Abstract
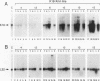
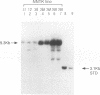
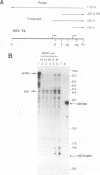
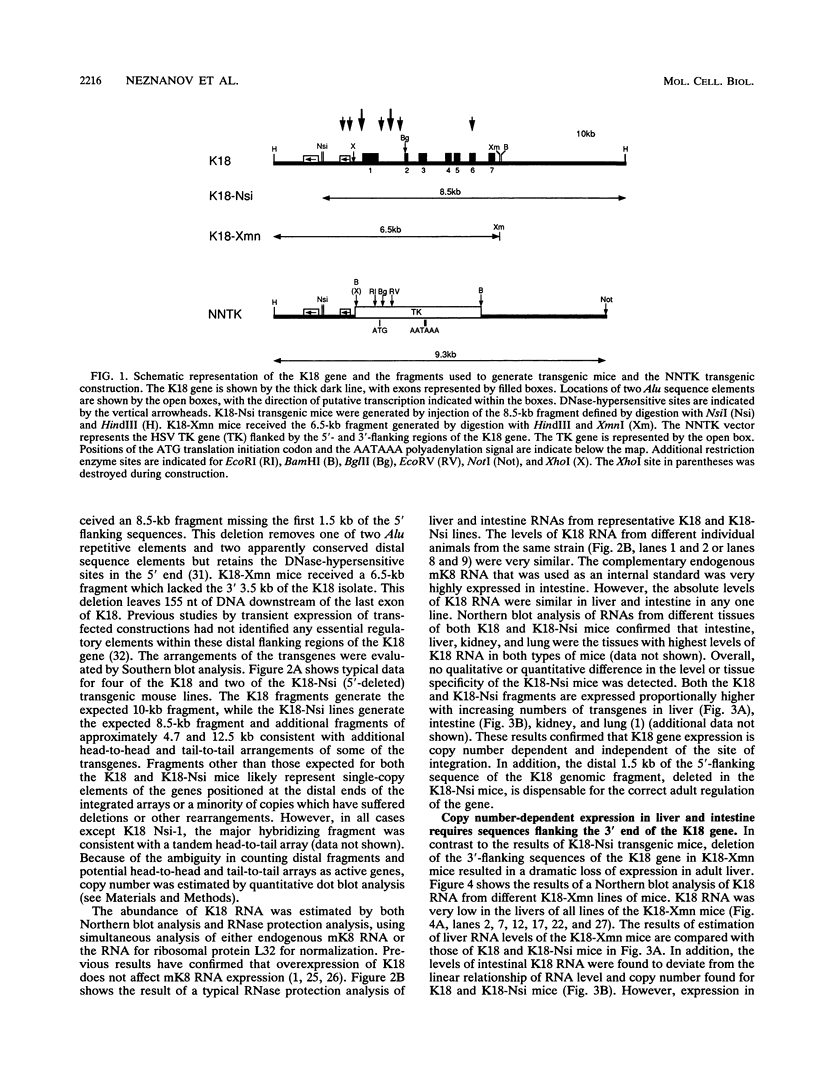
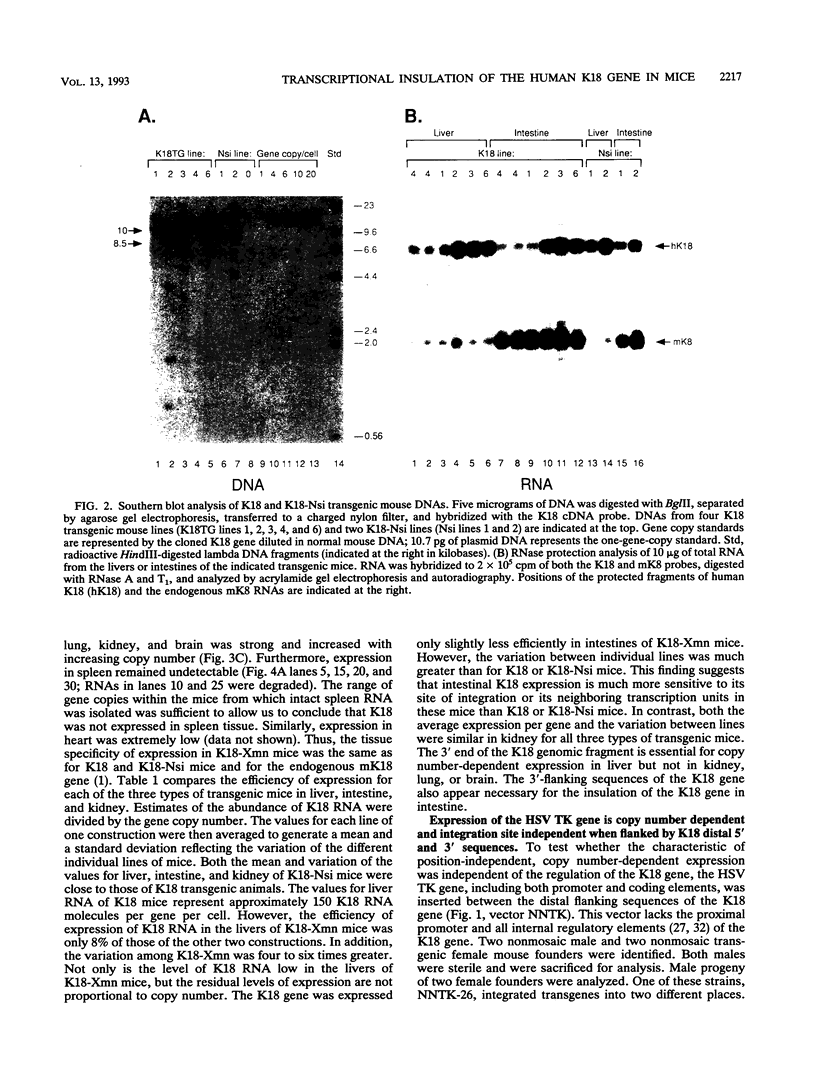
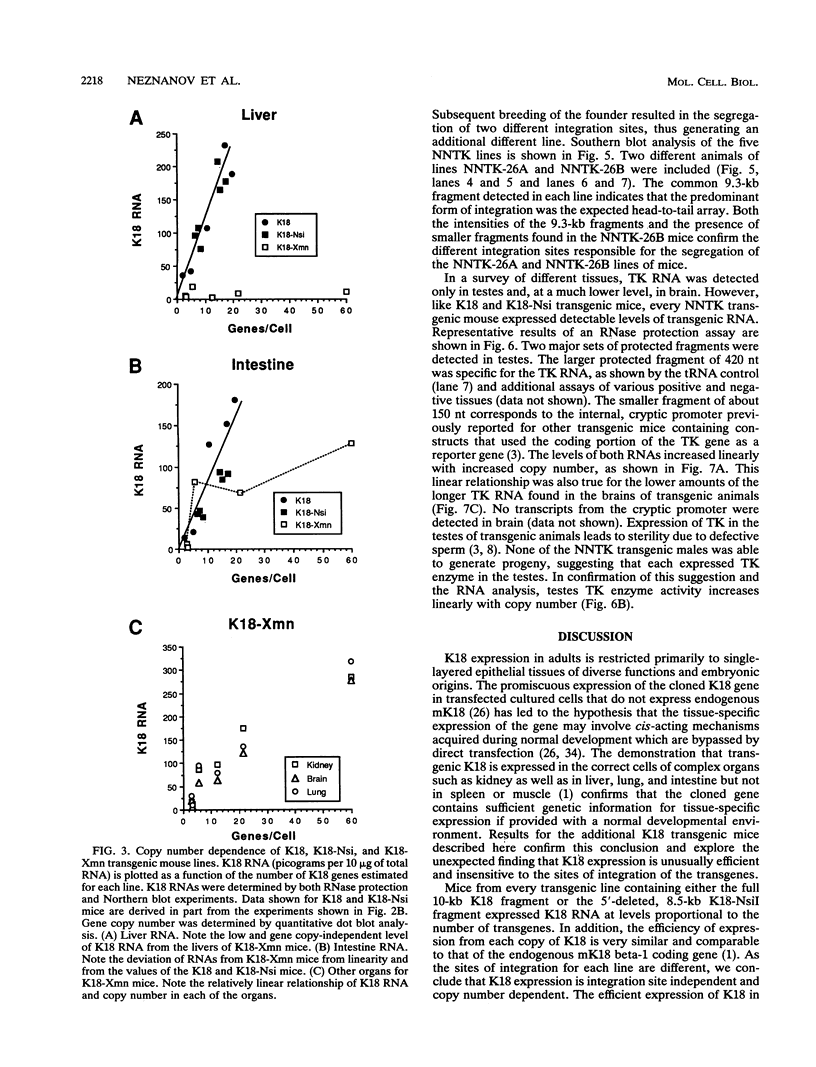
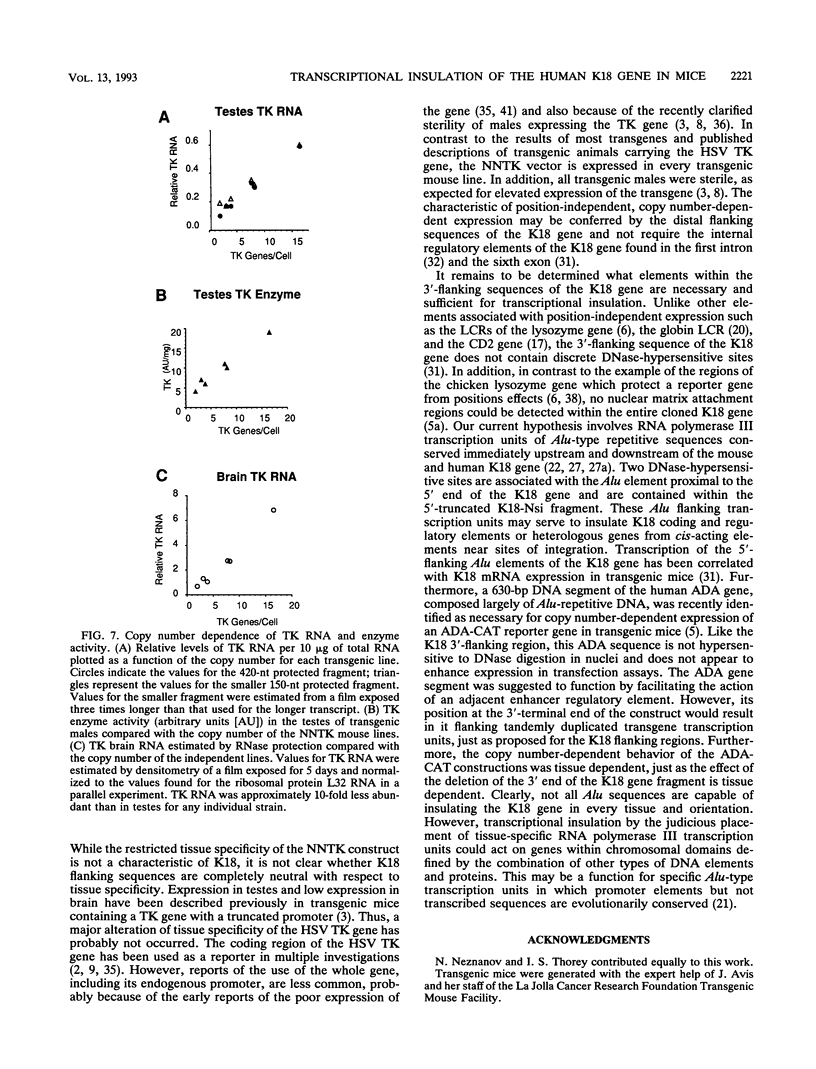
Expression of the 10-kb human keratin 18 (K18) gene in transgenic mice results in efficient and appropriate tissue-specific expression in a variety of internal epithelial organs, including liver, lung, intestine, kidney, and the ependymal epithelium of brain, but not in spleen, heart, or skeletal muscle. Expression at the RNA level is directly proportional to the number of integrated K18 transgenes. These results indicate that the K18 gene is able to insulate itself both from the commonly observed cis-acting effects of the sites of integration and from the potential complications of duplicated copies of the gene arranged in head-to-tail fashion. To begin to identify the K18 gene sequences responsible for this property of transcriptional insulation, additional transgenic mouse lines containing deletions of either the 5' or 3' distal end of the K18 gene have been characterized. Deletion of 1.5 kb of the distal 5' flanking sequence has no effect upon either the tissue specificity or the copy number-dependent behavior of the transgene. In contrast, deletion of the 3.5-kb 3' flanking sequence of the gene results in the loss of the copy number-dependent behavior of the gene in liver and intestine. However, expression in kidney, lung, and brain remains efficient and copy number dependent in these transgenic mice. Furthermore, herpes simplex virus thymidine kinase gene expression is copy number dependent in transgenic mice when the gene is located between the distal 5'- and 3'-flanking sequences of the K18 gene. Each adult transgenic male expressed the thymidine kinase gene in testes and brain and proportionally to the number of integrated transgenes. We conclude that the characteristic of copy number-dependent expression of the K18 gene is tissue specific because the sequence requirements for transcriptional insulation in adult liver and intestine are different from those for lung and kidney. In addition, the behavior of the transgenic thymidine kinase gene in testes and brain suggests that the property of transcriptional insulation of the K18 gene may be conferred by the distal flanking sequences of the K18 gene and, additionally, may function for other genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Oshima R. G. A single human keratin 18 gene is expressed in diverse epithelial cells of transgenic mice. J Cell Biol. 1990 Sep;111(3):1197–1206. doi: 10.1083/jcb.111.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shawi R., Burke J., Jones C. T., Simons J. P., Bishop J. O. A Mup promoter-thymidine kinase reporter gene shows relaxed tissue-specific expression and confers male sterility upon transgenic mice. Mol Cell Biol. 1988 Nov;8(11):4821–4828. doi: 10.1128/mcb.8.11.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronow B. J., Silbiger R. N., Dusing M. R., Stock J. L., Yager K. L., Potter S. S., Hutton J. J., Wiginton D. A. Functional analysis of the human adenosine deaminase gene thymic regulatory region and its ability to generate position-independent transgene expression. Mol Cell Biol. 1992 Sep;12(9):4170–4185. doi: 10.1128/mcb.12.9.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifer C., Vidal M., Grosveld F., Sippel A. E. Tissue specific and position independent expression of the complete gene domain for chicken lysozyme in transgenic mice. EMBO J. 1990 Sep;9(9):2843–2848. doi: 10.1002/j.1460-2075.1990.tb07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braun R. E., Lo D., Pinkert C. A., Widera G., Flavell R. A., Palmiter R. D., Brinster R. L. Infertility in male transgenic mice: disruption of sperm development by HSV-tk expression in postmeiotic germ cells. Biol Reprod. 1990 Oct;43(4):684–693. doi: 10.1095/biolreprod43.4.684. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M., Senear A. W., Warren R., Palmiter R. D. Somatic expression of herpes thymidine kinase in mice following injection of a fusion gene into eggs. Cell. 1981 Nov;27(1 Pt 2):223–231. doi: 10.1016/0092-8674(81)90376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo P. D., Martin G. R., Yamada Y. Alpha 1(IV) and alpha 2(IV) collagen genes are regulated by a bidirectional promoter and a shared enhancer. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9679–9682. doi: 10.1073/pnas.85.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada A., Maki R. A. The expression of I-A correlates with the uptake of interferon-gamma by macrophages. Eur J Immunol. 1989 Jan;19(1):205–208. doi: 10.1002/eji.1830190134. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. W., Vasavada H. A., Ganguly S., Weissman S. M. Identification of cis sequences controlling efficient position-independent tissue-specific expression of human major histocompatibility complex class I genes in transgenic mice. Mol Cell Biol. 1991 Jul;11(7):3564–3572. doi: 10.1128/mcb.11.7.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Dudov K. P., Perry R. P. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell. 1984 Jun;37(2):457–468. doi: 10.1016/0092-8674(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Forrester W. C., Novak U., Gelinas R., Groudine M. Molecular analysis of the human beta-globin locus activation region. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5439–5443. doi: 10.1073/pnas.86.14.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves D. R., Wilson F. D., Lang G., Kioussis D. Human CD2 3'-flanking sequences confer high-level, T cell-specific, position-independent gene expression in transgenic mice. Cell. 1989 Mar 24;56(6):979–986. doi: 10.1016/0092-8674(89)90631-4. [DOI] [PubMed] [Google Scholar]
- Greer P., Maltby V., Rossant J., Bernstein A., Pawson T. Myeloid expression of the human c-fps/fes proto-oncogene in transgenic mice. Mol Cell Biol. 1990 Jun;10(6):2521–2527. doi: 10.1128/mcb.10.6.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Howard B. H., Sakamoto K. Alu interspersed repeats: selfish DNA or a functional gene family? New Biol. 1990 Sep;2(9):759–770. [PubMed] [Google Scholar]
- Ichinose Y., Morita T., Zhang F. Y., Srimahasongcram S., Tondella M. L., Matsumoto M., Nozaki M., Matsushiro A. Nucleotide sequence and structure of the mouse cytokeratin endoB gene. Gene. 1988 Oct 15;70(1):85–95. doi: 10.1016/0378-1119(88)90107-2. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Subak-Sharpe J. H. Biochemical studies on the herpes simplex virus-specified deoxypyrimidine kinase activity. J Gen Virol. 1974 Sep;24(3):481–492. doi: 10.1099/0022-1317-24-3-481. [DOI] [PubMed] [Google Scholar]
- Kawamoto T., Makino K., Niwa H., Sugiyama H., Kimura S., Amemura M., Nakata A., Kakunaga T. Identification of the human beta-actin enhancer and its binding factor. Mol Cell Biol. 1988 Jan;8(1):267–272. doi: 10.1128/mcb.8.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum R., Schedl P. A position-effect assay for boundaries of higher order chromosomal domains. Cell. 1991 Mar 8;64(5):941–950. doi: 10.1016/0092-8674(91)90318-s. [DOI] [PubMed] [Google Scholar]
- Kulesh D. A., Ceceña G., Darmon Y. M., Vasseur M., Oshima R. G. Posttranslational regulation of keratins: degradation of mouse and human keratins 18 and 8. Mol Cell Biol. 1989 Apr;9(4):1553–1565. doi: 10.1128/mcb.9.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesh D. A., Oshima R. G. Complete structure of the gene for human keratin 18. Genomics. 1989 Apr;4(3):339–347. doi: 10.1016/0888-7543(89)90340-6. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lai E., Darnell J. E., Jr Transcriptional control in hepatocytes: a window on development. Trends Biochem Sci. 1991 Nov;16(11):427–430. doi: 10.1016/0968-0004(91)90169-v. [DOI] [PubMed] [Google Scholar]
- Mayo K. E., Warren R., Palmiter R. D. The mouse metallothionein-I gene is transcriptionally regulated by cadmium following transfection into human or mouse cells. Cell. 1982 May;29(1):99–108. doi: 10.1016/0092-8674(82)90094-0. [DOI] [PubMed] [Google Scholar]
- Neznanov N. S., Oshima R. G. cis regulation of the keratin 18 gene in transgenic mice. Mol Cell Biol. 1993 Mar;13(3):1815–1823. doi: 10.1128/mcb.13.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima R. G., Abrams L., Kulesh D. Activation of an intron enhancer within the keratin 18 gene by expression of c-fos and c-jun in undifferentiated F9 embryonal carcinoma cells. Genes Dev. 1990 May;4(5):835–848. doi: 10.1101/gad.4.5.835. [DOI] [PubMed] [Google Scholar]
- Oshima R. G., Millán J. L., Ceceña G. Comparison of mouse and human keratin 18: a component of intermediate filaments expressed prior to implantation. Differentiation. 1986;33(1):61–68. doi: 10.1111/j.1432-0436.1986.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Oshima R. G., Trevor K., Shevinsky L. H., Ryder O. A., Ceceña G. Identification of the gene coding for the Endo B murine cytokeratin and its methylated, stable inactive state in mouse nonepithelial cells. Genes Dev. 1988 May;2(5):505–516. doi: 10.1101/gad.2.5.505. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Wilkie T. M., Chen H. Y., Brinster R. L. Transmission distortion and mosaicism in an unusual transgenic mouse pedigree. Cell. 1984 Apr;36(4):869–877. doi: 10.1016/0092-8674(84)90036-9. [DOI] [PubMed] [Google Scholar]
- Ryan T. M., Behringer R. R., Martin N. C., Townes T. M., Palmiter R. D., Brinster R. L. A single erythroid-specific DNase I super-hypersensitive site activates high levels of human beta-globin gene expression in transgenic mice. Genes Dev. 1989 Mar;3(3):314–323. doi: 10.1101/gad.3.3.314. [DOI] [PubMed] [Google Scholar]
- Stief A., Winter D. M., Strätling W. H., Sippel A. E. A nuclear DNA attachment element mediates elevated and position-independent gene activity. Nature. 1989 Sep 28;341(6240):343–345. doi: 10.1038/341343a0. [DOI] [PubMed] [Google Scholar]
- Tamai Y., Takemoto Y., Matsumoto M., Morita T., Matsushiro A., Nozaki M. Sequence of EndoA gene encoding mouse cytokeratin and its methylation state in the CpG-rich region. Gene. 1991 Aug 15;104(2):169–176. doi: 10.1016/0378-1119(91)90247-9. [DOI] [PubMed] [Google Scholar]
- Vasseur M., Duprey P., Brûlet P., Jacob F. One gene and one pseudogene for the cytokeratin endo A. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1155–1159. doi: 10.1073/pnas.82.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F., Stewart T. A., Mintz B. The human beta-globin gene and a functional viral thymidine kinase gene in developing mice. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5016–5020. doi: 10.1073/pnas.78.8.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Costa R. H., Darnell J. E., Jr, Chen J. D., Van Dyke T. A. Distinct positive and negative elements control the limited hepatocyte and choroid plexus expression of transthyretin in transgenic mice. EMBO J. 1990 Mar;9(3):869–878. doi: 10.1002/j.1460-2075.1990.tb08184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Shawi R., Burke J., Wallace H., Jones C., Harrison S., Buxton D., Maley S., Chandley A., Bishop J. O. The herpes simplex virus type 1 thymidine kinase is expressed in the testes of transgenic mice under the control of a cryptic promoter. Mol Cell Biol. 1991 Aug;11(8):4207–4216. doi: 10.1128/mcb.11.8.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Shawi R., Kinnaird J., Burke J., Bishop J. O. Expression of a foreign gene in a line of transgenic mice is modulated by a chromosomal position effect. Mol Cell Biol. 1990 Mar;10(3):1192–1198. doi: 10.1128/mcb.10.3.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]