Abstract
Purpose
Stage III designation in NWTS-5 (National Wilms Tumor Study–5) was determined by four pathologic criteria: positive lymph nodes (LNs), peritoneal implants, residual disease, and tumor rupture. The objective of this study was to determine the prognostic significance of each of the stage III criteria.
Patients and Methods
Children with stage III Wilms tumor (WT) treated in NWTS-5 were assessed for event-free (EFS) and overall survival (OS). Sites of relapse and molecular status of tumors are reported. EFS and OS are reported 8 years after diagnosis.
Results
There were 569 patients with local stage III favorable-histology (FH) WT in this analysis, of whom 109 had overall stage IV disease. LN involvement alone was the most frequent criterion for stage III designation (38%), followed by microscopic residual disease alone (20%), microscopic residual disease and LN involvement (14%), and spill or soilage alone (9%). The 8-year EFS and OS estimates for all patients with local stage III FHWT were 82% and 91%, respectively. Multivariate analysis demonstrated that both LN involvement (relative risk, 1.89; P = .005) and microscopic residual disease (relative risk, 1.87; P = .007) were predictive of EFS, and OS results were similar. There was no apparent difference in pattern of relapse according to stage III subtype. The rate of loss of heterozygosity was higher (6%) for those with positive LNs than for those without (2%; P = .05).
Conclusion
LN involvement and microscopic residual are the stage III criteria highly predictive of EFS and OS for patients with stage III FHWT. It is possible that in future studies, patients with different stage III criteria may receive different therapies.
INTRODUCTION
Risk-based treatment for children with Wilms tumor (WT) involves balancing maximum tumor control while minimizing treatment-related toxicity. Treatment is determined by several factors, including age, tumor weight, histopathology, disease stage, and loss of heterozygosity (LOH) for chromosomes 1p and 16q.1 In the National Wilms Tumor Group and Children's Oncology Group unilateral WT protocols, staging is determined after an initial surgical procedure (either tumor biopsy or unilateral nephrectomy and lymph node [LN] sampling).1,2 Tumor stage is a major determinant of therapy, with a significant augmentation of therapy in children with stage III tumors compared with stage I or II. The increased treatment includes both doxorubicin and abdominal irradiation, increasing the toxicity of therapy while improving event-free survival (EFS).3,4
The factors included in stage III designation in NWTS-5 (National Wilms Tumor Study-5) were as follows: LN involvement by tumor, peritoneal implants, residual disease (gross or microscopic), and tumor rupture or spill.2 Whereas previous studies have shown that these factors are associated with adverse outcome, the prognostic significance of an individual criterion or combinations of the criteria for patients with stage III disease who receive contemporary therapy has not been evaluated. This could further guide risk-based therapy in children with WT.5 It could also identify which factors are most critical to establish accurate staging. The objective of this study was to determine the prognostic significance of the stage III criteria in favorable-histology (FH) WT.
PATIENTS AND METHODS
NWTS-5 was a prospective study of the treatment and biology of WT and other renal cancers of childhood.2,6,7 Each institution obtained local institutional review board approval before enrolling patients onto this study. The primary hypotheses for NWTS-5 have been described previously.2,6–8 Patients underwent nephrectomy before chemotherapy using previously described surgical guidelines, unless the primary tumor was considered to be unresectable by the treating surgeon, in which case a biopsy was obtained followed by initiation of chemotherapy. A tumor stage was assigned using the NWTS Group (NWTSG) surgical-pathologic staging system. Several criteria led to the designation of stage III disease: LN involvement by tumor, peritoneal implants, residual disease (gross or microscopic), and tumor rupture or spill. Patients were assigned a local stage according to the locoregional extent of tumor and an overall stage based on the presence or absence of distant metastases. For example, a patient could have local stage II disease (completely resected tumor with no LN involvement, tumor spill, and negative surgical margins) and overall stage IV disease if lung metastasis was present. Patients received chemotherapy, flank or whole abdominal radiation therapy (XRT) according local stage, and XRT to other sites according to overall stage, as previously described.7,8
NWTS-5 opened in January 1996 and closed in June 2002; 2,596 patients were enrolled, 2,397 of whom had FH tumors. All patients registered in NWTS-5 with local stage III disease, including those with overall stage IV disease, were identified and reviewed. Patients who were administered prenephrectomy chemotherapy were considered to have local stage III disease but were not included in this analysis because the prognostic significance of other stage III criteria (LN involvement, tumor spillage) may have differed between the immediate nephrectomy and preoperative chemotherapy groups. Data maintained at the Data and Statistical Center of the NWTSG in Seattle, Washington, were reviewed after institutional review board approval.
Statistical Analysis
EFS and overall survival (OS) at 8 years after diagnosis were estimated using actuarial Kaplan-Meier methods.9 Univariate comparisons of EFS and OS between patient subgroups were made using the log-rank test.10 The joint effect of predictors on EFS and OS was estimated using Cox proportional hazards regression analyses.11 Patients with local stage III/disease stage III and patients with local stage III/disease stage IV were analyzed both as separate cohorts and combined. Descriptive statistics are presented for the sites of relapse and molecular status of the tumors.
RESULTS
There were 717 cases of patients with local stage III renal tumors enrolled onto NWTS-5. Histologic subtype in these patients was as follows: FH (n = 581), focal anaplasia (n = 8), diffuse anaplasia (n = 73), clear-cell sarcoma of the kidney (n = 41), rhabdoid tumor of the kidney (n = 13), and unknown (n = 1). Analysis of outcome data was restricted to those patients with FHWT. Twelve of the 581 patients were missing information on one of the five criteria for classification as local stage III disease: positive LNs, microscopic residual disease, macroscopic residual disease, diffuse spill, or peritoneal implants. These patients were excluded from the analysis, leaving 569 patients. Among these patients, 109 had overall stage IV disease. Median follow-up time of patients still undergoing follow-up was 7.2 years (minimum, 0.61 years; maximum, 13.3 years).
Different criteria leading to stage III designation were assessed. Overall, positive LNs occurred in 347 (61%), microscopic residual disease in 273 (48%), gross residual disease in 133 (23%), soilage or spill in 133 (23%), and peritoneal implants in 24 (4%) of 569 patients. Stage III criteria occurred in isolation or in combination with other stage III criteria. LN involvement alone was the most frequent criterion (38%), followed by microscopic residual disease alone (20%), combined microscopic residual disease and LN involvement (14%), and spill or soilage alone (9%). Other factors such as macroscopic residual disease were too uncommon to enable a meaningful assessment of prognostic significance.
Eight-year EFS and OS estimates for the 569 patients with local stage III FHWT were 82% and 91.0%, respectively (Fig 1A). Patients with distant metastatic disease (overall stage IV, n = 109) fared worse than patients with overall stage III disease (n = 460; Fig 1B). Eight-year EFS was 83% for overall stage III disease and 76% for overall stage IV (P = .048). Likewise, 8-year OS was 94% for overall stage III and 82% for overall stage IV (P < .001).
Fig 1.
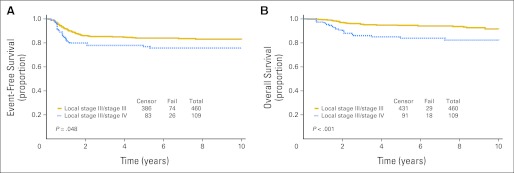
Kaplan-Meier curves for (A) event-free and (B) overall survival for local stage III favorable-histology Wilms tumor by overall stage.
Table 1 summarizes the univariate analysis of each individual criterion of all 569 patients with stage III disease. Univariate analysis did not detect any criterion that was statistically significantly associated with EFS or OS, applying a 5% significance level. Table 1 also summarizes EFS and OS estimates for subsets of patients with local stage III/overall stage III disease (n = 460). Univariate analysis did not detect a criterion that was predictive of outcome by the conventional P = .05 level of statistical significance. However, EFS (87% v 80%; P = .066) and OS (97% v 91%; P = .057) were less favorable in patients with LN involvement compared with those without LN involvement. In contrast, multivariate analysis demonstrated that the joint effect of LN involvement and microscopic residual disease was most predictive of outcome. For EFS, increased risk of failure was associated with LN involvement (relative risk, 1.89; P = .005) and microscopic residual disease (relative risk, 1.87; P = .007). Estimates of EFS for patients classified by the presence or absence of both positive LNs and microscopic residual disease are provided in Table 2 and Figure 2A. This difference was also seen in the subset of patients with local stage III/stage III disease (n = 460; LN involvement: relative risk, 2.24; P = .003; microscopic residual disease: relative risk, 2.08; P = .005). In contrast, LN involvement and microscopic residual disease were not predictive of EFS in the subset of patients with local stage III/stage IV disease (N = 109; LN involvement: relative risk, 0.64; P = .38; microscopic residual disease: relative risk, 1.31; P = .52), although the number of failures in this subset was small.
Table 1.
Univariate Analysis of EFS and OS Estimates
| Criterion | No. of Patients | 8-Year EFS (%) | P | 8-Year OS (%) | P |
|---|---|---|---|---|---|
| All patients with stage III disease (overall stage III and IV) | |||||
| All patients | 569 | 82 | 91 | ||
| Lymph nodes | .11 | .10 | |||
| Negative | 222 | 85 | 94 | ||
| Positive | 347 | 79 | 90 | ||
| Microscopic residual disease | .08 | .33 | |||
| Negative | 296 | 84 | 92 | ||
| Positive | 273 | 79 | 91 | ||
| Gross residual disease | .55 | .60 | |||
| Negative | 521 | 81 | 91 | ||
| Positive | 48 | 85 | 91 | ||
| Soilage-diffuse spill | .28 | .30 | |||
| Negative | 436 | 81 | 91 | ||
| Positive | 133 | 85 | 94 | ||
| Peritoneal implants | .24 | .48 | |||
| Negative | 545 | 81 | 91 | ||
| Positive | 24 | 92 | 96 | ||
| Subsets of patients with local stage III/overall stage III disease | |||||
| All patients | 460 | 83 | 94 | ||
| Lymph nodes | .066 | .057 | |||
| Negative | 205 | 87 | 97 | ||
| Positive | 255 | 80 | 91 | ||
| Microscopic residual disease | .14 | .82 | |||
| Negative | 236 | 86 | 93 | ||
| Positive | 224 | 81 | 94 | ||
| Gross residual disease | .22 | .12 | |||
| Negative | 426 | 82 | 93 | ||
| Positive | 34 | 90 | 100 | ||
| Diffuse spill | .35 | .26 | |||
| Negative | 339 | 82 | 93 | ||
| Positive | 121 | 86 | 97 | ||
| Peritoneal implants | .18 | .23 | |||
| Negative | 440 | 83 | 93 | ||
| Positive | 20 | 95 | 100 |
Abbreviations: EFS, event-free survival; OS, overall survival.
Table 2.
Combined Effect of LN Involvement and Microscopic Residual Disease
| Status (microscopic residual disease/LNs) | No. of Patients | 8-Year EFS (%) | P | 8-Year OS (%) | P |
|---|---|---|---|---|---|
| Stage III (with and without distant metastatic disease) | .008 | .07 | |||
| Negative/negative | 52 | 90 | 95 | ||
| Negative/positive | 244 | 83 | 91 | ||
| Positive/negative | 170 | 84 | 94 | ||
| Positive/positive | 103 | 71 | 86 | ||
| Stage III (no distant metastatic disease) | .004 | .14 | |||
| Negative/negative | 49 | 91 | 94 | ||
| Negative/positive | 187 | 84 | 92 | ||
| Positive/negative | 156 | 86 | 96 | ||
| Positive/positive | 68 | 69 | 89 |
Abbreviations: EFS, event-free survival; LN, lymph node; OS, overall survival.
Fig 2.
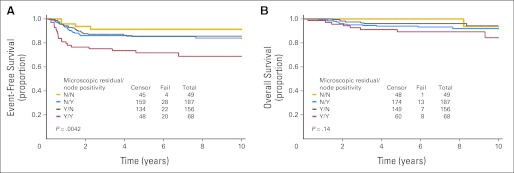
Kaplan-Meier curves for (A) event-free and (B) overall survival for local stage III favorable-histology Wilms tumor by microscopic disease, nonmetastatic only.
A generally similar pattern was observed for OS for all patients with local stage III disease (LN involvement: relative risk, 2.25; P = .02; microscopic residual disease: relative risk, 1.85; P = .05) and for the local stage III/stage III disease subset (LN involvement: relative risk, 2.79; P = .02; microscopic residual disease: relative risk, 1.71; P = .19). The small number of deaths observed, even in this large group of patients, makes the power to detect modest differences in survival small. Kaplan-Meier estimates of survival for the groups defined by LN involvement and microscopic residual disease are shown in Figure 2B.
The sites of relapse for the stage III subgroups were assessed (Table 3). There were 155 relapses, of which 86 (55%) were in the lung, 22 (14%) were in abdomen or pelvis, 17 were in the liver, and 30 were in other sites. There was no apparent difference in pattern of relapse according to stage III subtype. Of 125 relapses in patients with LN involvement and/or microscopic residual disease, only 17 (14%) involved the abdomen or pelvis.
Table 3.
Sites of Relapse
| Stage III Characteristic | No. of Treatment Failures | Abdomen or Pelvis |
Liver |
Lung ± Other* |
Other |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| Gross residual disease | 7 | 2 | 29 | 1 | 14 | 3 | 43 | 1 | 14 |
| LN positivity | 68 | 8 | 12 | 7 | 10 | 43 | 63 | 10 | 15 |
| Microscopic residual disease | 57 | 9 | 16 | 7 | 12 | 30 | 53 | 11 | 19 |
| Diffuse spill | 20 | 2 | 10 | 2 | 10 | 9 | 45 | 7 | 39 |
| Peritoneal implants | 3 | 1 | 1 | 1 | |||||
| Total | 155 | ||||||||
Abbreviation: LN, lymph node.
None of these other sites included abdominal or pelvic relapses.
A primary objective of NWTS-5 was to evaluate the prognostic significance of LOH at chromosomes 1p and 16q. LOH at both loci was found to be predictive of relapse and death.8 LOH status in the stage III subsets was assessed, as summarized in Table 4. The rate of LOH at both 1p and 16q was higher (6%) for those with positive LNs than for those without (2%; P =.05).
Table 4.
Molecular Status of Stage III Tumors
| Stage III Characteristic | No. of Patients | 1p+/16q+ |
1p+/16q− |
1p−/16q+ |
1p−/16q− |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| Gross residual disease | 43 | 0 | 0 | 1 | 2 | 7 | 16 | 35 | 81 |
| LN positivity | 299 | 18 | 6 | 27 | 9 | 37 | 12 | 217 | 73 |
| Microscopic residual disease | 228 | 7 | 3 | 18 | 8 | 32 | 14 | 171 | 75 |
| Diffuse spill | 107 | 2 | 2 | 4 | 4 | 20 | 19 | 81 | 76 |
| Peritoneal implants | 21 | 1 | 5 | 0 | 0 | 3 | 14 | 17 | 81 |
Abbreviation: LN, lymph node.
DISCUSSION
Current treatment protocols for children with WT were developed through a series of multidisciplinary cooperative group trials.4,7,12,13 These prospective randomized studies have provided a large body of data for the definition of optimal surgical, radiotherapy, and chemotherapy treatments. Treatment is based on factors associated with an increased risk of relapse, on stage and histology, and more recently on LOH at 1p and 16q. The goal of treatment (ie, risk-based management) is identification of approaches that maintain excellent outcomes for children with low-risk tumors without the use of anthracycline chemotherapy or XRT or, in some cases, without chemotherapy at all. Conversely, therapy may be augmented for children with high-risk tumors in an effort to improve their survival.
Treatment is augmented significantly on NWTS/Children's Oncology Group (COG) protocols for children with stage III local disease with the addition of doxorubicin and abdominal or flank irradiation.1,2,7 If a child's tumor demonstrates LOH for chromosome 1p and 16q, even more agents are added. The addition of therapy substantially increases the risk of short- and long-term toxicities, such as congestive heart failure, coronary artery disease, impaired renal function, second malignancies, and adverse pregnancy outcomes.15–21 Analysis of outcomes based on risk factors is a dynamic process. It is possible that as knowledge and therapies evolve, risk factors may become more or less prognostically important. In the NWTSG/COG staging, there are several clinicopathologic criteria that result in stage III designation. These are LN involvement by tumor, peritoneal implants, residual disease (gross or microscopic), and tumor rupture. The presence of any or all of these criteria results in the same therapy. Earlier NWTS studies showed that LN involvement was among the most important prognostic factors, but whether any of the individual or combinations of stage III criteria is more prognostic of outcome has not been assessed in the context of modern therapy.5
Our univariate analysis indicated that EFS and OS were not significantly associated with individual stage III criteria among patients with local stage III FHWT. In contrast, the combination of LN positivity and microscopic residual disease had a significant negative impact on EFS and OS. Although the P values differed slightly according to whether patients with distant metastatic disease were included in the analysis (Table 2), the magnitude of the effect was similar whether or not patients with overall stage IV disease were included.
The finding from the multivariate analysis that combined LN positivity and microscropic residual disease is associated with poorer EFS is thought provoking, but caution must be exercised before changing treatment recommendations. If a patient with stage III disease does not have positive LNs and microscopic residual disease, reducing anthracylines or XRT may be considered to reduce long-term complications.22,23 However, it is possible that the excellent survival rates were achieved because of the administration of doxorubicin and XRT. Conversely, one could consider augmenting therapy in patients with both positive LNs and microscropic residual disease. However, the differences in OS between patients with and without positive LNs and microscopic residual disease were not statistically significant. It is not clear whether the changes in mortality are large enough to warrant changes in our well-established protocols.
It is curious that macroscopic residual disease was not associated with prognosis yet microscopic residual disease was. Gross residual disease after primary nephrectomy is rare. Only one patient of 569 had gross residual disease as the only reason for stage III designation, and only 41 of 569 patients had gross residual disease in combination with another criterion. It is likely that there are too few patients with gross residual disease to adequately assess the prognostic significance of this stage III criterion.
Patients who received prenephrectomy chemotherapy were considered to have stage III disease; however, we chose not to include this group in our analysis for several reasons. The NWTSG staging system is surgical, based on pathology, with primary nephrectomy as initial treatment if possible. In patients who received prior chemotherapy and then underwent nephrectomy, there would have been a treatment effect on the tumor as well as the LNs, and one could not be certain whether the nodes were originally negative. Although the International Society of Pediatric Oncology uses radiographic imaging to stage patients, the NWTSG and COG are not comfortable with this type of staging, especially in analyzing spill and LN status. Reports by Gow et al25 and Otherson et al26 clearly highlight this problem. A recent study by Khanna et al27 using the COG AREN03B2 database of 3,000 patient cases found the preoperative imaging of tumor rupture to have a sensitivity and specificity of detecting WT rupture of only 53.7% and 88.4%, respectively, compared with the gold standard at operative finding. A preliminary report with LNs found the same thing. This would clearly affect staging; thus, we excluded these patients.
If a stage III criterion predicted worse outcome, it is possible that the site of relapse may relate to that criterion. For example, if spill or soilage resulted in poorer outcome, there might be more local recurrences with spill or soilage as compared with the other stage III criteria. Our analysis did not support this premise; there were 155 relapses but no correlation between relapse site and stage III criteria (Tables 1 and 3).
One aim of NWTS-5 was to understand the significance of LOH at 1p and 16q. Grundy et al8 showed that presence of LOH at 1p and 16q was associated with worse EFS and OS. We analyzed molecular differences in the tumors from the different subgroups of stage III and saw no major differences in frequency of LOH at 1p and 16q, although the rate of LOH positivity was higher for those with positive LNs (6%) than for those without (2%; P = .05).
LN sampling has been the subject of prior manuscripts. A previous study identified failure to sample LNs as the most common protocol deviation.28 Shamberger et al29 reported that patients who did not have LNs sampled had an increased risk of abdominal recurrence. The risk was greatest for patients with stage I disease, suggesting that some patients were undertreated. Furthermore, studies have demonstrated a higher risk of recurrence in children who did not have their LN status documented at time of nephrectomy.29–31
The limitations of this study are inherent in its design. This study is retrospective in nature, and the outcomes need to be validated in a prospectively collected data set. The current COG renal tumor study AREN03B2 data set will allow this question to be addressed. This study also includes real-time central review, which will help with accuracy of staging. The data presented here are from patients treated in NWTS-5. We did not evaluate the relationship between number of LNs sampled and outcome. Because the outcomes for children with WT are still favorable, we do not recommend altering therapy at this time. However, the results do suggest further investigation to determine if therapy should be adjusted for different types of stage III local disease.
In summary, LN involvement and microscopic residual disease are the stage III criteria combination most predictive of EFS and OS in stage III FHWT. Future studies that prescribe different therapies for different types of stage III disease should be considered.
Acknowledgment
We thank the many pathologists, surgeons, pediatricians, radiation oncologists, and other health professionals who managed the children enrolled onto the National Wilms Tumor Studies.
Footnotes
Supported by Grants No. CA42326 (to the National Wilms Tumor Study) and CA98543 (to the Children's Oncology Group) from the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00002610.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Peter F. Ehrlich, James R. Anderson, Jeffrey S. Dome, Daniel M. Green, Paul E. Grundy, Elizabeth J. Perlman
Provision of study materials or patients: Norman E. Breslow
Collection and assembly of data: Peter F. Ehrlich, James R. Anderson, Daniel M. Green, Norman E. Breslow
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Grundy PE, Dome JS, Ehrlich PF, et al. Renal tumors classification, biology and banking studies. http://www.childrensoncologroup.org.
- 2.Green DM. National Wilms Tumor Study Group–5 protocol, 1994. http://www.childrensoncologroup.org.
- 3.Breslow N, Ou SS, Beckwith JB, et al. Doxorubicin for favorable histology, stage II-III Wilms tumor: Results from the National Wilms Tumor Studies. Cancer. 2004;101:1072–1108. doi: 10.1002/cncr.20433. [DOI] [PubMed] [Google Scholar]
- 4.Green DM. The treatment of stages I-IV favorable histology Wilms' tumor. J Clin Oncol. 2004;22:1366–1372. doi: 10.1200/JCO.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Breslow N, Sharples K, Beckwith JB, et al. Prognostic factors in nonmetastatic, favorable histology Wilms' tumor: Results of the Third National Wilms' Tumor Study. Cancer. 1991;68:2345–2353. doi: 10.1002/1097-0142(19911201)68:11<2345::aid-cncr2820681103>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Green DM, Breslow N, Evans I, et al. Treatment of children with stage IV favorable histology Wilms tumor: A report from the National Wilms Tumor Study Group. Med Pediatr Oncol. 1996;26:147–152. doi: 10.1002/(SICI)1096-911X(199603)26:3<147::AID-MPO1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Dome JS, Cotton CA, Perlman EJ. Treatment of anaplastic histology Wilms' tumor: Results from the fifth National Wilms' Tumor Study. J Clin Oncol. 2006;24:2352–2358. doi: 10.1200/JCO.2005.04.7852. [DOI] [PubMed] [Google Scholar]
- 8.Grundy PE, Breslow NE, Li S, et al. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol. 2005;23:7312–7321. doi: 10.1200/JCO.2005.01.2799. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 10.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A. 1972;135:185–206. [Google Scholar]
- 11.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 12.Green DM, Beckwith JB, Breslow NE, et al. Treatment of children with stages II to IV anaplastic Wilms tumor: A report from the National Wilms' Tumor Study Group. J Clin Oncol. 1994;12:2126–2131. doi: 10.1200/JCO.1994.12.10.2126. [DOI] [PubMed] [Google Scholar]
- 13.Green DM, Breslow NE, Beckwith JB, et al. Treatment with nephrectomy only for small, stage I/favorable histology Wilms' tumor: A report from the National Wilms' Tumor Study Group. J Clin Oncol. 2001;19:3719–3724. doi: 10.1200/JCO.2001.19.17.3719. [DOI] [PubMed] [Google Scholar]
- 14. Reference deleted.
- 15.Breslow NE, Lange JM, Friedman DL, et al. Secondary malignant neoplasms after Wilms tumor: An international collaborative study. Int J Cancer. 2010;127:657–666. doi: 10.1002/ijc.25067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotton CA, Peterson S, Norkool PA. Early and late mortality after diagnosis of Wilms tumor. J Clin Oncol. 2009;27:1304–1309. doi: 10.1200/JCO.2008.18.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green DM, Grigoriev YA, Nan B, et al. Congestive heart failure after treatment for Wilms' tumor: A report from the National Wilms' Tumor Study group. J Clin Oncol. 2001;19:1926–1934. doi: 10.1200/JCO.2001.19.7.1926. [DOI] [PubMed] [Google Scholar]
- 18.Green DM, Grigoriev YA, Nan B, et al. Correction to “Congestive heart failure after treatment for Wilms' tumor.”. J Clin Oncol. 2003;21:2447–2448. doi: 10.1200/JCO.2003.99.005. 2447-8. [DOI] [PubMed] [Google Scholar]
- 19.Warwick AB, Kalapurakal JA, Ou SS. Portal hypertension in children with Wilms' tumor: A report from the National Wilms' Tumor Study Group. Int J Radiat Oncol Biol Phys. 2010;77:210–216. doi: 10.1016/j.ijrobp.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Dijk IW, Oldenburger F, Cardous-Ubbink MC. Evaluation of late adverse events in long-term Wilms' tumor survivors. Int J Radiat Oncol Biol Phys. 2010;78:370–378. doi: 10.1016/j.ijrobp.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Green DM, Lange JM, Peabody EM. Pregnancy outcome after treatment for Wilms tumor: A report from the national Wilms tumor long-term follow-up study. J Clin Oncol. 2010;28:2824–2830. doi: 10.1200/JCO.2009.27.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Dalen E, Raphaël MF, Caron HN, et al. Treatment including anthracyclines versus treatment not including anthracyclines for childhood cancer. Cochrane Database Syst Rev. 2011;1:CD006647. doi: 10.1002/14651858.CD006647.pub3. [DOI] [PubMed] [Google Scholar]
- 23.van Dalen EC, van den Berg H, Raphaël MF, et al. Should anthracyclines and dexrazoxane be used for children with cancer? Lancet Oncol. 2011;12:12–13. doi: 10.1016/S1470-2045(10)70301-6. [DOI] [PubMed] [Google Scholar]
- 24. Reference deleted.
- 25.Gow K, Roberts IF, Jamieson DH, et al. Local staging of Wilms' tumor: Computerized tomography correlation with histological findings. J Pediatr Surg. 2000;35:677–679. doi: 10.1053/jpsu.2000.5941. [DOI] [PubMed] [Google Scholar]
- 26.Othersen HB Jr, DeLorimer A, Hrabovsky E, et al. Surgical evaluation of lymph node metastases in Wilms tumor. J Pediatr Surg. 1990;25:330–331. doi: 10.1016/0022-3468(90)90079-o. [DOI] [PubMed] [Google Scholar]
- 27.Khanna G, Rosen N, Anderson JR, et al. Evaluation of diagnostic performance of CT for detection of tumor thrombus in children with Wilms tumor: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2012;58:551–555. doi: 10.1002/pbc.23222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehrlich PF, Ritchey ML, Hamilton TE, et al. Quality assessment for Wilms' tumor: A report from the National Wilms' Tumor Study-5. J Pediatr Surg. 2005;40:208–212. doi: 10.1016/j.jpedsurg.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 29.Shamberger RC, Guthrie KA, Ritchey ML, et al. Surgery related factors and local reccurance of Wilms tumor in the National Wilms Tumor Study 4. Ann Surg. 1999;229:292–297. doi: 10.1097/00000658-199902000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross RE. Embryoma of the kidney (Wilms tumor) In: Gross RE, editor. The Surgery of Infancy and Childhood. Philadelphia, PA: WB Saunders; 1953. pp. 588–605. [Google Scholar]
- 31.Raval MV, Bilimoria KY, Bentrem DJ, et al. Nodal evaluation in Wilms' tumors: Analysis of the National Cancer Data Base. Ann Surg. 2010;251:559–565. doi: 10.1097/SLA.0b013e3181cc95d7. [DOI] [PubMed] [Google Scholar]


