Abstract
Monitoring of patients taking antihypertensive treatment can identify potential adverse drug reactions (ADRs). However, published guidelines give divergent or incomplete recommendations on monitoring for ADRs. Using a predetermined strategy, we undertook a systematic review to identify hypertension guidelines published from January 2001 to October 2011 with recommendations for monitoring for ADRs. We screened 88 abstracts and 187 web-based guidelines, and identified 19 published guidelines on monitoring the biochemical effects of antihypertensive drug therapy. We then produced a set of practical clinical guidelines, synthesized from those recommendations. Our recommendations are designed to provide efficient monitoring. They reduce the number of tests to a minimum consistent with safe practice and align monitoring schedules, so that creatinine, potassium and sodium concentrations are measured at the same times in all cases. The instructions for biochemical monitoring in current guidelines differ greatly, both in the extent of advice and in the detail provided. The current lack of consistent and workable instructions poses serious difficulties for practitioners. The recommendations distilled from this systematic review should help practitioners when they monitor therapy with antihypertensive drugs.
Introduction
Biochemical monitoring during antihypertensive drug therapy can identify changes related to potential adverse drug reactions (ADRs) before they have caused serious or permanent effects, and so avert harm. Specific guidance on monitoring for ADRs for healthcare professionals is available from several sources, such as publications from government organizations, professional societies, and independent researchers or research groups.
Ideally, monitoring instructions should provide details on several factors: the purpose of monitoring, the appropriate frequency of monitoring and how to act on the results of a monitoring test.1 However, published guidelines can provide divergent or incomplete recommendations on monitoring for ADRs. We have previously shown that monitoring, as recommended by published guidelines, is not commonly undertaken in primary care.2 We hypothesized that that might be in part because the available guidelines are incomplete and inconsistent.3 We therefore set out to determine, by systematic review, the nature and extent of recommendations in published guidelines on biochemical monitoring for adverse reactions to drugs used in the treatment of hypertension, and to synthesize practicable recommendations based on the findings.
Methods
We searched for published guidelines that described methods of monitoring for adverse reactions in patients being treated for hypertension. We defined monitoring as a description of measurement of serum creatinine, potassium or sodium concentrations during treatment with angiotensin-converting enzyme (ACE) inhibitors, angiotensin-II receptor antagonists or diuretics.
We searched Medline using the OVID interface from January 2001 to October 2011 using the MeSH term ‘Hypertension/drug therapy’, limiting the results to the publication type ‘practice guideline’. We also searched the National Guideline Clearinghouse (NGC) database (www.guideline.gov) and the TRIP database (www.tripdatabase.com) using the search term ‘hypertension’.
Guidelines relating specifically to the treatment of hypertension in pregnant women, children or teenagers were excluded, as were guidelines relating specifically to the use of antihypertensive drugs in the treatment of heart failure and chronic kidney disease, for which monitoring is often more relevant to the condition rather than the treatment. We did not identify any guideline dealing specifically with hypertension in liver disease. The full search strategy is given in Supplementary Appendix A; please see http://jrs.rsmjournals.com/lookup/suppl/doi:10.1258/jrsm.2012.120137/-/DC1.
Data synthesis
We examined all the guidelines selected for inclusion, and determined the recommendations that were made and where there was disagreement between them, also noting the absence of recommendations. We also determined what evidence had been cited in support of the recommendations. We then synthesized a set of guidelines based on the recommendations for which there was general or majority agreement and resolved inconsistencies by discussion. We predicated our discussions on the principle that monitoring should be of the lowest intensity consistent with basic principles of safe practice.
Results
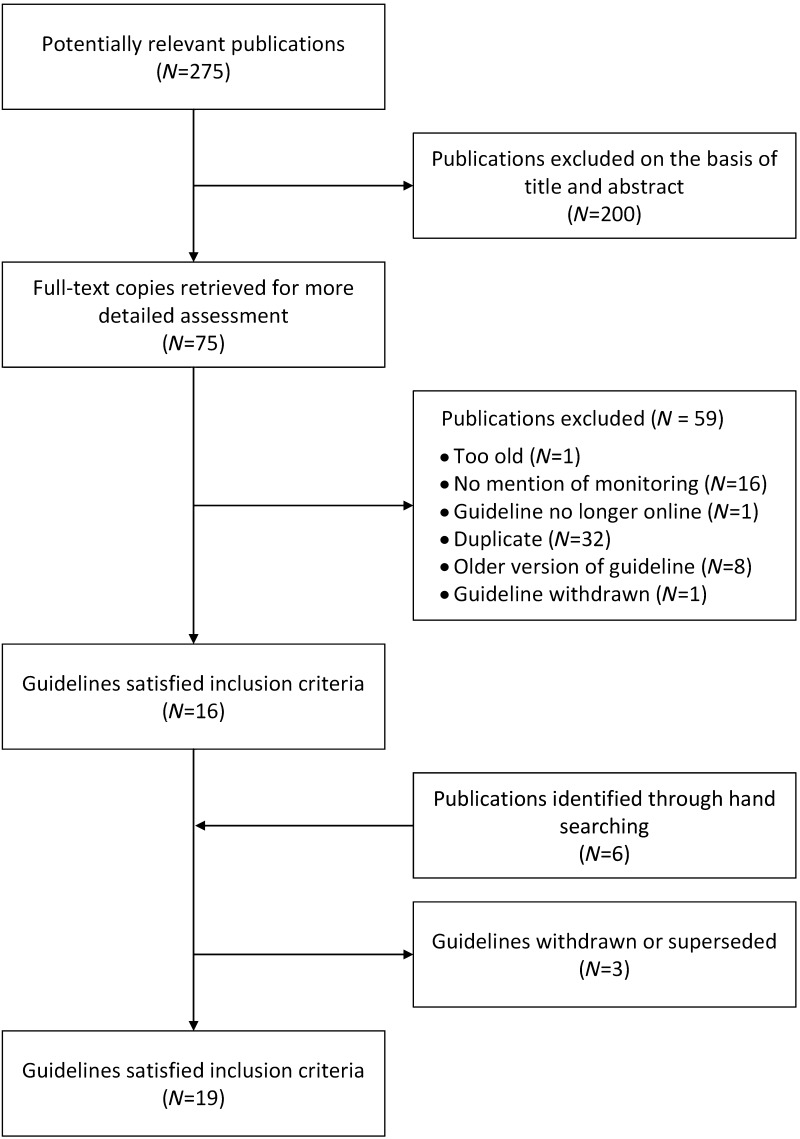
We screened 88 abstracts from Medline, 105 guidelines from the TRIP database and 82 guidelines from the NGC database (Figure 1). We identified 19 sets of published guidelines in which details on biochemical monitoring were provided (Supplementary Appendices B and C; http://jrs.rsmjournals.com/lookup/suppl/doi:10.1258/jrsm.2012.120137/-/DC1).4–22 Three published guidelines identified by the search have subsequently been withdrawn or superseded and were therefore excluded (Supplementary Appendix D; http://jrs.rsmjournals.com/lookup/suppl/doi:10.1258/jrsm.2012.120137/-/DC1).23–25 Tables 1–3 summarize the published guidelines, giving information on monitoring serum creatinine concentration in patients taking ACE inhibitors or angiotensin-II receptor antagonists, and electrolytes in patients taking ACE inhibitors, angiotensin-II receptor antagonists or diuretics.
Table 2.
Published recommendations for monitoring serum creatinine and potassium concentrations in patients taking ACE inhibitors or angiotensin-II receptor antagonists
| Timing | Guideline recommendations (creatinine) | N* | Guideline recommendations (potassium) | N† |
|---|---|---|---|---|
| Before starting therapy | Measure serum creatinine concentration | 13 | Measure serum potassium concentration before starting therapy | 12 |
| Do not start these agents if serum potassium concentration is over 5.0 mmol/L | 1 | |||
| During the first eight weeks of therapy | Check at four and 10 days for high-risk patients | 3 | Check within one week of starting therapy | 3 |
| Check within one week of starting therapy | 2 | Check within 1–2 weeks of starting therapy | 3 | |
| Check within 1–2 weeks of starting therapy | 3 | Check at 4 and 10 days for high risk patients | 3 | |
| After the first 8 weeks of therapy | Check every 3–6 months | 1 | Check every 3–6 months | 1 |
| Check every 6–12 months | 2 | Check every 6–12 months | 2 | |
| Check every 12 months | 7 | Check every 12 months | 5 | |
| More frequent monitoring of serum creatinine concentration than 3–6 months is recommended if the patient's clinical condition worsens or additional treatment is started | 1 | |||
| After a dosage change | Check | 3 | Check | 3 |
| If a change in the measurement occurs | If serum creatinine concentration increases by more than 30% from baseline, consider possible contributory factors (e.g. hypovolaemia, renal artery stenosis, NSAIDs); if none present, consider stopping treatment | 3 | If serum potassium concentration rises >6 mmol/L, all drugs that may increase potassium (including ACE inhibitors and ARBs) and concomitant nephrotoxic drugs should be stopped and specialist advice sought | 2 |
| Serum potassium concentration rises to 5.5–5.9 mmol/L should prompt more frequent monitoring | 3 | |||
| When other drugs are added | Take extra care in patients taking combinations of drugs or patients with susceptibility factors | 7 | Take extra care in patients taking combinations of drugs or patients with susceptibility factors | 8 |
NSAID, non-steroidal anti-inflammatory drugs; ACE, angiotensin-converting enzyme; ARB, angiotensin-II receptor antagonist
*Number of guidelines that include the recommendation (total 18)
†Number of guidelines that include the recommendation (total 17)
Figure 1.
Flow chart of selection of guidelines into the systematic review
Table 1.
Summary of guidelines making monitoring recommendations
| Drugs | Monitoring requirement | Number (percent) of guidelines making monitoring recommendations | ||||
|---|---|---|---|---|---|---|
| Before starting therapy | Within a time period after starting therapy | At each dose increment | Frequency of monitoring for continued therapy | Total number of guidelines identified | ||
| ACE inhibitors or angiotensin-II receptor antagonists | Creatinine | 13 (72%) | 5 (28%) | 3 (17%) | 3 (17%) | 18 |
| Potassium | 12 (71%) | 6 (35%) | 3 (18%) | 8 (47%) | 17 | |
| Diuretics | Electrolytes | 10 (59%) | 4 (24%) | 0 (0%) | 8 (47%) | 17 |
ACE, angiotensin-converting enzyme
Table 3.
Published guideline recommendations for monitoring serum electrolyte concentrations in patients taking diuretics
| Timing | Guideline recommendations | N* |
|---|---|---|
| Before starting therapy | Measure electrolytes before starting therapy | 10 |
| Do not start treatment if serum potassium concentration is over 5.0 mmol/L | 1 | |
| During the first eight weeks | Check after 5–7 days in high risk patients | 1 |
| Check within one week of starting therapy | 1 | |
| Check within 4–6 weeks of starting therapy | 2 | |
| Check within 6–8 weeks of starting therapy | 1 | |
| After the first eight weeks | Check every 6–12 months | 3 |
| Check every 12 months | 5 | |
| If a change in the measurement occurs | If serum potassium concentration rises >6 mmol/L, all drugs that may increase potassium and concomitant nephrotoxic drugs should be stopped and specialist advice sought | 1 |
| Serum potassium concentration rises to 5.5–5.9 mmol/L should prompt more frequent monitoring | 1 | |
| Review diuretic therapy if serum potassium concentration falls below 3 mmol/L (or 4 mmol/L in high-risk patients) | 1 | |
| Obtain nephrology advice if the serum sodium concentration falls to <132 mmol/L | 1 | |
| After a dosage change | (No guideline advice) | 0 |
| When other drugs are added | Take extra care in patients taking combinations of drugs | 2 |
*Number of guidelines that include the recommendation (total 17)
Monitoring recommendations from the guidelines can be divided into those that are required pretreatment, following treatment initiation and during continued therapy (see Table 1). For all three drug classes, the guidelines were more likely to recommend baseline monitoring of the selected parameters before treatment, than they were for early and continued therapy.
There was a difference in the recommendations made for continued ACE inhibitor or angiotensin- II receptor antagonist therapy, with more emphasis placed on the need to monitor the potassium concentration (n = 8/17) than the creatinine concentration (n = 3/18).
Guidelines differed in their advice on actions to take when electrolyte concentrations changed.
For ACE inhibitors and angiotensin-II receptor antagonists, five guidelines recommended a course of action should the potassium concentration exceed a defined value and three guidelines for creatinine. For diuretic therapy, three guidelines advised on the course of action for a raised potassium concentration and one for a low sodium concentration.
Finally, only three guidelines advised monitoring following a change in dose of ACE inhibitors or angiotensin-II receptor antagonists, and none for diuretic therapy.
Thirteen guidelines made no reference to any primary research supporting the monitoring guidance. Five referenced previously published guidelines, reviews or guidelines reporting the epidemiology of the ADRs during antihypertensive drug treatment. One guideline made no explicit reference to primary evidence, but instead graded the evidence as Grade D (i.e. based on expert opinion alone).
Table 4 shows the practical recommendations that we have distilled from a synthesis of the findings of this review. For each drug class, we have suggested baseline monitoring before treatment, followed by monitoring during early treatment, at incremental dose changes, and during continued therapy. These recommendations are designed to provide efficient monitoring, by reducing the number of tests to a minimum and aligning monitoring schedules, allowing creatinine, potassium and sodium concentrations to be measured at the same times in all cases. In addition, we have provided advice to assist the practitioner should a change in measurement occur.
Table 4.
Summary recommendations for monitoring based on a synthesis of the recommendations in published guidelines
| Timing | In patients taking ACE inhibitors or angiotensin receptor In patients taking diuretics antagonists | ||
|---|---|---|---|
| Serum creatinine | Serum potassium | Serum electrolytes | |
| Before starting therapy | Measure serum creatinine concentration | Measure serum potassium concentration | Measure electrolytes before starting therapy |
| If creatinine is raised, avoid or treat as high risk* | Do not start therapy if serum potassium concentration is over 5.0 mmol/L; hyperkalaemia and hypokalaemia both indicate the need to investigate secondary causes of hypertension | Do not start therapy if serum potassium concentration is over 5.0 mmol/L; hyperkalaemia and hypokalaemia both indicate the need to investigate secondary causes of hypertension | |
| During the first two weeks of therapy | Check at four and 10 days in high-risk patients (as defined*) | Check at four and 10 days for high-risk patients (as defined*) | Check at four and 10 days for high-risk patients (as defined*) |
| Otherwise check within two weeks | Otherwise, check within two weeks | Otherwise, check within two weeks | |
| After the first two weeks of therapy | Check if the patient's condition changes (e.g. during an intercurrent illness or if another treatment is added) | Check if the patient's condition changes (e.g. during an intercurrent illness or if another treatment is added) | Check if the patient's condition changes (e.g. during an intercurrent illness or if another treatment is added) |
| Otherwise check every 12 months | Otherwise check every 12 months | Otherwise check every 12 months | |
| After a dosage change | Check within two weeks, as above | Check within two weeks, as above | No guideline advice; prudent to check within two weeks, as above |
| If a change in the measurement occurs | If eGFR falls to 70% or less, review clinical status or treatment | If serum potassium concentration rises to >6 mmol/L, withhold nephrotoxic drugs and any drug that may increase potassium and seek specialist advice | If serum potassium concentration rises to >6 mmol/L, withhold nephrotoxic drugs and any drug that may increase potassium and seek specialist advice |
| If serum potassium concentration rises to 5.5–5.9 mmol/L, re-measure and re-assess; then, if serum potassium concentration is still >5.5 mmol/L, review clinical status and therapy | If serum potassium concentration rises to 5.5–5.9 mmol/L, re-measure and re-assess | ||
| If serum potassium concentration is still >5.5 mmol/L, review clinical status and therapy | |||
| Review diuretic therapy if serum potassium concentration falls below 3 mmol/L (or 4 mmol/L in high-risk* patients) | |||
| Obtain nephrology advice if the serum sodium concentration falls to <132 mmol/L | |||
| When other drugs are added | Review† | Review† | Review‡ |
eGFR, estimated glomerular filtration rate; ACE, angiotensin-converting enzyme
*Patients at high risk are those with renal disease, heart failure, diabetes mellitus, peripheral vascular disease, renal transplant or old age; those taking spironolactone or combination therapy
†Important combinations include any combinations of ACE inhibitors, angiotensin receptor antagonists and potassium-sparing diuretics
‡Important combinations include medications with the potential to alter electrolyte balance (e.g. selective serotonin re-uptake inhibitors, NSAIDs)
Discussion
We have identified a corpus of published guidelines on the treatment of patients with ACE inhibitors, angiotensin-II receptor antagonists and diuretics for hypertension, which differ in their advice on monitoring for ADRs, both in the extent of advice and in the details. Many give only rudimentary guidance or none at all. Where present, details differed substantially between guidelines. Some described monitoring as a ‘routine investigation’ or suggested that laboratory tests should be ‘measured every 6–12 months’. Such poorly specified recommendations are challenging for clinicians to apply in clinical practice.
A few guidelines gave far more detailed recommendations, specifying biochemical testing before the start of therapy, the frequency and nature of follow-up monitoring, and actions to be taken should the laboratory results be outside a stated range.9 Where details were given, they were inconsistent between guidelines. For example, of the 12 guidelines suggesting monitoring of serum potassium concentration during maintenance therapy with ACE inhibitors or angiotensin-II receptor antagonists, three described them as routine, one recommended monitoring every 3–6 months, two every 6–12 months and five annually. This lack of consensus is bound to result in confusion.
Finally, we found that guidelines rarely referenced primary evidence supporting the monitoring recommendations, as has been shown previously.26 Sica27 described guidelines for monitoring serum potassium concentrations in patients treated with antihypertensive drugs as ‘at best makeshift and often drawn from the know-how of the treating physician’.
The aim of this systematic review was to determine the nature and extent of recommendations in published guidelines on biochemical monitoring for adverse reactions to drugs used in the treatment of hypertension, and to synthesize practicable recommendations based on the findings. Our monitoring recommendations are designed to minimize the risk of patient harm, optimize therapy and provide physicians with actionable changes should adverse effects occur. We recommend baseline monitoring of parameters for each drug class, so that any fluctuations in concentrations can be more easily attributable to the treatment. Baseline testing also ensures that the patient starts treatment at an appropriate dose, and that the drug is not already contraindicated. We then recommend repeated monitoring within two weeks of starting treatment, so that any adverse reactions can be identified early, and any incremental dose increases can be made safely. In each case, if measurements have changed from baseline tests, actionable changes in the monitoring or dosing of treatment ensure an alternative management plan for the patient.
Clinicians often face the difficulty of divergent or incomplete recommendations in clinical guidelines. Here we have shown how systematic review and synthesis can yield more complete guidance in one specific case. This process may be more generally applicable.
Limitations of this review
We selected guidelines published in English or French, but we nevertheless believe that the results are widely applicable. Although formal recommendations on how to assess guidelines have been published,28 we did not evaluate the quality of the guidelines, because most of them were not written with the specific goal of describing monitoring in patients treated with antihypertensive drugs. Instead, most were written on the treatment of hypertension, and monitoring for ADRs was incidentally included. We have based our recommendations on the published guidelines, but these are not themselves based on clear evidence.
Conclusions
There is a need for detailed information on what, when and how to monitor in the management of hypertension and the adverse effects of antihypertensive drugs; specifically, we need more evidence on the ideal frequencies of monitoring, the circumstances in which extra monitoring is indicated and guidance on the thresholds at which actions are required. However, in the absence of any evidence of the outcomes of different monitoring schemes, there is no underlying knowledge of the relevant detail, which relies on consensus and is of unproven value. Only good outcome data can resolve this. Meanwhile, without better evidence, the recommendations distilled from this systematic review should help practitioners when they monitor drug therapy in hypertension.
DECLARATIONS
Competing interests
SMcD, SKT and JJC have no non-financial interests that may be relevant to the submitted work. REF and JJC are members of Medicines and Healthcare products Regulatory Agency (MHRA) advisory committees. JKA and REF are members of committees of the National Institute for Health and Clinical Excellence (NICE). JKA is President Emeritus of the British Pharmacological Society (BPS). The views expressed here do not represent the views of the MHRA or its committees or those of NICE, the BPS, the NHS, the NIHR or the UK Department of Health
Funding
SMcD, SKT and JJC are funded by the National Institute for Health Research (NIHR) through the Collaborations for Leadership in Applied Health Research and Care for Birmingham and Black Country (CLAHRC-BBC) programme. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The Antidote Trust Fund of the Sandwell and West Birmingham Hospitals NHS Trust also supported this work
Ethical approval
Not applicable
Guarantor
REF
Contributorship
SMcD contributed substantially to the conception and design of the paper, extracted and analysed the data, drafted and revised the paper. SKT extracted and analysed the data, and revised the paper. JJC, JKA and REF contributed substantially to the conception and design of the paper and revised the paper. All authors gave final approval of the version to be published
Acknowledgments
None
References
- 1.Ferner RE, Coleman J, Pirmohamed M, Constable SA, Rouse A The quality of information on monitoring for haematological adverse drug reactions . Br J Clin Pharmacol 2005;60:448–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDowell SE, Ferner RE Biochemical monitoring of patients treated with antihypertensive therapy for adverse drug reactions: a systematic review. Drug Saf 2011;34:1049–59 [DOI] [PubMed] [Google Scholar]
- 3.Coleman JJ, McDowell SE, Evans SJ, Gill PS, Ferner RE Oversight: a retrospective study of biochemical monitoring in patients beginning antihypertensive drug treatment in primary care. Br J Clin Pharmacol 2010;70:109–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knight EL, Avorn J Quality indicators for appropriate medication use in vulnerable elders. Ann Intern Med 2001;135:703–10 [DOI] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–52 [DOI] [PubMed] [Google Scholar]
- 6.Palmer BF Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 2004;351:585–92 [DOI] [PubMed] [Google Scholar]
- 7.Seedat YK, Croasdale MA, Milne FJ, et al. South African hypertension guideline 2006. S Afr Med J 2006;96:337–62 [PubMed] [Google Scholar]
- 8.Mancia G, De Backer G, Dominiczak A, et al. Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007;28:1462–536 [DOI] [PubMed] [Google Scholar]
- 9.Smellie WS, Forth J, Coleman JJ, et al. Best practice in primary care pathology: review 6. J Clin Pathol 2007;60:225–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidelines and Audit Implementation Network, Clinical Resource Efficiency Support Team Guidelines on the Management of Hypertension – FAQs 2007. see http://www.gain-ni.org/Publications/Guidelines/crest_hypertension_guidelines-faq_s-july_2007.pdf (last checked 24 April 2012)
- 11.Rotaeche del Campo R, Aguirrezabala JJ, Balagué GL, et al. Clinical Practice Guidelines on Arterial Hypertension – 2007 Update 2008. See http://www9.euskadi.net/sanidad/osteba/datos/gpc_07_04_ingles.pdf (last checked 24 April 2012)
- 12.British Columbia Ministry of Health Services Hypertension – Detection, Diagnosis and Management 2008. See http://www.bcguidelines.ca/pdf/hypertension.pdf (last checked 24 April 2012)
- 13.London and South East Regional Medicines Information Service, South West Medicines Information, Croydon Primary Care Trust Suggestions for Drug Monitoring in Adults in Primary Care 2008. See http://www.nelm.nhs.uk/en/NeLM-Area/Evidence/Drug-Monitoring/Suggestions-for-Drug-Monitoring-in-Adults-in-Primary-Care/ (last checked 24 April 2012)
- 14.University of Michigan Health System Essential Hypertension 2009. See http://cme.med.umich.edu/pdf/guideline/htn.pdf (last checked 24 April 2012)
- 15.Royal Infirmary of Edinburgh Renal Unit How to Start an ACE Inhibitor 2009. See http://renux.dmed.ed.ac.uk/edren/Unitbits/ACEIstart.html (last checked 24 April 2012)
- 16.Ogihara T, Kikuchi K, Matsuoka H, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertens Res 2009;32:3–107 [PubMed] [Google Scholar]
- 17.Australian Heart Foundation Guide to Management of Hypertension 2008 – Assessing and Managing Raised Blood Pressure in Adults Updated December 2010. See http://www.heartfoundation.org.au/SiteCollectionDocuments/HypertensionGuidelines2008to2010Update.pdf (last checked 24 April 2012)
- 18.Institute for Clinical Systems Improvement Hypertension Diagnosis and Treatment 2010. See http://www.icsi.org/hypertension_4/hypertension_diagnosis_and_treatment_4.html (last checked 24 April 2012)
- 19.Chiang CE, Wang TD, Li YH, et al. 2010 guidelines of the Taiwan Society of Cardiology for the management of hypertension. J Formos Med Assoc 2010;109:740–73 [DOI] [PubMed] [Google Scholar]
- 20.Michigan Quality Improvement Consortium Medical Management of Adults with Hypertension 2011. See http://www.uphpplus.com/docs%5CHypertensionMQIC.pdf (last checked 24 April 2012)
- 21.Rabi DM, Daskalopoulou SS, Padwal RS, et al. The 2011 Canadian Hypertension Education Program recommendations for the management of hypertension: blood pressure measurement, diagnosis, assessment of risk, and therapy. Can J Cardiol 2011;27:415–33 [DOI] [PubMed] [Google Scholar]
- 22.National Institute for Health and Clinical Excellence NICE Clinical Guideline 127. Hypertension: Management of Hypertension in Adults in Primary Care 2011. See http://www.nice.org.uk/nicemedia/live/13561/56007/56007.pdf (last checked 24 April 2012)
- 23.Scottish Intercollegiate Guidelines Network SIGN 49: Hypertension in Older People 2001. See http://www.sign.ac.uk/guidelines/fulltext/49/index.html (last checked 24 April 2012)
- 24.Williams B, Poulter NR, Brown MJ, et al. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ 2004;328:634–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.French Haute Autorité de Santé [Management of Adults with Eessential Hypertension – 2005 Update] 2005. See http://www.has-sante.fr/portail/upload/docs/application/pdf/2011-09/hta_2005_recommandations.pdf (last checked 24 April 2012)
- 26.Coleman JJ, McDowell SE, Ferner RE Where does the evidence for guidelines come from? An example of monitoring for the adverse renal effects of ACE inhibitors. Drug Saf 2008;3:905–6 [Google Scholar]
- 27.Sica DA Antihypertensive therapy and its effects on potassium homeostasis. J Clin Hypertens (Greenwich) 2006;8:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AGREE Advancing the Science of Practice Guidelines 2010. http://www.agreetrust.org/ (last checked 24 April 2012)