Abstract
RAS mutations occur frequently in human cancer and activated RAS signalling contributes to tumour development and progression. Apart from its oncogenic effects on cell growth, active RAS has tumour-suppressive functions via its ability to induce cellular senescence and apoptosis. RAS is known to induce p53-dependent cell cycle arrest, yet its effect on p53-dependent apoptosis remains unclear. We report here that apoptosis-stimulating protein of p53 (ASPP) 1 and 2, two activators of p53, preferentially bind active RAS via their N-terminal RAS-association domains (RAD). Additionally, ASPP2 colocalises with and contributes to RAS cellular membrane localisation and potentiates RAS signalling. In cancer cells, ASPP1 and ASPP2 cooperate with oncogenic RAS to enhance the transcription and apoptotic function of p53. Thus, loss of ASPP1 and ASPP2 in human cancer cells may contribute to the full transforming property of RAS oncogene.
Keywords: ASPP2, ASPP1, RAS, p53, apoptosis
The evolutionarily conserved RAS proto-oncogenes encode 21 kDa guanine nucleotide-binding proteins. RAS–GDP is inactive, whereas RAS–GTP is active and binds effectors to activate RAS/RAF/ERK or RAS/PI3K/AKT signalling cascades that are important in cell growth and death.1, 2 RAS activation takes place primarily at the plasma membrane. As one of the first oncogene identified in human cancer, activation of mutations at residues 12, 13 or 61 result in a mutant RAS that constitutively binds GTP. Activation of RAS can also be achieved by overexpression or mutation of its upstream activators such as epidermal growth factor receptor (EGFR).3, 4 Apart from its oncogenic effect, it is emerging that RAS also has tumour-suppressive functions through its ability to induce senescence and apoptosis.5 The final outcome of these contradictory signals depends largely on the cell type and context. It is therefore important to identify molecules that dictate cellular response to RAS activation.
Oncogenes, such as RAS, Myc and E1A are also known to activate the tumour-suppressor p53 via their ability to induce the expression of Arf and prevent Mdm2-mediated protein degradation of p53.6, 7, 8 Oncogenic signalling to p53 leads to one of two responses: cell cycle arrest or apoptosis. Oncogenic RAS induces senescence mediated by p53 and its downstream target gene p21.9, 10, 11 However, the tumour-suppressive function of p53 is closely linked to its ability to induce apoptosis. Therefore, it remains unclear whether oncogenic RAS has a role in regulating p53-mediated apoptosis.
Consistent with p53 as an important mediator of oncogenic RAS-induced senescence in primary fibroblasts, mutations of p53 and RAS are found at high frequency in colorectal and pancreatic tumours. In colorectal cancer, RAS mutations are an early event, whereas p53 mutations occur predominantly in metastatic tumours.12 Therefore, there might be a selective advantage in tumours expressing oncogenic RAS to inactivate the tumour-suppression function of p53. Regulators of p53 may thus also have a role in affecting cellular responses to RAS. Among those, apoptosis-stimulating protein of p53 (ASPP)1 and ASPP2 are two such potential candidates.
ASPP1 and ASPP2 belong to the evolutionarily conserved ASPP family of proteins, including ASPP1, ASPP2 and iASPP, which contain signature sequences in their C-termini; ankyrin repeats, a SH3 domain and proline-rich sequences. The evolutionarily conserved RAS-association domains (RAD) of ASPP1 and ASPP2 are located at their N-termini (first 100 amino acids) and this sequence does not exist in iASPP's N-terminus. In mouse primary fibroblasts, ASPP2 is a key mediator of RAS-induced senescence via its ability to inhibit RAS-induced autophagy, and nuclear accumulation of small ubiquitin-like modifier (SUMO) modified cyclin D1.13, 14 Mechanistically, the N-terminus of ASPP2 binds ATG5 and inhibits RAS-induced autophagy, independently of p53.13
In addition to inducing cellular senescence in primary cells, RAS activation induces apoptosis in certain cancer cells. Similarly, DNA damage- or oncogene-induced p53 mainly induces senescence in primary cells, but apoptosis in cancer cells. One explanation for this selective action of p53 in different cell types is the expression levels and activities of the ASPP family of proteins. In cancer cells, ASPP1 and ASPP2 stimulate, whereas iASPP inhibits, apoptosis induced by p53 and its family members, p63 and p73.15, 16, 17, 18 ASPP1 and ASPP2 are also transcriptional targets of E2F, and elevated E2F activity mainly occurs in cancer cells due to inactivation of the Rb pathway. Moreover, E2F1 binds and cooperates with p53 to induce apoptosis.19 Therefore, in cancer cells, enhanced activities of E2F, ASPP1 and ASPP2 may sensitise cancer cells to p53-induced apoptosis.19, 20 Importantly, the ability of ASPP1 and ASPP2 to stimulate p53-dependent apoptosis in cancer cells also requires their first 120 amino acids, as mutants lacking them are unable to coactivate with p53, even though they interact with p53.16
Recently, ASPP2 was shown to bind and colocalise with PAR3 via its N-terminus to maintain the integrity of cell polarity and adherence junctions.21, 22 RAS activation mainly occurs at the cellular membrane, and the RAD-containing N-terminus of ASPP2 is crucial in mediating oncogenic RAS-induced senescence in primary fibroblasts. Therefore, it is possible that ASPP1 and ASPP2 may interact with RAS and regulate RAS signalling. As a result, the status of ASPP1 and ASPP2 may dictate the cellular response to RAS signalling in both primary fibroblasts and cancer cells. Here we show that ASPP1 and ASPP2 bind active RAS. Endogenous ASPP2 contributes to endogenous RAS cellular membrane localisation, and potentiates RAS signalling. Additionally, we demonstrate that ASPP1 and ASPP2 cooperate with oncogenic RAS to enhance the transcription and apoptotic function of p53 in cancer cells.
Results
ASPP1 and ASPP2 preferentially bind active RAS
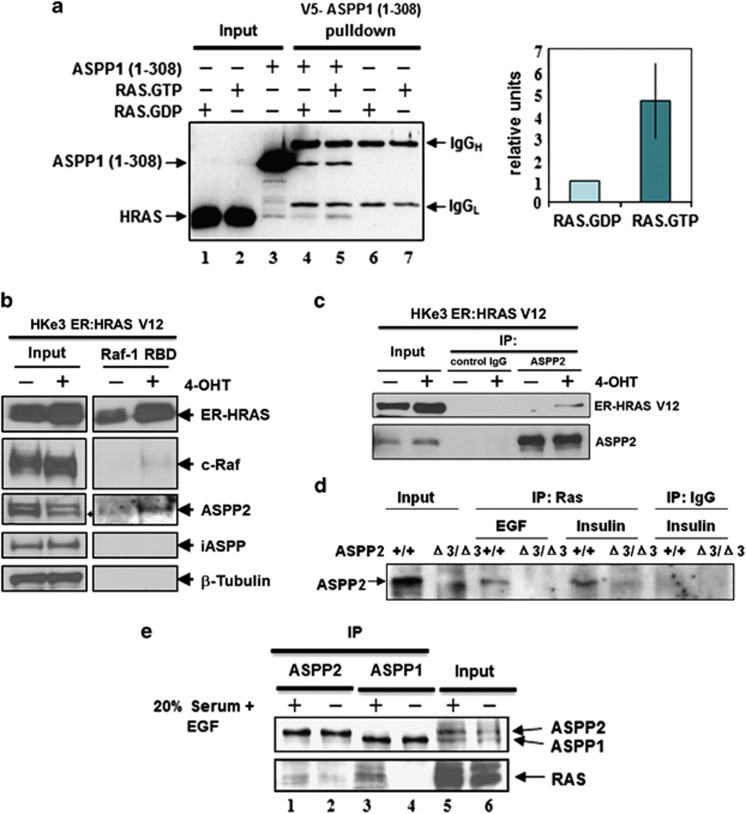
The evolutionarily conserved N-terminal 100 amino acids of ASPP1 and ASPP2 share high sequence homology with RAD present in c-Raf, PI3K and RasGDL,23 suggesting that ASPP1 and ASPP2 may interact with RAS. Purified N-terminal ASPP1 and purified RAS loaded with [3H]-labelled GTP or GDP were used to investigate whether ASPP1 RAD has a binding preference towards either RAS–GTP or RAS–GDP. We observed that purified ASPP1 fragment (1–310), containing the RAD, interacted with purified RAS in vitro. The ASPP1 N-terminus bound RAS–GTP with a four-fold higher affinity than RAS–GDP (Figure 1a, compare lanes 4 and 5). To illustrate that only the N-terminal region of ASPP1 binds RAS, various truncated ASPP1 mutants were constructed. All truncated ASPP1 mutants containing RAD complexed with endogenous RAS except ASPP1 (310–1090) lacking RAD (Supplementary Figures 1a and b).
Figure 1.
ASPP1 and ASPP2 preferentially bind active RAS. (a) ASPP1's N-terminus preferentially binds RAS–GTP in vitro. RAS protein, loaded with either tritium-labelled GDP or GTP, was added to V5-tagged recombinant ASPP1 (1–310) and immunoprecipitated with V5 antibody. As a negative control, RAS.GDP and RAS–GTP were immunoprecipitated with V5 antibody in the absence of ASPP1 (1–310). Co-immunoprecipitated RAS was quantified according to the presence of 3H-GTP or 3H-GDP. Values are shown as a bar graph. Standard deviation represents the mean of three independent experiments. (b) ASPP2 binds activated RAS in HKe3 cells. HKe3 ER:HRASV12 cells were treated with 100 nM 4-OHT for 2 days and Raf-1–RBD agarose pull-down assays were performed to pull down GTP-bound active RAS. (c) ASPP2 binds activated ER:HRASV12. Total cell lysates from HKe3 ER:HRASV12 cells treated with or without 4-OHT were immunoprecipitated with an anti-ASPP2 antibody, or control IgG as indicated. (d) ASPP2 binds activated RAS in MEFs. Total cell lysates from ASPP2(+/+) or ASPP2(Δ3/Δ3) MEFs treated with EGF (20 ng/ml) or insulin (1 μg/ml) for 15 min were immunoprecipitated with an anti-HRAS antibody or control IgG. (e) Saos2 cells were either starved in 0.5%, or stimulated with 20%, FCS plus 20 ng/ml EGF overnight. Lysates were collected and immunoprecipitated with rabbit anti-ASPP1 (ASPP1.88) or anti-ASPP2 (ASPP2/77) antibodies, respectively. The ASPP proteins were detected with mAbASPP1.54.1, which is known to crossreact with both ASPP1 and ASPP2
Activated RAS has a higher interaction affinity with the RAS-binding domain (RBD) of Raf1. Agarose beads linked to the Raf1–RBD can be used to pull down proteins associated with RAS–GTP. In this assay, the presence of small but detectable amounts of c-Raf served as a positive control for the assay. We thus used this as an alternative assay to investigate whether ASPP2 also selectively binds RAS–GTP. Under the same conditions, the percentage of ASPP2 pulled down by Raf1–RBD is clearly higher than that of c-Raf, illustrating that endogenous ASPP2 also complexes with active RAS (Figure 1b). Raf1–RBD failed to pull down iASPP, which does not contain RAD at its N-terminus. These results illustrate that ASPP1 and ASPP2 can bind active RAS, and this interaction is likely mediated through their N-termini.
An ASPP2 inducible U2OS cell line that expresses wild-type p53 was first used to test whether endogenous wild-type RAS binds ASPP2 with different efficiency upon EGF stimulation. Endogenous RAS was immunoprecipitated with or without ASPP2 induction in the presence or absence of EGF treatment (Supplementary Figure 1c). We found that there is very little binding between RAS and ASPP2 in starved cells. The RAS–ASPP2 complex was mainly detected in EGF-treated cells that express active RAS (Supplementary Figure 1c, compare lanes 7 and 8). It is important to note that the expression levels of RAS and ASPP2 are similar in cells before or after EGF treatment. Moreover, an interaction was also observed between endogenous ASPP2 and HRASV12 in a human colon cancer cell line HKe3 ER:HRASV12 cells, in which RAS activation is induced upon the addition of 4-hydroxytamoxifen (4-OHT) (Figure 1c).24, 25 The ability of endogenous ASPP2 to bind with endogenous active RAS was further tested in mouse embryonic fibroblasts (MEFs) derived from ASPP2 wild-type and ASPP2(Δ3/Δ3) embryos. Growth factors such as EGF or insulin were used to stimulate serum-starved ASPP2(+/+) and ASPP2(Δ3/Δ3) MEFs to induce RAS activation. Endogenous ASPP2 and RAS were co-immunoprecipitated with an anti-RAS antibody in ASPP2(+/+) MEFs. However, no co-immunoprecipitation was detected in ASPP2(Δ3/Δ3) MEFs, which occasionally express reduced amounts of N-terminal-truncated ASPP2 (Figure 1d). These data demonstrate that ASPP2 preferentially binds RAS when it is activated.
To provide further evidence that ASPP1 and ASPP2 preferentially interact with RAS–GTP, a p53-null osteosarcoma cell line Saos2 was starved for 20 h and then stimulated with 20% foetal calf serum (FCS) and EGF. More RAS was detected in the ASPP1 and ASPP2 immunoprecipitates derived from serum- and EGF-stimulated cell lysates than in non-stimulated ones (Figure 1e, compare lanes 1–2 for ASPP2 and 3–4 for ASPP1). All these suggest that N-termini of ASPP1 and ASPP2 selectively bind active RAS. This new property of ASPP1 and ASPP2 is independent of p53 because p53 binds the C-termini of the ASPPs and Saos2 cells are p53-null.
ASPP2 colocalises with and contributes to RAS activation at cell membrane
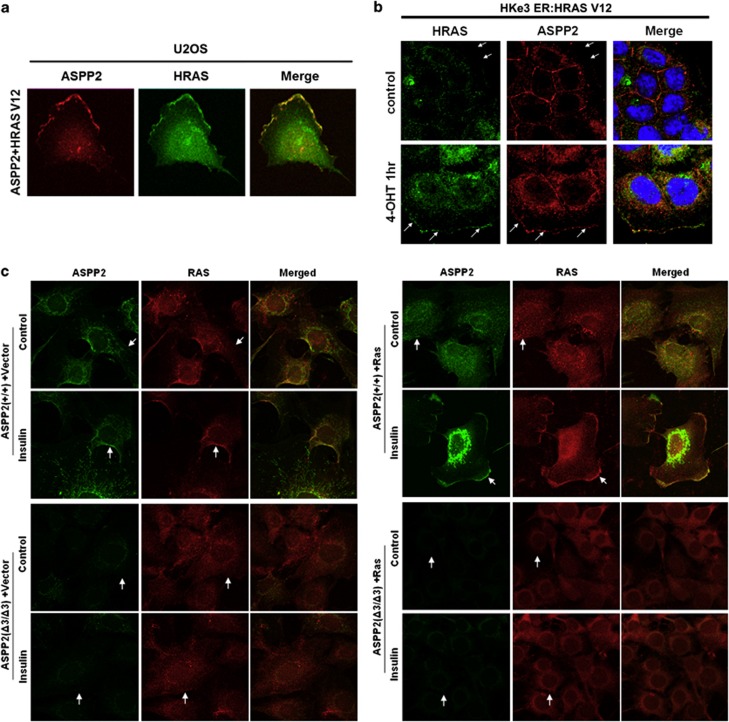
Activated RAS is mainly located at cellular membranes. To test the biological importance of the observed RAS–ASPP interactions, we first examined the cellular localisation of exogenously coexpressed HRASV12 and ASPP2, and observed that they colocalise to the cell membrane in U2OS cells (Figure 2a). In HK-e3ER:HRASV12 cells, ASPP2 is normally expressed at cell/cell junctions.22 One hour after RAS induction, endogenous ASPP2 colocalised with induced HRASV12 at the cell membrane in HKe3 cells (Figure 2b). Importantly, ASPP2 depletion by RNA interference (RNAi) largely abolished the membrane localisation of HRASV12 after RAS induction in these cells (Supplementary Figure 2).
Figure 2.
ASPP2 colocalises with and contributes to RAS activation at the cell membrane. (a) U2OS cells co-transfected with ASPP2 (red) and HRASV12 (green) show colocalisation of both at the cell membrane (yellow). (b) Immunofluorescence staining of HRAS in HKe3 ER:HRASV12 cells transfected with control or ASPP2 siRNA for 3 days, followed by treatment without (control) or with 100 nM 4-OHT for 1 h. Arrows indicate cell membrane. (c) Immunofluorescence staining of ASPP2 or HRAS in ASPP2(+/+) or ASPP2(Δ3/Δ3) MEFs, with or without HRASV12 expression, before or after treatment as indicated. Cells were treated with 1 μg/ml insulin for 15 min. Arrows indicate cell membrane
The ability of endogenous ASPP2 to bind and colocalise with endogenous active RAS was tested in a physiological setting. In ASPP2(+/+) MEFs, insulin-induced active RAS and ASPP2 colocalised at the cell membrane. The majority of the cells expressing membrane-associated ASPP2 also had membrane-localised RAS and vice versa (Figure 2c, left panel with arrows). RAS was not detected at the cell membrane in ASPP2(Δ3/Δ3) MEFs (Figure 2c) under the same conditions, consistent with the results in HKe3 cells (Supplementary Figure 2). Similar results were observed in HRASV12-expressing ASPP2(+/+) and ASPP2(Δ3/Δ3) MEFs (Figure 2c, right panel). Together, these data suggest that ASPP2 colocalises with RAS and facilitates the membrane localisation of RAS in human cancer cells and MEFs.
ASPP2's N-terminus is required and sufficient to potentiate RAS signalling
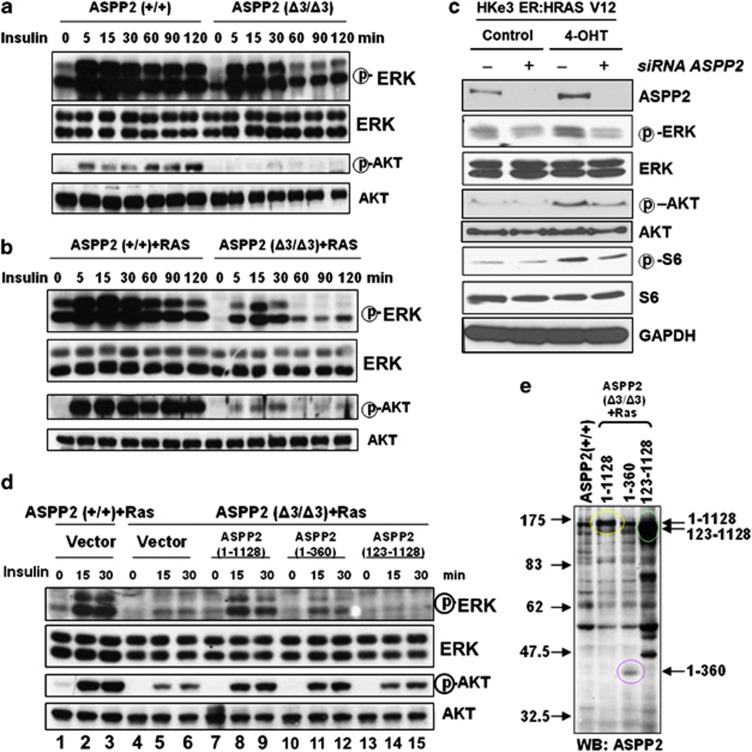
The biological implications of ASPP2–RAS binding and colocalisation were investigated by examining the RAS signalling cascade in ASPP2(+/+) and ASPP2(Δ3/Δ3) MEFs. The basal phosphorylation level of ERK in ASPP2(+/+) MEFs was higher than that in ASPP2(Δ3/Δ3) MEFs (Figure 3a; Supplementary Figure 3a). This difference was more notable in HRASV12-expressing ASPP2(+/+) than in ASPP2(Δ3/Δ3) MEFs (Figure 3b; Supplementary Figure 3b). Upon insulin treatment, a stronger response was observed in ASPP2(+/+) compared with ASPP2(Δ3/Δ3) MEFs, reflected by the phosphorylation level of both ERK and AKT (Figures 3a and b; Supplementary Figures 3a and b). These data suggest that by binding to and colocalising with active RAS at the cell membrane, ASPP2 potentiates RAS signalling.
Figure 3.
ASPP2's N-terminus is required and is sufficient to potentiate RAS signalling. (a and b) ASPP2(+/+) or ASPP2(Δ3/Δ3) MEFs, in the absence (a), or presence of HRASV12 (b) were serum-starved overnight, followed by stimulation with 1 μg/ml insulin for the indicated times. The activation status of ERK or AKT was determined by western blots. (c) Western blots showing the expression levels of endogenous ASPP2, phosphorylated ERK, ERK, phosphorylated AKT, AKT, phosphorylated S6 and S6 in HKe3 ER:HRASV12 cells with indicated treatments. GAPDH was used as a loading control. (d) Oncogenic HRASV12-expressing ASPP2(+/+) or ASPP2(Δ3/Δ3) MEFs infected with retroviruses expressing full-length ASPP2 or truncated mutants were serum-starved overnight before stimulation with 1 μg/ml insulin for the indicated times. The activation status of ERK or AKT was determined by western blot analysis. (e) Western blots showing expression levels of full-length ASPP2 and its mutants in HRASV12-expressing ASPP2(Δ3/Δ3) MEFs as indicated
The effects of ASPP2 on RAS signalling were further confirmed with human phospho-MAPK arrays. As expected, phosphorylation levels of several downstream effectors of RAS were increased to various extents following RAS activation in HKe3 ER:HRASV12 cells. ASPP2 knockdown dampened the RAS-induced activation of some effectors such as MSK2, p38s and JNKs (Supplementary Figure 3d). Consistent with this, RAS-induced phosphorylation of ERK and AKT is compromised in these cells upon ASPP2 depletion (Figure 3c; Supplementary Figure 3c).
Finally, to confirm whether the RAD in the N-terminus of ASPP2 is critical for its potentiation of RAS signalling, two ASPP2 mutants were generated: ASPP2 (1–360) and ASPP2 (123–1128), representing a naturally occurring ASPP2 splice variant.26 Retroviruses expressing full-length or truncated ASPP2 were infected into HRASV12-expressing ASPP2(Δ3/Δ3) MEFs. The impaired RAS–ERK and RAS–AKT signalling in HRASV12-expressing ASPP2(Δ3/Δ3) MEFs was largely rescued by the expression of full-length ASPP2 (Figure 3d, lower panel, lanes 7–9). The expression of ASPP2 (1–360) also partially restored the activities of ERK and AKT (Figure 3d, lower panel, lanes 10–12). This may be explained by the reduced expression of ASPP2 (1–360) relative to full-length ASPP2 (Figure 3e). The expression of ASPP2 (123–1128) failed to stimulate ERK and AKT pathways, although the amount of protein was similar to that of ASPP2 (1–1128) (Figure 3d (lower panel, lanes 13–15) and Figure 3e). This result illustrates that the N-terminus of ASPP2 (1–123) is sufficient to potentiate RAS signalling.
ASPP1 and ASPP2 cooperate with RAS to enhance the transcriptional activity of p53
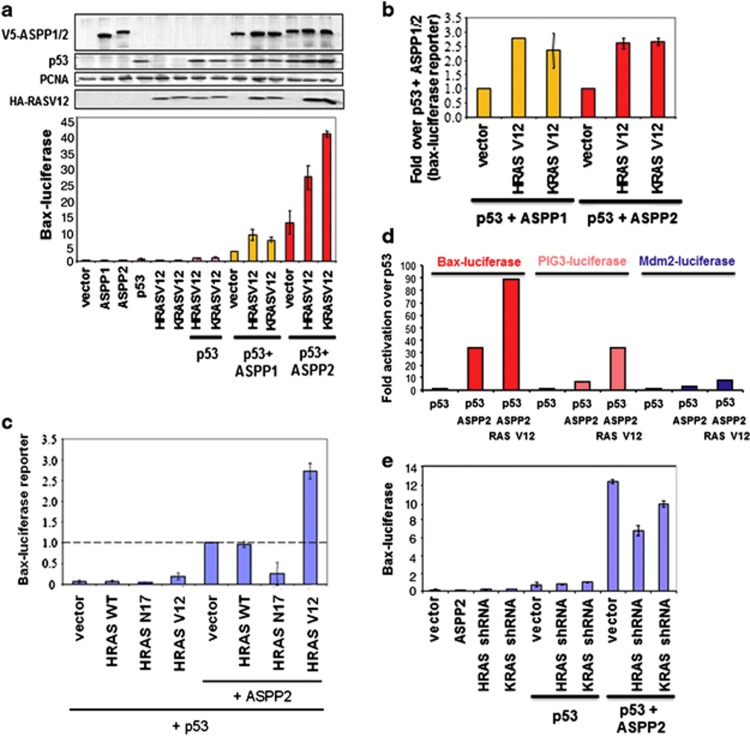
We showed previously that the N-termini of ASPP1 and ASPP2 are required for full enhancement of p53 activity.16 We thus tested whether ASPP1 and ASPP2 cooperate with RAS to stimulate the apoptotic function of p53. HRASV12 or KRASV12 was co-transfected with ASPP1, ASPP2 and p53 into Saos2 cells. The results shown in Figure 4a illustrate that HRASV12 or KRASV12 alone had a small stimulatory effect on p53 transactivity. As expected, coexpression of ASPP1 or ASPP2 with p53 enhanced the transcriptional activity of p53 on the Bax promoter–luciferase reporter by 3 and 10 times, respectively. In the presence of ASPP1, expression of HRASV12 or KRASV12 stimulated the transactivation function of p53 by ∼10-fold (Figure 4a). Similarly, the coexpression of ASPP2 and HRASV12 or KRASV12 stimulated the transcriptional activity of p53 by around 30–35-fold. When the transcriptional activities of p53+ASPP1 and p53+ASPP2 were set as 1, we observed that both HRASV12 and KRASV12 were able to further enhance the transcriptional activity of p53 by around three-folds (Figure 4b).
Figure 4.
ASPP1 and ASPP2 cooperates with RAS to enhance the transcriptional activity of p53. (a) Saos2 cells were transfected as indicated with a Bax–luciferase reporter and the luciferase activity shown (lower left panel). The expression of the proteins was verified by western blot (upper left panel). (b) The value of ASPP1+p53 or ASPP2+p53 were taken as 1.0 to reflect the fold increase of ASPP1/ASPP2 and p53 in the presence of mutant RAS. The mean values were derived from three independent experiments. (c) Saos2 cells were transfected with a Bax–luciferase reporter, p53, wild-type or mutant RAS, in the presence or absence of ASPP2 as indicated. The value of ASPP2+p53 was taken as 1.0 to reflect the fold increase. The mean values were derived from three independent experiments. (d) Saos2 cells were transfected as indicated with a Bax–luciferase or PIG3–luciferase or Mdm2–luciferase reporter; luciferase activity is reported here. (e) Saos2 cells were co-transfected with the p53 reporter Bax–luciferase, p53, ASPP2, HRAS shRNA or KRAS shRNA, as indicated
To demonstrate further that RAS activation is required to enhance the transcriptional activity of p53 via ASPP1 or ASPP2, a wild-type RAS or a dominant negative form of HRAS–HRASN17 was co-transfected with ASPP2 and p53 into Saos2 cells. The results shown in Figure 4c illustrate that HRASV12 alone had a small stimulatory effect on the transactivation function of p53. In the presence of ASPP2, however, HRASV12 stimulated the transactivation function of p53 by approximately 3-fold. When HRASN17 was co-transfected with ASPP2 and p53, the transactivation activity of p53 was inhibited. This inhibition was via ASPP2, as HRASN17 transfected only with p53 did not have such a strong inhibitory effect: HRASN17 inhibited p53 and ASPP2 activity more than 4-fold, whereas it inhibited p53 via endogenous ASPP about 1.5-fold. In contrast, wild-type RAS had very little effect on the transactivation function of p53. This may be explained by the fact that increased RAS expression does not necessarily lead to increased levels of RAS–GTP in cells.
We tested whether the observed ability of RAS oncogene to cooperate with ASPP2 to enhance p53 transcriptional activity is promoter-specific. Three p53 target gene promoter-containing reporters, PIG3-, Bax- and Mdm2-luciferases, were used. We observed that HRASV12 had the most profound effect on the transcriptional activity of p53 on PIG3 and BAX promoters. Under the same conditions, a reduced stimulatory effect was seen on Mdm2 promoter (Figure 4d). This agrees with our previous finding that ASPP2 has minimal impact on the transcriptional activity of p53 on the promoters of Mdm2 and p21waf1.16 Consistent with this, we also observed that p21waf1 mRNA expression induced by activated RAS in HKe3 cells is independent of ASPP2 status, as ASPP2 knockdown in these cells did not alter p21waf1 expression (Supplementary Figure 4a).
The requirement of endogenous RAS to stimulate the transcriptional activity of p53 via ASPP2 was tested using shRNA against HRAS or KRAS. Their ability to specifically reduce the expression level of HRAS or KRAS was confirmed (Supplementary Figure 4b). We found that RNAi against HRAS or KRAS dampened the ability of ASPP2 to stimulate the transactivation function of p53 on the promoters of the pro-apoptotic gene Bax (Figure 4e) and PIG3 (Supplementary Figure 4c). All these support the notion that ASPP1 and ASPP2 cooperate with RAS to enhance the transcriptional activity of p53 for apoptotic genes. This cooperation is likely to be mediated by the identified interaction between active RAS and RAD of the ASPPs, which also explains why the RAD of the ASPPs is required for their full potential to enhance the transcriptional activity of p53.
RAS oncogene enhances the apoptotic function of p53 via ASPP1 and ASPP2
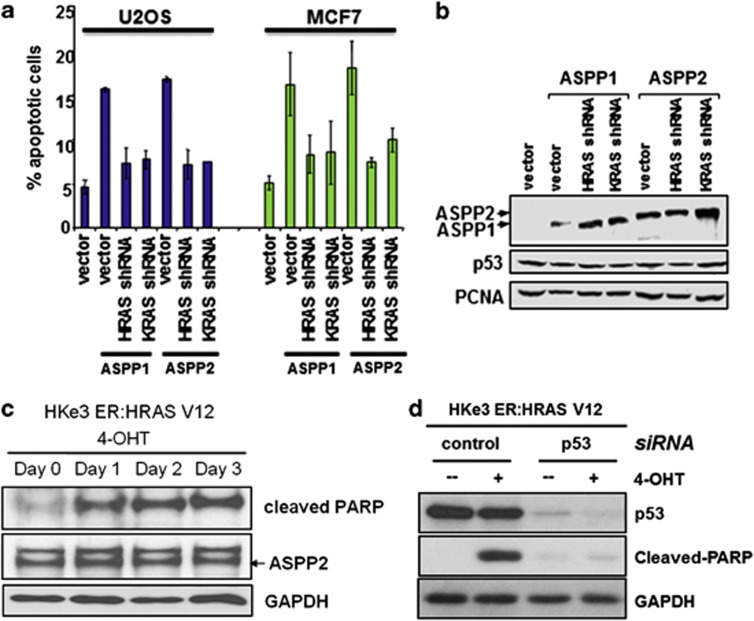
The requirement of RAS activation to potentiate the pro-apoptotic function of ASPP1 and ASPP2 was tested in two human cancer cell lines with wild-type p53: MCF7 and U2OS. As published previously, increased expression of ASPP1 or ASPP2 induces p53-mediated apoptosis in both cell lines.16 Using shRNAs to reduce the expression of endogenous HRAS or KRAS, we observed a reduction in the percentage of apoptosis in cells induced by ASPP1 or ASPP2 overexpression (Figure 5a). These findings suggest that endogenous HRAS and KRAS are required for the full activities of ASPP1 and ASPP2.
Figure 5.
RAS oncogene enhances the apoptotic function of p53 via ASPP1 and ASPP2. (a) ASPP1 and ASPP2 work with endogenous RAS to stimulate apoptosis. shRNA against HRAS or KRAS was co-transfected with ASPP1 or ASPP2 in U2OS or MCF7 cells. FACS analysis was used to determine apoptotic cells. (b) Western blots showing the expression levels of p53 and transfected ASPP1 and ASPP2. PCNA was used as a loading control. (c) Apoptotic activity is detected following oncogenic RAS activation. HKe3 ER:HRASV12 cells were treated with 4-OHT (100 nM) for the indicated time. Western blot showing expression levels of cleaved PARP and ASPP2. (d) p53 depletion reduces oncogenic RAS-induced apoptosis. HKe3 ER:HRASV12 cells transfected with control siRNA or siRNA against p53 for 3 days were treated with or without 4-OHT (100 nM) for another day. Western blots showing expression levels of cleaved PARP and p53
To further confirm this, we used HKe3 ER:HRASV12 cells, in which RAS activation is induced upon the addition of 4-OHT. Importantly, oncogenic RAS activation induced apoptosis in these cells indicated by the presence of cleaved-PARP (Figure 5c). To test whether p53 has a role in mediating the observed RAS-induced apoptosis in HKe3 cells, RNAi against p53 was used. Depletion of p53 abolished RAS-induced apoptosis in these cells. This is evident from a lack of PARP cleavage in these cells (Figure 5d).
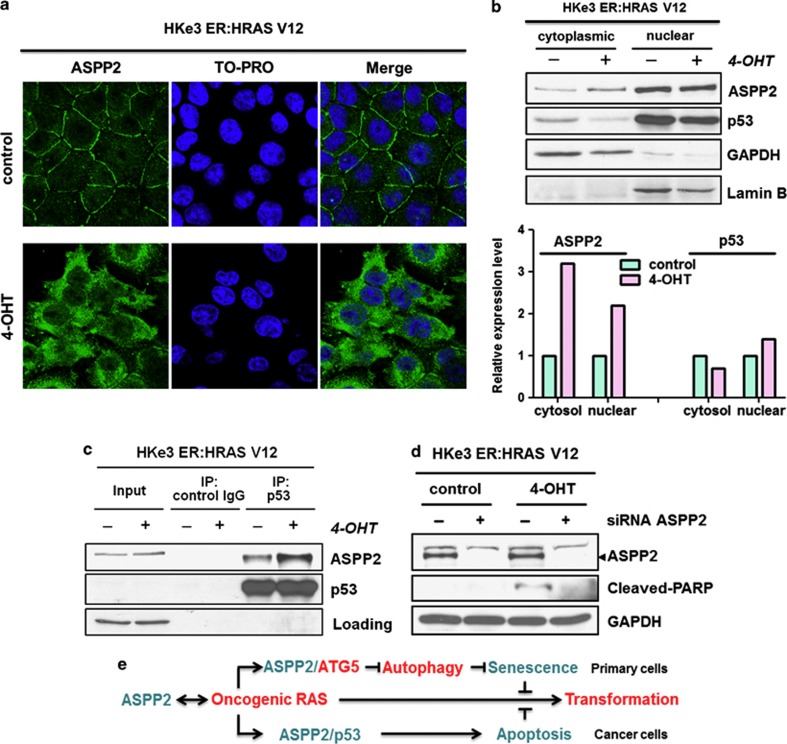
RAS activation induces ASPP2 translocation and enhances p53 binding in colon cancer cells
To understand how ASPP2 cooperates with p53 to induce apoptosis in cancer cells, we examined the cellular localisation of ASPP2 in HKe3 ER:HRASV12 cells upon RAS induction. We observed that endogenous ASPP2 translocates from cell/cell junctions to the cytosol/nucleus following RAS activation (Figure 6a). This is also confirmed by the detection of an increase in the expression levels of ASPP2 in the nuclear and cytoplasmic fractions in these cells upon RAS activation, with around two–three-folds increase in the level of nuclear and cytoplasmic ASPP2, respectively (Figure 6b). Moreover, we also detected an increase in the amount of p53–ASPP2 complex in these cells following RAS activation (Figure 6c). To test whether endogenous ASPP2 mediates the observed RAS-induced apoptosis in HKe3 cells, ASPP2 RNAi was used. ASPP2 knockdown abolished RAS-induced apoptosis indicated by a lack of PARP cleavage (Figure 6d). These data identify ASPP2 as an important mediator of oncogenic RAS-induced apoptosis. This may be achieved via the ability of the active RAS to induce the cellular translocation of ASPP2, and enhance the ability of ASPP2 to bind p53 and to enhance its activity.
Figure 6.
RAS activation induces ASPP2 translocation and enhances p53 binding in colon cancer cells. (a) RAS activation induces ASPP2 translocation. Immunofluorescence staining of ASPP2 in HKe3 ER:HRASV12 cells treated without (control) or with 100 nM 4-OHT for 3 days. (b) RAS activation induces cytoplasmic and nuclear accumulation of ASPP2. Cytoplasmic and nuclear fractions in HKe3 ER:HRASV12 cells with indicated treatments were isolated. GAPDH was used as a loading control for the cytoplasmic fraction, whereas Lamin B was used as a loading control for the nuclear fraction. The levels of ASPP2 and p53 were calculated by densitometry. Fold increase was calculated by normalising control group. (c) RAS activation enhances the binding between ASPP2 and p53. Total cell lysates from HKe3 ER:HRASV12 cells treated with or without 4-OHT were immunoprecipitated with an anti-p53 antibody or control IgG as indicated. (d) ASPP2 depletion reduces oncogenic RAS-induced apoptosis. HKe3 ER:HRASV12 cells transfected with control siRNA or siRNA against ASPP2 for 3 days were treated with or without 4-OHT (100 nM) for another day. Western blots showing expression levels of cleaved PARP and ASPP2. (e) Diagram summarises the interactions between ASPP2 and RAS for their regulation and functions (details are provided in Discussion)
Discussion
In addition to its well-established oncogenic properties, it is emerging that RAS also has growth-suppressive functions by its ability to induce senescence in primary fibroblasts and apoptosis in cancer cells. Recently, we showed that ASPP2 is a potent mediator of RAS-induced senescence. This property is p53-independent, and is achieved partly through the ability of the N-terminus of ASPP2 to bind ATG5 and to inhibit RAS-induced autophagy, as elevated autophagy activity bypasses oncogenic RAS-induced senescence in fibroblasts.13
We show here that ASPP1 and ASPP2 bind active RAS via their N-termini. ASPP2 potentiates RAS signalling and activates RAS at the cellular membrane. Moreover, the observed RAS–ASPP interactions enhance the transcription and apoptotic function of p53 in cancer cells. In primary fibroblasts, ASPP2 mediates RAS-induced senescence independent of p53, whereas in cancer cells, ASPP1 and ASPP2 mediate RAS-induced p53-dependent apoptosis. Therefore, ASPP1 and ASPP2 are the key mediators of RAS signalling in both primary and cancer cells. The status of ASPP1 and ASPP2 may therefore dictate the cellular response to RAS signalling. Therefore, the ability of ASPP2 to be located at cell junctions to bind and colocalise with RAS on the one hand, and located in the cytoplasm and bind p53 upon RAS activation on the other, places ASPP2 in an ideal position to survey the intensity of RAS signalling and to coordinate appropriate cellular responses accordingly. One possible explanation for this property of ASPP2 is that, when RAS is activated, it binds active RAS and controls the intensity of the RAS signal. When RAS signalling is elevated to a certain level, high enough to disrupt cell/cell junctions for example, it may then displace ASPP2 from cell/cell junctions. This redistribution of ASPP2 would thus enable the interaction with ATG5 to inhibit autophagy and to mediate RAS-induced senescence or to bind p53 to promote apoptosis (Figure 6e). As the levels of RAS activation induced by growth factors such as EGF or insulin in primary cells in normal growth conditions is lower than that in cancer cells caused by mutation or amplifications of RAS or EGFR genes, this cellular localisation of ASPP2 and its binding partners may differ between primary cells and cancer cells. Future studies are needed to test whether cellular localisation of ASPP1 and ASPP2, and their ability to interact with ATG5 or p53 may be an underline reason for why RAS or p53 activation predominately induces senescence in primary cells but apoptosis in cancer cells.
Although ASPP1 and ASPP2 share sequence similarity and have many overlapping functions in vitro, including the interaction with active RAS, they have distinct biological functions in vivo. ASPP2 binds PAR3 and locates at cell/cell junctions,21, 22 whereas ASPP1 binds YAP and locates in cytosol.27, 28 ASPP1 deficiency in mice is predominantly tolerated in mouse development, although lymphatic vessel leakage occurs.29 This differs from ASPP2-deficient mice, where a loss of cell polarity and excessive proliferation of neural progenitors are common features. Importantly, ASPP2 heterozygosity is sufficient to predispose mice to develop tumours,30, 31 establishing ASPP2's tumour-suppressive function. These differences may explain why ASPP2 depletion alone is sufficient to affect RAS signalling. Future studies are therefore needed to elucidate which of the in vivo functions of ASPP1 are influenced by the identified ASPP1–RAS interaction.
There have been many reports demonstrating that RAS activation inhibits apoptosis and promotes survival. Paradoxically, increasing numbers of studies have also shown that RAS activation induces apoptosis. The effect of RAS on survival versus apoptosis depends largely on cell types and concurrent activation of other signalling pathways. The most prominent pathway involved in RAS-mediated apoptosis is the Raf–ERK pathway.32, 33, 34 p53 is also thought to be involved in RAS-mediated apoptosis, as RAS is no longer able to induce apoptosis following p53 loss.34, 35, 36 RAS is known to stimulate p53 activity through the induction of Arf and PML.37, 38, 39 However, under the conditions used in these studies, RAS is likely to enhance the apoptotic function of p53 independently of Arf, as UO2S and MCF7 cells do not express Arf.40 The overall effect of RAS on p53 activity may be dependent on a balance between the different pathways linking the two proteins.
The ability of oncogenes to stimulate cell cycle arrest or apoptosis allows the cell to have a fail-proof system: oncogene activation would, instead of inducing the cell to proliferate in an uncontrolled manner, activate tumour-suppressor proteins, thereby preventing the cell from replicating its damage. Although cell cycle arrest and apoptosis are both efficient ways for cells to prevent propagation of their damage, apoptosis is irreversible and therefore more effective. The identification of ASPP1 and ASPP2 as mediators of RAS and p53 in regulating apoptosis suggests that oncogenic RAS would put pressure on p53 to induce apoptosis. Therefore, the discovery that ASPP1 and ASPP2 cooperate with RAS to enhance p53-induced apoptosis suggests that loss of ASPP1 or ASPP2 expression may be a frequent event in human cancers with mutant RAS. This finding may provide a novel molecular explanation for why RAS and p53 mutations are so tightly associated in human cancer.
Materials and Methods
Protein purification
Both recombinant RAS and the N-terminus of ASPP1 were purified from BL21 bacteria. Protein expression was induced by IPTG (0.2 mM) for 3 h, and the cells were sonicated and then lysed. The lysate was spun at 10 000 × g and the supernatant recuperated. The recombinant proteins were purified by Glutathionine Sepharose 4B beads (Pharmacia Biotech AB, Stockholm, Sweden) as described by the manufacturer in the case of RAS or in the case of N-terminus ASPP1 by nickel columns (Qiagen, West Sussex, UK). The GST tag of RAS was cleaved off with bovine thrombin (Sigma, Dorset, UK) at a concentration of 5 units/ml and the thrombin removed by P-aminobenzamidine-agarose beads (Sigma, West Sussex, UK). The GST-tagged ASPP2 fragment (693–1128) was purified by Glutathionine Sepharose 4B beads (Pharmacia Biotech AB). The protein was concentrated and re-suspended in 1 × kinase buffer (50 mM Tris-HCl, pH 7.5, 270 mM sucrose, 0.1 mM EGTA and 0.1% mercaptoethanol).
RAS GDP/GTP loading
2.5 μg of recombinant protein purified from Escherichia coli was incubated in a total volume of 320 μl assay buffer containing 2 μCi [3H] GDP or [3H] GTP in a water bath for 10 min at 30 °C. An aliquot of each sample had its tritium content measured to check the equal loading of GDP and GTP.
Reporter assay
Saos2 cells (7 × 105) were plated 24 h prior to transfection in 6-cm-diameter dishes. All transactivation assays contained 1 μg of reporter plasmid. Fifty nanograms of p53, 4 μg of ASPP2 or ASPP1 and 1.5 μg of wild-type or mutant HRAS expression plasmids were used as indicated. Cells were lysed in reporter lysis buffer 16–24 h after transfection and assayed using the luciferase assay kit (Promega, Madison, WI, USA). The fold increase of p53 and ASPP by wild-type or mutant HRAS was determined by the activity of p53 and ASPP in combination with wild-type or mutant HRAS divided by the activity of p53 and ASPP alone.
Active RAS pull-down assay
Active RAS pull-down assay was performed using the RBD of Raf-1–RBD, which were linked agarose (Millipore, Billerica, MA, USA). HKe3 ER:HRASV12 cells were treated with or without OHT (100 nM) for 2 days. Indicated cell lysates were incubated with 10 μl of a 50% slurry of Raf-1–RBD agarose. Subsequent blotting was done with indicated antibodies.
In vivo immunoprecipitation
1–4 mg of lysate was pre-cleared with protein G beads for 30 min at 4 °C and subsequently incubated with antibody prebound to protein G beads for 2–16 h at 4 °C. The beads were washed three times with NP40 buffer. Immunoprecipitations were analysed by western blots as indicated.
Immunofluorescence
Cells were fixed in 4% PBS–paraformaldeyde for 15 min, incubated in 0.2% TritonX-100 for 5 min, then in 0.2% Fish Skin Gelatine in PBS for 10 min and stained for 1 h with anti-RAS (RAS clone 10, Upstate Millipore Corporate Headquarters) or anti-ASPP2 (DX54.10 for human or S32 for mouse). Antibodies were used at 1 : 100 dilution in 0.2% Fish Skin Gelatine–PBS, respectively. Staining with the secondary antibody and Hoechst was performed as described before, followed by visualisation under a fluorescence microscope.
Flow cytometry
For FACS analysis, 106 cells were plated 24–48 h prior to transfection in 10-cm-diameter plates. All cells were transfected with 2 μg of a plasmid expressing CD20 as a transfection marker, 10 μg ASPP1 or ASPP2, 9 μg HRAS shRNA or KRAS shRNA as indicated. Both attached and floating cells were harvested using 4 mM EDTA–PBS and stained with FITC-conjugated anti-CD20 antibody. The cells were then fixed and stained with propidium iodide. The DNA content of all the cells expressing CD20 was analysed using a flow cytometer (Becton Dickinson, San Jose, CA USA).
Acknowledgments
We would like to thank the Ludwig Institute for Cancer Research Ltd for supporting this work, and Evelyn Harvey and Dr. Claire Beveridge for critical reading of the manuscript. We are grateful to Dr. Julian Downward for providing HKe3 ER:HRASV12 cells and Senji Shirasawa for HKe3 cells. We thank Dr. Richard Marais and Dr. Chris Marshall for providing us with the expression plasmids for HRAS, KRAS, HRASV12 and HRASN17, and Safia Ali for purifying the ASPP1 (1–308 amino acid) fragment.
Glossary
- 4-OHT
4-hydroxytamoxifen
- ASPP
apoptosis-stimulating protein of p53
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ER
oestrogen receptor
- ERK
extracellular signal-regulated kinases
- FCS
foetal calf serum
- GSK-3
glycogen synthase kinase 3
- iASPP
inhibitor of apoptosis-stimulating protein of p53
- MAPK
mitogen-activated protein kinase
- MEFs
mouse embryonic fibroblasts
- PBS
phosphate-buffered saline
- PI3K
phosphoinositide 3 kinase
- RAD
RAS-association domain
- RNAi
RNA interference
- siRNA
small interfering RNA
- shRNA
short hairpin RNA
- SUMO
small ubiquitin-like modifier
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by G Melino
Supplementary Material
References
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Buday L, Downward J. Many faces of Ras activation. Biochim Biophys Acta. 2008;1786:178–187. doi: 10.1016/j.bbcan.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Okines A, Cunningham D, Chau I. Targeting the human EGFR family in esophagogastric cancer. Nat Rev Clin Oncol. 2011;8:492–503. doi: 10.1038/nrclinonc.2011.45. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7:493–507. doi: 10.1038/nrclinonc.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AD, Der CJ. The dark side of Ras: regulation of apoptosis. Oncogene. 2003;22:8999–9006. doi: 10.1038/sj.onc.1207111. [DOI] [PubMed] [Google Scholar]
- Palmero I, Pantoja C, Serrano M. p19ARF links the tumour suppressor p53 to Ras. Nature. 1998;395:125–126. doi: 10.1038/25870. [DOI] [PubMed] [Google Scholar]
- Zindy F, Eischen C, Randle D, Kamijo T, Cleveland J, Sherr C, et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Stanchina E, McCurrach ME, Zindy F, Shieh S-Y, Ferbeyre G, Samuelson AV, et al. E1A signaling to p53 involves the p19ARF tumour suppressor. Genes Dev. 1998;12:2434–2442. doi: 10.1101/gad.12.15.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Weber JD, Taylor LJ, Roussel MF, Sherr CJ, Bar-Sagi D. Nucleolar Arf sequesters Mdm2 and activates p53. Nat Cell Biol. 1999;1:20–26. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- Pantoja C, Serrano M. Murine fibroblasts lacking p21 undergo senescence and are resistant to transformation by oncogenic Ras. Oncogene. 1999;18:4974–4982. doi: 10.1038/sj.onc.1202880. [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang XD, Lapi E, Sullivan A, Jia W, He YW, et al. Autophagic activity dictates the cellular response to oncogenic RAS. Proc Natl Acad Sci USA. 2012;109:13325–13330. doi: 10.1073/pnas.1120193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Lapi E, Sullivan A, Ratnayaka I, Goldin R, Hay R, et al. SUMO-modified nuclear cyclin D1 bypasses Ras-induced senescence. Cell Death Differ. 2011;18:304–314. doi: 10.1038/cdd.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi D, Samuels Y, Jin B, Duraisingham S, Crook T, Lu X. ASPP1 and ASPP2: common activators of p53 family members. Mol Cell Biol. 2004;24:1341–1350. doi: 10.1128/MCB.24.3.1341-1350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels-Lev Y, O'Connor DJ, Bergamaschi D, Trigiante G, Hsieh JK, Zhong S, et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell. 2001;8:781–794. doi: 10.1016/s1097-2765(01)00367-7. [DOI] [PubMed] [Google Scholar]
- Bergamaschi D, Samuels Y, O'Neil NJ, Trigiante G, Crook T, Hsieh JK, et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet. 2003;33:162–167. doi: 10.1038/ng1070. [DOI] [PubMed] [Google Scholar]
- Bergamaschi D, Samuels Y, Sullivan A, Zvelebil M, Breyssens H, Bisso A, et al. iASPP preferentially binds p53 proline-rich region and modulates apoptotic function of codon 72-polymorphic p53. Nat Genet. 2006;38:1133–1141. doi: 10.1038/ng1879. [DOI] [PubMed] [Google Scholar]
- Hsieh JK, Fredersdorf S, Kouzarides T, Martin K, Lu X. E2F1-induced apoptosis requires DNA binding but not transactivation and is inhibited by the retinoblastoma protein through direct interaction. Genes Dev. 1997;11:1840–1852. doi: 10.1101/gad.11.14.1840. [DOI] [PubMed] [Google Scholar]
- Fogal V, Kartasheva NN, Trigiante G, Llanos S, Yap D, Vousden KH, et al. ASPP1 and ASPP2 are new transcriptional targets of E2F. Cell Death Differ. 2005;12:369–376. doi: 10.1038/sj.cdd.4401562. [DOI] [PubMed] [Google Scholar]
- Cong W, Hirose T, Harita Y, Yamashita A, Mizuno K, Hirano H, et al. ASPP2 regulates epithelial cell polarity through the PAR complex. Curr Biol. 2010;20:1408–1414. doi: 10.1016/j.cub.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Sottocornola R, Royer C, Vives V, Tordella L, Zhong S, Wang Y, et al. ASPP2 binds Par-3 and controls the polarity and proliferation of neural progenitors during CNS development. Dev Cell. 2010;19:126–137. doi: 10.1016/j.devcel.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Tidow H, Andreeva A, Rutherford TJ, Fersht AR. Solution structure of ASPP2 N-terminal domain (N-ASPP2) reveals a ubiquitin-like fold. J Mol Biol. 2007;371:948–958. doi: 10.1016/j.jmb.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ngo VN, Marani M, Yang Y, Wright G, Staudt LM, et al. Critical role for transcriptional repressor Snail2 in transformation by oncogenic RAS in colorectal carcinoma cells. Oncogene. 2010;29:4658–4670. doi: 10.1038/onc.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajee M, Tarutani M, Deng H, Cai T, Khavari PA. Epidermal Ras blockade demonstrates spatially localized Ras promotion of proliferation and inhibition of differentiation. Oncogene. 2002;21:1527–1538. doi: 10.1038/sj.onc.1205287. [DOI] [PubMed] [Google Scholar]
- Naumovski L, Cleary ML. The p53-binding protein 53BP2 also interacts with Bc12 and impedes cell cycle progression at G2/M. Mol Cell Biol. 1996;16:3884–3892. doi: 10.1128/mcb.16.7.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron AM, Ludwig RL, Vousden KH. Cytoplasmic ASPP1 inhibits apoptosis through the control of YAP. Genes Dev. 2010;24:2430–2439. doi: 10.1101/gad.1954310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon Y, Ofir-Rosenfeld Y, Yabuta N, Lapi E, Nojima H, Lu X, et al. The Lats2 tumor suppressor augments p53-mediated apoptosis by promoting the nuclear proapoptotic function of ASPP1. Genes Dev. 2010;24:2420–2429. doi: 10.1101/gad.1954410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima M, Sano K, Morisada T, Murakami K, Rossant J, Suda T. Lymphatic vessel assembly is impaired in Aspp1-deficient mouse embryos. Dev Biol. 2008;316:149–159. doi: 10.1016/j.ydbio.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Vives V, Su J, Zhong S, Ratnayaka I, Slee E, Goldin R, et al. ASPP2 is a haploinsufficient tumor suppressor that cooperates with p53 to suppress tumor growth. Genes Dev. 2006;20:1262–1267. doi: 10.1101/gad.374006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa KM, Acoba JD, Chen D, Gay J, Lee H, Beemer K, et al. Apoptosis-stimulating protein of p53 (ASPP2) heterozygous mice are tumor-prone and have attenuated cellular damage-response thresholds. Proc Natl Acad Sci USA. 2009;106:4390–4395. doi: 10.1073/pnas.0809080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, et al. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- Navarro P, Valverde AM, Benito M, Lorenzo M. Activated Ha-ras induces apoptosis by association with phosphorylated Bcl-2 in a mitogen-activated protein kinase-independent manner. J Biol Chem. 1999;274:18857–18863. doi: 10.1074/jbc.274.27.18857. [DOI] [PubMed] [Google Scholar]
- Fukasawa K, Vande Woude GF. Synergy between the Mos/mitogen-activated protein kinase pathway and loss of p53 function in transformation and chromosome instability. Mol Cell Biol. 1997;17:506–518. doi: 10.1128/mcb.17.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater CA, Bartle LM, Dionne CA, Littlewood TD, Goldmacher VS. Induction of apoptosis by tamoxifen-activation of a p53-estrogen receptor fusion protein expressed in E1A and T24 H-ras transformed p53−/− mouse embryo fibroblasts. Oncogene. 1996;13:739–748. [PubMed] [Google Scholar]
- Nikiforov MA, Hagen K, Ossovskaya VS, Connor TM, Lowe SW, Deichman GI, et al. p53 modulation of anchorage independent growth and experimental metastasis. Oncogene. 1996;13:1709–1719. [PubMed] [Google Scholar]
- Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- Pomerantz J, Schreiber-Agus N, Liegeois NJ, Silverman A, Alland L, Chin L, et al. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- Ferbeyre G, de Stanchina E, Querido E, Prives C, Lowe SW. PML is induced by oncogenic ras and promotes premature senescence. Genes Dev. 2000;14:2015–2027. [PMC free article] [PubMed] [Google Scholar]
- Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, et al. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. Embo J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.