Abstract
Background:
Longitudinal studies drawn from high-income countries demonstrate long-term associations of early childhood socioeconomic deprivation with increased adiposity in adulthood. However, there are very few data from resource-poor countries where there are reasons to anticipate different gradients. Accordingly, we sought to characterise the nature of the socioeconomic status (SES)-adiposity association in Brazil.
Methods:
We use data from the Ribeirao Preto Cohort Study in Brazil in which 9067 newborns were recruited via their mothers in 1978/79 and one-in-three followed up in 2002/04 (23–25years). SES, based on family income (salaries, interest on savings, pensions and so on), was assessed at birth and early adulthood, and three different adiposity measures (body mass index (BMI), waist circumference (WC), waist-to-hip ratio (WHR)) ascertained at follow-up. The association between childhood SES, adult SES and social mobility (defined as four permutations of SES in childhood and adulthood: low–low, low–high, high–low, high–high), and the adiposity measures was examined using linear regression.
Results:
There was evidence that the association between SES and the three markers of adiposity was modified by gender in both adulthood (P<0.02 for all outcomes) and childhood SES (P<0.02 for WC and WHR). Thus, in an unadjusted model, linear regression analyses showed that higher childhood SES was associated with lower adiposity in women (coefficient (95% confidence intervals) BMI: −1.49 (−2.29,−0.69); WC: −3.85 (−5.73,−1.97); WHR: −0.03 (−0.04,−0.02)). However, in men, higher childhood SES was related to higher adiposity (BMI: 1.03 (0.28,−1.78); WC: 3.15 (1.20, 5.09); WHR: 0.009 (−0.001, 0.019)) although statistical significance was not seen in all analyses. There was a suggestion that adult SES (but not adult health behaviours or birthweight) accounted for these relationships in women only. Upward mobility was associated with protection against greater adiposity in women but not men.
Conclusion:
In the present study, in men there was some evidence that both higher childhood and adulthood SES was related to a higher adiposity risk, while the reverse gradient was apparent in women.
Keywords: life course, Brazil, socioeconomic status, gender, adiposity, body mass index
Introduction
Globally, an estimated one in ten individuals are now obese.1 Between 1980 and 2008, the age-standardized prevalence of obesity rose from 4.8 to 9.8% in men, and from 7.9 to 13.8% in women worldwide.1 As has been well documented, overweight and obesity have been linked to several major chronic diseases, including type II diabetes, selected cancers, cardiovascular disease, asthma, gallbladder disease, osteoarthritis and chronic back pain.2 An estimated 2.6 million people die each year from non-communicable disease as a result of being overweight or obese, the large majority of deaths occurring in resource-poor countries.3
Several risk factors for overweight and obesity (environmental, genetic, physiologic, metabolic and behavioural) have been identified during different periods of the life course, with special attention given to those occurring early in life. A review of the effects of early-life socioeconomic status (SES) based on 30 studies, almost all from high-income countries, found that low childhood SES was typically associated with an elevated risk of adult obesity, particularly in women.4
There have been conflicting reports as to the direction of the SES–adiposity relation in low- and middle-income countries (LMICs): some investigators report that higher SES is associated with higher adiposity,5, 6, 7 and others that higher SES is associated with lower adiposity.8, 9 This may be due to methodological limitations including the SES indicator used. In addition, most populations comprise only women, yet there may be differential SES–adiposity effects according to gender as evident in a cohort from China.10 However, longitudinal data from LMICs are scarce. This is in part because of the high loss to follow-up resulting from rudimentary administrative tracking systems and internal migration in these societies.
Herein, we address these methodological shortcomings and paucity of data by examining the association between early-life SES and adult adiposity, gender differentials, social mobility and the effect of mediators including adult SES and behavioural risk factors in the Ribeirao Preto Cohort, the earliest established cohort study in Brazil.11
Materials and methods
Sample and participants
In the present analyses, we use data collected between 1978 and 2004 as part of the Ribeirao Preto Cohort Study (RPCS). Ribeirao Preto is a city of over half-a-million inhabitants in the South East of Brazil. The RPCS was designed specifically to investigate the contribution of early-life exposures to adult risk of non-communicable disease. A full description of the study member characteristics and methodology has been published elsewhere.12
In brief, a total of 9067 newborn babies were recruited through their mothers from 1 June 1978 to 31 May 1979 (98% of all live births in the city over that period). Babies of mothers who were not from Ribeirão Preto and did not reside in the city at the time of delivery (N=2094) were excluded. The additional exclusion of twins (146 pairs), resulted in 6827 single liveborns in the cohort.
In 2004, when the study members were aged 23–25 years (average age 23.9 years), the procedures used to identify surviving study members included accessing the original birth charts, which held addresses of the liveborns, and updating this information from a series of registers. The latter included the Unified Health System electronic database, lists of users of private health plans, school charts and military recruitment charts. From the original cohort of 6827 liveborns, 343 participants were found to be deceased and 819 could not be traced, leaving 5665 singletons.
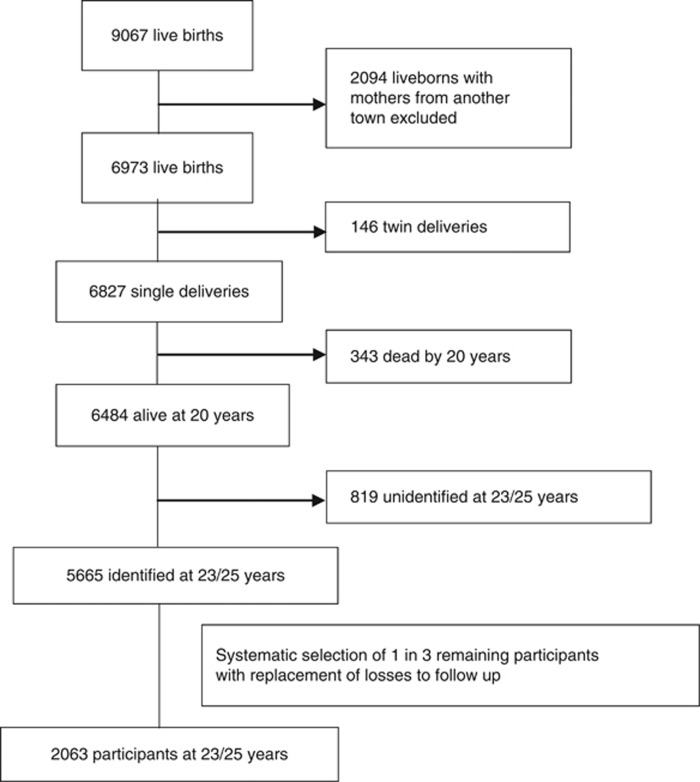
Attempts were then made to invite for medical examination a non-random sample based on one-in-three of this group. The first of every three names was selected from a list sorted by birth date in each geographic region and if unavailable, the next name down was selected. In this traced group, losses to follow-up (N=705) occurred because of refusal to participate, imprisonment, death after 20 years of age or failure to attend interview. Losses were replaced using the same sampling frame, resulting in 2063 young adults (1068 females).11, 12 Figure 1 illustrates the flow of participants over 25 years of follow-up.
Figure 1.
Sample characteristics of the RPCS at birth and at follow-up at 23/25 years.
Outcomes
Three indices of adiposity were used: body mass index (BMI), waist circumference (WC) and waist-to-hip ratio (WHR). These were calculated from anthropometric measurements obtained at follow-up. Trained doctors and nurse technicians took these measurements from individuals in light clothing without shoes, based on a standard protocol, using a precision scale that was periodically calibrated (for weight) and a non-stretch metric tape (for waist and hip circumference). Height was measured to the nearest 0.1 cm using a freestanding wooden stadiometer made at the University of Sao Paulo, Brazil, based on the Harpenden model. Weight was measured to the nearest 50 g using mechanical scales (Filizola, Brazil). WC was measured as the smallest circumference between the ribs and the iliac crest, while the participant was standing with the abdomen relaxed, at the end of a normal expiration. Where there was no natural waistline, the measurement was taken at the level of the umbilicus. Hip circumference was measured at the maximum circumference between the iliac crest and the crotch while the participant was standing. All the circumference measures were recorded to the nearest 0.1 cm. BMI was calculated as weight in kilograms divided by height in metres squared (kg m−2).
SES and other variables
Total family income was recorded at birth (1979) and then again at follow-up (2004). Participants were asked to report their family income using the question: ‘How much did you and all the members of your family earn last month?' Interviewers specified that sources of income should include salaries, money earned from investments including interest, property rentals, pensions, donations from parents and any other source. Absolute income values were converted into multiples of the Brazilian minimum wage in each period (US$ 84.0 in 1978; US$ 89.8 in 2003) and categorised into tertiles for the purposes of the present analyses. This overcame issues of inflation, changes in the minimum wage and the fact that Brazil changed currency during the course of the study. To examine the effect of social mobility on adiposity, a variable comprising the four different permutations of childhood and adult SES categories was created: (low–low=1, low–high=2, high–low=3, high–high=4); ‘low–low' was the reference group.
The role of a number of adult health behaviours as key mediators was also investigated: total caloric intake, percent fat intake and physical activity, current smoking (number of cigarettes smoked in the last 30 days) and current alcohol consumption. Total caloric intake and percent dietary fat were calculated by the Dietsys software, version 4.0 (National Cancer Institute, Bethesda, MD, USA) based on the information derived from a food frequency questionnaire validated for the Brazilian population.13 Physical activity was measured by the short version of the International Physical Activity Questionnaire.14 These data were then classified as low, moderate and high based on the metabolic cost or unit of resting metabolic rate (MET). Study members had been weighed at birth within 30 min of delivery, naked, using mechanical scales that were calibrated weekly and with a measurement accuracy of 10 g (Filizola, Brazil).
Statistical analysis
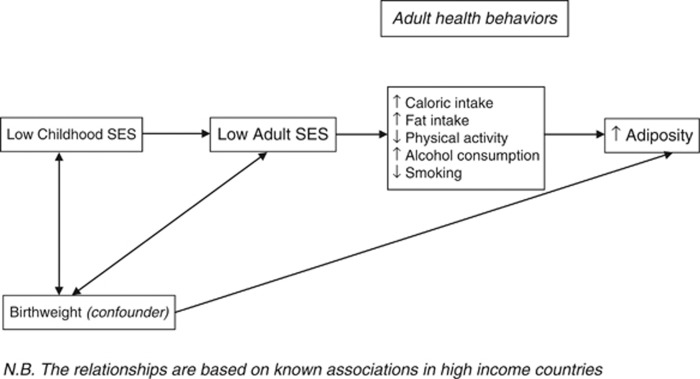
The sample characteristics were examined by SES and by gender. Linear regression was used to estimate the coefficients and 95% confidence intervals (CIs) for the association between childhood SES and the three markers of adiposity. In model 1, the unadjusted SES–adiposity relationship was estimated. In model 2, adult SES was added. Model 3 builds on model 2 by adding the potentially mediating health behaviour variables (total caloric intake, percentage dietary fat, alcohol consumption and smoking), as well as birthweight (a confounder). A path diagram of the association between childhood SES and adult adiposity based on known associations in developed countries is shown in Figure 2. The regression results were examined in the light of the expected associations depicted in this diagram.
Figure 2.
Path diagram of the association between childhood SES and adult adiposity.
In preliminary analyses, it became apparent that gender was an effect modifier (interacting variable) in the SES–adiposity relationship. The P-values for a gender interaction for childhood and adulthood SES in relation to WC and WHR were<0.02 in all models. For BMI, the equivalent P-value was <0.001 in the crude model. On this basis, analyses were performed separately for men and women. All the analyses were conducted using the STATA 10 statistical package (College Station, TX, USA).
Results
Table 1 shows the distribution of cohort characteristics by childhood and adult SES. Higher SES in childhood and adulthood was associated with higher birthweight for men and women. Birthweight was normally distributed in all income groups. Higher SES in both men and women was associated with lower current caloric intake, while higher SES was associated with a higher frequency of alcohol consumption and smoking.
Table 1. Distribution of study member characteristics by socioeconomic status—Ribeirão Preto Cohort Study, 1978/79 and 2004/06.
| Socioeconomic statusa | n | Calorie intake mean (s.d.) | % Fat in diet mean (s.d.) | Birthweight mean (s.d.) | Alcohol consumption in the last month % | Smoking last month % | Physical activity % low activity |
|---|---|---|---|---|---|---|---|
| Women | |||||||
| Childhood | |||||||
| Low (1.6–2.4) | 315 | 1991 (675) | 35.5 (5.2) | 3152 (465) | 49.2 | 12.1 | 60.8 |
| Medium (3.2–4.4) | 275 | 1985 (675) | 36.2 (5.5) | 3226 (498) | 58.9 | 13.5 | 59.1 |
| High (6.4–12.8) | 268 | 1773 (518) | 36.4 (5.8) | 3237 (468) | 71.6 | 14.6 | 53.4 |
| P-value for trend | <0.001 | 0.092 | 0.067 | <0.001 | 0.674 | 0.373 | |
| Adulthood | |||||||
| Low (2.1–4.4) | 379 | 1977 (691) | 36.2 (5.6) | 3120 (485) | 48.8 | 14.3 | 60.7 |
| Medium (4.7–13.7) | 343 | 1881 (659) | 36.2 (5.5) | 3225 (486) | 59.2 | 11.7 | 57.9 |
| High (13.9–20) | 256 | 1795 (483) | 36.2 (5.6) | 3322 (442) | 74.6 | 14.8 | 51.4 |
| P-value for trend | 0.002 | 0.997 | <0.001 | <0.001 | 0.456 | 0.224 | |
| Men | |||||||
| Childhood | |||||||
| Low (1.6–2.4) | 268 | 2366 (677) | 35.8 (6.0) | 3239 (533) | 71.3 | 15.7 | 39.6 |
| Medium (3.2–4.4) | 293 | 2367 (700) | 35.9 (5.6) | 3353 (527) | 74.4 | 18.1 | 43.4 |
| High (6.4–12.8) | 273 | 2334 (833) | 36.6 (5.4) | 3435 (445) | 82.4 | 26.7 | 41.0 |
| P-value for trend | 0.833 | 0.220 | <0.001 | 0.007 | 0.003 | 0.906 | |
| Adulthood | |||||||
| Low (2.1–4.4) | 302 | 2370 (720) | 35.7 (6.0) | 3250 (542) | 71.2 | 20.9 | 43.9 |
| Medium (4.7–13.7) | 288 | 2362 (696) | 36.1 (5.6) | 3360 (497) | 75.0 | 18.1 | 40.3 |
| High (13.9–20) | 344 | 2327 (797) | 36.2 (5.4) | 3414 (466) | 81.4 | 20.6 | 39.5 |
| P-value for trend | 0.736 | 0.459 | <0.001 | 0.009 | 0.635 | 0.785 | |
Abbreviation: SES, socioeconomic status.
SES represented as tertiles of income calculated as multiples of the minimum wage. Figures in brackets for each tertile represent the 25th percentile and 75th percentile of the range of multiples of the minimum wage.
Table 2 shows the unadjusted and adjusted linear regression coefficients for the association between SES and adult adiposity as measured by BMI. As described, there was some evidence of a difference in these associations in men and women. Thus, higher childhood SES was associated with lower adult BMI in women (regression coefficient for high vs low childhood SES; 95% CI: −1.49; −2.29, −0.69), whereas men from a more affluent background had an elevated adult BMI relative to their lower SES counterparts (1.03; 0.28, 1.88). After adjustment for adult SES, the association was attenuated in women (−0.44; −1.38, 0.50). Further adjustment for health behaviours and birthweight did not markedly change the association between childhood SES and BMI in either women or men.
Table 2. Unadjusted and adjusted linear regression coefficients for the association between SES and BMI by gender—Ribeirão Preto Cohort Study, 1978/79 and 2004/06.
| n | Mean (s.d.) | Unadjusted Coefficient (95% CI) (model 1)a | P for trend | Adjusted coefficient (95% CI) (model 2)b | P for trend | Adjusted coefficient (95% CI) (model 3)c | P for trend | |
|---|---|---|---|---|---|---|---|---|
| BMI (kg m−2) | ||||||||
| Women | ||||||||
| Childhood SES | <0.001 | 0.314 | 0.211 | |||||
| Low (1.6–2.4) | 314 | 24.2 (5.3) | Referent | Referent | Referent | |||
| Medium (3.2–4.4) | 273 | 23.7 (4.9) | −0.52 (−1.32; 0.27)) | −0.20 (−1.05; 0.64) | −0.29 (−1.15; 0.56) | |||
| High (6.4–12.8) | 267 | 22.7 (4.4) | −1.49 (−2.29;−0.69) | −0.44 (−1.38; 0.50) | −0.56 (−1.51; 0.39) | |||
| P for gender interaction | <0.001 | 0.074 | 0.076 | |||||
| Adult SES | <0.001 | <0.001 | <0.001 | |||||
| Low (2.1–4.4) | 376 | 24.4 (5.8) | Referent | Referent | Referent | |||
| Medium (4.7–13.7) | 343 | 23.8 (4.7) | −0.61 (−1.35; 0.13) | −0.47 (−1.30; 0.36) | −0.49 (−1.32; 0.35) | |||
| High (13.9–20) | 256 | 22.3 (4.2) | −2.02 (−2.83;−1.22) | −2.15 (−3.11;−1.19) | −2.32 (−3.31;−1.33) | |||
| P for gender interaction | <0.001 | 0.004 | 0.003 | |||||
| Men | ||||||||
| Childhood SES | 0.007 | 0.015 | 0.031 | |||||
| Low (1.6–2.4) | 268 | 24.5 (4.1) | Referent | Referent | Referent | |||
| Medium (3.2–4.4) | 291 | 25.0 (4.4) | 0.45 (−0.29; 1.19) | 0.54 (−0.24; 1.32) | 0.51 (−0.27; 1.29) | |||
| High (6.4–12.8) | 273 | 25.5 (4.8) | 1.03 (0.28; 1.78) | 1.03 (0.18; 1.88) | 0.92 (0.06; 1.78) | |||
| P for gender interaction | <0.001 | 0.074 | 0.076 | |||||
| Adult SES | 0.679 | 0.501 | 0.43 | |||||
| Low (2.1–4.4) | 301 | 25.0 (4.7) | Referent | Referent | Referent | |||
| Medium (4.7–13.7) | 286 | 24.9 (4.5) | −0.11 (−0.84; 0.62) | −0.48 (−1.29; 0.33) | −0.51 (− 1.32; 0.29) | |||
| High (13.9–20) | 344 | 25.1 (4.3) | 0.14 (−0.56; 0.83) | −0.29 (−1.11; 0.53) | −0.34 (−1.17; 0.49) | |||
| P for gender interaction | <0.001 | 0.004 | 0.003 | |||||
Abbreviations: BMI, body mass index; SES, socioeconomic status.
Unadusted model (Model 1 (childhood SES or adult SES)).
Mutually adjusted model (Model 2=Model 1 for Childhood SES+model 1 for adult SES).
Fully adjusted model (Model 3=Model 2+health behaviours+birthweight). Health behaviours include total calorie intake, percent fat in diet, alcohol consumption, smoking and physical activity.
Table 3 shows the results of the analyses with WC and WHR as the outcomes of interest. The pattern of association with SES was similar to that for BMI, except that the association between childhood SES and WHR remained significant after adjustment for the covariates. A model adjusting the childhood SES estimates for health behaviours and birthweight (omitting adult SES) was run, but did not result in any important differences. For example, the coefficient comparing high with low childhood SES in relation to BMI; 95%CI was −1.49; −2.29, −0.69 before adjustment and −1.50; −2.33, 0.67 after adjustment (full results available on request). Controlling for each individual health behaviour separately did not have any further impact on the SES–adiposity estimates (full results also available on request).
Table 3. Unadjusted and adjusted linear regression coefficients for the association between SES and WC and WHR by gender—Ribeirão Preto Cohort Study, 1978/79 and 2004/06.
| n | Mean (s.d.) | Unadjusted coefficient (95% CI) (Model 1)a | P for trend | Adjusted coefficient (95% CI) (model 2)b | P for | Adjusted coefficient (95% CI) (model 2)c | P for trend | |
|---|---|---|---|---|---|---|---|---|
| WC (cm) | ||||||||
| Women | ||||||||
| Childhood SES | <0.001 | 0.195 | 0.16 | |||||
| Low (1.6–2.4) | 315 | 78.3 (12.1) | Referent | Referent | Referent | |||
| Medium (3.2–4.4) | 275 | 77.5 (12.0) | −0.77 (−2.64; 1.10) | −0.23 (−2.20; 1.73) | −0.44 (−2.42; 1.54) | |||
| High (6.4–12.8) | 267 | 74.4 (10.3) | −3.85 (−5.73;−1.97) | −1.38 (−3.56; 0.80) | −1.51 (− 3.72; 0.70) | |||
| P for gender interaction | <0.001 | 0.011 | 0.016 | |||||
| Adult SES | <0.001 | <0.001 | <0.001 | |||||
| Low (2.1–4.4) | 378 | 78.8 (13.2) | Referent | Referent | Referent | |||
| Medium (4.7–13.7) | 343 | 77.0 (13.2) | −1.75 (−3.45;−0.06) | −1.45 (−3.37; 0.46) | −1.41 (−3.35; 0.53) | |||
| High (13.9–20) | 256 | 73.1 (9.2) | −5.64 (−7.48;−3.80) | −5.51 (−7.74;−3.28) | −5.62 (−7.93;−3.31) | |||
| P for gender interaction | <0.001 | 0.005 | 0.004 | |||||
| Men | ||||||||
| Low (1.6–2.4) | 0.002 | 0.003 | 0.015 | |||||
| Medium (3.2–4.4) | 268 | 86.2 (11.0) | Referent | Referent | Referent | |||
| High (6.4–12.8) | 293 | 87.4 (11.4) | 1.2 (−0.71; 3.12) | 1.26 (−0.77; 3.29) | 1.00 (−1.02; 3.03) | |||
| Low (1.6–2.4) | 273 | 89.3 (12.2) | 3.15 (1.20; 5.09) | 3.29 (1.08; 5.50) | 2.73 (0.49; 4.96) | |||
| P for gender interaction | <0.001 | 0.011 | 0.016 | |||||
| Adult SES | 0.499 | 0.519 | 0.387 | |||||
| Low (2.1–4.4) | 301 | 87.5 (12.2) | Referent | Referent | Referent | |||
| Medium (4.7–13.7) | 288 | 87.5 (11.8) | −0.04 (−1.94; 1.85) | −0.88 (−2.99; 1.22) | −1.04 (−3.13; 1.06) | |||
| High (13.9–20) | 344 | 88.1 (11.1) | 0.61 (–1.20; 2.42) | −0.74 (−2.89; 1.40) | −0.99 (−3.14; 1.17) | |||
| P for gender interaction | <0.001 | 0.005 | 0.004 | |||||
| WHR | ||||||||
| Women | ||||||||
| Childhood SES | <0.001 | 0.003 | 0.003 | |||||
| Low (1.6–2.4) | 315 | 0.773 (0.062) | Referent | Referent | Referent | |||
| Medium (3.2–4.4) | 275 | 0.763 (0.063) | −0.01 (−0.020;−0.001) | −0.007 (− 0.017; 0.004) | −0.007 (−0.017; 0.004) | |||
| High (6.4–12.8) | 267 | 0.743 (0.057) | −0.03 (−0.040;−0.020) | −0.017 (−0.029;−0.006) | −0.018 (−0.029;−0.006) | |||
| P for gender interaction | <0.001 | 0.001 | 0.001 | |||||
| Adult SES | <0.001 | <0.001 | <0.001 | |||||
| Low (2.1–4.4) | 378 | 0.776 (0.061) | Referent | Referent | Referent | |||
| Medium (4.7–13.7) | 343 | 0.76 (0.064) | −0.015 (−0.024;−0.006) | −0.012 (−0.022;−0.002) | −0.010 (−0.020;−0.001) | |||
| High (13.9–20) | 256 | 0.736 (0.051) | −0.04 (−0.050;−0.031) | −0.032 (−0.044;−0.021) | −0.029 (−0.041;−0.017) | |||
| P for gender interaction | <0.001 | 0.001 | 0.016 | |||||
| Men | ||||||||
| Childhood SES | 0.083 | 0.029 | 0.098 | |||||
| Low (1.6–2.4) | 268 | 0.852 (0.058) | Referent | Referent | Referent | |||
| Medium (3.2–4.4) | 292 | 0.856 (0.059) | 0.004 (−0.006; 0.013) | 0.004 (−0.006; 0.014) | 0.003 (−0.008; 0.013) | |||
| High (6.4–12.8) | 273 | 0.861 (0.058) | 0.009 (−0.001; 0.019) | 0.012 (0.001; 0.024) | 0.009 (−0.002; 0.021) | |||
| P for gender interaction | <0.001 | 0.001 | 0.001 | |||||
| Adult SES | 0.295 | 0.09 | 0.084 | |||||
| Low (2.1–4.4) | 301 | 0.86 (0.062) | Referent | Referent | Referent | |||
| Medium (4.7–13.7) | 287 | 0.857 (0.06) | −0.002 (−0.011; 0.008) | −0.005 (−0.015; 0.006) | −0.005 (−0.015; 0.006) | |||
| High (13.9–20) | 344 | 0.855 (0.056) | −0.005 (−0.014; 0.004) | −0.009 (−0.020; 0.001) | −0.010 (− 0.020; 0.001) | |||
| P for gender interaction | <0.001 | 0.001 | 0.019 | |||||
Abbreviations: BMI, body mass index; CI, confidence interval; SES, socioeconomic status; WC, waist circumference; WHR, waist-to-hip ratio.
Unadusted model (Model 1 (childhood SES or adult SES)).
Mutually adjusted model (Model 2=Model 1 for childhood SES+Model 1 for adult SES).
Fully adjusted model (Model 3=Model 2+health behaviours+birthweight). Health behaviours include total calorie intake, percent fat in diet, alcohol consumption, smoking and physical activity.
Table 4 shows the relationship of social mobility with the three markers of adiposity. Among men, there was some suggestion that upward mobility might be associated with protection against increased adiposity (unadjusted coefficient for association between upward mobility (low–high) vs no mobility (low–low) for BMI; 95%CI: −0.48; −1.35, 0.39). Both downward mobility (high–low) and remaining in the high SES group (high–high) appeared to be associated with higher adiposity compared with remaining in the low SES group (low–low) in men. Among women, the coefficients showed that upward mobility (low–high) was protective against adult adiposity compared with remaining in the low SES (low–low) group (unadjusted coefficient: −2.30; −4.44, −0.15 for WC). Remaining in the high SES group (high–high) was also protective compared with remaining in the low SES group (low–low) (adjusted coefficient: −5.55; −7.56, −3.53 for WC).
Table 4. Unadjusted and adjusted linear regression coefficients for the association between social mobility and adult adiposity for women and men—Ribeirão Preto Cohort Study, 1978/79 and 2004/06.
| Variables | Childhood SES | Adult SES | Social mobility | n | Mean (SD) | Unadjusted coefficient (95% CI) | Unadjusted P-value | Adjusted coefficient1(95% CI) | Adjusteda P-value |
|---|---|---|---|---|---|---|---|---|---|
| Women | |||||||||
| BMI | Low | Low | − | 215 | 24.6 (5.8) | Referent | 0.001 | Referent | <0.001 |
| Low | High | Upward | 213 | 23.7 (4.8) | −0.93 (−1.86 to −0.01) | −0.95 (−1.89 to −0.01) | |||
| High | Low | Downward | 76 | 23.9 (5.0) | −0.75 (−2.03 to 0.53) | −0.76 (−2.05 to 0.53) | |||
| High | High | − | 278 | 22.6 (4.1) | −2.04 (−2.91 to −1.17) | −2.20 (−3.12 to −1.29) | |||
| WC | |||||||||
| Low | Low | − | 217 | 79.4 (13.1) | Referent | <0.001 | Referent | <0.001 | |
| Low | High | Upward | 213 | 77.1 (11.4) | −2.3 (−4.44 to −0.15) | −2.24 (−4.42 to −0.06) | |||
| High | Low | Downward | 76 | 77.7 (11.6) | −1.68 (−4.64 to 1.29) | −1.59 (−4.59 to 1.40) | |||
| High | High | − | 278 | 73.9 (9.7) | −5.55 (−7.56 to −3.53) | −5.56 (−7.68 to −3.44) | |||
| WHR | |||||||||
| Low | Low | − | 217 | 0.78 (0.06) | Referent | <0.001 | Referent | <0.001 | |
| Low | High | Upward | 213 | 0.76 (0.06) | −0.014 (−0.026 to −0.003) | −0.013 (−0.024 to −0.001) | |||
| High | Low | Downward | 76 | 0.77 (0.06) | −0.01 (−0.025 to 0.006) | −0.009 (−0.025 to 0.006) | |||
| High | High | − | 278 | 0.74 (0.06) | −0.405 (−0.051 to −0.030) | −0.037 (−0.048 to −0.026) | |||
| Men | |||||||||
| BMI | |||||||||
| Low | Low | − | 184 | 24.8 (4.6) | Referent | 0.03 | Referent | 0.09 | |
| Low | High | Upward | 224 | 24.4 (3.8) | −0.48 (−1.35 to 0.39) | −0.53 (11.41 to 0.35) | |||
| High | Low | Downward | 63 | 25.7 (4.9) | 0.86 (−0.42 to 2.14) | 0.66 (−0.64 to 1.96) | |||
| High | High | − | 313 | 25.4 (4.7) | 0.56 (−0.25 to 1.38) | 0.39 (−0.44 to 1.23) | |||
| WC | |||||||||
| Low | Low | − | 184 | 86.8 (12.0) | Referent | 0.007 | Referent | 0.051 | |
| Low | High | Upward | 225 | 85.9 (9.8) | −0.95 (−3.22 to 1.32) | −1.15 (−3.42 to 1.12) | |||
| High | Low | Downward | 63 | 89.8 (12.8) | 2.97 (−0.36 to 6.30) | 2.31 (−1.06 to 5.68) | |||
| High | High | − | 314 | 88.9 (12.4) | 2.11 (−0.01 to 4.23) | 1.38 (−0.79 to 3.56) | |||
| WHR | |||||||||
| Low | Low | − | 184 | 0.86 (0.06) | Referent | 0.177 | Referent | 0.476 | |
| Low | High | Upward | 224 | 0.85 (0.06) | −0.006 (−0.017 to 0.005) | −0.006 (−0.017 to 0.006) | |||
| High | Low | Downward | 63 | 0.87 (0.06) | 0.01 (−0.007 to 0.026) | 0.007 (−0.010 to 0.024) | |||
| High | High | − | 314 | 0.86 (0.06) | 0.002 (−0.009 to 0.012) | −0.001 (−0.012 to 0.010) | |||
Abbreviations: BMI, body mass index; CI, confidence interval; SES, socioeconomic status; WC, waist circumference; WHR, waist-to-hip ratio.
Fully adjusted model (social mobility+health behaviours+birthweight). Health behaviours include total calorie intake, percent fat in diet, alcohol consumption, smoking, and physical activity
Discussion
In the present study, we examine the relation of SES in childhood and early adulthood with adult adiposity in a country in economic transition by using data from the Ribeirao Preto Cohort. The main finding was that in women higher (adulthood) SES is associated with lower adiposity, whereas in men higher (childhood) SES is associated with higher adiposity. Upward mobility and retaining a high SES were found to be protective against adult adiposity in women with the results generally consistent across the adiposity measures.
Comparison with prior studies
In relation to the conflicting reports as to whether excess adiposity is becoming a problem of the poor or remains a problem of the higher social groups, this study contributes by using a different measure of SES (income). Most other studies are based on data for women, and use education or wealth to represent SES.5, 6, 8, 9, 15, 16 The findings presented in this study show that excess adiposity is a greater problem for low rather than high SES women, as found in high-income countries (HICs). The findings concur with the predictions from the Monteiro model (2004)8 that in countries with a gross national income per capita greater than US$ 2500 (in 2005) higher SES is associated with lower adiposity in women (Brazil's gross national income per capita in 2005—near the time of follow-up—was US$ 3960).
The opposite is true for men (higher SES is associated with higher adiposity). Where data for men are available, evidence suggests that higher SES men become protected from excess adiposity much later than higher SES women in the process of economic development,17 which could explain the gender differences observed in this study. Among men in the UK Whitehall II cohort, a negative association between SES and WHR appears to have emerged, suggesting men of higher SES may eventually become protected compared with men of lower SES at very high levels of gross national income per capita.
In terms of the relative importance of childhood vs adult SES, the findings in this study differ from those in HICs, where childhood SES appears to be important in determining adult adiposity in women18, 19, 20 and less so in men4, 21 There are however comparable gender differences in the childhood SES–adiposity relationship in HICs: higher childhood SES is associated with lower adiposity in women (as in this study), while a less consistent direction of association is reported in men (we report a positive association). Data from China—another LMIC—show an independent association between early-adulthood SES and metabolic risk after adjustment for childhood SES and health behaviours in women as reported in this study.22
We also found that upward mobility reduces the risk of excess adiposity in women, as reported in some of the cohorts from HICs20, 21, but not others such as the Whitehall II cohort23 and the British Birth Cohort.18 This may be a result of differences in cohort maturity. The much more mature UK cohorts may be subject to an attenuation of the protective effect of upward mobility due to the increased risk of overweight associated with ageing. In addition, mobility effects may be much more pronounced in rapidly changing economies.
Plausible mechanisms
Socially patterned childhood exposures are thought to influence later life outcomes in a number of ways including biological programming during critical periods of growth, and through the early acquisition of unhealthy lifestyles.24 While childhood and adult SES are correlated, the importance of adult SES in this study was greater in the SES–adiposity relationship in women, whereas childhood SES appears to be more important in men. One explanation for this gender differential may lie in the rapidly changing environment, in the early 1980s when the RPCS cohort participants were children, overweight and obesity were not yet a public health problem, and awareness of dietary habits and obesity may have been low. At that time, ensuring children had a sufficient quantity of food and enjoying any new commercial food products were the dietary priority. At follow-up, however, in 2004, it is likely that women of high SES would have experienced social pressure to lose weight and conform to a slim, western body shape preference.25 Men on the other hand, may not have experienced the pressure of these new norms, and may simply have retained the dietary habits and body shape expectations formed in their childhood.
This explanation is temporally consistent with observed long-term trends of obesity by SES in Brazil. From 1975 to 1997, a period of significant social and economic change in Brazil, obesity increased from 2.4 to 6.9% among men and from 7 to 12.5% among women. This change, however, was not observed across all socioeconomic groups. In men, all groups showed an increase in obesity over time, and the gradient remained positive (men in the higher SES group (higher income) had a higher prevalence of obesity). In women, obesity more than doubled in the lower SES group, while the prevalence in richer women started to drop from 12.8% in 1989 to 9.2% in 1997.7, 26 This suggests the social gradient had reversed in Brazil in women but not men, and is consistent with other reports.11
In further explaining why women who remain in the low SES group (low–low—see Table 4) are most at risk of increased adiposity (whereas men in the same social mobility category are not), a biology-environment interaction specific to transition settings may be at play. A rapid nutrition transition from a situation of calorie scarcity to one of high-energy density could have caused a mismatch between metabolic programming and the energy available for consumption, with a consequent increase in the susceptibility to excess adiposity, as formulated in Gluckman's match–mismatch hypothesis.27 Indeed, stunted Brazilian children have been found to have impaired oxidative stress, which combined with a later life exposure to energy-dense diets, increases the risk of obesity.28 This phenomenon is thought to only be observable in countries undergoing a rapid nutrition and economic transition.29
Furthermore, intra-uterine growth retardation was socially patterned in the RPCS with lower birthweights found among lower SES groups.30 A separate study of the RPCS found that severe intrauterine growth restriction (IUGR) was associated with increased preference for carbohydrate consumption in girls but not boys.13 A study of a Chinese cohort has also examined the effect of rapid transition in both genders (using a natural experiment). It compared long-term Hong Kong residents with residents who had migrated from a relatively undeveloped Chinese province, and found gender differences in lipid profile and fat distribution.31, 32 It was postulated that sex hormones in puberty among males experiencing an improved nutrition environment and higher socioeconomic development caused them to be more likely to be at risk.
Other possible explanations for the gender and SES differentials that link social and biological factors, include the large scale social changes experienced in Brazil particularly by women. There has been a dramatic decline in fertility rates, especially in wealthier households, 33, 34 where women of higher SES tend to have fewer children35 (through older age at marriage and contraceptive use).36 This may reduce their risk of excess adiposity compared with lower SES women who continue to have a relatively greater number of children. In addition, the adiposity risk associated with repeated pregnancies (correlated with limited access to contraceptive technologies and education) may explain why women appear to be generally more susceptible to excess adiposity than men in LMICs.37 These initial findings from LMICs including Brazil and China begin to illustrate how risk factors that have both critical and cumulative effects differ in salience in men and women, and how rapid economic transition can influence this salience.38
Strengths and limitations
The 1978/79 RPCS provides unique data for the investigation of cardiovascular risk in transition settings. The findings are hypothesis generating for future research in LMICs despite any limitations. Of the samples (comprising 36% of the original cohort), those followed up had a slightly higher overall education level, and occupational status compared with the overall sample.12 In addition, individuals of low SES had somewhat lower follow-up rates compared with those of high SES: 30% of women and 25% of men of low SES were followed up compared with 36% of women and men of high SES (P<0.05 for the difference between SES groups). This may have influenced the variation in the sample and account for the absence of social patterning of some of the important behavioural factors in Table 1. In particular, the fact that there was a significant difference between the two periods in the prevalence of low childhood SES may have affected the results. However, the fact that the findings are consistent with other cohorts from Brazil and data from Chinese cohorts supports their relevance in understanding the social patterning and mechanisms of excess adiposity in LMICs.
A single SES measure was used in the study, but the availability of income data provides an alternative to the wealth and education indicators commonly used in analyses of SES and adiposity, and contribute to resolving some of the questions surrounding the association in LMICs. Education was not taken into account although it has been shown to mediate between childhood SES and adult smoking and overweight.18 This may have had a role in producing the gender differences observed in the data as the health returns to educational attainment are likely to differ between the populations of men and women in Brazil. However, the majority of participants at follow-up had similar levels of education (over 12 years of schooling).13
The results were largely consistent across the three adiposity measures, displaying the same gender patterns. The availability of anthropometry at birth strengthens the conclusions regarding SES effects independently of biological factors. The investigation incorporated health behaviours and revealed very little role for these in mediating the SES effect in either gender. Although physical activity remains difficult to assess accurately, the International Physical Activity Questionnaire is one of the more reliable questionnaires. The cohort was not old enough to yield information about effects at older ages and therefore limits the generalisability of the findings to other age groups.
Finally, another possible source of uncertainty around the results comes from the fact that low SES participants were more often found to be heads of their household (53%) compared with high SES participants (16%), which may have affected the precision of the household-based SES measurement. The most likely explanation is that high SES 24-year-olds will have remained in education for longer and be living in their parental homes. Low SES individuals would more often be classified as head of the household either because they entered employment and started earning greater incomes than their parents, or may have started working, got married and left the parental home. In view of these contextual observations, it is unlikely that the precision of the SES measure would be significantly affected particularly in comparing low- and high-SES groups, because the overall aggregate household incomes would have been very different.
Conclusion
This study contributes to both the investigation of changing patterns of association between SES and adiposity, and the emerging understanding of life course effects of SES in low- and middle-income countries (LMICs), where the burden of excess adiposity is reaching alarming levels.39 It shows that the nature of the association and the effects of early-life SES on adiposity differ by gender. Among women, high SES is associated with lower adiposity and adult SES appears to be more important than childhood SES. In contrast, men displayed the opposite pattern with high SES being associated with higher adiposity, and adult SES being less important. These findings suggest women are experiencing the social transition (or reversal of the social gradient of excess adiposity) much faster than men.
LMICs are experiencing social changes affecting obesity risk at a much faster pace than populations in HICs. HICs experienced changes in dietary and physical activity patterns over several decades, affording them time for health services and public health systems to adapt to the changing disease profile in the population. The luxury of time may not be available for LMICs, and they may need to adapt much more rapidly to provide adequate prevention and health care delivery for excess adiposity and its complications.
In terms of research, the confounding structure of any given disease outcome in settings experiencing such rapid social and economic transition may differ from those in high-income countries, and gender specific effects of SES on adiposity appear to be more salient in LMICs.17, 19 This makes the investigation of the patterns and mechanisms of overweight and obesity in LMICs crucial.40, 41 Longitudinal data from LMICs can reveal important differences in the role of SES as period effects are captured within single cohorts. Finally, reducing health inequalities, gender or otherwise, is an important intervention in reducing poverty as set out in the Millennium Development Goals.42
Acknowledgments
We thank Aubrey Sheiham for his helpful comments on the manuscript. The RPCS project is supported by the Brazilian Research Council (CNPq), the University of Sao Paulo and the Sao Paulo Research Foundation grant number 00/09508-7. AA-S and GDB are Wellcome Trust fellows; MM is supported by a MRC professorship. MA Barbieri, H Bettiol and AAM Silva are CNPq senior research fellows.
The authors declare no conflict of interest.
References
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guh D, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis A. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Global Status Report On Noncommunicable Diseases 2010. Description of the Global Burden of NCDs, Their Risk Factors and Determinants. Geneva; April 2011. [Google Scholar]
- Senese LC, Almeida ND, Fath AK, Smith BT, Loucks EB. Associations between childhood socioeconomic position and adulthood obesity. Epidemiol Rev. 2009;31:21–51. doi: 10.1093/epirev/mxp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman M, Finlay JE, Davey Smith G, Subramanian S. The poor stay thinner: stable socioeconomic gradients in BMI among women in lower- and middle-income countries. Am J Clin Nutr. 2011;94:1348–1357. doi: 10.3945/ajcn.111.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Perkins JM, Özaltin E, Davey Smith G. Weight of nations: a socioeconomic analysis of women in low- to middle-income countries. Am J Clin Nutr. 2011;93:413–421. doi: 10.3945/ajcn.110.004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro CA, Conde WL, Popkin BM. Income-specific trends in obesity in Brazil. Am J Public Health. 2007;97:1808–1812. doi: 10.2105/AJPH.2006.099630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro CA, Conde WL, Lu B, Popkin BM. Obesity and inequities in health in the developing world. Int J Obes Relat Metab Disord. 2004;28:1181–1186. doi: 10.1038/sj.ijo.0802716. [DOI] [PubMed] [Google Scholar]
- Jones-Smith JC, Gordon-Larsen P, Siddiqi A, Popkin BM.Is the burden of overweight shifting to the poor across the globe? Time trends among women in 39 low- and middle-income countries (1991-2008) Int J Obes 2011. e-pub ahead of print 13 September 2011doi: 10.1038/ijo.2011.179 [DOI] [PMC free article] [PubMed]
- Schooling CM, Jiang CQ, Lam TH, Zhang WS, Cheng KK, Leung GM. Life-course origins of social inequalities in metabolic risk in the population of a developing country. Am J Epidemiol. 2008;167:419–428. doi: 10.1093/aje/kwm329. [DOI] [PubMed] [Google Scholar]
- Cardoso VC, Simões VMF, Barbieri MA, Silva AAM, Bettiol H, Alves MTSSB, et al. Profile of three Brazilian birth cohort studies in Ribeirão Preto, SP and São Luís, MA. Braz J Med Biol Res. 2007;40:1165–1176. doi: 10.1590/s0100-879x2006005000148. [DOI] [PubMed] [Google Scholar]
- Barbieri MA, Bettiol H, Silva AAM, Cardoso VC, Simões VMF, Gutierrez MRP, et al. Health in early adulthood: the contribution of the 1978/79 Ribeirão Preto birth cohort. Braz J Med Biol Res. 2006;39:1041–1055. doi: 10.1590/s0100-879x2006000800007. [DOI] [PubMed] [Google Scholar]
- Molina MC, Bettiol H, Barbieri MA, Silva AAM, Conceição SIO, Dos-Santos JE. Food consumption by young adults living in Ribeirão Preto, SP 2002/2004. Braz J Med Biol Res. 2007;40:1257–1266. doi: 10.1590/s0100-879x2006005000168. [DOI] [PubMed] [Google Scholar]
- IPAQ Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ) Short Form. 2004.
- Ziraba A, Fotso J, Ochako R. Overweight and obesity in urban Africa: A problem of the rich or the poor. BMC Public Health. 2009;9:465. doi: 10.1186/1471-2458-9-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell R, Khan L, Hughes M, Grummer-Strawn L. Obesity in women from developing countries. Eur J Clin Nutr. 2000;54:247–252. doi: 10.1038/sj.ejcn.1600931. [DOI] [PubMed] [Google Scholar]
- Molarius A, Seidell J, Sans S, Tuomilehto J, Kuulasmaa K. Educational level, relative body weight, and changes in their association over 10 years: an international perspective from the WHO MONICA Project. Am J Public Health. 2000;90:1260–1268. doi: 10.2105/ajph.90.8.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand BH, Murray ET, Guralnik J, Hardy R, Kuh D.Childhood social class and adult adiposity and blood-pressure trajectories 36–53 years: gender-specific results from a British birth cohort J Epidemiol Community Health 2010. e-pub ahead of print 23 November 2010; doi: 10.1136/jech.2010.115220 [DOI] [PMC free article] [PubMed]
- Chapman BP, Fiscella K, Duberstein P, Kawachi I, Coletta M. Can the influence of childhood socioeconomic status on men's and women's adult body mass be explained by adult socioeconomic status or personality? Findings from a national sample. Health Psychol. 2009;28:419–427. doi: 10.1037/a0015212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Mishra GD. Whose socioeconomic status influences a woman's obesity risk: her mother's, her father's, or her own. Int J Epidemiol. 2006;35:131–138. doi: 10.1093/ije/dyi216. [DOI] [PubMed] [Google Scholar]
- Langenberg C, Hardy R, Kuh D, Brunner E, Wadsworth M. Central and total obesity in middle aged men and women in relation to lifetime socioeconomic status: evidence from a national birth cohort. J Epidemiol Community Health. 2003;57:816–822. doi: 10.1136/jech.57.10.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooling CM, Jiang CQ, Lam TH, Zhang WS, Cheng KK, Leung GM. Life-course origins of social inequalities in metabolic risk in the population of a developing country. Am J Epidemiol. 2008;167:419–428. doi: 10.1093/aje/kwm329. [DOI] [PubMed] [Google Scholar]
- Heraclides A, Brunner E. Social mobility and social accumulation across the life course in relation to adult overweight and obesity: the Whitehall II study. J Epidemiol Community Health. 2010;64:714–719. doi: 10.1136/jech.2009.087692. [DOI] [PubMed] [Google Scholar]
- Kuh D, Ben-Shlomo Y. A Life Course Approach to Chronic Disease Epidemiology: Tracing the Origins of Ill-Health From Early to Adult Life. Oxford University Press: Oxford; 2004. [Google Scholar]
- Sobal J, Stunkard A. Socio-economic status and obesity: a review of the literature. Psychol Bull. 1989;105:260–275. doi: 10.1037/0033-2909.105.2.260. [DOI] [PubMed] [Google Scholar]
- Monteiro C, Mondini L, de Souza A, Popkin B. The nutrition transition in Brazil. Eur J Clin Nutr. 1995;49:101–113. [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Beedle AS, Raubenheimer D. Fetal and neonatal pathways to obesity. Front Horm Res. 2008;36:61–72. doi: 10.1159/000115337. [DOI] [PubMed] [Google Scholar]
- Hoffman D, Sawaya A, Verreschi I, Tucker K, Roberts S. Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shantytown children from Sao Paulo, Brazil. Am J Clin Nutr. 2000;72:702–707. doi: 10.1093/ajcn/72.3.702. [DOI] [PubMed] [Google Scholar]
- Eckhardt C. Micronutrient Malnutrition, Obesity, and Chronic Disease in Countries Undergoing the Nutrition Transition: Potential Links and Program/Policy Implications. International Food Policy Research Institute (IFPRI): Washington; 2006. [Google Scholar]
- Barbieri MA, Portella AK, Silveira PP, Bettiol H, Agranonik M, Silva AA, et al. Severe intrauterine growth restriction is associated with higher spontaneous carbohydrate intake in young women. Pediatr Res. 2009;65:215–220. doi: 10.1203/PDR.0b013e31818d6850. [DOI] [PubMed] [Google Scholar]
- Schooling CM. Risk factors for the metabolic syndrome in contemporary China. CVD Prev Control. 2009;4:41–50. [Google Scholar]
- Schooling CM, Lam TH, Thomas GN, Cowling BJ, Heys M, Janus ED, et al. Growth environment and sex differences in lipids, body shape and diabetes risk. PLoS One. 2007;2:e1070. doi: 10.1371/journal.pone.0001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J, Schmertmann C, Cavenaghi S. Fertility and development: evidence from Brazil. Demography. 2002;39:739–761. doi: 10.1353/dem.2002.0039. [DOI] [PubMed] [Google Scholar]
- Martine G. Brazil's fertility decline, 1965-95: A fresh look at key factors. Popul Dev Rev. 1996;22:47–75. [Google Scholar]
- International Encyclopedia of Sexuality—1997-2001Available at http://www2.hu-berlin.de/sexology/IES/index.html (accessed August 2011).
- Bollen K, Glanville J, Stecklov G. Socioeconomic status and class in studies of fertility and health in developing countries. Annu Rev Sociol. 2001;27:153–185. [Google Scholar]
- Brooks R, Maklakov A. Sex differences in obesity associated with total fertility rate. PLoS One. 2010;5:e10587. doi: 10.1371/journal.pone.0010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes F, Portella A, Barbieri M, Bettiol H, Agranonik M, Silveira P, et al. Risk factors for sedentary behaviour in young adults: similarities in the inequalities. J Dev Orig of Health Dis. 2010;1:255–261. doi: 10.1017/S204017441000019X. [DOI] [PubMed] [Google Scholar]
- Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol. 2006;35:93–99. doi: 10.1093/ije/dyi272. [DOI] [PubMed] [Google Scholar]
- Batty GD, Alves JG, Correia J, Lawlor DA. Examining life-course influences on chronic disease: the importance of birth cohort studies from low- and middle- income countries. An overview. Braz J Med Biol Res. 2007;40:1277–1286. doi: 10.1590/s0100-879x2007000900015. [DOI] [PubMed] [Google Scholar]
- Miranda JJ, Kinra S, Casas JP, Davey Smith G, Ebrahim S. Non-communicable diseases in low- and middle-income countries: context, determinants and health policy. Trop Med Int Health. 2008;13:1225–1234. doi: 10.1111/j.1365-3156.2008.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros FC, Matijasevich A, Requejo JH, Giugliani E, Maranhao AG, Monteiro CA, et al. Recent trends in maternal, newborn, and child health in Brazil: progress toward millennium development goals 4 and 5. Am J Public Health. 2010;100:1877–1889. doi: 10.2105/AJPH.2010.196816. [DOI] [PMC free article] [PubMed] [Google Scholar]