induced craving for alcohol in heavy drinkers: Preliminary evidence of genetic moderation by the OPRM1 and CRH-BP genes. Alcohol Clin Exp Res. 2011;35:166–174. doi: 10.1111/j.1530-0277.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–131. [PubMed] [Google Scholar]
- Rivier C, Imaki T, Vale W. Prolonged exposure to alcohol: effect on CRF mRNA levels, and CRF- and stress-induced ACTH secretion in the rat. Brain Res. 1990;520:1–5. doi: 10.1016/0006-8993(90)91685-a. [DOI] [PubMed] [Google Scholar]
- Rivier C, Lee S. Effect of repeated exposure to alcohol on the response of the hypothalamic-pituitary adrenal axis of the rat: II. Role of the length and regimen of alcohol treatment. Alcohol Clin Exp Res. 2001;25:106–111. [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res. 1998;22:1564–1569. [PubMed] [Google Scholar]
- Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 2010;52:244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004a;79:125–132. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004b;80:387–393. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- Seo DO, Rivier C. Interaction between alcohol and nitric oxide on ACTH release in the rat. Alcohol Clin Exp Res. 2003;27:989–996. doi: 10.1097/01.ALC.0000071737.84882.C4. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Silva SM, Madeira MD. Effects of chronic alcohol consumption and withdrawal on the response of the male and female hypothalamic-pituitary-adrenal axis to acute immune stress. Brain Res. 2012;1444:27–37. doi: 10.1016/j.brainres.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Silva SM, Santos-Marques MJ, Madeira MD. Sexually dimorphic response of the hypothalamo-pituitary-adrenal axis to chronic alcohol consumption and withdrawal. Brain Res. 2009;1303:61–73. doi: 10.1016/j.brainres.2009.09.099. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Characterizing the ontogeny of ethanol-associated increases in corticosterone. Alcohol. 2004;32:145–155. doi: 10.1016/j.alcohol.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res Dev Brain Res. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc Natl Acad Sci U S A. 2010;107:11104–11109. doi: 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ontogeny of acute tolerance to ethanol-induced social inhibition in Sprague-Dawley rats. Alcohol Clin Exp Res. 2006;30:1833–1844. doi: 10.1111/j.1530-0277.2006.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- von Bardeleben U, Heuser I, Holsboer F. Human CRH stimulation response during acute withdrawal and after medium-term abstention from alcohol abuse. Psychoneuroendocrinology. 1989;14:441–449. doi: 10.1016/0306-4530(89)90043-7. [DOI] [PubMed] [Google Scholar]
- Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- Willey AR, Anderson RI, Morales M, Ramirez RL, Spear LP. Effects of ethanol administration on corticosterone levels in adolescent and adult rats. Alcohol. 2012;46:29–36. doi: 10.1016/j.alcohol.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]