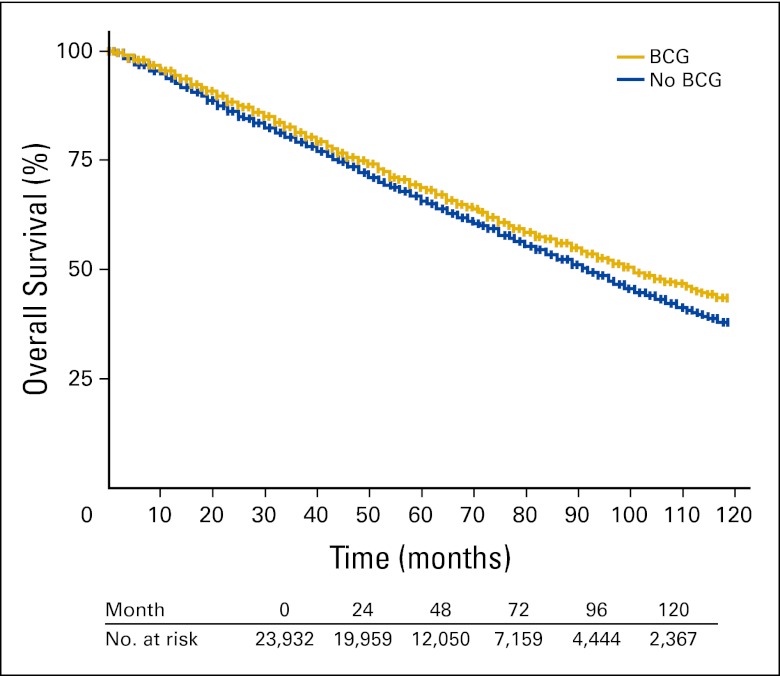
This large population-based study found improved OS and bladder cancer–specific survival associated with use of adjuvant intravesical BCG among older patients with NMIBC.
Abstract
Purpose:
National guidelines recommend adjuvant intravesical Bacillus Calmette-Guérin (BCG) therapy for higher-risk non–muscle-invasive bladder cancer (NMIBC). Although a survival benefit has not been demonstrated, randomized trials have shown reduced recurrence and delayed progression after its use. We investigated predictors of BCG receipt and its association with survival for older patients with NMIBC.
Patients and Methods:
We identified individuals with NMIBC registered in the Surveillance, Epidemiology, and End Results–Medicare database from 1991 to 2003. We used logistic regression to compare those treated with BCG within 6 months of initial diagnosis with those not treated, adjusting for demographic and clinical factors. Cox proportional hazards modeling was used to analyze the association between BCG and overall survival (OS) and bladder cancer–specific survival (BCSS) for the entire cohort and within tumor grades.
Results:
Of 23,932 patients with NMIBC identified, 22% received adjuvant intravesical BCG. Predictors of receipt were stages Tis and T1, higher grade, and urban residence. Age > 80 years, fewer than two comorbidities, and not being married were associated with decreased use. In the survival analysis, BCG use was associated with better OS (hazard ratio [HR], 0.87; 95% CI, 0.83 to 0.92) in the entire cohort and BCSS among higher-grade cancers (poorly differentiated: HR, 0.78; 95% CI, 0.72 to 0.85; undifferentiated: HR, 0.66; 95% CI, 0.56 to 0.77).
Conclusion:
Despite guidelines recommending its use, BCG is administered to less than one quarter of eligible patients. This large population-based study found improved OS and BCSS were associated with use of adjuvant intravesical BCG among older patients with NMIBC. Better-designed clinical trials focusing on higher-grade cancers are needed to confirm these findings.
Introduction
The management of non–muscle-invasive bladder cancer (NMIBC) remains a common and complex clinical challenge. Approximately 70,000 new cases of bladder cancer are diagnosed annually in the United States, with two thirds of cases occurring in persons older than age 65 years.1,2 Three quarters of incident cases are considered superficial or non–muscle invasive: stages Ta, T1, and Tis.3 The risk of recurrence and progression of NMIBC is influenced by tumor stage, grade, size, number of tumors, and previous history of and length of time to recurrence. Overall, 65% of non–muscle-invasive tumors will recur, and 25% of these will progress to a higher stage or grade.3 Ta lesions are usually low grade and rarely progress to a higher stage or grade. The clinical behavior of T1 cancers is heterogeneous, with some being indolent and others more aggressive, with a tendency to recur and progress to muscle-invasive disease (T2). Tis (carcinoma in situ [CIS]) lesions carry a much higher risk of recurrence and progression to muscle-invasive disease.
Various options are available for management of NMIBC, including transurethral resection (TUR), adjuvant intravesical therapy, and radical cystectomy. TUR is the initial step for the diagnosis and management of NMIBC. After TUR, 10-year disease-specific survival for persons with Ta and T1 tumors is 85% and 70%, respectively.4 NMIBC has a high likelihood of recurrence after treatment with TUR alone. For example, in one large cohort of T1 bladder tumors treated only with TUR, 75% and 90% recurred by 5 and 10 years of follow-up, respectively.5 One third progressed to muscle invasion within 5 years.6
Intravesical therapy has been investigated in the adjuvant setting after TUR in an attempt to improve the outcomes of patients with NMIBC. Bacillus Calmette-Guérin (BCG) is an immunotherapeutic agent that is effective in the treatment of NMIBC. Several clinical trials have demonstrated statistically significant reductions in short- and long-term recurrence rates with adjuvant BCG treatment after complete TUR of Ta and T1 lesions when compared with TUR alone or with intravesical chemotherapy.7–10 Adjuvant BCG is effective treatment for Tis, eradicating 80% of cases and improving disease-specific survival.11 A single randomized trial of 86 patients with high-risk disease and 15-year follow-up demonstrated a survival advantage for BCG in conjunction with TUR.4,12 Three meta-analyses concluded that BCG in conjunction with TUR reduced recurrence13–15 and progression16 of NMIBC but had no effect on overall (OS) or disease-specific survival.16 The inability to demonstrate a survival advantage likely results from the majority of studies having relatively short follow-up, given the prolonged natural history of NMIBC and the low risk of progression from non–muscle-invasive to invasive disease. BCG is considered safe as an intravesical agent, with fewer than 5% of patients experiencing a major adverse event.17
Thus, no consensus exists regarding the impact of adjuvant intravesical BCG on OS or disease-specific survival. Specifically, early treatment at first diagnosis of bladder cancer has not been consistently studied. Nevertheless, the National Comprehensive Cancer Network guideline currently recommends adjuvant BCG after TUR for all non–muscle-invasive tumors that are high grade and/or stage Tis or T1.18 Using a large population-based database, we sought to determine the predictors of receipt of intravesical therapy and investigate the influence of intravesical BCG on survival in a large cohort of elderly patients with NMIBC.
Patients and Methods
Data Source
We analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database linked with diagnostic and procedural billing claims from Medicare.19 SEER, under the auspices of the National Cancer Institute, has collected tumor registry data on selected populations across the United States, representing approximately 26% of the population, since 2000. SEER provides information on tumor histology, location, disease stage, treatment, and survival along with selected census tract–level sociodemographic information. The Medicare database includes Medicare A and B eligibility status, dates of enrollment in health maintenance organizations (HMOs), and billed claims, including inpatient and outpatient services, procedures, and diagnoses.
Sample Selection
We identified all individuals within the SEER-Medicare database age ≥ 65 years who received a pathologically confirmed primary diagnosis of bladder cancer (SEER site of cancer, 58) from January 1, 1991, to December 31, 2002. We excluded patients who were enrolled in an HMO from 12 months before to 12 months after diagnosis and who were not covered by Medicare Parts A or B over the same period, because those in HMOs are not billed within Medicare only. We limited our sample to those with stage I (according to the American Joint Committee on Cancer) NMIBC (N = 24,982). We excluded patients who died during the first 3 months after a diagnosis of NMIBC (n = 535), who had a claim for intravesical BCG or bladder cancer surgery more than 14 days before the date of bladder cancer diagnosis (n = 353), who died on the date of diagnosis (n = 1), and who had fewer than 30 days of follow-up (n = 161). This resulted in a cohort of 23,932 patients.
Tumor and Patient Characteristics
Data on clinical stage, histologic grade, age, race/ethnicity, sex, marital status, type of hospital, and area of residence were taken from the SEER database. We recoded extension codes for clinical stage into equivalent TNM stages: extension codes 00 and 06 were considered CIS; 01, 03, 06, and 10 were considered Ta; and 15 was considered T1. Patients without an extension code were considered AJCC stage I, not otherwise specified. Age at diagnosis was categorized in pentads starting at 65 years. We recoded the SEER marital status variable into married, not married, and unknown. Area-based socioeconomic (SES) status was estimated using a composite of three different variables from the 2000 census, using a method described previously by Chamie et al.20 Individuals were ranked into quintiles on the basis of their census tract median household income, percentage of persons age ≥ 25 years with at least a high school education, and percentage of people below the poverty level. Each patient was assigned a composite SES score based on the average of these three rankings.
Comorbid Disease
We computed a comorbidity score for each patient using the Klabunde adaptation of the Charlson comorbidity index.21,22 Medicare inpatient and outpatient claims were searched from 365 days before to 120 days after the diagnosis of cancer for all International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes corresponding to each of the following comorbid conditions: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, mild to severe liver disease, diabetes with or without end-organ damage, hemiplegia, moderate or severe renal disease, and AIDS. Each disease category was weighted based on the Charlson index, and patients were assigned a score of 0, 1, or > 1.
Treatment Characteristics
We searched the Medicare physician claims files (National Claims History database), hospital outpatient claims files (Outpat), and Medicare provider review files (Medicare Provider Analysis and Review database) for procedural and treatment codes related to adjuvant cancer therapy within the first 6 months from diagnosis. Instances of intravesical chemotherapy were identified on the basis of having claims for the following codes: instillation of intravesical BCG, Level II Health Care Procedure Coding System J9031, and instillation of intravesical anticarcinogenic agent, Current Procedural Terminology 51720. Although the ability of SEER-Medicare claims data to reliably identify a wide-ranging set of cancer treatments has been described previously, the validity of intravesical chemotherapy has not been evaluated.23,24
Mortality and Survival
Survival was calculated in months from the date of bladder cancer diagnosis to the date of death according to SEER. Survival was censored as of the last Medicare coverage month when patients were known to be alive or as of December 31, 2002. Bladder cancer–specific mortality was assessed with ICD-9 codes 188.0 to 188.9 for patients dying as a result of bladder cancer from 1991 to 1998 and ICD-10 codes C67.0 to C67.9 from 1999 to 2002.
Statistical Analyses
We used the χ2 test to compare the distributions of clinical and demographic characteristics (age at diagnosis, sex, year of diagnosis, race, stage, grade, marital status, comorbidity score, and area SES quintile), comparing those who received adjuvant intravesical BCG within 6 months with those who did not. We used univariate and multivariate logistic regression models to identify predictors of receipt of BCG from among the measured clinical and demographic characteristics. We adjusted these models for age, sex, year of diagnosis, race, marital status, comorbidity score, urban/rural location, and area SES quintile. Among patients who survived at least 12 weeks, we developed Cox proportional hazards models to analyze the association of BCG treatment with OS and bladder cancer–specific survival (BCSS). Finally, we generated adjusted Kaplan-Meier curves for each of the survival end points as well as curves stratified by tumor grade. All statistical analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC). The study protocol was approved by the Columbia University Medical Center Institutional Review Board.
Results
The sample was predominantly male (74%), white (93%), married (63%), and without comorbidities (57%); most resided in urban locales (91%); and the mean age was 77 years (Table 1). Sixty-two percent of patients had stage Ta disease, and 64% had well- or moderately differentiated tumors. Differences between the treated and untreated groups included younger age, more men, fewer blacks and other races, higher-grade tumors, stage CIS, married status, urban residence, and higher socioeconomic status in the treated group.
Table 1.
Characteristics and Multivariate* Predictors of Receipt of Adjuvant Intravesical BCG Among Patients Diagnosed With NMIBC Registered in the SEER-Medicare Database, 1991-2002 (N = 23,932)
| Category | BCG (n = 5,885) |
No BCG (n = 18,047) |
Multivariate Predictors of Receipt of BCG |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | OR | 95% CI | |
| Age, years† | ||||||
| Mean | 75.9 | 76.9 | ||||
| 65-69 | Referent | |||||
| 70-74 | 1.05 | 0.95 to 1.16 | ||||
| 75-79 | 0.95 | 0.86 to 1.05 | ||||
| 80-84 | 0.88 | 0.79 to 0.98 | ||||
| ≥ 85 | 0.51 | 0.45 to 0.58 | ||||
| Sex† | ||||||
| Male | 4,477 | 76 | 13,177 | 73 | Referent | |
| Female | 1,408 | 24 | 4,870 | 27 | 0.99 | 0.92 to 1.07 |
| Race/ethnicity† | ||||||
| White | 5,434 | 92 | 16,904 | 94 | Referent | |
| Black | 170 | 3 | 517 | 3 | 0.94 | 0.78 to 1.14 |
| Hispanic | 45 | 1 | 137 | 1 | 0.86 | 0.60 to 1.23 |
| Other | 223 | 4 | 438 | 22 | 1.42 | 1.20 to 1.68 |
| Grade† | ||||||
| Well differentiated | 505 | 9 | 4,111 | 23 | Referent | |
| Moderately differentiated | 2,018 | 34 | 8,606 | 48 | 1.85 | 1.66 to 2.05 |
| Poorly differentiated | 1,993 | 34 | 3,363 | 19 | 4.07 | 3.63 to 4.56 |
| Undifferentiated | 659 | 11 | 847 | 5 | 4.60 | 3.97 to 5.32 |
| Unknown | 710 | 12 | 1,120 | 6 | 3.88 | 3.37 to 4.47 |
| Stage† | ||||||
| Tis | 679 | 12 | 862 | 5 | Referent | |
| Ta | 2,706 | 46 | 12,178 | 67 | 2.67 | 2.37 to 3.02 |
| T1 | 2,257 | 38 | 3,750 | 21 | 1.91 | 1.77 to 2.05 |
| Other | 243 | 4 | 1,257 | 7 | 0.76 | 0.66 to 0.89 |
| No. of comorbidities | ||||||
| 0 | 3,342 | 57 | 10,262 | 57 | Referent | |
| 1 | 1,480 | 25 | 4,432 | 25 | 1.01 | 0.94 to 1.09 |
| ≥ 2 | 1,063 | 18 | 3,353 | 19 | 0.94 | 0.86 to 1.02 |
| Marital status† | ||||||
| Single/divorced | 1,708 | 29 | 6,048 | 34 | Referent | |
| Married | 3,941 | 67 | 11,242 | 62 | 0.86 | 0.80 to 0.92 |
| Unknown | 236 | 4 | 757 | 4 | 0.87 | 0.75 to 1.03 |
| Residence† | ||||||
| Urban | 5,390 | 92 | 16,331 | 90 | 1.16 | 1.03 to 1.30 |
| Rural | 495 | 8 | 1,716 | 10 | Referent | |
| SES quintile | ||||||
| 1st | 1,129 | 19 | 3,340 | 19 | 1.08 | 0.97 to 1.19 |
| 2nd | 1,103 | 19 | 3,521 | 20 | 0.99 | 0.90 to 1.10 |
| 3rd | 1,133 | 19 | 3,687 | 20 | 0.92 | 0.84 to 1.02 |
| 4th | 1,213 | 21 | 3,694 | 20 | 0.98 | 0.89 to 1.08 |
| 5th | 1,307 | 22 | 3,805 | 21 | Referent | |
Abbreviations: BCG, Bacillus Calmette-Guérin; NMIBC, non–muscle-invasive bladder cancer; OR, odds ratio; SEER, Surveillance, Epidemiology, and End Results; SES, socioeconomic.
Adjusted for all other variables in the table and year of diagnosis.
Statistically significant differences exist between BCG and no BCG groups for these variables (χ2 significance test).
Overall, 22% of patients received BCG within 6 months of being diagnosed with NMIBC. In multivariate analysis, the strongest predictors of receipt of BCG were grade: undifferentiated (odds ratio [OR], 4.60; 95% CI, 3.97 to 5.32), poorly differentiated (OR, 4.07; 95% CI, 3.63 to 4.56), and moderately differentiated (OR, 1.85; 95% CI, 1.66 to 2.05) versus well differentiated; stage: Tis (OR, 2.67; 95% CI, 2.37 to 3.02) and T1 (OR, 1.91; 95% CI, 1.77 to 2.05) versus Ta; age: 80 to 84 years (OR, 0.88; 95% CI, 0.79 to 0.98) and > 85 years (OR, 0.51; 95% CI, 0.45 to 0.58) versus 65 to 69 years; marital status: single or divorced (OR, 0.86; 95% CI, 0.80 to 0.92) versus married; and urban (OR, 1.16; 95% CI, 1.03 to 1.30) versus rural residence.
Table 2 summarizes the results of Cox proportional hazards models for OS and BCSS. A total of 10,598 patients (44% of the sample) died during the study period, including 2,303 who were treated with BCG and 8,295 who were not; 1,623 patients (7%) died as a result of bladder cancer, of whom 443 received BCG and 1,180 did not. After adjusting for demographic, SES, and clinical characteristics, we found that OS in the entire cohort (hazard ratio [HR], 0.87; 95% CI, 0.83 to 0.92) was improved among patients who received adjuvant intravesical therapy within 6 months of diagnosis of NMIBC. In a subgroup analysis stratified by stage (data not shown), we found that OS was improved in higher grades (poorly differentiated: OR, 0.78; 95% CI, 0.72 to 0.85 and undifferentiated: OR, 0.66; 95% CI, 0.56 to 0.77) but not in well- or moderately differentiated grades. Although there was only a trend toward a survival advantage for BCSS in the entire cohort (OR, 0.90; 95% CI, 0.80 to 1.01), adjuvant BCG conferred a significant survival advantage among subsets of higher-grade cancers (poorly differentiated: OR, 0.70; 95% CI, 0.59 to 0.84 and undifferentiated: OR, 0.56; 95% CI, 0.41 to 0.77). OS was worse among patients with well- and moderately differentiated tumors who received BCG. Older age, higher grade, stages T1 and Tis, more comorbidities, and not being married were risk factors for both all-cause and bladder cancer–specific mortality. Male sex was associated with a greater likelihood of all-cause mortality, and black and Hispanic races were risk factors for bladder cancer–specific mortality.
Table 2.
Estimates of Overall and Disease-Specific Survival Using Cox Proportional Hazards Models Among Patients Diagnosed With NMIBC in the SEER-Medicare Database, 1991-2002 (N = 23,932)
| Category | Overall Survival |
Disease-Specific Survival |
||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| BCG | 0.87 | 0.83 to 0.92 | 0.90 | 0.80 to 1.01 |
| Age, years | ||||
| 65-69 | Referent | Referent | ||
| 70-74 | 1.41 | 1.31 to 1.52 | 1.16 | 0.97 to 1.39 |
| 75-79 | 1.89 | 1.76 to 2.03 | 1.47 | 1.23 to 1.76 |
| 80-84 | 2.77 | 2.57 to 2.98 | 1.82 | 1.51 to 2.19 |
| ≥ 85 | 4.53 | 4.21 to 4.89 | 3.10 | 2.58 to 3.73 |
| Sex | ||||
| Male | Referent | Referent | ||
| Female | 0.74 | 0.71 to 0.78 | 1.07 | 0.95 to 1.20 |
| Race | ||||
| White | Referent | Referent | ||
| Black | 1.08 | 0.97 to 1.21 | 1.57 | 1.26 to 1.96 |
| Hispanic | 1.14 | 0.92 to 1.43 | 1.94 | 1.26 to 2.99 |
| Other | 0.75 | 0.66 to 0.84 | 0.86 | 0.64 to 1.15 |
| Grade | ||||
| Well differentiated | Referent | Referent | ||
| Moderately differentiated | 1.11 | 1.05 to 1.17 | 1.91 | 1.55 to 2.36 |
| Poorly differentiated | 1.41 | 1.32 to 1.50 | 4.21 | 3.40 to 5.23 |
| Undifferentiated | 1.72 | 1.57 to 1.89 | 5.91 | 4.62 to 7.56 |
| Unknown | 1.41 | 1.29 to 1.54 | 3.67 | 2.84 to 4.75 |
| Stage | ||||
| Ta | Referent | Referent | ||
| Tis | 1.09 | 1.00 to 1.22 | 1.50 | 1.20 to 1.87 |
| T1 | 1.25 | 1.19 to 1.31 | 2.18 | 1.94 to 2.46 |
| Other | 1.34 | 1.25 to 1.44 | 2.73 | 2.32 to 3.20 |
| Year of diagnosis | 0.97 | 0.96 to 0.98 | 0.93 | 0.92 to 0.95 |
| No. of comorbidities | ||||
| 0 | Referent | Referent | ||
| 1 | 1.39 | 1.33 to 1.46 | 1.02 | 0.90 to 1.15 |
| ≥ 2 | 2.13 | 2.03 to 2.24 | 1.28 | 1.12 to 1.46 |
| Marital status | ||||
| Married | Referent | Referent | ||
| Single/divorced | 1.26 | 1.20 to 1.31 | 1.21 | 1.08 to 1.35 |
| Unknown | 0.87 | 0.78 to 0.98 | 0.67 | 0.48 to 0.93 |
| Residence | ||||
| Urban | 1.01 | 0.94 to 1.08 | 1.07 | 0.89 to 1.30 |
| Rural | Referent | Referent | ||
| SES quintile | ||||
| 1st | 1.23 | 1.15 to 1.31 | 1.20 | 1.02 to 1.41 |
| 2nd | 1.17 | 1.10 to 1.24 | 1.13 | 0.96 to 1.33 |
| 3rd | 1.05 | 0.99 to 1.12 | 1.01 | 0.86 to 1.18 |
| 4th | 1.09 | 1.03 to 1.16 | 1.06 | 0.90 to 1.24 |
| 5th | Referent | Referent | ||
Abbreviations: BCG, Bacillus Calmette-Guérin; HR, hazard ratio; NMIBC, non–muscle-invasive bladder cancer; SEER, Surveillance, Epidemiology, and End Results; SES, socioeconomic.
Adjusted Kaplan-Meier survival curves comparing treatment groups are shown for the entire cohort in Figure 1 and stratified by tumor grade in Appendix Figures A1A to A1D (online only). Those receiving adjuvant intravesical BCG experienced a statistically significant survival advantage over the 10-year follow-up period compared with those not receiving therapy (HR, 0.87; 95% CI, 0.83 to 0.92). The absolute survival advantage at 5 years was approximately 4%. Median survival was approximately 2 years greater in the BCG group. In Figures A1A to A1D, OS stratified by histologic grade is plotted for the treatment groups. Statistically significant differences in OS and disease-specific survival were seen among the poorly and undifferentiated groups but not in the well- or moderately differentiated groups.
Figure 1.
Adjusted Kaplan-Meier 10-year overall survival. BCG, Bacillus Calmette-Guérin.
Discussion
Adjuvant intravesical BCG therapy has long been known to decrease the likelihood of bladder cancer recurrence and progression rates, but it has never been convincingly shown in a large randomized clinical trial to prolong OS or BCSS. Given the prolonged natural history of NMIBC and the attendant costs of a multiyear randomized clinical trial, the feasibility of a prospective study investigating a survival benefit in this disease may be limited. Thus, this observational study may represent the best we can do in a disease with late death. We found an association with improved survival among elderly patients with high-grade bladder cancer. The overall effect was moderate, increased substantially with increasing grade, and persisted for 10 years of follow-up. Furthermore, we demonstrated that BCG use among patients with low-grade tumors was not associated with survival.
In their recent guidelines, the National Comprehensive Cancer Network and American Urological Association both recommend the use of BCG and/or mitomycin C for adjuvant treatment after TUR for decreasing the risk of recurrence and progression, but not for a survival advantage.18,25 Indications include high grade and stage Tis or T1 disease. Although we found grade and stage to be the strongest predictors for receiving BCG, only 39% of those with stage Tis or T1 and 27% of those with grades higher than well differentiated received BCG within 6 months of diagnosis. Although the majority of Ta tumors are low grade, we chose not to eliminate this stage from the analysis to avoid biasing our results. In fact, when limiting the analysis to stages Tis and T1, we found that the protective effect of BCG was strengthened, with an HR for OS of 0.79 (95% CI, 0.74 to 0.84).
We also found that elderly patients and those with two or more comorbidities were less likely to undergo BCG treatment, which would seem to be appropriate. However, we performed a survival analysis stratified by age and found that BCG was protective among those age ≥ 80 years. Furthermore, a significant interaction between age and receipt of BCG was present. Other studies have documented the appropriateness of treating octogenarians with muscle-invasive bladder cancer.20 Identifying significant underuse of a guideline-recommended treatment, we have documented a lapse in quality of care. Although many of the untreated patients did receive BCG later in their disease courses, such treatment cannot be considered adjuvant therapy. This study suggests that early treatment is important.
Although this study and others suggest that the use of intravesical BCG is increasing,26 the overall rate remains low. Ways in which BCG use could be increased might include designating adjuvant intravesical BCG as a measure of quality among younger patients newly diagnosed with high-grade NMIBC. The Centers for Medicare and Medicaid Services Physician Quality Reporting Initiative ties the self-reporting of quality indicators to evidence-based practices and may represent an intervention that could improve compliance with adjuvant intravesical therapy for NMIBC. Potential quality indicators might include not only the early use of BCG within 6 months of diagnosis for high-grade tumors but also the absence of BCG use for low-grade tumors, thereby cost-effectively focusing treatment on those most likely to benefit.
There are several limitations to our study. Its retrospective observational nature prevents us from confirming a cause-and-effect relationship between BCG and improved survival; the association with survival may have been related to confounders rather than to the use of the drug itself. This Medicare claims–based study was limited to those age ≥ 65 years. Therefore, we cannot generalize about the effectiveness of BCG in younger patients. However, a recent study found that younger age was associated with more favorable tumor characteristics,2 and the risk of death as a result of bladder cancer increased with increasing age.27 Patients with a nonspecific intravesical therapy claim for Current Procedural Terminology code 51720 were included with the BCG group, because more than 97% of adjuvant intravesical therapy administered with a specified drug was for BCG. Only 3% received other drugs, including mitomycin C, thiotepa, or interferon. Therefore, we acknowledge that there may have been some misclassification of patients in the treatment groups. We did not have information regarding the dose of intravesical therapy used, nor did we quantify the number of instillations administered. However, the most effective dose of BCG remains unknown, with a recent randomized clinical trial demonstrating that one-third strength BCG was as effective as the standard strength.28 SEER does not report the number or size of tumors nor concomitant Tis, which are important risk factors for recurrence of NMIBC.29 We were also unable to adjust for the completeness for the TUR of the bladder cancer, a factor that is important for adjuvant BCG to be effective.30 We found that the effect on OS was slightly greater than that on disease-specific survival, suggesting that a small part of the survival benefit may represent unmeasured selection. Finally, a limitation of SEER is the inability to identify cancer recurrence. For our analysis, we assumed that patients who received BCG more than 6 months after diagnosis (n = 3,148) had suffered a recurrence, were treated with BCG as a salvage therapy, and were therefore included in the comparison untreated group. Our selection criteria attempted to avoid confounding by indication, which would have occurred if those who were treated with BCG later than 6 months were included in the treated group and if the benefit of treatment was ascribed to this group. Such cohort selection would have likely led to much greater bias. Because 37% of those who received BCG at any time did so after 6 months, the potential beneficial effect of BCG on survival was greatly attenuated and was a conservative estimate.
To our knowledge, this is the first large population-based study to suggest a survival advantage among patients with NMIBC who receive adjuvant intravesical BCG. Although our data are derived from a retrospective data set, this study may be more indicative of the true effect of intravesical BCG because of its large sample size, particularly of high-grade cancers, leading to less dilution of the benefit of BCG, and longer follow-up period than previous clinical trials and meta-analyses. It also reflects its use and effectiveness in the community rather than in highly selected trial patients.
Acknowledgment
Supported by American Cancer Society Grants No. RSGHP PBP-105710 and RSGT-01-024-04-CPHPS and National Cancer Institute Grants No. CA95597, CA09529, and CA89155. Presented in part at the 102nd Annual Meeting of the American Urological Association, Anaheim, CA, May 19-24, 2007. We acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, National Cancer Institute; Offices of Information Services and Strategic Planning, Health Care Financing Administration; Information Management Services; and Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Appendix
Figure A1.
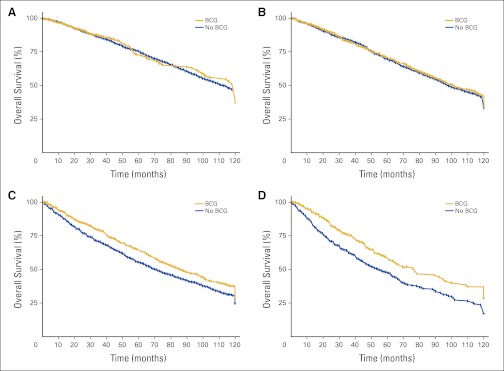
Adjusted Kaplan-Meier 10-year overall survival by grade: (A) well differentiated; (B) moderately differentiated; (C) poorly differentiated; (D) undifferentiated. BCG, Bacillus Calmette-Guérin.
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Benjamin A. Spencer, Dawn L. Hershman, Harry W. Herr, Mitchell C. Benson, Supriya Gupta-Mohile, Alfred I. Neugut
Financial support: Mitchell C. Benson, Alfred I. Neugut
Administrative support: Mitchell C. Benson
Collection and assembly of data: Donna Buono
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.American Cancer Society. What are the key statistics about bladder cancer? http://www.cancer.org/cancer/bladdercancer/detailedguide/bladder-cancer-key-statistics.
- 2.Cho KS, Hwang TK, Kim BW, et al. Differences in tumor characteristics and prognosis in newly diagnosed Ta, T1 urothelial carcinoma of bladder according to patient age. Urology. 2009;73:828–832. doi: 10.1016/j.urology.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 3.Amling CL. Diagnosis and management of superficial bladder cancer. Curr Probl Cancer. 2001;25:219–278. doi: 10.1067/mcn.2001.117539. [DOI] [PubMed] [Google Scholar]
- 4.Cookson MS, Herr HW, Zhang ZF, et al. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol. 1997;158:62–67. doi: 10.1097/00005392-199707000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Herr HW. High-risk superficial bladder cancer: Transurethral resection alone in selected patients with T1 tumor. Semin Urol Oncol. 1997;15:142–146. [PubMed] [Google Scholar]
- 6.Herr HW. Natural history of superficial bladder tumors: 10- to 20-year follow-up of treated patients. World J Urol. 1997;15:84–88. doi: 10.1007/BF02201977. [DOI] [PubMed] [Google Scholar]
- 7.Dalbagni G. The management of superficial bladder cancer. Nat Clin Pract Urol. 2007;4:254–260. doi: 10.1038/ncpuro0784. [DOI] [PubMed] [Google Scholar]
- 8.Krege S, Giani G, Meyer R, et al. A randomized multicenter trial of adjuvant therapy in superficial bladder cancer: Transurethral resection only versus transurethral resection plus mitomycin C versus transurethral resection plus bacillus Calmette-Guerin—Participating clinics. J Urol. 1996;156:962–966. doi: 10.1016/s0022-5347(01)65673-8. [DOI] [PubMed] [Google Scholar]
- 9.Herr HW. Intravesical BCG: Current results, natural history and implications for urothelial cancer prevention. J Cell Biochem Suppl. 1992;16I:112–119. doi: 10.1002/jcb.240501322. [DOI] [PubMed] [Google Scholar]
- 10.Nadler RB, Catalona WJ, Hudson MA, et al. Durability of the tumor-free response for intravesical bacillus Calmette-Guerin therapy. J Urol. 1994;152:367–373. doi: 10.1016/s0022-5347(17)32741-6. [DOI] [PubMed] [Google Scholar]
- 11.Hudson MA, Herr HW. Carcinoma in situ of the bladder. J Urol. 1995;153:564–572. doi: 10.1097/00005392-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Herr HW, Schwalb DM, Zhang ZF, et al. Intravesical bacillus Calmette-Guerin therapy prevents tumor progression and death from superficial bladder cancer: Ten-year follow-up of a prospective randomized trial. J Clin Oncol. 1995;13:1404–1408. doi: 10.1200/JCO.1995.13.6.1404. [DOI] [PubMed] [Google Scholar]
- 13.Han RF, Pan JG. Can intravesical bacillus Calmette-Guerin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology. 2006;67:1216–1223. doi: 10.1016/j.urology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Shelley MD, Court JB, Kynaston H, et al. Intravesical Bacillus Calmette-Guerin in Ta and T1 bladder cancer. Cochrane Database Syst Rev. 2000;4:CD001986. doi: 10.1002/14651858.CD001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shelley MD, Kynaston H, Court J, et al. A systematic review of intravesical bacillus Calmette-Guerin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 2001;88:209–216. doi: 10.1046/j.1464-410x.2001.02306.x. [DOI] [PubMed] [Google Scholar]
- 16.Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 17.Lamm DL. Efficacy and safety of Bacille Calmette-Guérin immunotherapy in superficial bladder cancer. Clin Infect Dis. 2000;31(suppl 3):S86–S90. doi: 10.1086/314064. [DOI] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Bladder cancer including upper tract tumors and urothelial carcinoma of the prostate. 2010;Volume 2 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 19.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 20.Chamie K, Hu B, Devere White RW, et al. Cystectomy in the elderly: Does the survival benefit in younger patients translate to the octogenarians? BJU Int. 2008;102:284–290. doi: 10.1111/j.1464-410X.2008.07636.x. [DOI] [PubMed] [Google Scholar]
- 21.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Lamont EB, Lauderdale DS, Schilsky RL, et al. Construct validity of medicare chemotherapy claims: The case of 5FU. Med Care. 2002;40:201–211. doi: 10.1097/00005650-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(suppl 8):IV-55–IV-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 25.American Urological Association. Guideline for the management of nonmuscle invasive bladder cancer: Stage Ta, T1, Tis—2007 update. http://www.auanet.org/content/clinical-practice-guidelines/clinical-guidelines.cfm?sub=bc. [DOI] [PubMed]
- 26.Strope SA, Ye Z, Hollingsworth JM, et al. Patterns of care for early stage bladder cancer. Cancer. 2010;116:2604–2611. doi: 10.1002/cncr.25007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang GJ, Hamilton AS, Lo M, et al. Predictors of intravesical therapy for nonmuscle invasive bladder cancer: Results from the surveillance, epidemiology and end results program 2003 patterns of care project. J Urol. 2008;180:520–524. doi: 10.1016/j.juro.2008.04.016. discussion 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez-Piñeiro JA, Martínez-Piñeiro L, Solsona E, et al. Has a 3-fold decreased dose of bacillus Calmette-Guerin the same efficacy against recurrences and progression of T1G3 and Tis bladder tumors than the standard dose? Results of a prospective randomized trial. J Urol. 2005;174:1242–1247. doi: 10.1097/01.ju.0000173919.28835.aa. [DOI] [PubMed] [Google Scholar]
- 29.Orsola A, Cecchini L, Raventos CX, et al. Risk factors for positive findings in patients with high-grade T1 bladder cancer treated with transurethral resection of bladder tumour (TUR) and bacille Calmette-Guerin therapy and the decision for a repeat TUR. BJU Int. 2010;105:202–207. doi: 10.1111/j.1464-410X.2009.08694.x. [DOI] [PubMed] [Google Scholar]
- 30.Herr HW. Restaging transurethral resection of high risk superficial bladder cancer improves the initial response to bacillus Calmette-Guerin therapy. J Urol. 2005;174:2134–2137. doi: 10.1097/01.ju.0000181799.81119.fc. [DOI] [PubMed] [Google Scholar]