The authors present a guide to the necessary infrastructure and institutional support that must be in place before considering a program like the Minority-Based Community Clinical Oncology Program.
Abstract
The Minority-Based Community Clinical Oncology Program (MB-CCOP) at University of Medicine and Dentistry of New Jersey, New Jersey Medical School–University Hospital Cancer Center was established to serve an unmet need in a medically, educationally, and socioeconomically underserved community of primarily African American and Latino patients in Newark and Essex County, New Jersey. The MB-CCOP was built on an existing infrastructure of multidisciplinary teams of cancer specialists who collaborated in patient care and an existing clinical research program, which included multilingual staff and a breast cancer navigator. This article highlights some of the unique opportunities and challenges involved in the startup of an MB-CCOP specifically relevant to an academic setting. We present a guide to the necessary infrastructure and institutional support that must be in place before considering such a program and some of the steps an institution can take to overcome barriers preventing successful enrollment of patients onto clinical trials.
Introduction
The National Cancer Institute (NCI) developed the Minority-Based Community Clinical Oncology Program (MB-CCOP) to overcome significant differences in minority enrollment onto and participation in cancer clinical trials.1 Overall patient enrollment rates in clinical trials in the United States are under 4%, but the rate of enrollment of minorities is significantly lower.2,3 The rationale for creating the MB-CCOP was based on the ethical and social imperatives to provide equal access to government-funded state-of-the-art cancer therapy for underserved populations in the United States and to reverse the exclusion of a segment of the overall population from randomly controlled research sampling frames, which tend to systematically distort data and decrease the validity of findings.
The MB-CCOP was established to foster the enrollment of minority patients onto cancer clinical trials at hospitals that provide care to a patient population with at least 40% representation of minority groups, as defined by the Office of Management and Budget Directive No. 15.4 Each group and its subgroups are endowed with individual characteristics that make them unique and provide specific opportunities and challenges regarding enrollment onto cancer clinical trials. The MB-CCOP has been successful by most measures, enrolling minority patients at a 65% higher rate than other CCOPs.1
The University of Medicine and Dentistry of New Jersey, New Jersey Medical School–University Hospital (NJMS-UH) Cancer Center exemplifies a prototypical setting for providing minority patients access to and enrollment onto cancer clinical trials. The patients with cancer served by the cancer center, primarily from Newark and Essex County, New Jersey, belong to an urban population consisting of approximately 31% who classify themselves as African American, 23% as Hispanic or Latino, and 2% as Asian or Pacific Islander. This report outlines some of the unique opportunities and challenges involved in the startup of an MB-CCOP specifically relevant to an academic setting.5 We present a guide to the necessary infrastructure and institutional support that must be in place before considering such a program and some of the steps an institution can take to overcome barriers preventing successful enrollment of patients onto clinical trials.
Methods
A research team at the University of North Carolina at Chapel Hill conducted in-depth interviews with a variety of MB-CCOP stakeholders, including the principal investigator (PI), the MB-CCOP manager, and the cancer center director. Interviews were intended to assess the infrastructure, personnel, workflow, patient-encounter setting, collaborating departments, multidisciplinary tumor boards, clinical research support, institutional support, and patient characteristics and challenges that preexisted and coexisted with the startup of the NJMS-UH Cancer Center MB-CCOP. The PI also discussed many of these issues during several interviews with the MB-CCOP manager, the senior associate dean for research of the NJMS, and MB-CCOP investigators. This report is a narrative description of the programmatic issues, strengths, challenges, and recommendations derived from the interviews. The issues are categorized at patient, physician, and organization and system levels.
Results
Program Description
Patients who are screened and enrolled at the NJMS-UH Cancer Center MB-CCOP are from University Hospital inpatient and outpatient units. The PI, physicians, and surgeons are full-time faculty members at the NJMS. The MB-CCOP office is located in the cancer center and occupies space and shares staff with the cancer center Clinical Research Office (CRO) and the medical school Clinical Research Unit. The MB-CCOP manager is also the director of the CRO and of the Clinical Research Unit. The PI is the associate director for clinical and translational research for the cancer center. The multispecialty physicians collaborate in the care of patients with cancer and participate in seven multidisciplinary disease-specific tumor boards, where most patients are presented and discussed. Patients have access to all modalities of therapy.
Strengths of the Program That Foster Success As an MB-CCOP
The program had a number of significant strengths at the time of application, making it an attractive candidate to become an MB-CCOP (Table 1). The principal strength was derived from the patients who depended on the cancer center for their care. Most patients were from a predominantly minority and medically underserved population. The patients and community developed trust in the oncology physicians from prior relationships and word of mouth and generally agreed to participate in clinical trials when they were offered. The physicians at the cancer center had extensive experience caring for patients from the community, were adept at sensitive, multilingual approaches to patient care, and had benefitted from using a bilingual breast cancer navigator. The program had a long-standing track record of enrolling minority patients onto clinical trials and experience obtaining informed consent from non–English speaking patients.
Table 1.
Strengths at NJMS-UH Cancer Center MB-CCOP Supporting Clinical Trial Enrollment
| Level of Categorization | Strengths |
|---|---|
| Patient-based issues | Dependence of local population on University Hospital for care |
| Large percentage of minority patients | |
| Trust of community in oncology division based on prior relationships | |
| Welcomed opportunity by patients to participate in NCI trials | |
| Physician-based issues | Competent, knowledgeable academic physicians |
| Long history of multidisciplinary approach to cancer care | |
| Seven multispecialty disease-specific tumor boards where most patients are discussed | |
| Philosophic buy-in from physician faculty for need to enroll patients onto clinical trials | |
| Rich scientific and intellectual environment in medical school and cancer center | |
| PI is physician-scientist with active laboratory research program | |
| Organization- and system-based issues | Prior experience in care and support of indigent population |
| Strong administrative support to obtain charity care and help enroll patients on Medicaid | |
| Prior experience with multilingual population with many members of staff who speak Spanish | |
| Prior successful externally funded breast navigator program demonstrating effectiveness of paradigm as applied to clinical trials | |
| Excellent cancer center CRO staff | |
| Strong organization of cancer center CRO with respect to work assignments, workflow, work meetings, and recent takeover of new patient appointment process, enabling identification of study candidates | |
| Active systematic process to identify and activate new available protocols for most diseases/stages |
Abbreviations: CRO, Clinical Research Office; MB-CCOP, Minority-Based Community Clinical Oncology Program; NCI, National Cancer Institute; NJMS-UH, New Jersey Medical School–University Hospital; PI, principal investigator.
The academic physicians at University Hospital and the cancer center worked well together and bought into the concept that excellent cancer care includes participation in research clinical trials. Almost every patient was presented and discussed at one of seven multidisciplinary tumor boards, which were attended by the relevant cancer specialists as well as members of the CRO. The faculty physicians were part of the larger, academic environment at the medical school and the cancer center, which fostered a natural curiosity conducive to clinical research. The PI was a physician scientist with an active basic research program in breast cancer.
At the time of application, the cancer center had an established CRO with a core group that had worked together for several years and built an organizational setup with respect to work assignments, workflow, and weekly work meetings. As part of the organizational structure, a system was in place to identify new cooperative group protocols as they opened, appropriate to the patients served.
Program Challenges to Address for Further Success As an MB-CCOP
The challenges for successful enrollment of minority patients onto cooperative group trials have been well documented and reflect primarily patient-based issues, physician communication and trust issues, and issues concerning availability of clinical trials.5 The challenges to patient enrollment at the NJMS-UH Cancer Center were somewhat different in order and scope of impact. The main reasons for not participating were the presence of comorbidities that precluded eligibility, late disease presentation, a unique disease spectrum in this patient population, and discouragement by family and loved ones (Table 2).
Table 2.
Challenges to Clinical Trial Enrollment at NJMS-UH Cancer Center MB-CCOP
| Level of Categorization | Challenges |
|---|---|
| Patient-based issues | High rates of comorbidities in population of patients with cancer |
| Late disease presentation | |
| Unique disease spectrum not addressed by NCI protocols | |
| Discouragement by some family members and loved ones to participate in clinical trials | |
| Physician-based issues | Lack of redundancy among physician subspecialists |
| Key positions left unfilled for many months because of turnover | |
| Systematic referral of insured patients to outside oncologists by University Hospital surgeons | |
| Frequent start of treatment off protocol by busy medical oncologists, without considering protocol availability | |
| Lack of impact on careers of clinical faculty of enrolling or not enrolling patients onto clinical trials | |
| Organization- and system-based issues | Progressive decline in number of patients with cancer because of referral patterns, lack of access to appointments, and administrative impediments at University Hospital |
| Modified policy that excludes undocumented residents from registering in outpatient practice | |
| Lack of adequate prior workup or available records on uninsured patients referred by regional hospitals, narrowing window for protocol eligibility | |
| Delay in tissue samples being sent out for molecular analysis, preventing some eligible patients from enrolling in time | |
| Support staff turnover and lack of knowledge, understanding, or interest in clinical research | |
| Financial constraints, resulting in staff cutbacks and expiration of support services and patient navigation program | |
| Time needed for personnel recruiting, institutional hiring delays, training, and initiating protocols and processes for recruitment | |
| MB-CCOP policy of initial support for 3-year term, resulting in logistic challenges for startup and unachievable reapplication expectations |
Abbreviations: MB-CCOP, Minority-Based Community Clinical Oncology Program; NCI, National Cancer Institute; NJMS-UH, New Jersey Medical School–University Hospital.
Overall, the number of patients with cancer has declined over the past few years at University Hospital in part because of limitations placed on undocumented residents by hospital administration to make oncology practice appointments and decreased referrals by surgical subspecialists. This latter pattern was a result of the resignation of several surgeons and the subsequent delay in finding replacements and of the increased referral of patients with cancer by subspecialty surgeons to outside medical oncologists, particularly if the patients had insurance.
Other challenges to successful enrollment for the NJMS-UH Cancer Center MB-CCOP included busy medical oncologists who frequently began treatment of their patients off protocol, not considering the availability of clinical trials. The lack of institutional incentives or disincentives provided to physicians for enrolling or not enrolling was also unhelpful. Delays in evaluation of patients because of inadequate records from referring hospitals or in analysis of tissue samples sometimes resulted in potentially eligible patients missing protocol windows for enrollment. Hospital financial constraints resulted in the inability to support the patient navigator once the external support expired. Staff turnover, lack of knowledge, understanding, or interest in clinical research by outpatient practice staff, and substantial delays in replacement of departed staff also significantly hindered the process of timely appointments for potential study candidates.
Another systemic impediment to the success of the program was the NCI MB-CCOP policy of initial support for a 3-year term. The reapplication had to be prepared 19 months after a delayed initial funding award. The shortened time period presented a logistical challenge for the startup of the program. The hiring and training of staff, establishment of policies and practices to improve patient identification, and operational preparation require several years, making the initial expectations set by NCI to enroll a minimum number of patients onto trials nearly impossible to achieve.
Progress and Outcomes
The program was initiated once funding was received in August 2009. Once the program was initiated at the NJMS-UH Cancer Center, the MB-CCOP core group took several steps to successfully implement the program. The group hired personnel, activated available treatment protocols for the most common diseases seen in the practice, and opened cancer control protocols that the population was likely to accept. Full consents and short forms were submitted for all protocols in Spanish (or other appropriate non-English languages) prospectively to help alleviate language barriers as an impediment to enrollment.
It took 5 months, from the beginning of the award to January 2010, to achieve a critical mass in personnel and activate a significant number of relevant protocols. The MB-CCOP team set up workflows to efficiently identify, enroll, and retain patients on study, manage data collection, and handle reporting. Because there was no budget for a navigator, we attempted to capture all eligible patients with cancer in the cancer center through other mechanisms. Coordinators, research nurses, and medical oncologists began attending all subspecialty tumor boards regularly to identify eligible patients for clinical trials. Coordinators began meeting with medical oncologists weekly to obtain lists of planned chemotherapy treatments for the next week. Eventually, the cancer center CRO took over scheduling of new patients with cancer for the clinical practice, allowing prospective identification of all potential treatment and cancer control candidates. The coordinators, who represented multiple ethnic backgrounds, continued to assume active roles in informing patients about clinical trials and serving as liaisons. The ethnic mix of the staff served as a significant contributor to patient comfort and acceptance of the concept of participating in clinical research.
The MB-CCOP team at the NJMS-UH Cancer Center generally found that previously published patient barriers6 were only minor factors in the lack of recruitment. Patients who were offered participation in clinical trials assented most of the time. As mentioned earlier, comorbidities were the primary barrier to enrollment of identified patients. Our experience is reflected in part by prior review of the data from the national MB-CCOP, which noted that minority enrollment was primarily affected by the availability of clinically relevant protocols, regulatory issues, characteristics of the patient population, and level of support from sponsoring institutions and community physicians.1 We addressed these issues by activating as many relevant protocols as were available, engaging our community with understanding, compassion, and care to earn their trust and provide supportive services, and focusing on institutional- and physician-based challenges as best as possible within the constraints of our administrative structure.
The faculty was reminded that part of its evaluation required academic endeavors. To that end, the PI met with the senior associate dean for faculty affairs to try to develop a school policy attributing academic credit for clinical faculty who enrolled patients onto cooperative group trials to be considered during promotion decisions. The PI, who is the chair of the Faculty Committee on Appointments and Promotions, initiated changes in the Faculty Guidelines for Promotions to include enrollment of patients onto clinical trials as scholarship credit toward consideration for promotion of clinical faculty.
The number of patients with cancer at University Hospital has continued to decline for a variety of financially driven reasons beyond the control of the caregivers in medical oncology. There was an attempt to partner with another inner-city hospital to increase the potential for enrollment, but after negotiations and site visits, the potential partner decided it could not participate because of financial and logistic constraints. Three other institutions declined to pursue attempts at partnering as a result of their own fiscal and regulatory challenges.
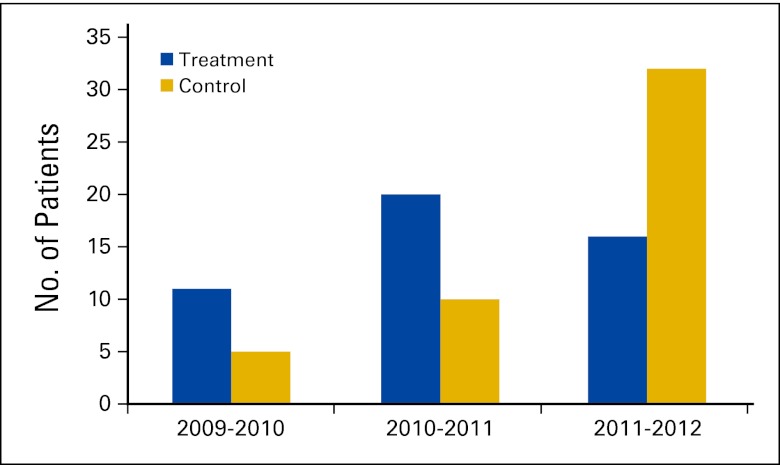
The MB-CCOP requires accrual of 60 patients in the first year, 80 in the second year, and 100 in the third year of the award, equally divided between treatment and control studies. The program at NJMS-UH Cancer Center accrued approximately 26% of the required patients in the first year, mostly to treatment trials (Appendix Fig A1, online only). During the second year, accruals increased to 37.5% of the minimum required. With availability and activation of additional cancer control studies, patient enrollment increased further in the third year; however, the MB-CCOP only reached 48% of the minimum number required for a program in its third year. As a result, the reapplication submitted in August 2011, 2 years after the initiation of funding, was not scored highly enough to be refunded for 5 years. Instead, a 1-year extension with funding was granted to permit reapplication. The program continues to increase minority accrual rates, particularly to control studies, and a second reapplication was submitted in August 2012 focusing on the increased trajectory of patient accrual rates to clinical trials over the past 3 years.
Lessons and Recommendations
A number of lessons were learned from our experience establishing an MB-CCOP (Table 3). First, it is important to keep in mind that minority patients represent different races and ethnicities, each with their own proud cultural history, and must be treated with the unique respect and cultural sensitivity that their communities require and deserve. A program considering application to become an MB-CCOP must have extensive experience providing care for patients from minority groups and must have earned the trust of the community from prior interactions. Physicians need extensive experience enrolling minority patients onto clinical trials and must have resolved issues with obtaining translated informed consent with their institutional review boards before considering application.
Table 3.
Lessons Learned and Recommendations for Success of Academic MB-CCOP
| Level of Categorization | Recommendations |
|---|---|
| Patient-based issues | All minority patient populations are different; it is necessary to address specific issues relevant to each group |
| Program must have experience enrolling minority patients onto clinical trials before application is considered | |
| Navigator who speaks patient's language is key element to successful enrollment of minority patients; navigator should attend surgical clinics to identify patients prospectively | |
| Approaches to non–English speaking consent and IRB procedures should be firmly in place before application is made | |
| Physician-based issues | Formal expectations and accountability of physicians for enrolling patients must be meaningful to meet goals |
| Rewards and remediation must be embedded in system | |
| Physician buy-in across cancer treatment subspecialties must occur; they must see value in program for MB-CCOP to be successful | |
| Reliable referral pattern from surgical subspecialties to medical oncology must be in place to ensure stable patient base | |
| Multidisciplinary tumor boards for most common malignancies must be established and functioning, with most patients being presented | |
| Organization- and system-based issues | Infrastructure, personnel, policies, procedures, and workflow must be in place, and enrollment should be near MB-CCOP goals before initiation of new program |
| MB-CCOP Executive Committee must be formed, consisting of key personnel, including cooperative group chairs, subspecialty division directors, other relevant physicians, and manager; its functions should include providing input on selection of trials and providing effective voice with administration | |
| Hospital, departmental, and divisional leadership should agree that clinical trial participation is basic condition of cancer care | |
| Stable projection of patients to hospital must be upheld, as much as assurances are possible, by policy outlines conducive to patient referrals | |
| Medical school administration and hospital management should have complete buy-in regarding program, in terms of both strategic and financial support | |
| Job vacancies need to be filled prospectively to ensure there are no lapses in key personnel | |
| Larger number of clinical trials appropriate to endemic patient populations should be open before application is made | |
| NCI should reconsider policy of awarding new MB-CCOPs 3-year initial grant, extending it beyond 3 years |
Abbreviations: IRB, institutional review board; MB-CCOP, Minority-Based Community Clinical Oncology Program; NCI, National Cancer Institute.
Second, physicians must have buy-in with regard to becoming an MB-CCOP and must be committed to referring all eligible patients for clinical trials. Physicians need to have demonstrated that they work well together, both with internal referrals for systemic therapy and with active participation in multidisciplinary tumor boards where most patients are discussed. This level of commitment from physicians translates to the understanding that their career progress at the medical school will be tied to their active participation in the program.
Third, institutional and system-wide support must be in place if a startup has a chance at succeeding. The infrastructure for a Cancer Clinical Research Program must predate any application for NCI status. A well-functioning core group made up of personnel with prior experience working together, existing policies and practices, and workflow patterns must predate an application. The hospital, department, and division must subscribe to the concept that excellent cancer care cannot be delivered without the opportunity to participate in state-of-the-art cooperative group trials and that establishing an MB-CCOP is an advantage to the institution, measured in more than the financial support provided to the CRO.
Fourth, the hospital must espouse policies to ensure stable patient volume and not allow patient numbers to diminish because of financial reasons. Hospital administration must commit to keeping subspecialties staffed with more than one physician each and provide an environment that is conducive to retention of excellent subspecialists. Outpatient practice staff must be educated as to the importance of the clinical research program and how to deal with patients seeking appointments in medical oncology.
Fifth, from a procedural standpoint, a significant number of clinical trials must be activated before application is made. Frequent and ongoing systematic reviews must be undertaken to ensure that the studies activated appropriately match the type of patients with cancer being served. To succeed in achieving the needed patient enrollment, many protocols must be kept open.
Finally, from a policy standpoint, the NCI MB-CCOP design requiring reapplication after fewer than 2 years as a new program is impractical. The initial award should be longer than 3 years. New programs will find it difficult to develop the level of functioning required for success in such a short time. It might be useful for the NCI to modify its evaluation of candidate programs to ensure the proposed elements are in place and accrual is near expected levels before granting an award.
Discussion
The MB-CCOP at the NJMS-UH Cancer Center, an ideal environment and catchment area for providing access to cooperative group trials to a significantly underserved urban population of primarily African American and Latino patients, faced significant challenges in meeting accrual goals after 3 years and was extended for only 1 year, permitting it time to reapply once again. The lessons learned during the startup of the MB-CCOP at the NJMS-UH Cancer Center follow the basic tenets of any successful business model. First, there must be an unmet need. Second, it is imperative that an individual with vision, talent, organizational ability, and intense dedication step forward to lead the cause. Third, the leader has to assemble a core team (physicians, surgeons and other subspecialists, clinical research associates, and others) committed to working toward the success of the program. Fourth, the leader must convince potential stakeholders, specifically the institution, to provide financial and programmatic support for the program. Fifth, the leadership must be able to keep the team together and have the authority to tangibly reward those team members who consistently enroll patients and remediate or separate from the team those members who do not enroll patients. Unfortunately, in an academic institution, particularly one that is state controlled, this business model becomes increasingly difficult to follow. Significant conflicting interests, daunting and disabling regulations, financial challenges, a primarily indigent patient population with a fiscally unsupportive third party–payer mix, physician subspecialists who cannot be retained or replaced or who send their insured patients to outside physicians and institutions, and a shrinking patient base all work against program success. Many of these issues are prevalent throughout medical centers in the country and represent fundamental challenges to the success of any program.
Acknowledgment
Supported by Grant No. 1U10CA128506-01A1 from the National Cancer Institute (R.W.).
Appendix
Fig A1.
Cancer treatment and cancer control patients enrolled onto cooperative group trials by the Minority-Based Community Clinical Oncology Program in the first 3 years of inception.
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Robert Wieder, Tracie Saunders, Bryan J. Weiner
Collection and assembly of data: Robert Wieder, Randall Teal
Data analysis and interpretation: Robert Wieder, Randall Teal, Bryan J. Weiner
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.McCaskill-Stevens W, McKinney MM, Whitman CG, et al. Increasing minority participation in cancer clinical trials: The Minority-Based Community Clinical Oncology Program experience. J Clin Oncol. 2005;23:5247–5254. doi: 10.1200/JCO.2005.22.236. [DOI] [PubMed] [Google Scholar]
- 2.Roberson NL. Clinical trial participation: Viewpoints from racial/ethnic groups. Cancer. 1994;74:2687–2691. doi: 10.1002/1097-0142(19941101)74:9+<2687::aid-cncr2820741817>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 4.Office of Management and Budget. Standards for the Classification of Federal Data on Race and Ethnicity. http://www.whitehouse.gov/omb/fedreg/1997standards.html.
- 5.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer. 2008;112:228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 6.Brown DR, Fouad MN, Basen-Engquist K, et al. Recruitment and retention of minority women in cancer screening, prevention, and treatment trials. Ann Epidemiol. 2000;10:S13–S21. doi: 10.1016/s1047-2797(00)00197-6. [DOI] [PubMed] [Google Scholar]