Abstract
The Saccharomyces cerevisiae Rad1/Rad10 complex is a multifunctional, structure-specific endonuclease that processes UV-induced DNA lesions, recombination intermediates, and inter-strand DNA crosslinks. However, we do not know how Rad1/Rad10 recognizes these structurally distinct target molecules or how it is incorporated into the protein complexes capable of incising divergent substrates. Here, we have determined the order and hierarchy of assembly of the Rad1/Rad10 complex, Saw1, Slx4, and Msh2/Msh3 complex at a 3′ tailed recombination intermediate. We found that Saw1 is a structure-specific DNA binding protein with high affinity for splayed arm and 3′-flap DNAs. By physical interaction, Saw1 facilitates targeting of Rad1 at 3′ tailed substrates in vivo and in vitro, and enhances 3′ tail cleavage by Rad1/Rad10 in a purified system in vitro. Our results allow us to formulate a model of Rad1/Rad10/Saw1 nuclease complex assembly and 3′ tail removal in recombination.
Keywords: double strand break, nuclease, Saccharomyces cerevisiae , Saw1, single-strand annealing
Introduction
Environmental and metabolic stress induces DNA damage that interferes with essential DNA transactions such as DNA replication and chromosome segregation. Multiple mechanisms and their affiliated DNA metabolizing enzymes are responsible for sensing and repairing DNA damage efficiently and faithfully. The yeast Rad1/Rad10 complex is a nuclease that participates in multiple repair pathways including nucleotide excision repair (NER), base excision repair (BER), inter-strand crosslink repair (ICLR), and homologous recombination (HR) (reviewed in Ciccia et al, 2008). In NER, Ssl2/Rad3 (XPB/D in humans) unwinds DNA containing DNA lesions, Rad1/Rad10 (XPF/ERCC1 in humans) then incises the damaged DNA strand on the 5′ side, concurrently with a 3′ incision made by Rad2 (XPG in humans), producing a gapped DNA intermediate that can be filled by a DNA polymerase (Sijbers et al, 1996; de Boer and Hoeijmakers, 2000; Prakash and Prakash, 2000). In HR, Rad1/Rad10 (and XPF/ERCC1) cleaves 3′ non-homologous tails after annealing of flanking repeats in single-strand annealing (SSA) or from the D-loop structure that harbours an invading DNA end with a non-homologous sequence (Schiestl and Prakash, 1990; Fishman-Lobell and Haber, 1992; Tomkinson et al, 1993; Adair et al, 2000). Removal of the 3′ non-homologous DNA tail is a prerequisite for the initiation of repair DNA synthesis. In BER, Rad1/Rad10 plays a redundant or back-up role to Apn1, Apn2, and Mus81/Mms4, since any of the above can cleave a 3′ blocked end as a 3′-flap endonuclease (Boiteux and Guillet, 2004; Guzder et al, 2004). In ICLR, Rad1/Rad10 is hypothesized to either unhook the crosslink or to remove a non-homologous DNA tail to restore the replication fork by HR (Bergstralh and Sekelsky, 2008).
The versatility of Rad1/Rad10 for a wide range of DNA lesions and structures poses a unique question: how does Rad1/Rad10 recognize these different substrate molecules and how is its nuclease activity regulated to enhance the specificity for these molecules? The available evidence has hinted that substrate specificity of Rad1/Rad10 might be dictated by its interaction partners, some of which recruit and position Rad1/Rad10 at the cleavage target. For instance, Rad14 (XPA in mammals) interacts with Rad1/Rad10 and targets it to UV lesions (Li et al, 1995; Guzder et al, 2006). However, the mechanisms for targeting Rad1/Rad10 (and XPF/ERCC1) to recombination, BER substrates and intermediates have not been determined.
Rad1/Rad10 interacts with many proteins during HR, some of which may target Rad1/Rad10 to recombination intermediates, while others may modulate the enzyme activity or the substrate stability. The Rad52 and Rad59 proteins likely contribute to 3′ tail cleavage by mediating the annealing of complementary single-stranded DNA (ssDNA) strands, a prerequisite for Rad1/Rad10 targeting (Lyndaker and Alani, 2009). In human cells, Rad52 directly interacts with ERCC1, and this interaction stimulates XPF/ERCC1 activity in processing 3′ non-homologous tailed substrates (Motycka et al, 2004). Msh2/Msh3 was initially proposed to recognize recombination intermediates containing a 3′-flap, thereby recruiting Rad1/Rad10 to the 3′ tail or stabilizing the complex of Rad1/Rad10 and DNA (Kirkpatrick and Petes, 1997). However, inactivating Msh2 or Msh3 has no effect on SSA between longer repeats (>1 kb). Instead, a role for Msh2/Msh3 in stabilizing annealed intermediates prior to 3′ tail cleavage has been invoked (Sugawara et al, 1997). Slx4 is a subunit of the Slx1/Slx4 complex that was originally identified in a genetic screen for synthetic lethality with the Sgs1 helicase. In addition, it has recently been implicated in SSA and 3′ non-homologous tail removal independent of Slx1 (Mullen et al, 2001; Flott et al, 2007). Interestingly, Slx4 is a phosphoprotein and its phosphorylation depends on DSBs and on the Mec1 and Tel1 kinases (Flott and Rouse, 2005; Flott et al, 2007). DNA damage-induced phosphorylation of Slx4 plays an essential role in SSA repair of DNA breaks but the precise mechanism is not yet known (Toh et al, 2010). Recently, Slx4 has been implicated in targeting ERCC1-XPF to ICL repair substrates by physical interaction (Andersen et al, 2009; Fekairi et al, 2009; Munoz et al, 2009; Svendsen et al, 2009; Crossan et al, 2011; Kim et al, 2011; Stoepker et al, 2011). Finally, Saw1 was identified from a genetic screen for novel proteins involved in SSA and was shown to physically interact with multiple factors involved in 3′ tail cleavage (Li et al, 2008). Saw1 is a strong candidate for targeting Rad1/Rad10 to 3′ tailed recombination intermediates because deletion of SAW1 or expression of saw1 mutants deficient in interaction with Rad1/Rad10 abrogates binding of Rad1/Rad10 at 3′ tails and blocks SSA (Li et al, 2008).
The recent identification of several new factors required for 3′ tail cleavage during HR raises questions about the precise function of these factors in Rad1/Rad10-mediated 3′ tail removal and the coordination among these proteins. How is the recruitment and assembly on the DNA substrate regulated and properly orchestrated to produce the specific cleavage pattern? Indeed, what are the components of the protein machinery that mediates 3′ tail removal? We have approached the above questions by determining the temporal and spatial protein recruitment patterns at 3′-flap recombination intermediates and also their association hierarchy using chromatin immunoprecipitation (ChIP) and live-cell imaging. We have assessed the DNA binding activity of Saw1 using electrophoretic mobility shift assay (EMSA) and by ChIP. We demonstrate that Saw1 contributes to 3′ tail removal as a mediator between a 3′ DNA flap substrate and Rad1/Rad10. Collectively, our results suggest a model for how the 3′ tail removal machinery is assembled onto DNA and shed light on the molecular steps in processing 3′ tailed DNA intermediates.
Results
Recruitment of nuclease deficient rad1 to 3′ tailed recombination intermediates requires Saw1, Msh2, but does not Slx4
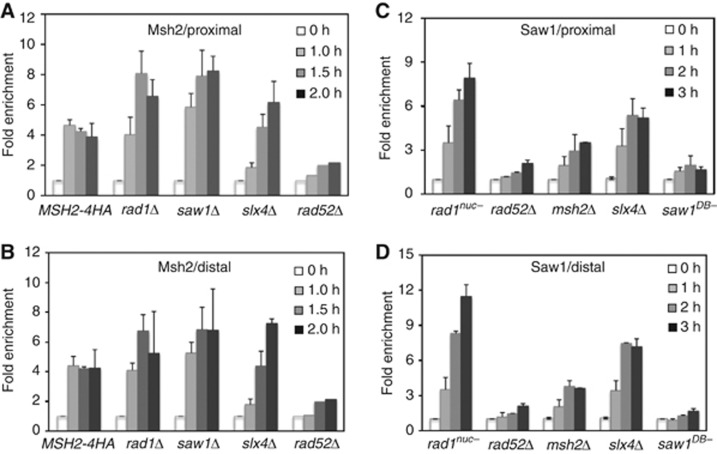
In vivo studies have shown that 3′ tail removal during recombination requires at least 12 proteins: Rad1, Rad10, Msh2, Msh3, Rad52, Rad59, Saw1, Slx4, Srs2, and Rpa1, 2, and 3. All but Saw1 have a known DNA binding activity and likely target, position, and/or regulate Rad1/Rad10-mediated 3′-flap cleavage. To elucidate their role(s) in the early steps of 3′ tail removal, we examined Rad1 recruitment to a 3′ tail in strains lacking Rad52, Rad10, Msh2, Saw1, or Slx4. Association of nuclease proficient Rad1 at 3′ tails in wild-type cells was undetectable by ChIP, presumably due to the rapid dissociation of Rad1/Rad10 following cleavage (see Supplementary Figure S3A). We therefore used ChIP to examine the association of nuclease-deficient variants of Rad1 (rad1nuc-) at the 3′-flap after the HO-endonuclease-mediated induction of a DSB that is flanked by 205 bp of ura3 repeats (Li et al, 2008) (Figure 1B). We predicted that the nuclease-deficient rad1 would stably associate with the target DNA to allow for its detection by ChIP.
Figure 1.
Recruitment of rad1nuc- to 3′ non-homologous tail carrying recombination intermediates. (A) Domain structure of Rad1 and the location of rad1 mutations that disrupt nuclease activity. (B) Schematic illustration of HO break and the flanking repeats in tNS1379/EAY1141 or YMV80 strain used for ChIP assay. Location of primers and the size of the repeats are shown. Paired arrows indicate primers used for ChIP assay. (C, D) The requirements of Rad10, Rad52, Msh2, Saw1, and Slx4 for the recruitment of rad1nuc- to the proximal (pJC1 and pJC2) (C) or distal (pJC3 and pJC4) (D) side of the 3′-flap site in tNS1379 strain. Association of rad1nuc- to the 3′-flap 6 h post HO induction in the msh2Δ derivative of YMV80 strains is also shown (D). Fold enrichment represents the ratio of the rad1 IP PCR signal before and after HO induction, normalized by the PCR signal of the MAT control. Data represent the mean±s.d. of three or more independent experiments.
To this end, we generated three rad1 mutants, all of which carry amino-acid substitutions at putative nuclease active sites that are conserved from yeast to human (Enzlin and Scharer, 2002) (Figure 1A). Two of the three mutant proteins (D825A, K865A) were purified and biochemically confirmed to be deficient in 3′-flap cleavage despite retaining DNA-binding activity (Supplementary Figure S1D). Expression of the mutant rad1 proteins in rad1Δ did not alleviate UV sensitivity (due to impaired NER) or restore SSA repair of a DNA break flanked by 205-bp ura3 repeats (Supplementary Figure S2). We also confirmed that these rad1 variants were expressed at the normal level and retain physical interactions with both Rad10 and Saw1 (Supplementary Figure S3B). Most importantly, all three rad1 mutants exhibited strong enrichment at 3′ tails within 1 h after HO expression and remain associated for up to 4 h, providing direct evidence that nuclease deficiency results in Rad1 accumulation at 3′ tailed intermediates (Supplementary Figure S3C and D).
We then used ChIP to examine the effect of deleting individual 3′ tail removal genes on the retention of rad1 mutants (rad1nuc-) at the HO break. We discovered that recruitment of rad1nuc- relies on Rad10, Rad52, and Saw1 (Figure 1C and D). The results suggest that dimerization with Rad10, annealing of complementary ssDNA by Rad52, and targeting of Rad1/Rad10 nuclease to recombination intermediates by Saw1 are important for 3′ non-homologous tail removal complex assembly. Interestingly, the effect of MSH2 gene deletion on rad1nuc- recruitment is complex and depends on the length of repeats; when repeats are 205-bp long, Msh2 is required (Figure 1C and D), but when they are 1-kb long, Msh2 is not needed (see Figure 1D, YMV80). The results are consistent with Msh2 not being required for SSA between 1-kb repeats (Supplementary Figure S4) and with the proposed role for Msh2/Msh3 in stabilizing products made by annealing short complementary DNA strands (Sugawara et al, 1997).
Slx4 is implicated in Rad1-dependent 3′ non-homologous tail cleavage and SSA independently of Slx1 (Flott et al, 2007; Li et al, 2008; Lyndaker et al, 2008). We discovered that rad1nuc- is recruited to recombination intermediates in slx4Δ cells with kinetics similar to wild type (Figure 1C and D). Interestingly, the recruitment of Rad1 is slightly faster on the proximal side of the break in slx4 mutant (Figure 1C). The results suggest that Slx4 is dispensable for Rad1 recruitment. Collectively, the results suggest that recruitment of Rad1 to 3′ tails requires the combined action of multiple tail removal factors, but not Slx4.
Recruitment of Msh2 to 3′ non-homologous tails depends on Rad52, but not other tail removal factors
We have previously shown that Msh2/Msh3 binds branched substrates in vitro, and hypothesized that it enhances the accessibility of DNA to cleavage by Rad1/Rad10 (Surtees and Alani, 2006). To test this model, we used ChIP to monitor Msh2 recruitment at 3′ tails formed by 205-bp ura3 or 1-kb leu2 repeats flanking the HO break. Msh2 (tagged with four HA epitopes) was rapidly recruited to both sides of the recombination intermediate, as previously observed (Evans et al, 2000; Lyndaker and Alani, 2009), whereas the deletion of RAD52 abrogated this binding (Figure 2). The deletion of RAD1, RAD10, SAW1, or SLX4 actually enhanced binding of Msh2 to the DNA tails, although the kinetics of localization appear altered in slx4Δ. We reasoned that enhanced Msh2 binding in these mutants stems from the lack of tail removal causing persistent accumulation of Msh2 (Evans et al, 2000; Lyndaker and Alani, 2009). Based on the above results, we could deduce that the assembly of tail removal complex requires binding of Rad52, then Msh2/Msh3, and is finally completed with the Saw1-dependent recruitment of Rad1/Rad10.
Figure 2.
Recruitment of Msh2 (A, B) and Saw1 (C, D) to 3′ non-homologous tail carrying recombination intermediates. The recruitment of Msh2 to the centromere distal (A) or the proximal (B) side of the 3′-flap in EAY1141 or its mutant derivatives. The recruitment of Saw1 to the proximal (C) or distal (D) side of the 3′-flap in the EAY1141 strain expressing wild-type or mutant saw1 proteins using ChIP assays. Fold enrichment is calculated as described in Figure 1. Data represent the mean±s.d. of three or more independent experiments.
Saw1 associates with 3′-flaps in vivo
The requirement of Saw1 for rad1nuc- recruitment to 3′ tails could be mediated through interaction between Saw1 and the DNA substrate. To address this question, we used ChIP to ask whether Saw1 associates with 3′ tailed intermediates. We found that Saw1 is recruited to 3′ tails with kinetics similar to that of rad1nuc- (Figure 2C and D).
We also examined Saw1 association at 3′-flaps in strains deleted for one of the other 3′ tail removal genes. We found that recruitment of Saw1 also depends on RAD52 and MSH2 (Figure 2C and D). Again, Slx4 is dispensable for enrichment of Saw1 at the 3′ tailed DNA. Finally, association of Saw1 with the 3′ intermediate is abolished by a mutation (saw1DB-) that disrupts its DNA-binding activity (Figure 2C and D, and see below). Importantly, this mutation prevents recruitment of rad1nuc- to the 3′ tails (Figure 5B). These results thus revealed that Saw1 targets Rad1/Rad10 to 3′ tailed recombination intermediates.
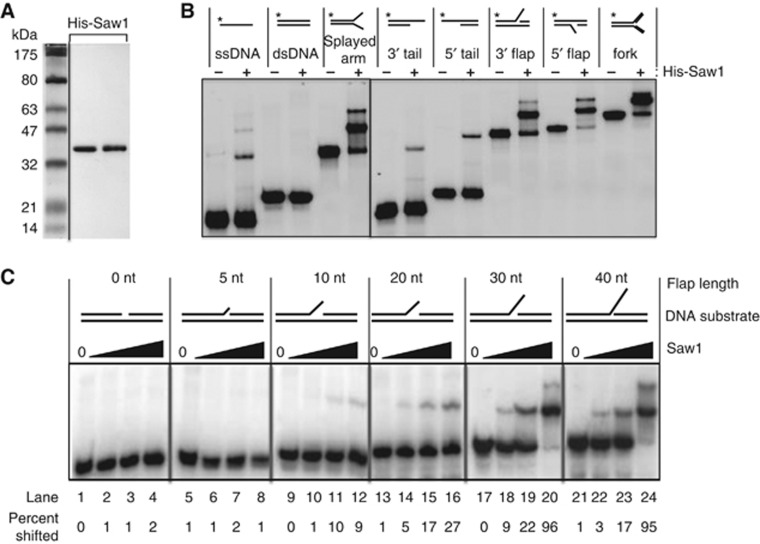
Saw1 is a structure-specific DNA binding protein
The Saw1 ChIP experiments indicated that an interaction between Saw1 and the DNA intermediate is critical for recruiting Rad1/Rad10 and for the completion of SSA. To define Saw1’s DNA-binding activity biochemically, we expressed Saw1 bearing a 6 × His tag in E. coli and purified it to near homogeneity (Figure 3A). Purified Saw1 interacts with Rad1 and Rad52, indicating that it is functional (Supplementary Figure S5A). We tested purified Saw1 for DNA binding in a DNA mobility shift assay with a variety of radiolabelled substrates. The results showed that Saw1 has the highest affinity for 5′- or 3′-flap DNA, a splayed arm structure, and a replication fork-like structure, but binds single-stranded DNA and 5′ or 3′ tailed DNA only weakly, and has no affinity for linear duplex DNA (Figure 3B). The presence of Saw1 in the nucleoprotein complex was validated by an antibody super-shift experiment using anti-6 × His or anti-Saw1 antibody and the splayed Y structure as substrate; antibody alone did not affect the DNA mobility (Supplementary Figure S5B). Saw1 also binds to a DNA substrate containing 14 bp bubble structure as efficiently as the splayed Y structure. Saw1 however did not bind to a substrate with 7 bp bubble or a hairpin with either a 10 base or 20 base loop (Supplementary Figure S6). The results thus revealed a structure-specific DNA binding activity in Saw1.
Figure 3.
Saw1 binds to DNA with the distinct structural motif. (A) 6 × His-tagged Saw1 protein was purified from E. coli as described in Materials and methods. Purified proteins were analysed by SDS–PAGE followed by Coomassie blue staining. (B) Electrophoretic mobility shift assay (EMSA) for 6 × His-Saw1. Reactions contained the indicated 32P-labelled substrates (0.1 pmol) and purified 6 × His-Saw1 (200 nM). Reactions were incubated on ice for 30 min, and protein–DNA complexes were analysed by 6% PAGE followed by autoradiography. 32P labels are indicated with asterisks. The splayed arm, 3′ and 5′ tail, 3′- and 5′-flap DNA all contain single-stranded DNA flap of 30 nucleotides. (C) EMSA to determine Saw1 binding to DNA substrates with various length of 3′-flaps. The indicated DNA substrates were incubated with or without purified Saw1 protein (10–200 nM) and the DNA-Saw1 complex was detected by acrylamide mini gel electrophoresis followed by autoradiography. Data were assembled by removal of irrelevant lanes but from the same gel or from multiple gels exposed in parallel.
Source data for this figure is available on the online supplementary information page.
Previous genetic studies demonstrated that a 3′ DNA tail is efficiently cleaved by Rad1/Rad10 only if it is longer than 20 nucleotides (Colaiacovo et al, 1999; Li et al, 2008). We therefore investigated whether Saw1’s affinity to 3′-flap DNA as a function of the length of the 3′ tails (Figure 3C). We found that the Saw1 binds to a 3′-flap only when the flap strand is at least 10 nucleotides in length, and that the protein clearly has a higher affinity for substrates with a longer flap strand.
DNA binding of Saw1 is required for Rad1/Rad10-dependent 3′-flap removal
To explore the biological significance of the structure-specific DNA binding activity of Saw1 in Rad1/Rad10-dependent 3′-flap cleavage, we screened for saw1 mutants that are deficient in DNA binding but retain physical interactions with Rad1, Msh2, and Rad52. To help identify such mutants, we aligned Saw1 and its fungal homologues. This analysis revealed high conservation of six positively charged amino acids at the carboxyl terminus of Saw1 across species (Supplementary Figure S7). We reasoned that these basic residues could be involved in DNA binding via interactions with the phosphodiester backbone of DNA. To test this premise, we produced saw1 mutants deleting these conserved amino acids or the surrounding amino acids (Materials and methods). The resulting saw1 mutants bearing 6 × His tag (saw1-Δ192–195, -Δ202–220, -Δ221–229, and -Δ244–250; Supplementary Figure S8B) were tested for their DNA binding by EMSA using 3′-flap DNA as substrate (Figure 4; Supplementary Figure S8C). Included in the EMSA were the saw1 mutants with deletions or substitution of the conserved amino acids at the N-terminus (saw1Δ18–24, or saw1-R19A, respectively) that were shown to have an impaired interaction with Rad1 (Li et al, 2008; Supplementary Figure S9A). Among the saw1 mutants, the variant lacking the six positively charged C-terminal residues (saw1-Δ244–250 or saw1DB-) is the only one that is almost completely devoid of 3′-flap DNA binding. GST-saw1DB- retains the ability to interact with TAP-tagged Rad1, Msh2, and Rad52 as determined in a GST pull-down experiment (Supplementary Figure S8A). We next tested the effect of the Δ244–250 mutation on 3′-flap cleavage in cells (Sugawara et al, 2000; Li et al, 2008). Importantly, we used two distinct assays testing either the HR mediated plasmid retention (Supplementary Figure S9B) or chromosome-based SSA (Supplementary Figure S9C), respectively, both of which depend on 3′ non-homologous tail removal. In both, the saw1DB- mutation causes a severe defect in Rad1/Rad10-dependent 3′-flap cleavage. The results thus provide genetic evidence that the structure-specific DNA binding activity of Saw1 is indispensable for Rad1/Rad10-mediated 3′-flap processing.
Figure 4.
Characterization of saw1 mutant defective in interaction with Rad1 or DNA binding. The 3′-flap DNA binding, physical interaction with Rad1, and efficiency of 3′-flap removal were assessed by EMSA, GST pull-down, and plasmid-based 3′-flap removal assays, respectively, as described in Materials and methods. Percent 3′-flap removal efficiency was calculated by scoring % plasmid retention in strains deleted for SAW1 supplemented with wild-type Saw1, or mutant saw1 proteins. NA, not available.
Saw1 is required for recruitment of Rad1/Rad10 to 3′-flaps
In order to determine whether Saw1 facilitates the binding of Rad1/Rad10 to 3′-flaps, we co-expressed 6 × His-Rad1, Rad10, and 6 × His-Saw1 or 6 × His-saw1 mutants in E. coli. We co-purified 6 × His-Rad1/Rad10 with 6 × His-Saw1 or saw1 mutant derivatives through a Cobalt agarose column. The three proteins co-eluted (Supplementary Figure S10) and the eluate was used to perform DNA gel mobility shift experiments. The strategy allowed us to purify His-Saw1 or His-saw1 mutants independent of its interaction with Rad1/Rad10 complex. Purified Rad1/Rad10 did not bind the 3′-flap DNA but became incorporated into a higher order nucleoprotein complex when Saw1 was present (Figure 5A). In contrast, fewer higher order species was seen when saw1-Δ18–24 or saw1DB- was used (Figure 5A; Supplementary Figure S11). The results provide support for the model that Saw1 targets Rad1/Rad10 to the 3′ tailed substrate in HR.
Figure 5.
Saw1 recruits Rad1/Rad10 complex to 3′-flap DNA substrate. (A) His-Rad1-Rad10 and His-Saw1 were co-expressed from E. coli and purified together with wild-type His-Saw1, His-saw1-Δ18–24, or His-saw1DB-, respectively, over a Cobalt column. The 5′-32P-labelled 3′-flap DNA substrate (0.1 pmol) was then incubated with increasing amounts of the His-Rad1/Rad10/His-Saw1 mixture, reported as μg/ml because the stoichiometry of any complexes is unclear The reaction mixtures were separated by acrylamide mini gel electrophoresis followed by autoradiography to detect Rad1/Rad10/Saw1-DNA or Saw1-DNA complex. Data were assembled by removal of irrelevant lanes and from two gels exposed in parallel. (B) The recruitment of rad1nuc- to the proximal to the 3′-flap site in EAY1141 and its saw1-R19A or saw1DB- mutant derivatives was examined by ChIP assays. Fold enrichment is calculated as described in Figure 1. Data represent the mean±s.d. of three or more independent experiments. (C) The top left panel shows a schematic illustration of the SSA substrate and TetO array located ∼15 kb from the HO cut site on chromosome III. The bottom left panel shows representative images of DIC merged with Venus-Rad1 and TetR-mRFP1 either before or after HO induction. Right panel shows percent co-localization of Venus-Rad1 with TetR-mRFP1 in wild-type or isogenic strain deleted for SAW1 and plotted at the indicated time post HO induction. Data represent % co-localization of Venus-Rad1 and TetR-mRFP1 from two independent experiments in each of which scored at least 150 cells and the individual data were plotted vertically together with a line denoting the mean. The size bar represents 0.5 μm.
Source data for this figure is available on the online supplementary information page.
To further validate the role of Saw1 in Rad1/Rad10 recruitment to 3′ tailed recombination intermediates, we examined the effect of expressing saw1DB- or saw1-R19A deficient in the interaction with Rad1 on the recruitment of rad1nuc- by ChIP. As shown in Figure 5B and Supplementary Figure S12, neither saw1DB- nor saw1-R19A could recruit rad1nuc- to 3′ tailed intermediates. Finally, we developed a live-cell imaging assay to visualize Rad1 localization to an HO endonuclease-induced DSB. A 128X tetO array, to which TetR-mRFP1 binds, was positioned ∼15 kb distal to HO cleavage site that was flanked by direct repeat sequences to mark the site of SSA repair. Venus-Rad1 forms nuclear foci and co-localizes with TetR-mRFP1 bound to the tetO array. There is an ∼10-fold increase in co-localization following induction of HO to create the DSB. When SAW1 was deleted, there was essentially no increase in co-localization of Venus-Rad1 with TetR-mRFP1 following induction of a DSB (Figure 5C). Taken together, the results strongly suggest that Saw1 targets Rad1/Rad10 to 3′-flap DNA through its ability to bind concurrently to Rad1 and DNA harbouring a 3′-flap.
Saw1 forms a stable complex with Rad1-Rad10
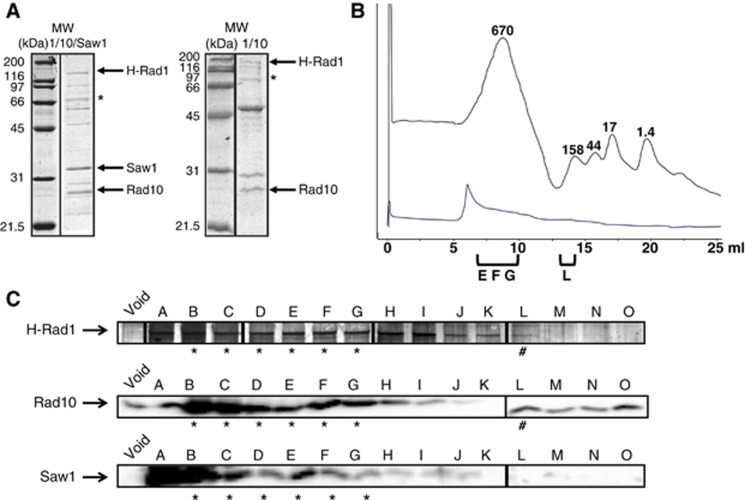
The gel mobility shift assays in Figure 5 indicated that Rad1/Rad10 and Saw1 formed a complex. We therefore purified this complex, by overexpressing His-Rad1, untagged Rad10, and untagged Saw1 in E. coli. All three proteins were co-purified by Cobalt chromatography (Figure 6A). Notably, purification of Saw1 is based on its affinity to His-Rad1/Rad10, indicating that Saw1 forms a complex with Rad1/Rad10. We tested the stability of the purified complex with the addition of gel filtration step, to determine whether Saw1 remained bound to Rad1/Rad10. The eluate from the Cobalt column (Figure 6A) was concentrated and loaded onto a Superose 6 column and the fractions were analysed for the presence of Rad1, Rad10, and Saw1. All three proteins coeluted in fractions B to H, corresponding to ∼500–700 kDa, consistent with a multimeric complex (Figure 6B and C). The rad1R825A (rad1nuc-)/Rad10/Saw1 complex was purified in the same way and had an elution profile very similar to that of Rad1/Rad10/Saw1 (Supplementary Figure S13A). The only Saw1 that we observed co-eluted with Rad1 (or rad1R825A) and Rad10, indicating that Saw1 exists exclusively in a higher molecular weight complex. In contrast, Rad1/Rad10 (without Saw1) purified over Cobalt and gel filtration columns co-eluted in a peak corresponding to ∼150–200 kDa, consistent with the expected molecular weight of the heterodimeric complex (∼150 kDa) (Figure 6B and C). His-Saw1 purified on its own, through the Cobalt and gel filtration steps, eluted in two peaks that corresponded to ∼40 and 90 kDa (Supplementary Figure S13C). These data indicate that Rad1, Rad10, and Saw1 form a multimeric complex (or complexes) that is stable through two purification steps. This is consistent with our observation that Saw1 is unstable in cells when Rad1/Rad10 is absent (Li et al, 2008).
Figure 6.
Rad1, Rad10, and Saw1 form a stable complex. (A) Purification of His-Rad1/Rad10/Saw1 (left) and His-Rad1/Rad10 (right) from Cobalt column. The asterisk indicates Rad1 degradation products, as determined by western blot. Irrelevant lanes were removed. The lanes in each sub-panel originate from the same gel. (B) Elution profile of broad range standards (grey) and Rad1/Rad10/Saw1 (blue) through the Superose 6 column. The molecular weights of the standards are indicated in kDa above the grey curve. (C) Analysis of representative His-Rad1/Rad10/Saw1 gel filtration fractions. Fractions were analysed for the presence of Rad1 (top; silver stain), Rad10 (middle; western blot), or Saw1 (bottom; western blot). Fractions that contain all three proteins are indicated by an asterisk. The fraction that contains only Rad1 and Rad10 is indicated by the pound sign. Depending on the preparation, Rad1/Rad10 alone elutes in fraction K through M. The Rad1 data are from two gels electrophoresed and stained in parallel. Molecular weight marker and empty lanes were removed. The western blot images for each protein were obtained from the same exposure of filters processed in parallel.
Source data for this figure is available on the online supplementary information page.
Saw1 stimulates endonuclease activity of Rad1/Rad10 in vitro
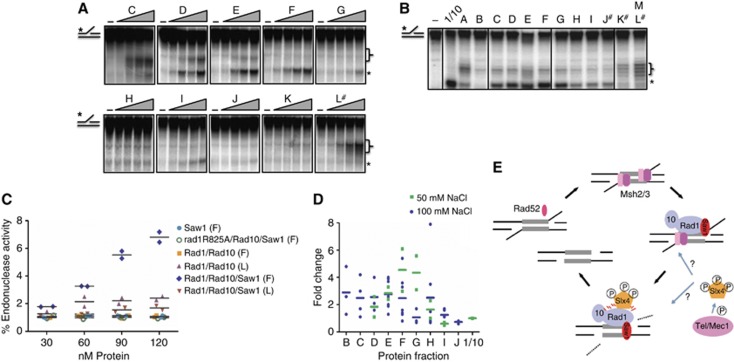
To determine whether the Rad1/Rad10/Saw1 complex was functional for Rad1/Rad10 endonuclease activity and to determine whether Saw1 might stimulate Rad1/Rad10′s nuclease activity, we tested the gel filtration column fractions under sub-optimal conditions (100 mM NaCl) for Rad1/Rad10 endonuclease activity with a 3′-flap substrate (Supplementary Figure S1B and C; Bastin-Shanower, 2003). Non-specific cleavage activity could be detected from several fractions including the void, but this was distinct from that of Rad1/Rad10 and did not cleave the DNA at the base of the flap. In the fractions containing Rad1/Rad10/Saw1, we observed a cleavage pattern consistent with that of Rad1/Rad10 (Figure 7). The fractions with peak activity corresponded to the fractions D to G, which contained similar levels of Rad1, Rad10, and Saw1. We also performed endonuclease activity assays at 50 mM NaCl and observed a similar peak in endonuclease activity from fractions D to G. At this lower salt concentration, we observed higher activity, consistent with our results with the purified Rad1/Rad10 (Supplementary Figure S1C). In contrast, equivalent fractions containing rad1R825A/Rad10/Saw1 exhibited no endonuclease activity (Supplementary Figure S13A), confirming that cleavage of the flap was specific and mediated by Rad1/Rad10. Similarly, when Rad1/Rad10 was purified alone (without Saw1), the equivalent gel filtration fractions (D–G) harboured no endonuclease activity (Supplementary Figure S13B). Together, these data indicated that the endonuclease activity observed in fractions D–G (MW∼500–700) is intrinsic to the Rad1/Rad10/Saw1 complex and requires the nuclease active site in Rad1.
Figure 7.
(A) Endonuclease assays of fractions from His-Rad1/Rad10/Saw1 gel filtration column performed at 100 mM NaCl. Increasing amounts of each fraction (0, 30, 60, 90, 120 nM of Rad1/Rad10/Saw1 assuming a 1:1:1 stoichiometry) were incubated with 3′-flap substrate to examine any endonuclease activity. The brackets indicate a non-specific activity seen in several preparations including those with a nuclease-dead rad1 mutant. The asterisks indicate the Rad1/Rad10-specific cleavage product that is observed with purified Rad1/Rad10 (Supplementary Figure S1). The fraction containing only Rad1/Rad10 in this preparation is indicated by the pound sign. Lanes containing irrelevant data were omitted. (B) Endonuclease assays of fractions from a different His-Rad1/Rad10/Saw1 gel filtration column, performed at 50 mM NaCl. For each fraction, 200 nM protein (assuming a 1:1:1 stoichiometry of Rad1/Rad10/Saw1) was incubated in the presence of the 3′-flap substrate. Purified Rad1/Rad10 was used as a control to determine the correct cleavage product, indicated by the asterisk. The bracket indicates likely non-specific activity. The pound sign indicates the fractions in this prep that contain only Rad1 and Rad10. Irrelevant lanes were omitted. All lanes originated from the same gel; no protein control lanes were removed. (C) Quantification of cleavage activity, at 100 mM NaCl, in fractions F and/or L, which correspond to Rad1/Rad10/Saw1 peak activity (F) and Rad1/Rad10 peak activity (L), respectively, from purification of untagged Saw1 (light blue), rad1R825A/Rad10/Saw1 (green), Rad1/Rad10 (purple and orange) or Rad1/Rad10/Saw1 (red and blue). Data points represent the % endonuclease activity of individual experiments and the mean for each fraction is represented by a horizontal bar. (D) Relative endonuclease activity of Rad1/Rad10/Saw1 fractions normalized to Rad1/Rad10 activity purified in the same preparation (set to 1 and indicated as 1/10 on the x axis), at 50 mM NaCl (green) or 100 mM NaCl (blue). Data points represent calculated fold changes of independent experiments and the mean is represented by a horizontal bar. (E) A model for 3′-flap removal during SSA. Direct repeats flanking a DSB is annealed by Rad52 and further stabilized by Msh2/Msh3. Saw1 recruits Rad1/Rad10 complex to recombination intermediates carrying 3′ non-homologous tails through both physical interaction with Rad1 and affinity to 3′-flaps. Slx4 is phosphorylated by Mec1/Tel1 and may stimulate nuclease activity of Rad1/Rad10 or coordinate cleavage complex assembly.
Source data for this figure is available on the online supplementary information page.
When Rad1/Rad10 was purified alone, fractions containing Rad1 and Rad10 (K and L) also showed some endonuclease activity (Figure 7B; Supplementary Figure S13B). The cleavage activity in these fractions was significantly lower (∼3- to 4-fold) than that observed in fractions containing Rad1/Rad10/Saw1 (Figure 7C). Similarly, within the same protein preparation, fractions comprising Rad1/Rad10/Saw1 (fractions D–G) exhibited higher activity than those containing only Rad1/Rad10 (fractions K and L); ∼3-fold higher at 100 mM NaCl and ∼5 fold higher at 50 mM NaCl (Figure 7D). Fractions D–G from cells expressing only untagged Saw1 had no endonuclease activity (Figure 7C). These data indicated that Rad1/Rad10 interacts with Saw1 to form a complex that has endonuclease activity higher than that observed with Rad1/Rad10 alone, supporting the idea that Saw1 targets the Rad1/Rad10 complex to 3′-flaps.
Since His-Saw1 bound efficiently to a fork substrate (Figure 3B), we also asked whether the presence of Saw1 would allow Rad1/Rad10 to cleave a fork substrate. Using the fractions from the Rad1/Rad10/Saw1 gel filtration purification described above, we examined possible cleavage of a labelled fork substrate (Supplementary Figure S13D). We observed no cleavage of this substrate under these conditions.
Discussion
Cleavage of 3′ non-homologous tails is an essential step in SSA and a subset of gene conversion processes (Lyndaker and Alani, 2009). This reaction requires a large number of protein factors, two of which, Saw1 and Slx4, were identified in our recent genetic screen (Li et al, 2008). How these proteins contribute to 3′ DNA tail removal, in particular the manner in which these proteins are recruited and assembled to form a nuclease complex at 3′-flaps, has not been determined. Here, we have defined the temporal order and assembly hierarchy among these tail removal factors at 3′-flap recombination intermediates using ChIP and live cell nuclear foci formation upon DNA damage. We have also provided biochemical and genetic evidence as to how Saw1 recruits and stimulates Rad1/Rad10 to process 3′ DNA intermediates. Based on these results, we propose a model for 3′-flap removal complex assembly and 3′ tail cleavage (Figure 7E).
Strand annealing and formation of 3′ non-homologous tails
Our ChIP results and those of previous genetic and biochemical analyses have uncovered at least three distinct steps in 3′ non-homologous tail cleavage: (1) annealing of complementary ssDNA catalysed by Rad52 and Rad59 (Bai et al, 1999; Sugawara et al, 2000; Wu et al, 2006); (2) recognition and binding of Msh2/Msh3 at branched DNA junctions for stabilization of the structure (Sugawara et al, 1997); (3) recruitment of the Rad1/Rad10/Saw1 nuclease ensemble, and possibly stimulation of cleavage by Slx4.
In the first step, Rad52 and Rad59 anneal the ssDNA to form 3′-flap intermediate structures (Paques and Haber, 1999) and may aid in the recruitment of Rad1/Rad10 (Motycka et al, 2004). Interestingly, Rad59 becomes particularly important when repeats are short (Sugawara et al, 2000). Msh2/Msh3 also becomes important when repeat lengths are <1 kb, indicating that this complex helps to stabilize the DNA intermediate structures. A similar function has been proposed for Msh2/Msh3 in the removal of large DNA loops during meiosis (Kirkpatrick, 1999). Our ChIP results also support the idea that Msh2/Msh3 stabilizes annealed DNA intermediates, indirectly impacting on the efficient recruitment of Rad1/Rad10 to 3′ tails (Figure 1C and D).
Saw1 is a structure-specific DNA binding protein
Following strand annealing and formation of 3′-flap intermediates, Rad1/Rad10 is recruited to the target site to mediate flap cleavage. Since Rad1/Rad10 itself possesses weak affinity for 3′-flap DNA (see Figure 5A), additional targeting factors, such as Saw1, are required for its recruitment to the cleavage substrate, analogous to the Rad14 recruiting Rad1/Rad10 to UV lesions in NER (Guzder et al, 2006). Indeed, deletion of SAW1 greatly impairs SSA and 3′-flap cleavage, and abrogates the recruitment of Rad1/Rad10 to the 3′-flap intermediate (Figure 1; Li et al, 2008). Since Saw1 can interact with Rad1, Msh2, and Rad52, we previously proposed that Saw1 targets Rad1/Rad10 to 3′ tailed substrates by bridging Rad1/Rad10 and Rad52, Msh2/Msh3 on recombination intermediates (Li et al, 2008; Lyndaker and Alani, 2009). Alternatively, Saw1 may recognize 3′-flap substrates directly and physically deliver Rad1/Rad10 to the substrate DNA. In support of the latter idea, we have shown that purified Saw1 binds 3′-flap or Y-form DNA with high affinity. Furthermore, we have been able to detect the association of Saw1 with 3′-flap intermediates using ChIP with kinetics that are congruent with a role in targeting Rad1/Rad10 to these molecules. We have also identified a saw1 DNA binding mutant that is deficient in 3′-flap cleavage and SSA. All these results are consistent with the model that Saw1 is a structure-specific DNA binding protein responsible for targeting Rad1/Rad10 to 3′-flaps.
We surmise that the high affinity of Saw1 to 3′-flap DNA can guide Rad1/Rad10 to locate its target, thereby stimulating its enzymatic activity. In support of this prediction, we were able to purify a stable Saw1/Rad1/Rad10 complex and found that this complex has greater endonuclease activity on 3′-flaps than Rad1/Rad10 alone. Of note, Saw1 is unstable in rad1 or rad10 deleted strains and thus likely rarely exists outside of Rad1/Rad10/Saw1 complex. The results thus suggest that the major biological role of Saw1 is to target Rad1/Rad10 to 3′-flap intermediate during recombination.
Surprisingly, Saw1 also binds efficiently to a bubble containing DNA that mimics NER substrate (Supplementary Figure S6). The results suggest that discrimination of Rad1/Rad10 from NER and HR substrates may not be solely dependent on the ability of Saw1 to bind unique DNA structure. We propose that targeting of Rad1/Rad10 to HR substrates rests on the Saw1’s ability to bind both DNA and other HR factors.
Slx4 is dispensable for Rad1/Rad10 recruitment to 3′ tails but needed for DNA cleavage
Our ChIP data indicate that Slx4 is not required for the recruitment of Rad1, Saw1, or Msh2. Then, how does Slx4 contribute to 3′ tail cleavage? The available evidence suggests that Slx4 itself is a DNA binding protein and forms a heterodimeric, structure-specific endonuclease complex with Slx1 (Fricke and Brill, 2003). Together with Slx1, Slx4 also resolves Holliday junctions (Andersen et al, 2009; Fekairi et al, 2009; Munoz et al, 2009; Svendsen et al, 2009) and stalled replication forks (Roberts et al, 2008). However, for 3′-flap cleavage, Slx4 works independently of Slx1 nuclease and is phosphorylated by Tel1-Mec1- in a DNA damage-dependent manner (Flott et al, 2007; Li et al, 2008).
It has been proposed that Slx4 acts as a molecular scaffold for Slx1, Rad1, and Mus81 nucleases (Munoz et al, 2009). Indeed, Slx4 exists as a high molecular weight complex with Slx1, Mus81, and XPF/Ercc1 in humans and stimulates the activity of Slx1 and Mus81 in cell lysates (Munoz et al, 2009). It is thus tempting to speculate that Slx4 stimulates the nuclease activity of Rad1/Rad10. If so, however, this stimulation is not essential; deletion of SLX4 does not lead to accumulation of Rad1 at 3′ tails as the nuclease-deficient variants of Rad1 do (JD and SEL, unpublished observations). Alternatively, Slx4 may participate in proper positioning of Rad1/Rad10 or directing proper assembly/disassembly of one or more tail removal proteins, such as Saw1, at 3′ tailed intermediates. Slx4 may also act to link recombination and DNA damage checkpoint control, enabling ongoing recombination to be coordinated with cell-cycle progression.
In summary, our results offer the most comprehensive picture of the order and hierarchy of 3′-flap removal enzyme assembly. In yeast, gene conversion involving completely homologous DNA partners does not require Rad1 or Saw1, suggesting that 3′-flap cleavage is dispensable in this context (Sugawara et al, 1997; Li et al, 2008). In mammals, however, inactivation of XPF causes a dramatic decrease in gene conversion, even when no apparent non-homology exists between the recombining DNA sequences (Al-Minawi et al, 2008). It has been proposed that repair DNA synthesis extends the invading strand in the D-loop structure sufficiently such that its dissociation from the D-loop and annealing with the other DNA end results in a 3′-flap structure that needs to be trimmed before the gene conversion event can be completed (Al-Minawi et al, 2008; Lyndaker and Alani, 2009). The genetic results suggest that the XPF/ERCC1 nuclease, the orthologue of Rad1/Rad10, mediates the DNA cleavage event. Defining the genetic and biochemical requirements of 3′ DNA tail removal in yeast and mammals has great relevance for understanding the mechanisms of genome maintenance and cancer avoidance in humans.
Materials and methods
Strains and plasmids
EAY 1141 (ho HML mat::leu2::hisG hmrΔ3 leu2-3,112 ura3-52 trp1 THR4-ura3-A(205bp)-HOcs-URA3-A ade3::GAL10-HO::NAT) and its derivatives were used for ChIP and SSA assays (Supplementary Table S1). Gene deletion mutants were constructed by using a PCR-derived KANMX module flanked by short terminal sequences homologous to the ends of each gene open reading frame (Wach et al, 1994). The SSA substrate labelled with a TetO array was constructed by first integrating TETR-mRFP1 in chromosome VII at the YGL119W gene locus. TETO array was integrated at the SNT1 locus on chromosome III, which is ∼15 kb away from the MATa HO cut site. A 527-bp sequence downstream of MATa HO cut site was amplified and integrated at PHO87 locus, generating two direct repeats flanking the HO cut site. The endogenous yeast RAD1 was fused in frame with the Venus to express the Venus-Rad1 by gene replacement technique. SAW1 was amplified and cloned into pET28A vector at BamHI-XhoI in frame with N-terminal 6 × His tag to generate pHIS-SAW1. RAD1 and RAD10 were co-expressed from the same plasmid pJAS21. To construct this plasmid, RAD1 was cloned into pET15b to encode a six histidine-tagged fusion of Rad1, creating pJAS18. RAD10 was cloned into pET11a, leaving Rad10 untagged, creating pJAS19. The BglII/BamHI fragment from pJAS19 was cloned into the BamHI site of pJAS18, yielding pJAS21. Both genes are in the same orientation and each is expressed from its own T7 promoter.
Chromatin immunoprecipitation
ChIP assay was performed as previously described (Li et al, 2008). Primers used for ChIP assay are pJC1: 5′-GCCCAGTATTCTTAACCCAACTGCAC-3′, pJC2: 5′-CAGCTGGCGTAATAGCGAAGAGGCCC-3′, pJC3: 5′-CCTTAGTAGTTGGTAACCTGACAAAGG-3′, pJC4: 5′-CCTTCTGTTCGGAGATTACCGAATC-3′
pJC7: 5′-GACCTGACCATTTGATGGAG-3′ and pJC8: 5′-TGATGATCTGGCCTCATGGA-3′.
SAW1 and RAD1 Mutagenesis
Mutant saw1 and rad1 were generated by PCR-mediated site-directed mutagenesis as previously described (Li et al, 2008). The presence of each mutation was confirmed by DNA sequencing.
Expression and purification of recombinant 6 × His-Saw1
E. coli carrying pHIS-SAW1 was induced to express 6 × His-Saw1 by addition of IPTG and was suspended in 50 ml cold breaking buffer (50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 10% glycerol, 0.5% Triton X-100, 5 mM beta-mercaptoethanol, protease inhibitor cocktail (Roche), 10 mM imidazole) prior to sonication on ice (20 s × 5, 30 s interval). The lysate was centrifuged at ∼48 000 g at 4°C for 20 min and the supernatant was loaded onto Ni-NTA agarose column equilibrated with breaking buffer. Column was washed sequentially with 50 ml T-600 (20 mM Tris–HCl (pH 8.0), 600 mM NaCl, 10% glycerol), 50 ml T-600 plus 100 mM imidazole, and finally with T-150 plus protease inhibitor cocktail. The 6 × His-Saw1 was then eluted with 10 ml elution buffer (50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 10% glycerol 300 mM imidazole, protease inhibitors cocktail). The eluted proteins were applied to 1 ml Mono-S column, and eluted with NaCl gradient from 150 to 1000, mM. The 6 × His-Saw1 was eluted at 600–700 mM NaCl, but adjusted to 150 mM NaCl, and stored at −80°.
Rad1-Rad10 expression and purification
Rad1-Rad10 was overexpressed as a complex from pJAS21 in E. coli BB101 [ara Δ(lac pro) halA argE (Am) rift hi-1 ΔslyD/F’ lacIq lacZ::TN5 pro+] (λDE3) transformed with the pRARE plasmid (chloramphenicol resistant) (Novagen). Cells carrying pJAS21 were grown at 37°C in LB medium containing ampicillin and chloramphenicol to an A600 of ∼0.5. IPTG was added to 0.5 mM and the culture was incubated for an additional 7 h at 25°C. The cells were harvested by centrifugation and resuspended in P1 buffer (25 mM sodium phosphate buffer, pH 7.6, 10% glycerol, 0.01% NP-40) with 150 mM NaCl to an A600 of ∼400. Induced cells were lysed by French press. The lysates was cleared by centrifugation at 30 000 g for 40 min. The cleared supernatant was loaded onto a 5-ml phosphocellulose column, equilibrated in P1 buffer+150 mM NaCl. The column was eluted with a 125-ml gradient, from 150 mM to 1 M NaCl. His-Rad1-Rad10 eluted over several fractions, from about 400 to 800 mM NaCl. The peak fractions were pooled and loaded directly onto a 5-ml Ni-NTA column equilibrated in P1 buffer+500 mM NaCl+10 mM imidazole. The Ni-NTA column was eluted in three steps: (1) 25 ml P1 buffer+500 mM NaCl+50 mM NaCl; (2) 25 ml P1 buffer+500 mM NaCl+100 mM NaCl; and (3) 25 ml P1 buffer+500 mM NaCl+200 mM NaCl. Fractions containing Rad1 and Rad10 were diluted down to P1 buffer+150 mM NaCl and loaded onto a 2-ml SP-Sepharose column. Rad1-Rad10 was eluted with a 17-ml gradient, from 150 mM to 1 M NaCl. His-Rad1-Rad10 eluted at around 500 mM NaCl. The protein was concentrated, aliquoted, flash frozen, and stored at −80°C.
Induction and purification of the mutant rad1-Rad10 complexes was exactly as described above.
Purification of high molecular weight complex
E. coli BL21 C+ cells carrying plasmids encoding His-Rad1 and Rad10 (pJAS21) or His-rad1-nuc and Rad10 (pJAS26), and pET28a-Saw1 were induced to express His-Rad1, Rad10, and Saw1 by addition of IPTG (0.5 mM final). The cell pellet was resuspended in SAW1 breaking buffer (50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 10% glycerol, 0.5% Triton X-100, 10 mM imidazole, and supplemented with protease inhibitors mix). The cell lysate was prepared by French Press three times at 12 000 p.s.i. and clarified by centrifugation at 30 000 g at 4°C for 45 min. The supernatant was incubated with 3 ml HisPurTM Cobalt Resin (Pierce) at 4°C for at least 1 h with constant rocking. The protein bound resin was loaded onto a column and washed with 50 ml washing buffer (50 mM Tris–HCl (pH 8.0), 250 mM NaCl, 10% glycerol, and 30 mM imidazole) and the bound proteins were eluted with 5 ml elution buffer (50 mM Tris–HCl (pH 8.0), 100 mM NaCl, 10% glycerol, and 300 mM imidazole). The eluted proteins were concentrated by Amicon Ultra with a 10-kD size cutoff (Millipore) to a final volume of 150 μl. The concentrated proteins were loaded on a Superose 6 10/300 GL (GE Healthcare) gel filtration column. Fractions were collected and concentrated by Amicon Ultra (10 kDa) and analysed for the presence of Saw1, Rad1, and Rad10 by silver stained gels and by western blot.
Electrophoretic mobility shift assay
Reactions (10 μl) containing 5′-32P-labelled DNA substrate (0.1 pmol) in EMSA buffer (20 mM Tris–HCl (pH 8.0), 5 mM EDTA, 1 mM DTT, 100 μg/ml BSA, 10 μg/ml poly dI/Dc, and 5% glycerol) were supplemented with different amount of Saw1 protein ranging from 10 to 200 nM and incubated at room temperature for 30 min before loading onto acrylamide mini-gel buffered with 25 mM Tris-glycine (pH 8.3) containing 2% glycerol. After gel running on 135V under 4°C for 50 min, gel was dried and subjected to autoradiography.
DNA substrates for EMSA
All DNA substrates for EMSA were end-labelled by γ-32P-ATP and purified by gel electrophoresis. Sequences for each oligonucleotide are listed in Supplementary Table S2.
Live-cell imaging
Cell cultures were grown in YEPD for ∼24 h and then transferred to YEPG (YEP+glycerol) media at ratio of 1:10, and incubated at 30°C overnight. The next morning, HO was induced by adding 2% galactose (v/v). Aliquots of cultures were removed at indicated time points and centrifuged briefly (13 200 g for ∼3 s), resuspended in residual media and mixed on a glass microscope slide with equal volumes of 42°C YEP-galactose media containing 1.5% (w/v) low melting agarose (Agarose Unlimited, Gainesville, Florida). Images were captured using an Electron Multiplying CCD (EM-CCD) camera mounted to an Olympus IX71 inverted microscope with a 100 × , 1.4 NA oil immersion objective. The fluorescent light source was a 250W Xenon light source. Fluorophores were imaged using the Live Cell Filter Set (Applied Precision, Issaquah, Washington) including mCherry 572/632 nm and YFP 500/535 nm fluorescent filters. Image acquisition times for fluorophore-fusion proteins are as follows: 1000, ms for Venus-Rad1 and 1000, ms for TetR-mRFP1. Images were acquired for ∼11 different focal planes at 0.3 μm intervals along the Z-axis of the cells. Images were obtained, deconvoluted and analysed using SoftWorx Suite software (Applied Precision, Issaquah, Washington). Contrast enhancement was optimized for quantification of each fluorophore and all images were analysed with the same optimized parameters. To reduce background, the contrast enhancement was adjusted for Venus-Rad1 so that only the most prominent Rad1 foci were visible during quantitation. The same optimized parameters for both Venus-Rad1 and TetR-mRFP1 were applied to both HO-induced and non-induced images. Both representative images were re-adjusted for publication using Adobe Photoshop (Adobe Systems, Mountain View, CA) and using the same Photoshop parameters. Graphs depict the average of two experiments and standard deviation for each set of experiments. Approximately 150 cells were counted for each individual experiment.
Endonuclease assays
Reactions (10 μl) were performed in 50 mM Tris–HCl (pH 8.0), 5 mM MgCl2, 5 mM DTT with 0.1 pmol (10 nM) end-labelled 3′-flap substrate (LS1/LS3/LS16) (Surtees and Alani, 2006). Protein fractions were added as indicated in each experiment. The mixture was incubated at 37°C for 1 h. The reaction was deproteinized by the addition of 100 μg Proteinase K and 0.1% SDS followed by incubation at 37°C for an additional 15 min. The reactions were then electrophoresed through a 10% native acrylamide gel, buffered by 1 × TBE, for 1 h 30 min and 250 V. The gel was dried and then exposed to a PhosphorImager screen (Molecular Dynamics) and quantified by ImageQuant (GE).
Supplementary Material
Acknowledgments
We thank E Alani, J Haber, S Prakash, J Rouse, and A Tomkinson for reagents and the plasmids in the study. We are also grateful to E Alani, J Haber, P Hasty and the members of Lee, Surtees and Sung labs for helpful discussions. This work is supported by NIH research grants GM71012 (to SEL), GM57814 (to PS), and GM87549 (to JAS). SEL is a scholar of the Leukemia and Lymphoma Society.
Author contributions: FL, EYS, JAS, and SEL designed experiments. FL performed biochemical experiments shown in Figures 3, 4 and 5A, Supplementary Figures S5, S6, S7, S8, S9A, B, S10, and S11. JD carried out ChIP assays in Figures 1, 2, and 5B and Supplementary Figures S2, S3, S4, S9C, and S12A. RE performed biochemical experiments shown in Figures 6 and 7 and Supplementary Figures S1 and S13. CH carried out live-cell imaging in Figure 5C and Supplementary Figure S12B. EM performed experiments in Supplementary Figure S1B, DRP and PS devised methods to purify recombinant Saw1 and Rad1/Rad10 proteins used in throughout the paper. SEL, JAS, and PS wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adair GM, Rolig RL, Moore-Faver D, Zabelshansky M, Wilson JH, Nairn RS (2000) Role of ERCC1 in removal of long non-homologous tails during targeted homologous recombination. EMBO J 19: 5552–5561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Minawi AZ, Saleh-Gohari N, Helleday T (2008) The ERCC1/XPF endonuclease is required for efficient single-strand annealing and gene conversion in mammalian cells. Nucleic Acids Res 36: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Bergstralh DT, Kohl KP, LaRocque JR, Moore CB, Sekelsky J (2009) Drosophila MUS312 and the vertebrate ortholog BTBD12 interact with DNA structure-specific endonucleases in DNA repair and recombination. Mol Cell 35: 128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Davis AP, Symington LS (1999) A novel allele of RAD52 that causes severe DNA repair and recombination deficiencies only in the absence of RAD51 or RAD59. Genetics 153: 1117–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin-Shanower SA, Fricke WM, Mullen JR, Brill SJ (2003) The mechanism of Mus81-Mms4 cleavage site selection distinguishes it from the homologous endonuclease Rad1-Rad10. Mol Cell Biol 23: 3487–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstralh DT, Sekelsky J (2008) Interstrand crosslink repair: can XPF-ERCC1 be let off the hook? Trends Genet 24: 70–76 [DOI] [PubMed] [Google Scholar]
- Boiteux S, Guillet M (2004) Abasic sites in DNA: repair and biological consequences in Saccharomyces cerevisiae. DNA Repair 3: 1–12 [DOI] [PubMed] [Google Scholar]
- Ciccia A, McDonald N, West SC (2008) Structural and functional relationships of the XPF/MUS81 family of proteins. Annu Rev Biochem 77: 259–287 [DOI] [PubMed] [Google Scholar]
- Colaiacovo MP, Paques F, Haber JE (1999) Removal of one nonhomologous DNA end during gene conversion by a RAD1- and MSH2-independent pathway. Genetics 151: 1409–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossan GP, van der Weyden L, Rosado IV, Langevin F, Gaillard PH, McIntyre RE, Gallagher F, Kettunen MI, Lewis DY, Brindle K, Arends MJ, Adams DJ, Patel KJ (2011) Disruption of mouse Slx4, a regulator of structure-specific nucleases, phenocopies Fanconi anemia. Nat Genet 43: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J, Hoeijmakers JH (2000) Nucleotide excision repair and human syndromes. Carcinogenesis 21: 453–460 [DOI] [PubMed] [Google Scholar]
- Enzlin JH, Scharer OD (2002) The active site of the DNA repair endonuclease XPF-ERCC1 forms a highly conserved nuclease motif. EMBO J 21: 2045–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Sugawara N, Haber JE, Alani E (2000) The Saccharomyces cerevisiae Msh2 mismatch repair protein localizes to recombination intermediates in vivo. Mol Cell 5: 789–799 [DOI] [PubMed] [Google Scholar]
- Fekairi S, Scaglione S, Chahwan C, Taylor ER, Tissier A, Coulon S, Dong MQ, Ruse C, Yates JR 3rd, Russell P, Fuchs RP, McGowan CH, Gaillard PH (2009) Human SLX4 is a Holliday junction resolvase subunit that binds multiple DNA repair/recombination endonucleases. Cell 138: 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman-Lobell J, Haber JE (1992) Removal of nonhomologous DNA ends in double-strand break recombination: the role of the yeast ultraviolet repair gene RAD1. Science 258: 480–484 [DOI] [PubMed] [Google Scholar]
- Flott S, Alabert C, Toh GW, Toth R, Sugawara N, Campbell DG, Haber JE, Pasero P, Rouse J (2007) Phosphorylation of slx4 by mec1 and tel1 regulates the single-strand annealing mode of DNA repair in budding yeast. Mol Cell Biol 27: 6433–6445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flott S, Rouse J (2005) Slx4 becomes phosphorylated after DNA damage in a Mec1/Tel1-dependent manner and is required for repair of DNA alkylation damage. Biochem J 391: 325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke WM, Brill SJ (2003) Slx1-Slx4 is a second structure-specific endonuclease functionally redundant with Sgs1-Top3. Genes Dev 17: 1768–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzder SN, Sommers CH, Prakash L, Prakash S (2006) Complex formation with damage recognition protein Rad14 is essential for Saccharomyces cerevisiae Rad1-Rad10 nuclease to perform its function in nucleotide excision repair in vivo. Mol Cell Biol 26: 1135–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzder SN, Torres-Ramos C, Johnson RE, Haracska L, Prakash L, Prakash S (2004) Requirement of yeast Rad1-Rad10 nuclease for the removal of 3′-blocked termini from DNA strand breaks induced by reactive oxygen species. Genes Dev 18: 2283–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Lach FP, Desetty R, Hanenberg H, Auerbach AD, Smogorzewska A (2011) Mutations of the SLX4 gene in Fanconi anemia. Nat Genet 43: 142–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick DT (1999) Roles of the DNA mismatch repair and nucleotide excision repair proteins during meiosis. Cell Mol Life Sci 55: 437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick DT, Petes TD (1997) Repair of DNA loops involves DNA-mismatch and nucleotide-excision repair proteins. Nature 387: 929–931 [DOI] [PubMed] [Google Scholar]
- Li F, Dong J, Pan X, Oum JH, Boeke JD, Lee SE (2008) Microarray-based genetic screen defines SAW1, a gene required for Rad1/Rad10-dependent processing of recombination intermediates. Mol Cell 30: 325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Peterson CA, Lu X, Legerski RJ (1995) Mutations in XPA that prevent association with ERCC1 are defective in nucleotide excision repair. Mol Cell Biol 15: 1993–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyndaker AM, Alani E (2009) A tale of tails: insights into the coordination of 3′ end processing during homologous recombination. Bioessays 31: 315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyndaker AM, Goldfarb T, Alani E (2008) Mutants defective in Rad1-Rad10-Slx4 exhibit a unique pattern of viability during mating-type switching in Saccharomyces cerevisiae. Genetics 179: 1807–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motycka TA, Bessho T, Post SM, Sung P, Tomkinson AE (2004) Physical and functional interaction between the XPF/ERCC1 endonuclease and hRad52. J Biol Chem 279: 13634–13639 [DOI] [PubMed] [Google Scholar]
- Mullen JR, Kaliraman V, Ibrahim SS, Brill SJ (2001) Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics 157: 103–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz IM, Hain K, Declais AC, Gardiner M, Toh GW, Sanchez-Pulido L, Heuckmann JM, Toth R, Macartney T, Eppink B, Kanaar R, Ponting CP, Lilley DM, Rouse J (2009) Coordination of structure-specific nucleases by human SLX4/BTBD12 is required for DNA repair. Mol Cell 35: 116–127 [DOI] [PubMed] [Google Scholar]
- Paques F, Haber JE (1999) Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 63: 349–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S, Prakash L (2000) Nucleotide excision repair in yeast. Mutat Res 451: 13–24 [DOI] [PubMed] [Google Scholar]
- Roberts TM, Zaidi IW, Vaisica JA, Peter M, Brown GW (2008) Regulation of rtt107 recruitment to stalled DNA replication forks by the cullin rtt101 and the rtt109 acetyltransferase. Mol Biol Cell 19: 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl RH, Prakash S (1990) RAD10, an excision repair gene of Saccharomyces cerevisiae, is involved in the RAD1 pathway of mitotic recombination. Mol Cell Biol 10: 2485–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijbers AM, de Laat WL, Ariza RR, Biggerstaff M, Wei YF, Moggs JG, Carter KC, Shell BK, Evans E, de Jong MC, Rademakers S, de Rooij J, Jaspers NG, Hoeijmakers JH, Wood RD (1996) Xeroderma pigmentosum group F caused by a defect in a structure-specific DNA repair endonuclease. Cell 86: 811–822 [DOI] [PubMed] [Google Scholar]
- Stoepker C, Hain K, Schuster B, Hilhorst-Hofstee Y, Rooimans MA, Steltenpool J, Oostra AB, Eirich K, Korthof ET, Nieuwint AW, Jaspers NG, Bettecken T, Joenje H, Schindler D, Rouse J, de Winter JP (2011) SLX4, a coordinator of structure-specific endonucleases, is mutated in a new Fanconi anemia subtype. Nat Genet 43: 138–141 [DOI] [PubMed] [Google Scholar]
- Sugawara N, Ira G, Haber JE (2000) DNA length dependence of the single-strand annealing pathway and the role of Saccharomyces cerevisiae RAD59 in double-strand break repair. Mol Cell Biol 20: 5300–5309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N, Paques F, Colaiacovo M, Haber JE (1997) Role of Saccharomyces cerevisiae Msh2 and Msh3 repair proteins in double-strand break-induced recombination. Proc Natl Acad Sci USA 94: 9214–9219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees JA, Alani E (2006) Mismatch repair factor MSH2-MSH3 binds and alters the conformation of branched DNA structures predicted to form during genetic recombination. J Mol Biol 360: 523–536 [DOI] [PubMed] [Google Scholar]
- Svendsen JM, Smogorzewska A, Sowa ME, O'Connell BC, Gygi SP, Elledge SJ, Harper JW (2009) Mammalian BTBD12/SLX4 assembles a Holliday junction resolvase and is required for DNA repair. Cell 138: 63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh GW, Sugawara N, Dong J, Toth R, Lee SE, Haber JE, Rouse J (2010) Mec1/Tel1-dependent phosphorylation of Slx4 stimulates Rad1-Rad10-dependent cleavage of non-homologous DNA tails. DNA Repair (Amst) 9: 718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson AE, Bardwell AJ, Bardwell L, Tappe NJ, Friedberg EC (1993) Yeast DNA repair and recombination proteins Rad1 and Rad10 constitute a single-stranded-DNA endonuclease. Nature 362: 860–862 [DOI] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pöhlmann R, Philippsen P New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10: 1793–1808 [DOI] [PubMed] [Google Scholar]
- Wu Y, Sugiyama T, Kowalczykowski SC (2006) DNA annealing mediated by Rad52 and Rad59 proteins. J Biol Chem 281: 15441–15449 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.