Abstract
Autism is a neurodevelopmental disorder characterized by abnormal reciprocal social interactions, communication deficits, and repetitive behaviours with restricted interests. Autism-relevant phenotypes in the inbred mouse strain BTBR T+tf/J (BTBR) offer translational tools to discover biological mechanisms underlying unusual mouse behaviours analogous to symptoms of autism. Two of the most consistent findings with BTBR are lack of sociability as measured by the three-chamber social approach task and increased amount of time engaged in self-grooming in an empty cage. Here we evaluated BTBR as compared to two typical inbred strains with high sociability and low self-grooming, C57BL/6J (B6) and FVB/AntJ (FVB), on both the automated three-chambered social approach task and repetitive self-grooming assays. Brains from the behaviourally tested mice were analyzed using magnetic resonance imaging and diffusion tensor imaging to investigate potential neuroanatomical abnormalities throughout the brain; specifically, to discover neuroanatomical mechanisms which could explain the autism-relevant behavioural abnormalities. Significant differences in volume and white matter microstructure were detected in multiple anatomical regions throughout the brain of BTBR compared to B6 and FVB. Further, significant correlations were found between behavioural measures and areas of the brain known to be associated with those behaviours. For example, striatal volume was strongly correlated to time spent in self-grooming across strains. Our findings suggest that neuropathology exists in BTBR beyond the previously reported white matter abnormalities in the corpus callosum and hippocampal commissure and that these brain differences may be related to the behavioural abnormalities seen in BTBR.
Keywords: BTBR T+tf/J mice, magnetic resonance imaging, diffusion tensor imaging, behaviour
1. Introduction
Autism is a neurodevelopmental disorder for which diagnosis is based on three domains of behavioural symptoms: 1) abnormal reciprocal social interactions, 2) deficits in communication, and 3) repetitive behaviours with restrictive interests (APA 2001). The BTBR T+tf/J (BTBR) inbred mouse strain exhibits behavioural phenotypes analogous to all three diagnostic symptoms of autism including deficits on tests of sociability and reciprocal social interactions, reduced ultrasonic vocalizations and scent marking in the presence of social cues, and high levels of repetitive self-grooming (Bolivar et al., 2007; Moy et al., 2007; Yang et al., 2007a; 2007b; McFarlane et al., 2008; Scattoni et al., 2008a; Yang et al., 2009; Pobbe et al., 2010; Silverman et al., 2010a; 2010c; Chadman, 2011; Defensor et al., 2011; Pearson et al., 2011; Pobbe et al., 2011; Roullet et al., 2011; Scattoni et al., 2011; Wöhr et al., 2011; Yang et al., 2011b; Silverman et al., 2012; Yang et al., 2012). The BTBR strain provides an opportunity to investigate the biological mechanisms underlying behavioural abnormalities relevant to the symptoms of autism.
Prior histological evaluation has shown pathology exists in the brains of BTBR: a complete agenesis of the corpus callosum and a smaller hippocampal commissure (Wahlsten et al., 2003). Clinical evidence suggests that pathology exists in the brains of individuals with autism, and one of the most consistent findings in clinical studies is a reduction or thinning of the corpus callosum (Frazier and Hardan, 2009; Stanfield et al., 2008; Radua et al., 2010; Frazier et al., 2012; Paul et al., 2007; Casanova et al., 2011; Keller et al., 2007). Structural imaging studies have also found volumetric differences in regions of the frontal lobe, amygdala and caudate nucleus (Amaral et al., 2008; Stigler et al., 2011). Pathology in these areas may be particularly relevant to the behavioural symptoms of autism. Given the known white matter deficits in BTBR and the potential relevance of additional neuropathology to previous clinical findings in autism, we pursued a comprehensive evaluation of the BTBR brains to discover the full extent of neuroanatomical abnormalities.
Magnetic resonance imaging has been used for examining both volume changes and white matter structural integrity in the human (Verhoeven et al., 2010; Beacher et al., 2012) and animal brain (Ellegood et al., 2011; Kumar et al., 2012). In human autism research, volumetric findings are quite heterogeneous with confounds, such as age, IQ, environment and genetics, which leads to multiple regions in the brain being identified or discounted in autism (Stanfield et al., 2008). Robust anatomical phenotyping, which can be performed in the mouse, permits one to control both the environmental and genetic uniformity within groups. Anatomical phenotyping with MRI has been valuable in detecting subtle changes in the mouse brain (Johnson et al., 2002; Nieman et al., 2006). These measurements can detect left/right asymmetries (Spring et al., 2010), and differences in the male and female brain (Spring et al., 2007). Further, specific changes in the mouse brain have been detected after 5 days of training on the Morris water maze (Lerch et al., 2011b), as well as in disease specific models of Huntington’s (Lerch et al., 2008), Alzheimer’s disease (Lau et al., 2008), dopaminergic dysfunction (Cyr et al., 2005), and prenatal alcohol exposure (O'Leary-Moore et al., 2010). Recently, volume changes have been examined in several mouse models related to autism (Ellegood et al., 2010; 2011; Horev et al., 2011; Ellegood et al., 2012).
Diffusion Tensor Imaging (DTI) has become a staple in the investigation of the neuropathology associated with autism, especially due to the abnormal connectivity theory proposed as a potential mechanism underlying the behavioural symptoms of autism (Belmonte et al., 2004). DTI studies have shown increased diffusivity in the white matter of socio-emotional circuits in individuals with autism (Ameis et al., 2011). In the mouse, DTI has examined the developing mouse brain (Mori et al., 2001; Zhang et al., 2002) and genetic mouse models (Wang et al., 2006; Ren et al., 2007). Recently, DTI has been used to examine connectivity in different mouse models of autism (Ellegood et al., 2010; 2011; Kumar et al., 2012). The DTI related changes found in two of these three studies were minimal (Ellegood et al., 2011) or non-existent (Ellegood et al., 2010). The Kumar study, however, reported several changes in both Fractional Anisotropy (FA) and Mean Diffusivity in the BALB/cJ mouse compared to the C57BL6/J in the limited number of regions examined.
Here we aimed to explore volumetric differences in BTBR as compared to two typical inbred strains with high sociability and low incidence of repetitive behaviours, C57BL/6J (B6) and FVB/AntJ (FVB), using magnetic resonance imaging. FVB/AntJ is the FVB strain with normal vision, derived from the conventional FVB/NJ to eliminate the retinal degeneration gene (Errijgers et al., 2007). Further, we investigated whether volumetric differences related to specific behavioural abnormalities in individual mice tested on standardized behavioural assays relevant to the symptoms of autism. Finally, we assessed white matter microstructure using DTI, to determine if white matter abnormalities exist beyond the reported deficits in corpus callosum and hippocampal commissure volumes.
2. Methods
2.1 Mice
Inbred strains C57BL/6J(B6), BTBR T+ tf/J (BTBR), and FVB/AntJ (FVB) were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in a conventional vivarium at the National Institute of Mental Health (NIMH). Pups were weaned at 21 days of age, and group housed by sex in cages of 2–4 littermates per cage. Specially formulated NIH rodent chow (Quality Lab Products, Elkridge MD) and tap water were available ad libitum. In addition to Betachip bedding (Quality Lab Products Elkridge, MD), a Nestlet square (Amcare Inc., Bellmore NY) and a cardboard tube (Jonesville Paper tube company, Jonesville NY) were provided in each cage. The colony room was maintained at approximately 20°C and 55% humidity and with a 12:12 light/dark cycle with lights on at 7:00 AM. All housing and procedures were conducted in strict compliance with the National Institute of Health Guidelines for Care and Use of Laboratory Animals and were approved by the National Institute of Mental Health Animal Care and Use Committees. Twelve adult males from each strain were used in our experiments.
2.2 Behavioural Assays
Behavioural assays were conducted in dedicated behavioural testing rooms during the standard light phase, usually between 1000h and 1500h. The social approach task was run on postnatal day 75 (P75) for all experimental subjects and the self-grooming assay was run on P76 for all subjects.
2.2.1 Automated three-chambered social approach task
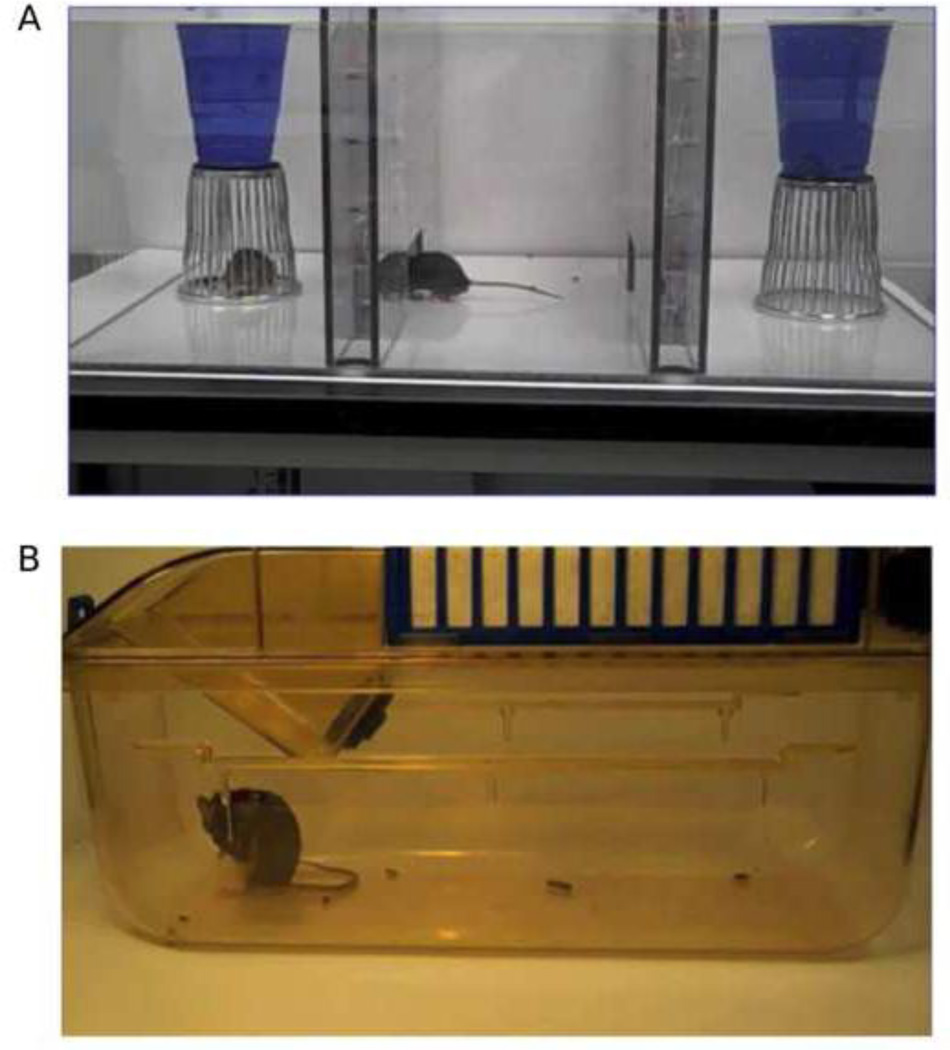
Social approach was assayed in our automated three-chambered apparatus (NIMH Research Services Branch, Bethesda, MD; Figure 1A) using methods previously described (Crawley, 2007; Chadman et al., 2008; McFarlane et al., 2008; Moy et al., 2008; Yang et al., 2009; Silverman et al., 2010a; 2010b; 2011; Yang et al., 2011b; 2011a). Briefly, the apparatus was a rectangular, three-chambered box made of clear polycarbonate. Retractable doorways built into the two dividing walls controlled access to the side chambers. Number of entries and time spent in each chamber were automatically detected by photocells embedded in the doorways and tallied by the software. The test session began with a 10 min habituation session in the center chamber only, followed by a 10 min habituation to all three empty chambers. The subject was then briefly confined to the center chamber while the clean novel object (an inverted stainless steel wire pencil cup, Galaxy, Kitchen Plus, http://www.kitchen-plus.com) was placed in one of the side chambers. A novel mouse, 129S1/SvImJ (purchased from The Jackson Laboratory, Bar Harbor, ME), between 8 and 16 weeks of age, previously habituated to the enclosure, and the same sex as the target mouse, was placed in an identical wire cup located in the other side chamber. After both stimuli were positioned, the two side doors were simultaneously lifted and the subject was allowed access to all three chambers for 10 min. Time spent in each chamber and entries into each chamber were automatically tallied. Time spent sniffing the novel object and time spent sniffing the novel mouse during the 10 min test session was later scored by a trained observer from videos recorded during the test session. The apparatus was cleaned with 70% ethanol and water between subjects. Up to four subject mice were tested in the same room at the same time, using a high-throughput multi-unit arrangement of the 4 test chambers.
Figure 1.
Behavioural assays. (A) In the three-chambered social approach box the subject mouse is allowed to freely explore all three chambers. One side chamber contains a novel object (empty overturned wire cup). The other side chamber contains a novel mouse confined within an overturned wire cup. Weighted plastic cups hold down the wire cups. Sociability is defined as more time spent in the chamber with the novel mouse as compared to the chamber with the novel object or more time spent sniffing the novel mouse as compared to time spent sniffing the novel object (B) In the empty cage observations, trained observers recorded the amount of time the subject mouse spent grooming themselves.
2.2.2 Repetitive self-grooming
Mice were scored for spontaneous grooming behaviours as described previously (Yang et al., 2007b; McFarlane et al., 2008). Briefly, each mouse was given a 10 minute habituation period in a clean, empty mouse cage (Figure 1B) and then video recorded for an additional 10 minutes. Videos were subsequently scored for cumulative time spent grooming all body regions by trained observers. Differences in color and markings between the inbred strains prevented fully blind ratings, however the distinguishing features of the strain were less prominent in the video recordings, which is why this method was chosen over live scoring.
2.2.3 Statistical Analysis
For the automated social approach task, time spent in the two side chambers was compared using within-group Repeated Measures ANOVAs, with chamber side (novel mouse side vs. novel object side) as the within-group factor. Time spent in the center chamber was included for illustrative purposes, but was not included in the statistical analyses. Time spent sniffing was similarly analyzed using within-group Repeated Measures ANOVAs, with novel mouse vs. novel object as the within group factor. A one-way ANOVA was used to compare the total number of entries into the side chambers in the social approach task across strains. Time spent self-grooming in an empty cage was similarly analyzed by using a one-way ANOVA to compare the total amount of time spent grooming across strains.
2.3 Brain Specimen Preparation
Mice were sacrificed on postnatal day 77, one day following the completion of the behavioural testing. An imaging perfusion protocol was followed as previously described (Cahill et al., 2012; Spring et al., 2007; Lerch et al., 2011a). Briefly, mice were anesthetized with a ketamine/xylazine mix then intracardially perfused with 30mL of 0.1 M PBS containing 10 U/mL heparin (Sigma) and 2mM ProHance (a Gadolinium contrast agent) followed by 30 mL of 4% paraformaldehyde (PFA) containing 2mM ProHance. After perfusion, mice were decapitated and the skin, lower jaw, ears and cartilaginous nose tip were removed as previously described. The brain within the skull was incubated in 4% PFA containing 2mM ProHance overnight at 4 degrees Celsius then transferred to 0.1M PBS containing 2mM ProHance and .02% sodium azide for at least 7 days prior to MRI scanning. Perfusions were conducted at the National Institute of Mental Health and brains were shipped to the Mouse Imaging Centre in Toronto, Ontario for imaging.
2.4 Image Acquisition
Images were acquired on a 7 Tesla MRI scanner (Varian Inc., Palo Alto, CA) with a 40 cm inner bore diameter as previously described (Nieman et al., 2006; 2005).
2.4.1 Anatomical MRI
An in-house custom built 16-coil solenoid array was used to acquire the anatomical images in parallel, allowing acquisition of the MRI images for 16 samples in one overnight session (Dazai et al., 2004; Lerch et al., 2011a). Parameters used in the anatomical MRI were optimized for high efficiency and gray/white matter contrast. The sequence was a T2-weighted 3D fast spin echo (FSE), with TR=2000 ms, echo train length = 6, TEeff=42 ms, field of view (FOV) of 25 mm × 28 mm × 14 mm, and a matrix size of 450 × 504 × 250. This yields an isotropic (3D) resolution of 56 µm. In the first phase encode dimension, consecutive k-space lines were assigned to alternating echoes to move discontinuity related ghosting artifacts to the edges of the FOV (Thomas et al., 2004). This sequence involves oversampling in the phase encoding direction by a factor of 2 to avoid the interference of these artifacts. This FOV direction was subsequently cropped to 14 mm after reconstruction. Total imaging time was 11.7 hours.
2.4.2 Diffusion Tensor Imaging
For the DTI scanning, an in-house custom built 3-coil solenoid array was used to acquire the DTI images from 3 brains in parallel (Nieman et al., 2007). The DTI scanning used a 6-cm inner bore diameter insert gradient, which was required for the increased gradient strength needed in the DTI sequence design. The DTI sequence used was a 3D diffusion-weighted FSE, with TR=350 ms, echo train length = 6, first TE = 30 ms, TE = 6 ms for the remaining 5 echoes, two averages, FOV = 25 mm × 14 mm × 14 mm, and a matrix size of 192 × 108 × 108, which yielded an image with 130 µm isotropic voxels. Five initial b=0 s/mm2 images were acquired and 30 high b-value (b = 1917 s/mm2) in 30 different directions were acquired corresponding to the Jones30 scheme (Jones et al., 1999). Total imaging time was ~ 14 hours. After acquisition the images are analyzed using the FSL software package (FMRIB, Oxford UK), which was used to create Fractional Anisotropy (FA) maps for each of the 36 brains used in this study.
2.5 Registration and Analysis
To visualize and compare the mouse brains, the images from the volume scans or the averaged b=0 s/mm2 images from the diffusion scan were linearly (6 parameter followed by a 12 parameter) and nonlinearly registered together. All scans were then resampled with the appropriate transform and averaged to create a population atlas representing the average anatomy of the study sample. All registrations were performed using a combination of the mni_autoreg tools (Collins et al., 1994) and ANTS (Avants et al., 2010). The result of the registration was to have all scans deformed into exact alignment with each other in an unbiased fashion. For the volume measurements, this allowed for the analysis of the deformations needed to take each individual mouse’s anatomy into this final atlas space, the goal being to model how the deformation fields relate to genotype (Nieman et al., 2006; Lau et al., 2008). The Jacobian determinants of the deformation fields are then calculated as measures of volume at each voxel. For the diffusion measurements, the registration allows for the analysis of the intensity differences of all measures (FA, MD, AD, or RD) between genotypes. Significant volume changes and intensity differences were then calculated by warping a pre-existing classified MRI atlas onto the population atlas, which allowed for either the volume or diffusion measure (FA, MD, AD, or RD) of 62 segmented structures encompassing cortical lobes, large white matter structures (i.e. corpus callosum), ventricles, cerebellum, brain stem, and olfactory bulbs (Dorr et al., 2008) to be assessed in all brains. Further, these measurements were examined on a voxel-wise basis in order to localize the differences found within regions or across the brain. Multiple comparisons were controlled for by using the False Discovery Rate (FDR) (Genovese et al., 2002).
The pre-existing classified atlas (Dorr et al., 2008) was manually segmented previously on a sample of 40 B6 mouse brains. The population atlas used in this study was composed of all 36 brains, and since 24 of the 36 brains that were scanned had a corpus callosum that crossed the midline (all the FVB and B6 mice), the population atlas had a crossing corpus callosum. The automated registration of the classified atlas, therefore, was re-segmented manually for the BTBR brains such that the BTBR brains were correctly labeled without a crossing corpus callosum. An example image of this re-segmentation can be seen in Supplementary Figure 1. Axial and coronal slices of the BTBR population atlas and the FVB/B6 population atlas illustrate the changes made to the classified atlas. In the BTBR mouse the area where the corpus callosum would be, if it crossed, was replaced with two new structures that were defined in our BTBR atlas: Probst bundles, which were composed of anterior/posterior travelling white matter bundles (Ren et al., 2007), and additional cortical gray matter.
2.6 Behavioural Correlations
For all mice individually, correlations between behaviour and size of specific brains regions were investigated, this was preformed on both a voxel-wise basis and then subsequently on the 62 different regions examined. Linear models were performed on every voxel or region within the brain of the 36 mice (relative volume vs. sniff difference + self groom time). This allowed the comparison of the relative volume to either the individual sociability scores, operationally defined for the purposes of the present study as the difference between time spent sniffing the novel mouse and time spent sniffing the novel object in the three-chambered social approach assay, with the grooming measure held constant, or self groom time, with the sociability score kept constant. Significant correlations were corrected for multiple comparisons with the false discovery rate (FDR) (Genovese et al., 2002).
3. Results
3.1 Behavioural results
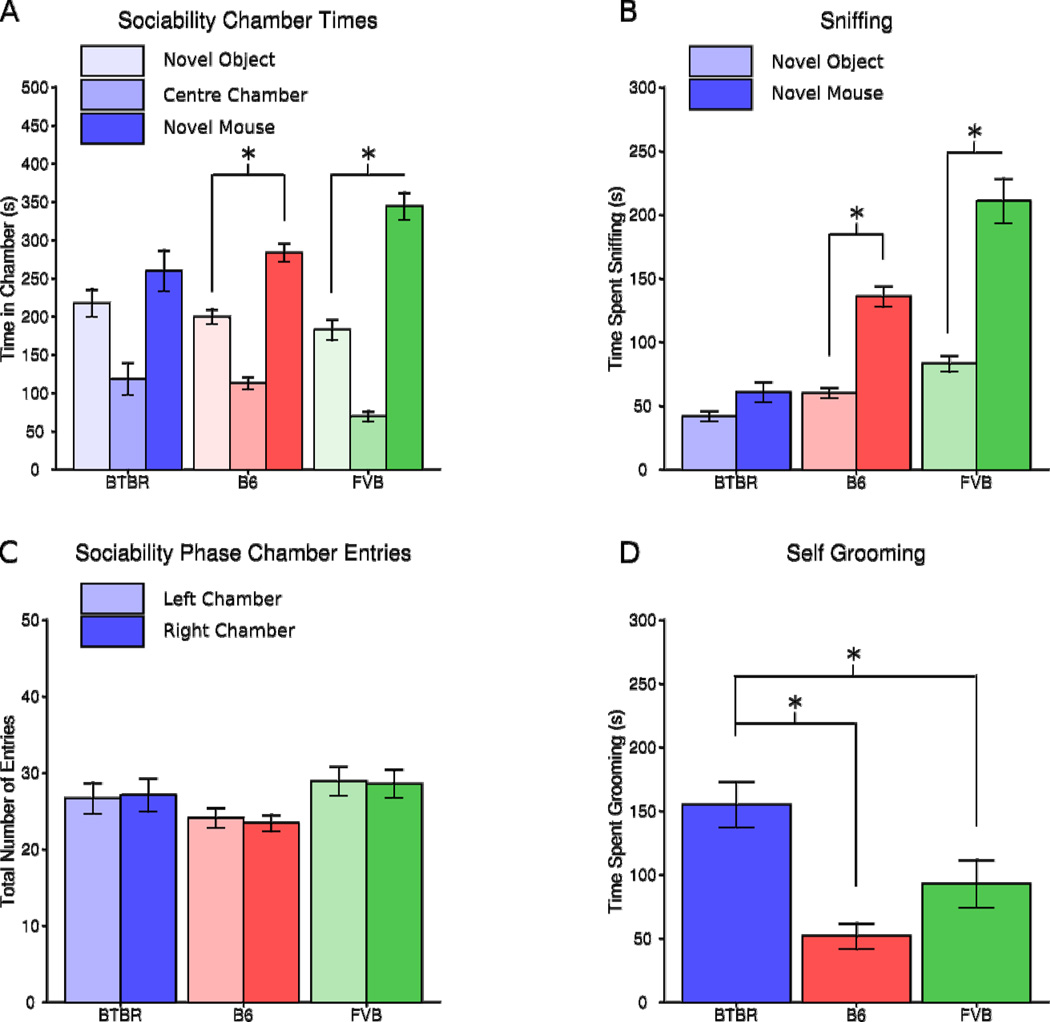
Two parameters of sociability were measured in our automated three-chamber social approach task prior to specimen preparation: difference in time spent in the chamber with the novel mouse versus with the novel object and difference in time spent sniffing the novel mouse versus the novel object. B6 and FVB spent significantly more time in the chamber containing the novel mouse as compared to the chamber containing the novel object (B6: F(1,11) =18.588, p =0.0012; FVB: F(1,11) = 25.096, p =0.0005 Figure 2A) meeting the standard definition of sociability for this task (Yang et al., 2011b; Silverman et al., 2010b). BTBR spent equal time in both chambers (BTBR F(1,11) = 1.135, NS; Figure 2A), meeting the standard definition of lack of sociability. Similarly, B6 and FVB spent more time sniffing the novel mouse as compared to the novel object (B6: F(1,11) = 72.404, p <0.0001; FVB: F(1,11) = 32.732, p < 0.0001 Figure 2B). BTBR displayed no significant differences in time sniffing the novel mouse as compared to the novel object (BTBR F(1,11) = 4.574, NS; Figure 2B). There were no strain differences in total number of transitions during the sociability phase (F(2,33) = 2.604, NS; Figure 2C), nor did any strain appear to have an innate preference for either side chamber during the habituation session (data not shown, B6: F(1,11) = 0.540, NS; FVB: F(1,11) = .121, NS; BTBR: F(1,11) = 0.114, NS; Figure 2C). Strain differences in total time engaged in self-grooming (F(1,11) = 10.568, p =0.0003; Figure 2D) confirmed that BTBR spent significantly more time self-grooming as compared to both B6 and FVB (p<0.05; Figure 2D), as previously described (Yang et al., 2009; 2011b).
Figure 2.
Behavioural results. B6 and FVB displayed species typical sociability in the social approach task as measured both by chamber time (A) and sniff time (B). BTBR did not display sociability on either measure. C) None of the strains had a side bias or significantly higher activity levels. D) BTBR spent significantly more time grooming themselves in the empty cage observations as compared to both B6 and FVB. The error bars represent the standard error of the mean. Significant comparisons are indicated with asterisks, the lines below the asterisks show what is being compared.
3.2 Volume Measurements
Two measurements were calculated to assess the volume in the brain of the BTBR mouse as compared to the two typical strains: absolute volume, measured in mm3, and relative volume, measured as a percentage of the total brain volume. Both are equally valid measures of comparison to understand the difference between two or more groups of mice. Since, the total brain volume was significantly reduced in BTBR (BTBR – 446 ± 12 mm3, B6 - 466 ± 19 mm3, FVB - 483 ± 14 mm3), despite BTBR having a larger body size than both the B6 and FVB (BTBR – 33.3 ± 1.8 g, B6 – 27.5 ± 1.27 g, FVB – 31.6 ± 1.9 g), we focused our examination on the relative volume. The relative volume differences for all regions are listed in Supplemental Table 1. Absolute volume differences for all regions are listed in Supplemental Table 2.
3.2.1 Regional volumes
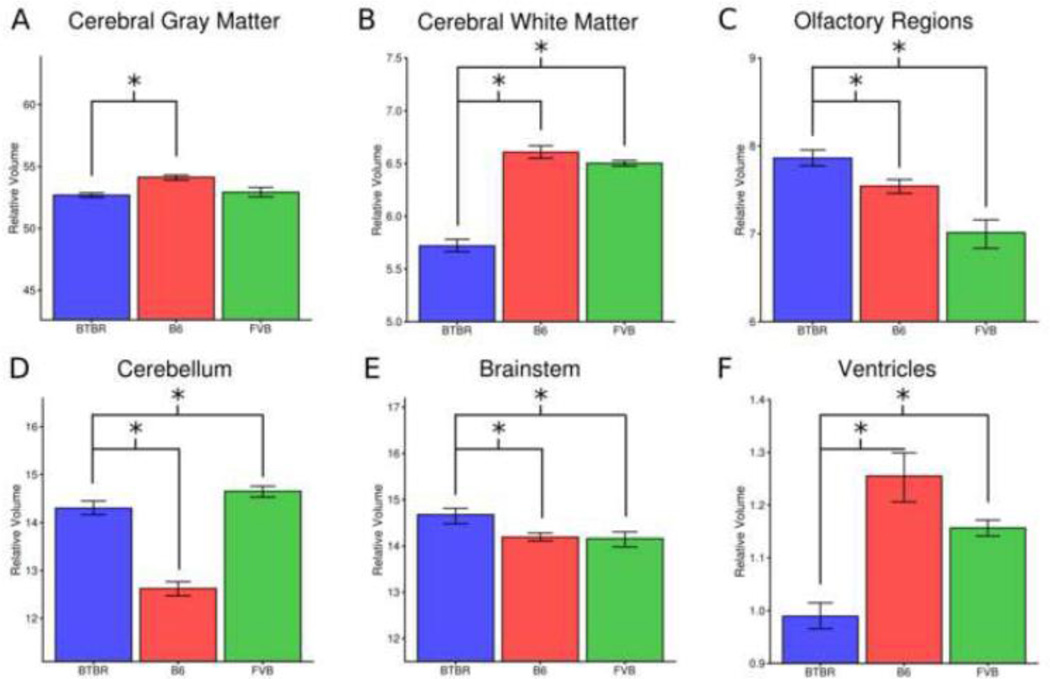
The 62 regions examined were divided into 6 summary regions (Figure 3): cerebral gray matter (CGM – 20 regions), cerebral white matter (CWM – 14 regions), olfactory regions (OLF – 4 regions), cerebellum (5 regions), ventricles (4 regions), and brainstem (15 regions). These regions were selected based on the original B6 atlas paper (Dorr et al., 2008). Interestingly, the relative volume of the CGM region in the BTBR was only slightly smaller compared to B6 (−2% FDR <1%) and not significantly different from FVB (FDR=27%). The CWM region, on the other hand remained significantly smaller compared to both B6 (−13%, FDR <1%) and FVB (−12%, FDR <1%). The olfactory region was significantly larger in the BTBR brain as compared to B6 (4%, FDR<1%) and FVB (12%, FDR<1%). The relative volume of the BTBR cerebellum was larger than relative volume of the B6 cerebellum (13%, FDR<1%) but smaller than the FVB cerebellum (−2%, FDR<1%). The relative volume of the brainstem was increased in BTBR as compared to both B6 (3%, FDR<1%) and FVB (4%, FDR<1%). Relative volume of the ventricles was smaller in BTBR as compared to both B6 (−21%, FDR<1%) and FVB (−14%, FDR<1%).
Figure 3.
Relative volume differences by region. Bars represent the average relative volume of each region with error bars representing the 95% confidence interval. The relative volume of cerebral white matter (B) and the ventricles (F) were significantly reduced in BTBR as compared to both typical strains. The relative volume of the olfactory regions (C) and brainstem regions (E) were increased in BTBR as compared to both strains. Relative volume of cerebral gray matter was reduced in BTBR as compared to B6 but not FVB (A) and relative volume of the cerebellum was increased as compared to B6 but not FVB (D). The y-axis is scaled such that it is centered on the mean value of the summary region +/− 20% of that mean. Significant comparisons are indicated with asterisks, the lines below the asterisks show what is being compared.
3.2.2 Cerebral grey matter regions
Of the 41 grey matter regions, twelve were found to be significantly different between BTBR and B6 at an FDR of 1% when relative volumes were compared (Supplementary table 1). Eleven regions that differed all had decreased relative volume in the BTBR as compared to B6. The bed nucleus of the stria terminalis and the hippocampus both had greater relative volume in the BTBR as compared to B6. Fifteen regions differed between BTBR and FVB at an FDR of 1% (Supplementary table 1). Nine regions had significantly smaller relative volume in BTBR as compared to FVB and six regions had significantly greater relative volume in BTBR as compared to FVB. Regions that were significantly different in BTBR as compared to both control strains include bed nucleus of the stria terminalis, frontal lobe, parietal lobe, dentate gyrus of the hippocampus, globus pallidus, nucleus accumbens, pre para subicular, striatum granulosum of the hippocampus and the striatum.
3.2.3 Cerebral white matter regions
Of the 14 white matter regions, ten were found to be significantly different between BTBR and B6 at an FDR of 1% (Supplementary table 1). Of these 10 regions all were significantly smaller in BTBR as compared to B6 except for the anterior commissure pars anterior which had a greater relative volume in the BTBR mouse. Nine regions differed between BTBR and FVB at an FDR of 1% (Supplementary table 1). Five regions had significantly smaller relative volumes in BTBR as compared to FVB, and five regions had significantly greater volumes. Regions that were significantly different in BTBR as compared to both typical strains include the anterior commissure pars anterior, cerebral peduncle, corpus callosum, fimbria, habenular commissure and the internal capsule.
3.2.4 Olfactory regions
Three of the four olfactory regions had larger relative volumes in BTBR as compared to B6 (Supplementary table 1). All four olfactory regions had larger relative volumes in BTBR as compared to FVB (Supplementary table 1).
3.2.5 Cerebellar regions
The relative volumes of two of the five cerebellar regions significantly differed between BTBR and B6 (Supplementary table 1). The arbor vita of the cerebellum and the cerebellar cortex were both larger in BTBR as compared to B6. Three of the five cerebellar regions had smaller relative volumes in the BTBR as compared to FVB. The arbor vita of the cerebellum differed significantly between BTBR and both strains; however this region was differed in opposing directions, it was larger in the BTBR compared to B6 and smaller in BTBR compared to FVB.
3.2.6 Ventricular regions
The lateral ventricle was the only ventricular region that significantly differed in relative volume between BTBR and B6 (Supplementary table 1). The lateral ventricle had a smaller relative volume in BTBR as compared to B6. The lateral ventricle also had smaller relative volume in BTBR as compared to FVB (Supplementary table 1). The smaller lateral ventricle in BTBR compared to B6 and FVB was caused by the relocation of the crossing corpus callosum white matter fibers to form the Probst bundles (Supplementary Figure 1), which consist of white matter fibers travelling anterior/posterior through the lateral ventricles decreasing the ventricular space. The fourth ventricle had larger relative volume in BTBR as compared to FVB (Supplementary table 1).
3.2.7 Brainstem regions
Seven of the 15 brainstem regions significantly differed in relative volume between B6 and BTBR (Supplementary table 1). Only one of these areas was significantly smaller in BTBR as compared to B6. Seven brainstem regions significantly differed in relative volume between BTBR and FVB. Five of the seven regions that differed were significantly larger, relatively in BTBR as compared to FVB.
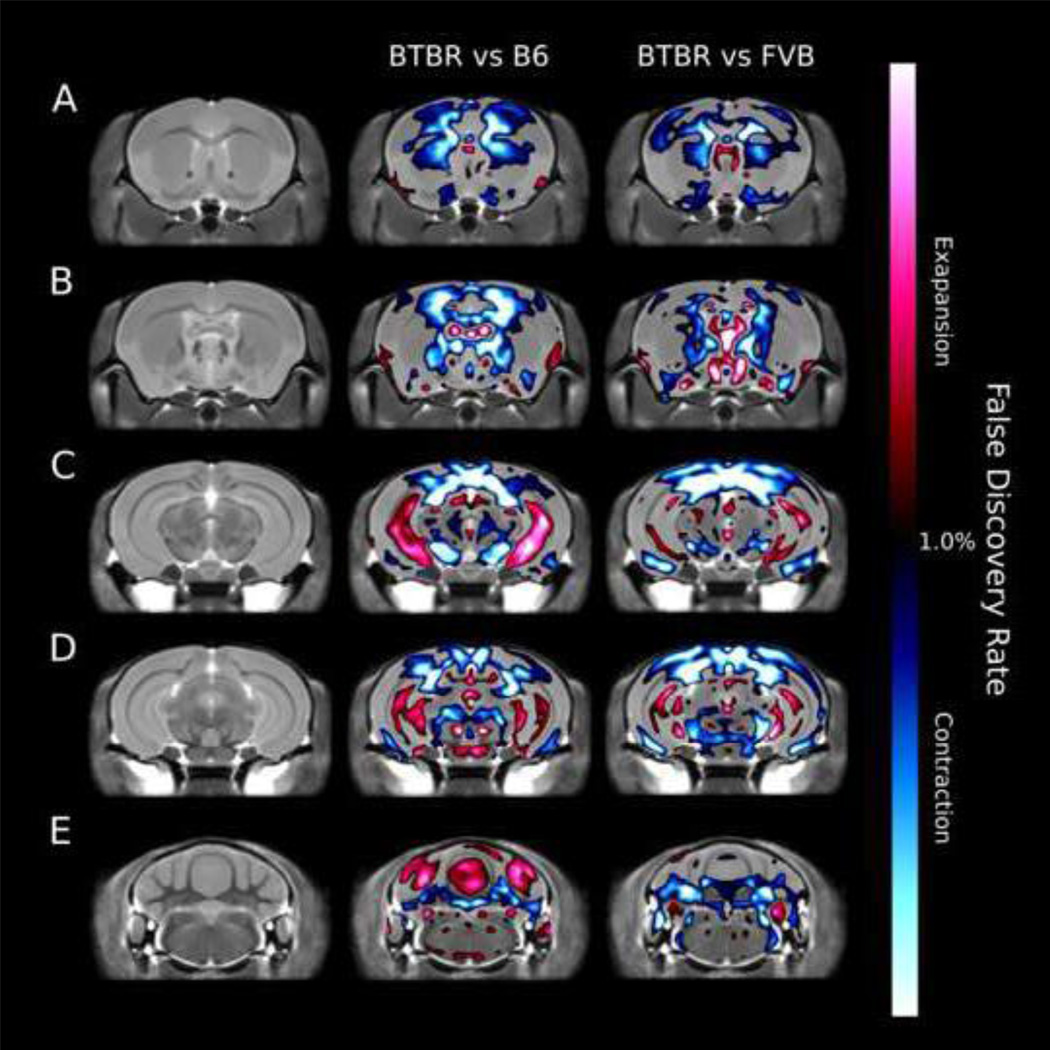
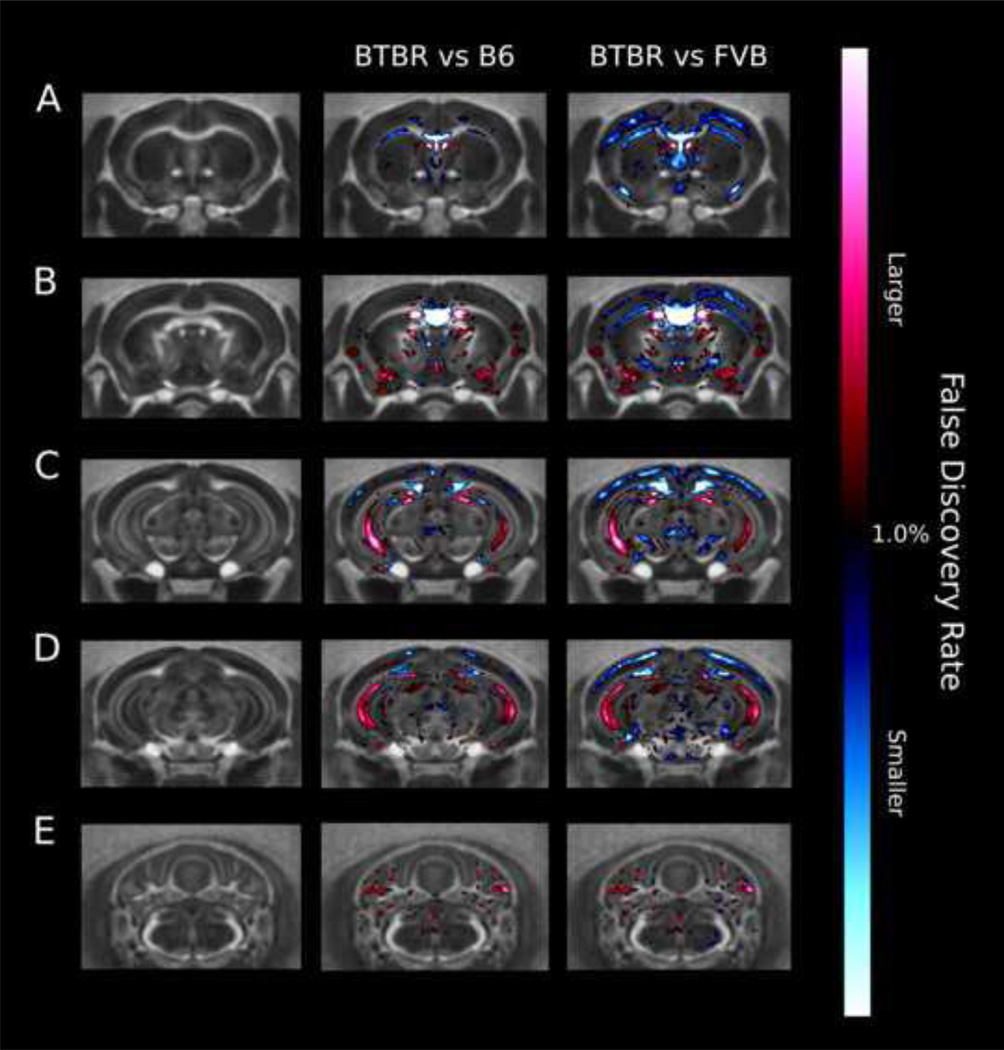
3.2.8 Voxelwise measurements - Volume
Figure 4 highlights the relative volume difference between the BTBR and both the B6 and FVB strains. Voxels highlighted in either blue (BTBR is smaller) or red (BTBR is larger) were significant at an FDR of <1%. Please note that unlike the regional measurements that were manually segmented to account for the lack of a crossing corpus callosum in BTBR, this is not possible for the voxel-wise volume measurements, and thus the voxelwise volume changes located where there should be a crossing corpus callosum are somewhat unreliable.
Figure 4.
Fly through of coronal slices in the brain highlighting the voxel-wise significant relative volume differences between BTBR and B6, or between BTBR and FVB. Anything highlighted in red is significantly larger in the BTBR compared to the typical strain and anything highlighted in blue is significantly smaller in the BTBR. All changes highlighted are significant at an FDR value of <1%.
3.3 Behaviour Correlations with Volume
3.3.1 Correlations between volume and sociability
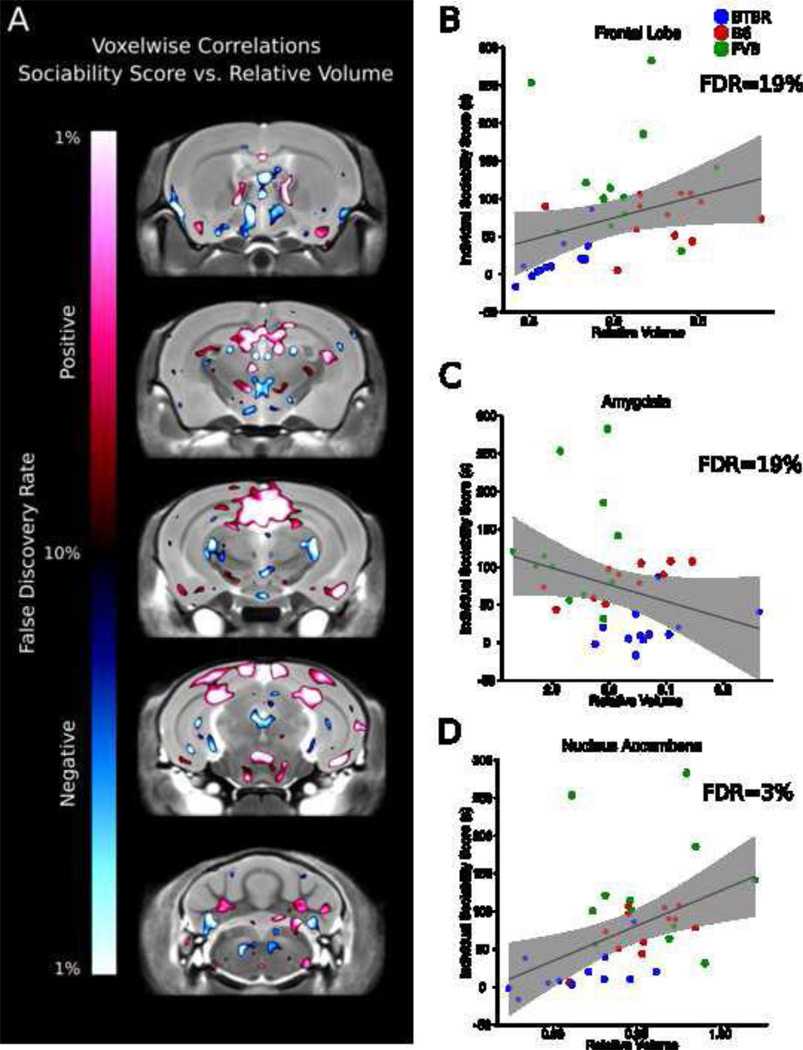
Figure 5A displays the voxelwise correlations in five different coronal slices highlighting other areas of interest, which may or may not be significant when taking into account the entire region. Significant correlations between relative volume of individual brain regions and individual sociability scores (difference between time spent sniffing the mouse and time spent sniffing the object) are listed in supplementary table 4, in which significance was defined at an FDR threshold of less than 10%. Negative correlations, wherein higher relative volume correlated with absence of sociability, were found in the 17 individual regions, for example, the anterior commissure, hypothalamus, olfactory bulbs, and the pons. Positive correlations, where higher relative volume was associated with normal sociability, were found in 10 individual regions (Supplementary Table 4), for example, the corpus callosum, nucleus accumbens, and the occipital lobe. Correlations between sociability scores and individual brain regions with relevance to social behaviour are shown in Figure 5B, C and D. Figure 5B shows the correlation between the entire relative volume of the frontal lobe and sociability score. While this correlation across all strains is not significant, there is a strong correlation with BTBR individual sociability scores and the relative volume of the frontal lobe (p=0.003). Other regions such as the amygdala (Figure 5C) were not significantly correlated, however areas within the amygdala were positively correlated with sociability (Figure 5A). The relative volume of the nucleus accumbens was positively correlated with individual sociability scores across all three strains (Figure 5C).
Figure 5.
Relationship of brain volume and individual sociability score (sniff time difference). A) Voxelwise comparison between individual sociability score and relative volume measurements. Areas highlighted in red indicate a positive correlation, and areas highlighted in blue represent a negative correlation. Three specific regions that are relevant to sociability are highlighted: the frontal lobe (B), amygdala (C), and nucleus accumbens (D). The shaded gray area represents the 95% confidence interval.
3.3.2 Correlations between volume and repetitive self-grooming
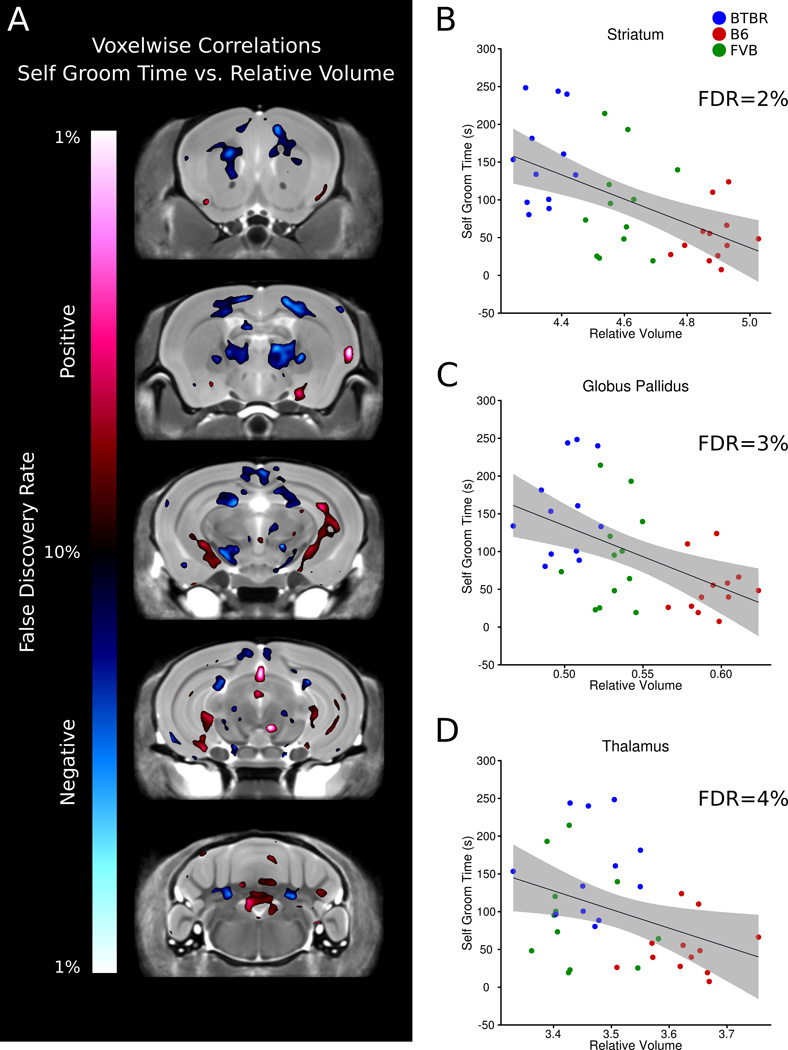
Voxelwise maps of five different coronal slices highlight significant correlations in specific areas throughout the brain (Figure 6A). Significant correlations between relative volume of individual regions and self-grooming are listed in supplementary table 4, in which significance was defined at an FDR threshold of less than 10%. Negative correlations were found in 15 individual regions across all three strains, indicating that as self-grooming was higher the relative volume of these regions was lower. Regions such as the striatum, globus pallidus, and thalamus known to be associated with repetitive behaviours were significantly correlated with time spent self-grooming (Figure 6B,C and D). Positive correlations were found in 8 regions, indicating the as self-grooming was higher the relative volume of these regions was also higher. Example regions with positive correlations with respect to self-grooming were the arbor vita of the cerebellum, the cerebellar cortex, and the hippocampus.
Figure 6.
Relationship of brain volume and time spent grooming. A) Voxelwise comparison between repetitive self-grooming and relative volume measurements. Areas highlighted in red indicate a positive correlation, and areas highlighted in blue represent a negative correlation. Three specific regions that are relevant to repetitive behaviours are highlighted: the striatum (B), globus pallidus (C), and thalamus (D). The shaded gray area represents the 95% confidence interval.
3.4 Diffusion Measurements
3.4.1 Fractional anisotropy differences in white matter regions
When BTBR was compared to B6 in the 14 white matter regions, the Fractional anisotropy (FA) value only differed in one region, the corpus callosum, which was found to have a significantly smaller FA, at an FDR of <1% (Supplementary table 3). It should be noted again for clarity that the corpus callosum region in our classified atlas includes the external capsule; therefore, in the BTBR brains there is still a region labeled “corpus callosum”, which is composed of the remaining external capsule. When BTBR was compared to FVB, 4 regions were found to have significantly different FA values at an FDR of <1%. FA values in the anterior commissure pars posterior, corpus callosum, fornix and stria medullaris of BTBR were significantly smaller compared to FVB (Supplementary table 3). Only the corpus callosum region differed at an FDR of <1% between the BTBR and both typical strains.
3.4.2 Voxelwise measurements of Fractional Anisotropy
Similar to the voxelwise volume measurements, the same coronal slices highlighting changes in FA are shown in Figure 7. Figure 7 displays differences in FA between the BTBR and B6 or FVB. Voxels highlighted in blue indicate that BTBR has a smaller FA and voxels highlighted in red indicate that BTBR has a larger FA in those corresponding areas.
Figure 7.
Fly through of coronal slices in the brain highlighting the voxelwise significant FA differences between BTBR and B6, or between BTBR and FVB. Anything highlighted in red is significantly larger in the BTBR compared to the typical strain and anything highlighted in blue is significantly smaller in the BTBR. All changes highlighted are significant at an FDR value of <1%.
4. Discussion
The BTBR mouse model of autism was compared to two typical inbred strains, B6 and FVB on behavioural assays and magnetic resonance imaging (MRI), which composed of both an anatomical scan to measure volume differences and diffusion tensor imaging (DTI) to assess the white matter. The behavioural assays utilized in these experiments examined two of the autism relevant phenotypes seen consistently in BTBR mice: lack of sociability and repetitive self-grooming (Bolivar et al., 2007; Moy et al., 2007; Yang et al., 2007a; 2007b; McFarlane et al., 2008; Scattoni et al., 2008b; Yang et al., 2009; Pobbe et al., 2010; Silverman et al., 2010a; 2010c; Chadman, 2011; Defensor et al., 2011; Pearson et al., 2011; Pobbe et al., 2011; Roullet et al., 2011; Scattoni et al., 2011; Wöhr et al., 2011; Yang et al., 2011b; Silverman et al., 2012; Yang et al., 2012). B6 and FVB displayed typical mouse sociability as measured by two variables in the social approach task, as expected. Both B6 and FVB spent more time in a chamber with a novel mouse as compared to the chamber with the novel object and spent more time sniffing the novel mouse as compared to the novel object. As expected, BTBR mice failed to spend more time in the chamber with the novel mouse or spend more time sniffing the novel mouse as compared to the novel object, indicating a lack of sociability. BTBR also spent significantly more time self-grooming in an empty cage compared to B6 or FVB. These behavioural results were consistent with previously published reports (Bolivar et al., 2007; Moy et al., 2007; Yang et al., 2007a; McFarlane et al., 2008; Yang and Crawley, 2009; Pobbe et al., 2010; Chadman, 2011; Defensor et al., 2011; Silverman et al., 2011; Yang et al., 2011b; Silverman et al., 2012; Yang et al., 2012; Pearson et al., 2011; Amodeo et al., 2012). Results from the present behavioural assays confirmed that our subject mice used for the neuroanatomical studies were displaying the expected phenotypes.
Volumetric MRI revealed robust differences in the brains of BTBR mice as compared to each of the typical strains. Significant differences in relative volume in BTBR were highlighted in almost all of the 6 summary regions when relative volumes were compared to both B6 and FVB. The only regional comparison that was not significantly different was the comparison between BTBR and FVB in the cerebral gray matter. The most robust difference in the summary regions was the smaller cerebral white matter found in BTBR as compared to both B6 (−13%) and FVB (−12%). In other regions such as the cerebellum and olfactory bulbs there were also marked differences, though not as consistent or robust as the differences seen in the white matter of BTBR as compared to B6 or FVB. The BTBR cerebellum was larger when compared to B6 (13%) and a smaller when compared FVB (−2%). The olfactory region in BTBR was larger than both B6 (4%) and FVB (12%). As expected the corpus callosum region was significantly smaller compared to both B6 (−24%) and FVB (−25%) (Wahlsten et al., 2003; Yang et al., 2009; Bolivar et al., 2007). In contrast, the anterior commissure was larger in BTBR compared to both typical strains. Other white matter structures, which were smaller in BTBR compared to the typical strains, included some of the largest white matter tracts in the mouse brain, i.e. the cerebral peduncle, fimbria, and internal capsule were decreased in size from 4–13%. These various white matter differences in BTBR are not only similar to previous mouse models of autism, such as the neuroligin3 R451C knockin (Ellegood et al., 2011) and the intergrinβ3 (Ellegood et al., 2012) knockout models, but white matter differences have been a consistent finding in clinical studies of individuals with autism (Travers et al., 2012).
Although comparisons of the relative volume of cerebral gray matter as a summary region only significantly differed between BTBR and B6, there were significant differences between BTBR and both typical strains in individual grey matter regions/structures. For example, three out of the four cortical areas revealed significant reductions in BTBR as compared to the typical strains, indicating perhaps a significant reduction in cortical volume in the BTBR. However, BTBR brains appear to have an additional cortical region (Supplementary Figure 1), which, in addition to the Probst bundles, replaces the lack of a corpus callosum. If we assume this tissue is in fact cortical and include this additional cortical gray matter in the BTBR cortex measurements, then the BTBR cortex remains smaller than B6 (−1.7%), but not smaller than FVB, indicating that this additional cortical gray matter may be making up for the losses in the other cortical regions in BTBR. The significance of reductions of other gray matter structures, such as the nucleus accumbens and the striatum, are discussed in their relationship to behavioural variables.
Total volume differences in the cerebellum revealed an interesting intermediate phenotype for the BTBR. While the cerebellum in BTBR was significantly larger than B6, it was significantly smaller compared to FVB. It has been previously shown that FVB mice have a more complex foliation pattern as compared to B6, although volume differences were not discussed (Sillitoe and Joyner, 2007). Foliations can be seen in Supplementary Figure 2, which displays sagittal slices of the cerebellum highlighting the different foliation pattern between the 3 groups used in this study. Overall the volumetric findings suggest the BTBR brain has significant structural differences from the two typical strains; again confirming that volume differences exist throughout the BTBR brain beyond the previously reported agenesis of the corpus callosum.
Fractional anisotropy (FA) measurements of white matter areas revealed a significantly smaller FA between BTBR and both typical strains only in the corpus callosum, which corresponds to the known agenesis. From the voxelwise images in Figure 7B, the loss of the corpus callosum, as measured by FA, is strongly highlighted. Also in Figure 7A and B, in the comparison of BTBR versus B6 or FVB, there is a smaller FA along the length of the external capsule away from the corpus callosum. This FA difference is stronger versus FVB. A loss in FA can be indicative of a number of factors, such as less myelination, permeability changes, or a differing axonal organization, which could be composed of changes in axonal size, density or organization (Beaulieu, 2002). It is unclear which of these factors is driving the change in FA in the external capsule, but the volume loss in that region (Figure 4A and B) and the smaller FA is indicative of less, possibly disorganized, white matter fiber tracts. In Figure 7 there are also a number of areas in which a larger FA was found. Despite the inherent difficulty in interpretation of FA changes in gray matter, one particular area of interest was within the hippocampus in which BTBR was found to have a larger FA than both B6 and FVB (Figure 7C and D). This same area was also found to be larger in BTBR compared to both typical strains (Figure 4C and D). The increase in FA seems to be localized to the CA1 region of the hippocampus (Figure 7D). The larger size of this area combined with an increased FA may indicate an increase in the number of pyramidal neurons. An important point to note here is that these hypotheses are speculative. Histological examination of these areas would be required to answer these questions but was beyond the scope of this study. There is, however, a clear difference in the hippocampus and formation of the external capsule in BTBR as compared to the typical mice.
BALB/cJ, another inbred strain mouse model of autism with a deficit in sociability, has been examined recently using DTI (Kumar et al., 2012). The Kumar study examined the social behaviour of the BALB/cJ mouse and also performed in vivo DTI. Kumar et al. reported a trend of a reduced FA in the external capsule of the BALB/cJ mice, which is consistent with the reduction of the corpus callosum region we see in BTBR. Although the Kumar paper attributes their FA trend in the external capsule to a loss in myelination, it is entirely possible that this strain difference is caused by the axonal organization or structure.
A number of different genetic mouse models of autism have previously been investigated to determine how the brain is altered by genetic manipulation (Ellegood et al., 2010; 2011; Horev et al., 2011; Ellegood et al., 2012). As compared to these previous mouse models, the BTBR strain has aspects of the differences found in neuroligin3 R451C knockins (NL3 KI) (Ellegood et al., 2011) and intergrinβ3 knockouts (ITGβ3 KO) (Ellegood et al., 2012). The total brain volume of the BTBR brain was found to be decreased by 4.5% compared to B6 and 7.9% compared to FVB. The total brain volume in the NL3 KI and ITGβ3 mice was also decreased in comparison to their corresponding wild-type (WT) mouse, 8% and 11% respectively. In these models the corpus callosum was present and connected between the two cortical hemispheres, but it was considerably decreased in size (−14% in the NL3 KI and –17% in the ITGβ3 KO). Furthermore, many other white matter regions in the NL3 KI and ITGβ3 KO models were significantly decreased in size, consistent with the comparison of BTBR with two social strains. In the NL3 KI mouse the white matter regions were decreased by 10% in comparison to the WT mouse. In the ITGβ3 the white matter regions had substantial decreases in both absolute and relative volume.
An additional component of the study was to evaluate all the mice behaviourally prior to perfusion and MRI scanning which allowed comparisons between the animal’s performance on the behavioural tasks and its brain measurements obtained through imaging. Relative volume measurements on voxel by voxel basis as well as the 62 individual regions were compared to both sociability and repetitive behaviour across the 3 strains used. There was some initial concern with performing these behavioural correlations across all 3 groups simultaneously, as it was felt that the correlation plots may be dominated by the differences in volume between groups and subjected to a “barbell” effect, in which two distinct groupings were at opposite ends of a correlation plot. This was seen in the comparison between the relative volume of the corpus callosum and sniff time difference (Supplementary Figure 3); however, other areas of the brain did not suffer from a similar “barbell” effect as seen in Figure 5B, C and D as well as Figure 6B, C, and D. Relative volume correlated with sociability in multiple areas throughout the brain (Figure 5A). Specific regions are highlighted in Figure 5B, C, and D, namely the frontal lobe, amygdala, and nucleus accumbens, all which have been linked to the “social brain” (Insel and Fernald, 2004; Young, 2002; Adolphs, 2009). Despite this implication a significant correlation was not found across all three strains for relative volume of the frontal lobe (FDR=19%), but the relative volume of the frontal lobe and sniff time difference did correlate when BTBR were considered separately (data not shown). The amygdala (Figure 5C) also did not have a significant correlation (FDR=19%), but there were specific areas within the amygdala where a correlation was found (Figure 5A). The nucleus accumbens has been linked to the formation of partner preferences (Young et al., 2001) and was positively correlated with sociability (Figure 5D), indicating that the larger the nucleus accumbens the more social the mice were. The striatum has been linked with repetitive behaviours in humans (Langen et al., 2011a) and mice (Langen et al., 2011b). We found a strong correlation between smaller relative volume of the striatum (FDR=2%, Figure 6B) and increased grooming time. In contrast, a larger size of the caudate nucleus has been reported in autism (Stanfield et al., 2008; Sears et al., 1999). However, in obsessive compulsive disorder (OCD), which is characterized by high repetitive behaviours, the striatum has been reported to be smaller (Robinson et al., 1995). Similar negative correlations were found in the frontal lobe, globus pallidus (FDR=3%, Figure 6C) and thalamus (FDR=4%, Figure 6D) all of which have been implicated previously in repetitive behaviours (Langen et al., 2011b). These behavioural correlations highlight the power of our imaging method, in detecting a predicted anatomical correlation for mouse behaviour, and for discovering a previously unknown area that may be implicated in the behaviour.
In sum, neuroanatomical imaging and DTI have revealed striking differences between BTBR and two typical inbred strains, B6 and FVB. Based on these findings, it appears that white matter volume deficits in the BTBR brain are not specific to the corpus callosum. Rather, BTBR mice have strong white matter deficits throughout the brain based on both volume and DTI measures. Further studies will be necessary to understand the mechanisms underlying these neuroanatomical differences, and developmental time course at which they manifest, in mouse models of autism.
Supplementary Material
Acknowledgements
We would like to thank Christine Laliberté for her assistance with the MRI scanning, Matthijs van Eede and Jan Scholz for help with different stages of the analysis, and Mu Yang and Jill Silverman for their assistance with the behavioural tasks and perfusions. We also acknowledge the Ontario Mental Health Foundation (OMHF) for salary support (Jacob Ellegood). This research was conducted with the support of the Canadian Institute for Health Research (CIHR), the National Institute of Mental Health Intramural Research Program, and the Ontario Brain Institute (OBI). OBI was created to become an internationally recognized centre of excellence in brain and neuroscience research. This independent non-profit corporation, funded partially by the Ontario government, is dedicated to improving approaches to the prevention, early diagnosis, treatment and management of neurological, and psychiatric disorders. The opinions, results, and conclusions are those of the authors and no endorsement by any of the agencies is intended or should be inferred.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Ameis SH, Fan J, Rockel C, Voineskos AN, Lobaugh NJ, Soorya L, Wang AT, Hollander E, Anagnostou E. Impaired structural connectivity of socioemotional circuits in autism spectrum disorders: a diffusion tensor imaging study. PLoS ONE. 2011;6:e28044. doi: 10.1371/journal.pone.0028044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behavioural Brain Research. 2012;227:64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edn. Washington DC: APA; 2001. [Google Scholar]
- Avants BB, Yushkevich P, Pluta J, Minkoff D, Korczykowski M, Detre J, Gee JC. The optimal template effect in hippocampus studies of diseased populations. Neuroimage. 2010;49:2457–2466. doi: 10.1016/j.neuroimage.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacher FD, Minati L, Baron-Cohen S, Lombardo MV, Lai M-C, Gray MA, Harrison NA, Critchley HD. Autism attenuates sex differences in brain structure: a combined voxel-based morphometry and diffusion tensor imaging study. AJNR Am J Neuroradiol. 2012;33:83–89. doi: 10.3174/ajnr.A2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. Journal of Neuroscience. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behavioural Brain Research. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill LS, Laliberté CL, Ellegood J, Spring S, Gleave JA, Eede MCV, Lerch JP, Henkelman RM. Preparation of fixed mouse brains for MRI. Neuroimage. 2012;60:933–939. doi: 10.1016/j.neuroimage.2012.01.100. [DOI] [PubMed] [Google Scholar]
- Casanova MF, El-Baz A, Elnakib A, Switala AE, Williams EL, Williams DL, Minshew NJ, Conturo TE. Quantitative analysis of the shape of the corpus callosum in patients with autism and comparison individuals. Autism. 2011;15:223–238. doi: 10.1177/1362361310386506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol. Biochem. Behav. 2011;97:586–594. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Crawley JN. Mouse Behavioral Assays Relevant to the Symptoms of Autism. Brain Pathology. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr M, Caron MG, Johnson GA, Laakso A. Magnetic resonance imaging at microscopic resolution reveals subtle morphological changes in a mouse model of dopaminergic hyperfunction. Neuroimage. 2005;26:83–90. doi: 10.1016/j.neuroimage.2005.01.039. [DOI] [PubMed] [Google Scholar]
- Dazai J, Bock NA, Nieman BJ, Davidson LM, Henkelman RM, Chen XJ. Multiple mouse biological loading and monitoring system for MRI. Magn Reson Med. 2004;52:709–715. doi: 10.1002/mrm.20215. [DOI] [PubMed] [Google Scholar]
- Defensor EB, Pearson BL, Pobbe RLH, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behavioural Brain Research. 2011;217:302–308. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr AE, Lerch JP, Spring S, Kabani N, Henkelman RM. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage. 2008;42:60–69. doi: 10.1016/j.neuroimage.2008.03.037. [DOI] [PubMed] [Google Scholar]
- Ellegood J, Henkelman RM, Lerch JP. Neuroanatomical Assessment of the Integrin β3 Mouse Model Related to Autism and the Serotonin System Using High Resolution MRI. Front Psychiatry. 2012;3:37. doi: 10.3389/fpsyt.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegood J, Lerch JP, Henkelman RM. Brain abnormalities in a Neuroligin3 R451C knockin mouse model associated with autism. Autism Res. 2011;4:368–376. doi: 10.1002/aur.215. [DOI] [PubMed] [Google Scholar]
- Ellegood J, Pacey LK, Hampson DR, Lerch JP, Henkelman RM. Anatomical phenotyping in a mouse model of fragile X syndrome with magnetic resonance imaging. Neuroimage. 2010;53:1023–1029. doi: 10.1016/j.neuroimage.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Errijgers V, Van Dam D, Gantois I, Van Ginneken CJ, Grossman AW, D'Hooge R, De Deyn PP, Kooy RF. FVB.129P2-Pde6b(+) Tyr(c-ch)/Ant, a sighted variant of the FVB/N mouse strain suitable for behavioral analysis. Genes, Brain and Behavior. 2007;6:552–557. doi: 10.1111/j.1601-183X.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Hardan AY. A Meta-Analysis of the Corpus Callosum in Autism. BPS. 2009;66:935–941. doi: 10.1016/j.biopsych.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Keshavan MS, Minshew NJ, Hardan AY. A Two-Year Longitudinal MRI Study of the Corpus Callosum in Autism. J Autism Dev Disord. 2012 doi: 10.1007/s10803-012-1478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Horev G, Ellegood J, Lerch JP, Son Y-EE, Muthuswamy L, Vogel H, Krieger AM, Buja A, Henkelman RM, Wigler M, et al. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc. Natl. Acad. Sci. U.S.A. 2011;108:17076–17081. doi: 10.1073/pnas.1114042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu. Rev. Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Cofer GP, Fubara B, Gewalt SL, Hedlund LW, Maronpot RR. Magnetic resonance histology for morphologic phenotyping. J Magn Reson Imaging. 2002;16:423–429. doi: 10.1002/jmri.10175. [DOI] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42:515–525. [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kumar M, Kim S, Pickup S, Chen R, Fairless AH, Ittyerah R, Abel T, Brodkin ES, Poptani H. Longitudinal in-vivo diffusion tensor imaging for assessing brain developmental changes in BALB/cJ mice, a model of reduced sociability relevant to autism. Brain Res. 2012;1455:56–67. doi: 10.1016/j.brainres.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M, Durston S, Kas MJH, van Engeland H, Staal WG. The neurobiology of repetitive behavior: …and men. Neurosci Biobehav Rev. 2011a;35:356–365. doi: 10.1016/j.neubiorev.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Langen M, Kas MJH, Staal WG, van Engeland H, Durston S. The neurobiology of repetitive behavior: of mice…. Neurosci Biobehav Rev. 2011b;35:345–355. doi: 10.1016/j.neubiorev.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Lau JC, Lerch JP, Sled JG, Henkelman RM, Evans AC, Bedell BJ. Longitudinal neuroanatomical changes determined by deformation-based morphometry in a mouse model of Alzheimer's disease. Neuroimage. 2008;42:19–27. doi: 10.1016/j.neuroimage.2008.04.252. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Carroll JB, Dorr A, Spring S, Evans AC, Hayden MR, Sled JG, Henkelman RM. Cortical thickness measured from MRI in the YAC128 mouse model of Huntington's disease. Neuroimage. 2008;41:243–251. doi: 10.1016/j.neuroimage.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Sled JG, Henkelman RM. MRI phenotyping of genetically altered mice. Methods Mol. Biol. 2011a;711:349–361. doi: 10.1007/978-1-61737-992-5_17. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Yiu AP, Martinez-Canabal A, Pekar T, Bohbot VD, Frankland PW, Henkelman RM, Josselyn SA, Sled JG. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. Neuroimage. 2011b;54:2086–2095. doi: 10.1016/j.neuroimage.2010.09.086. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes, Brain and Behavior. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Mori S, Itoh R, Zhang J, Kaufmann WE, van Zijl PC, Solaiyappan M, Yarowsky P. Diffusion tensor imaging of the developing mouse brain. Magn Reson Med. 2001;46:18–23. doi: 10.1002/mrm.1155. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behavioural Brain Research. 2008;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behavioural Brain Research. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman BJ, Bishop J, Dazai J, Bock NA, Lerch JP, Feintuch A, Chen XJ, Sled JG, Henkelman RM. MR technology for biological studies in mice. NMR Biomed. 2007;20:291–303. doi: 10.1002/nbm.1142. [DOI] [PubMed] [Google Scholar]
- Nieman BJ, Bock NA, Bishop J, Chen XJ, Sled JG, Rossant J, Henkelman RM. Magnetic resonance imaging for detection and analysis of mouse phenotypes. NMR Biomed. 2005;18:447–468. doi: 10.1002/nbm.981. [DOI] [PubMed] [Google Scholar]
- Nieman BJ, Flenniken AM, Adamson SL, Henkelman RM, Sled JG. Anatomical phenotyping in the brain and skull of a mutant mouse by magnetic resonance imaging and computed tomography. Physiol. Genomics. 2006;24:154–162. doi: 10.1152/physiolgenomics.00217.2005. [DOI] [PubMed] [Google Scholar]
- O'Leary-Moore SK, Parnell SE, Godin EA, Dehart DB, Ament JJ, Khan AA, Johnson GA, Styner MA, Sulik KK. Magnetic resonance microscopy-based analyses of the brains of normal and ethanol-exposed fetal mice. Birth Defects Res. Part A Clin. Mol. Teratol. 2010;88:953–964. doi: 10.1002/bdra.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat. Rev. Neurosci. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- Pearson BL, Pobbe RLH, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, Blanchard RJ. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes, Brain and Behavior. 2011;10:228–235. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RLH, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+ tf/J mouse strain. Behavioural Brain Research. 2011;216:446–451. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RLH, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behavioural Brain Research. 2010;214:443–449. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J, Via E, Catani M, Mataix-Cols D. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol. Med. 2010;41:1539–1550. doi: 10.1017/S0033291710002187. [DOI] [PubMed] [Google Scholar]
- Ren T, Zhang J, Plachez C, Mori S, Richards LJ. Diffusion Tensor Magnetic Resonance Imaging and Tract-Tracing Analysis of Probst Bundle Structure in Netrin1- and DCC-Deficient Mice. Journal of Neuroscience. 2007;27:10345–10349. doi: 10.1523/JNEUROSCI.2787-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D, Wu H, Munne RA, Ashtari M, Alvir JM, Lerner G, Koreen A, Cole K, Bogerts B. Reduced caudate nucleus volume in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 1995;52:393–398. doi: 10.1001/archpsyc.1995.03950170067009. [DOI] [PubMed] [Google Scholar]
- Roullet FI, Wöhr M, Crawley JN. Female urine-induced male mice ultrasonic vocalizations, but not scent-marking, is modulated by social experience. Behavioural Brain Research. 2011;216:19–28. doi: 10.1016/j.bbr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS ONE. 2008a;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L, Crawley JN. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behavioural Brain Research. 2008b;187:371–378. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes, Brain and Behavior. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1999;23:613–624. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Sillitoe RV, Joyner AL. Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu. Rev. Cell Dev. Biol. 2007;23:549–577. doi: 10.1146/annurev.cellbio.23.090506.123237. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Smith DG, Rizzo SJS, Karras MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH, et al. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci Transl Med. 2012;4:131ra51. doi: 10.1126/scitranslmed.3003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010a;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, Sheng M, Crawley JN. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010b;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010c;171:1197–1208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring S, Lerch JP, Henkelman RM. Sexual dimorphism revealed in the structure of the mouse brain using three-dimensional magnetic resonance imaging. Neuroimage. 2007;35:1424–1433. doi: 10.1016/j.neuroimage.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Spring S, Lerch JP, Wetzel MK, Evans AC, Henkelman RM. Cerebral asymmetries in 12-week-old C57Bl/6J mice measured by magnetic resonance imaging. Neuroimage. 2010;50:409–415. doi: 10.1016/j.neuroimage.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: A systematic review and meta-analysis of structural magnetic resonance imaging studies. European Psychiatry. 2008;23:289–299. doi: 10.1016/j.eurpsy.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Stigler KA, McDonald BC, Anand A, Saykin AJ, McDougle CJ. Structural and functional magnetic resonance imaging of autism spectrum disorders. Brain Res. 2011;1380:146–161. doi: 10.1016/j.brainres.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DL, De Vita E, Roberts S, Turner R, Yousry TA, Ordidge RJ. High-resolution fast spin echo imaging of the human brain at 4.7 T: implementation and sequence characteristics. Magn Reson Med. 2004;51:1254–1264. doi: 10.1002/mrm.20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Adluru N, Ennis C, Tromp DPM, Destiche D, Doran S, Bigler ED, Lange N, Lainhart JE, Alexander AL. Diffusion Tensor Imaging in Autism Spectrum Disorder: A Review. Autism Res. 2012 doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven JS, De Cock P, Lagae L, Sunaert S. Neuroimaging of autism. Neuroradiology. 2010;52:3–14. doi: 10.1007/s00234-009-0583-y. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Crabbe JC. Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T/+ tf/tf has severely reduced hippocampal commissure and absent corpus callosum. Brain Res. 2003;971:47–54. doi: 10.1016/s0006-8993(03)02354-0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang J, Mori S, Nathans J. Axonal growth and guidance defects in Frizzled3 knock-out mice: a comparison of diffusion tensor magnetic resonance imaging, neurofilament staining, and genetically directed cell labeling. Journal of Neuroscience. 2006;26:355–364. doi: 10.1523/JNEUROSCI.3221-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes, Brain and Behavior. 2011;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Abrams DN, Zhang JY, Weber MD, Katz AM, Clarke AM, Silverman JL, Crawley JN. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiol Behav. 2012 doi: 10.1016/j.physbeh.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009;Chapter 8(Unit 8):24. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur. J. Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2011a;4:17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007a;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Curr Protoc Neurosci. 2011b;Chapter 8(Unit 8):26. doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int. J. Dev. Neurosci. 2007b;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ. The neurobiology of social recognition, approach, and avoidance. BPS. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Zhang J, van Zijl PCM, Mori S. Three-Dimensional Diffusion Tensor Magnetic Resonance Microimaging of Adult Mouse Brain and Hippocampus. Neuroimage. 2002;15:892–901. doi: 10.1006/nimg.2001.1012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.