Abstract
Objectives
Although serotonergic mechanisms have been implicated in pathological gambling (PG), no ligand-based imaging studies have assessed serotonin receptors in individuals with PG. Given its role in substance addictions and its abundance in brain regions implicated in PG, we evaluated serotonin 1B receptors (5-HT 1BRs) in PG.
Methods
Ten medication-free subjects with PG (mean ± SD age = 36.3 ± 9.4 years, nine men) and ten control comparison (CC) subjects (mean ± SD age = 35.8 ± 9.9 years, nine men) underwent [11C]P943 positron emission scanning on a high resolution research tomograph.
Results
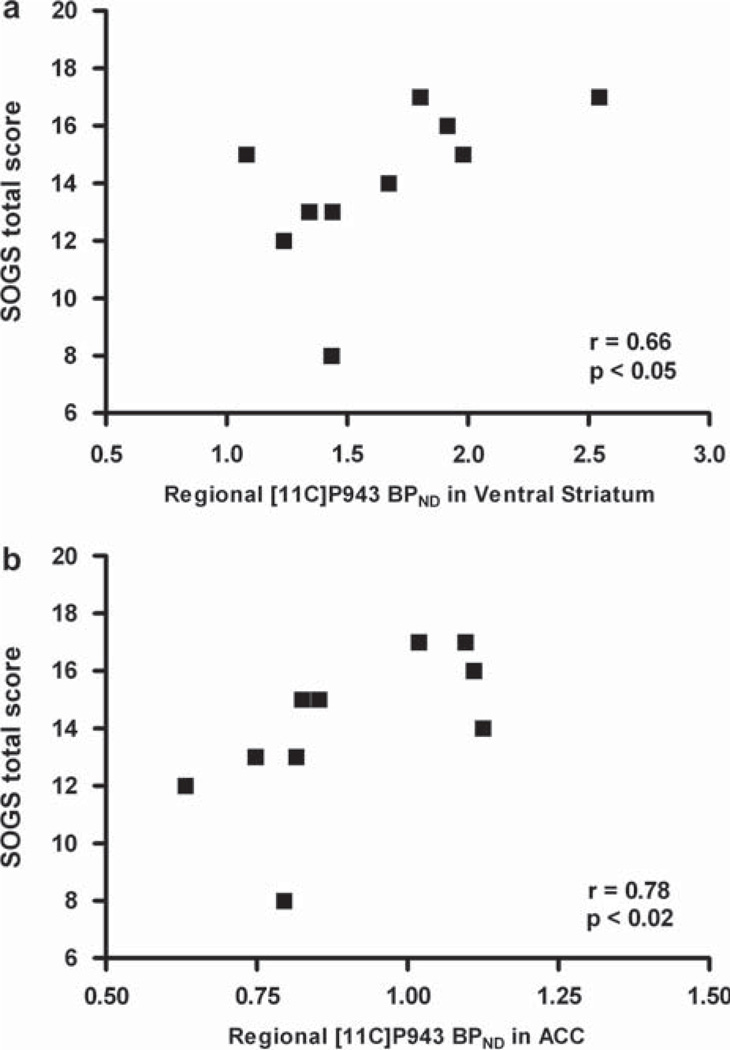
5-HT 1BR BPND values were similar in PG and CC subjects (P > 0.1). Among PG subjects, scores on the South Oaks Gambling Screen (SOGS) correlated positively with 5-HT 1BR BPND values in the ventral striatum (r = 0.66; P = 0.04), putamen (r = 0.67; P = 0.03) and anterior cingulate cortex (r = 0.73; P = 0.02).
Conclusions
These findings provide the first evidence that PG severity in humans is linked to increased levels of 5-HT 1BRs in regions previously implicated in functional neuroimaging studies of PG. These findings indicate a potential role for serotonergic function in the ventral striatum and anterior cingulate cortex contributing to problem gambling severity and warrant further studies to investigate whether numbers of available 5-HT 1BRs might represent a vulnerability factor for PG or develop in relationship to problem gambling.
Keywords: Pathological gambling, brain imaging, serotonin 1B receptor, positron emission tomography, South Oaks Gambling Screen
Introduction
Pathological gambling (PG), a disorder with prevalence estimates of about 1%, shares clinical and biological features with substance addictions (Potenza 2008). However, in comparison to substance addictions, less is known regarding the neurobiology of PG as animal models of the disorder have only recently been developed and comparably few human investigations have been performed (Potenza 2009). An improved understanding of the neurobiology of PG could lead to the development of improved treatment strategies for PG, a significant need as no medications are currently approved by the Food and Drug Administration for the disorder.
Genetic, clinical, neural and neurochemical data link PG with substance addictions like alcohol dependence. Shared genetic contributions partly underlie the co-occurrence of these two disorders, opioid antagonists have been found to be helpful in randomized clinical trials for each disorder, and individuals with each disorder demonstrate relatively diminished ventral striatal activation during reward anticipation or simulated gambling (Potenza 2008; Beck et al. 2009). Studies of PG and alcohol dependence have implicated ventral striatal and anterior cingulate function in each disorder (Potenza et al. 2003; Reuter et al. 2005; Beck et al. 2009). Individuals with alcohol dependence and PG each have shown low levels of the serotonin metabolite 5-hydroxy-indole acetic acid in some but not all studies (Bergh et al. 1997; Nordin and Eklundh 1999), and the serotonergic ligand meta-chlorophenyl piperazine (m-CPP), a drug which agonizes multiple 5-HTRs including the 5-HT 1BR, similarly generates a more positive subjective response in individuals with these disorders than in those without (Potenza 2008). Together, these findings suggest a possible role for 5-HT 1BR function in PG.
5-HT 1BR function in cortico-limbic regions has been implicated in other psychiatric disorders, including ones characterized by mood dysregulation, stress vulnerability and impaired impulse control. Individuals with major depression have shown relatively diminished 5-HT 1BR binding potential in the ventral striatum (Murrough et al. 2011) and individuals with post-traumatic stress disorder (PTSD), particularly those with co-occurring major depression, have shown relatively diminished 5-HT 1BR binding potentials in multiple cortico-limbic regions, including the anterior cingulate cortex (Murrough et al. in press). In the latter study, 5-HT 1BR binding potential in the anterior cingulate cortex correlated with age of first trauma, suggesting that markers associated with illness severity in psychiatric disorders might correlate with regional 5-HT 1BR binding potentials. In contrast to PTSD and major depression, elevated 5-HT 1BR binding potential was observed in the ventral striatum in alcohol dependence (Hu et al. 2010). These findings indicate that 5-HT 1BR function may contribute in differential fashions to specific psychiatric disorders. Alcohol dependence has multiple similarities with PG, including with respect to biological and behavioral responses to m-CPP (Potenza 2008) and in demonstrating shared genetic and environmental contributions (Slutske et al. 2000). The findings linking the biological underpinnings of alcohol dependence and PG may be particularly relevant to men as participants in these studies were predominantly or solely male and PG in women has shown stronger links to trauma (Petry et al. 2005a), gambling to escape dysphoria (Blanco et al. 2006) and internalizing disorders like depression (Desai and Potenza 2008).
In the current study, we assessed 5-HT 1BRs in a largely male cohort of individuals with PG. Given the data summarized above and findings that: (1) show expression of the 5-HT 1BR in the ventral striatum and anterior cingulate (Varnas et al. 2005); (2) similarly link impulsivity with both ventral striatal and anterior cingulate responses during reward processing in alcohol dependence (Beck et al. 2009); (3) implicate anterior cingulate cortex and ventral striatum dysfunction in PG (Potenza 2008), and (4) link gambling severity with activations in brain regions including the ventral striatum (Reuter et al. 2005), we hypothesized that: (1) PG subjects (versus control comparison (CC) subjects) would show elevated [11C]P943 BPND measures in ventral striatum and anterior cingulate cortex; and (2) [11C]P943 BPND measures in these regions would correlate positively with South Oaks Gambling Screen scores in the PG group.
Methods and materials
Subjects
Ten PG subjects (mean ± SD age = 36.3 ± 9.4 years, nine men, seven white) and 10 matched CC subjects (mean ± SD age = 35.8 ± 9.9 years, nine men, eight white) provided written informed consent for this study that was approved by the Yale Human Investigation Committee. Subjects were recruited through advertising and were evaluated via Structured Clinical Interview for DSM-IV (First et al. 1995) and Structured Clinical Interview for Pathological Gambling (Grant 2004). Subjects were medication-free. Three PG and two CC were current daily tobacco smokers. With the exception of PG for the PG group, no subjects met criteria for any current psychiatric diagnoses. No CC subjects met criteria for any lifetime psychiatric diagnoses. One PG subject met criteria for remitted panic disorder without agarophobia. With the exception of tobacco, no PG subjects met lifetime dependence criteria for any substance. Four PG subjects met criteria for remitted alcohol abuse, one of whom also abused cannabis previously. Subjects provided written, informed consent to participate in the protocol that was approved by the Yale Human Investigations Committee. Evaluations included physical examinations, electrocardiograms, urine toxicologies and other standard laboratory tests, as described previously (Hu et al. 2010). Individuals were excluded for histories of medical or neurological illnesses, head trauma with loss of consciousness, or metal in body precluding MRI. Subjects (all PG and a minority of CC subjects (n = 2)) completed the South Oaks Gambling Screen (Lesieur and Blume 1987), a widely used screening instrument with a range extending from 0 to 20 and a score of 5 or more suggesting pathological gambling (Lesieur and Blume 1987) and whose German correlate has been used previously as a measure of problem gambling severity in neuroimaging analyses (Reuter et al. 2005).
Imaging
Imaging methodologies followed those employed in our prior work (Hu et al. 2010). MRI scans for localization and detection of structural anomalies were performed with a 3T Trio Siemens magnet. PET imaging procedures involved indwelling intravenous (IV) catheter placement and a transmission scan with a 137Cs point source. Subsequent emission scans were obtained for 120 min at rest with a single IV injection of high specific activity [11C]P943, a selective 5-HT 1BR antagonist radiotracer (Nabulsi et al. 2010), using a high-resolution research tomography (HHRT) scanner (207 slices, resolution at < 3 mm full-width-at-half-maximum in 3-D acquisition mode). Dynamic scan data were reconstructed with corrections (attenuation, normalization, scatter, randoms, and dead time). Motion-correction was performed by co-registering each reconstructed frame to an early summed image (0 – 10 min after injection) with 6-parameter mutual information algorithm and FMRIB ' s Linear Image Registration Tool (FLIRT, FSL 3.2, Analysis Group, FMRIB, Oxford, UK).
For each individual, a summed image (0 – 10 min following injection) derived from motion-corrected PET data was co-registered with their MR image, which then was registered (12-parameter affine transformation) to an MR template in Montreal Neurological Institute space. The regions of interest for the ventral striatum, involving the nucleus accumbens and globus pallidum, and anterior cingulate cortex, involving its subgenual and pregenual segments, were taken from the template (Anatomical Automatic Labeling (Tzourio-Mazoyer et al. 2002) for SPM2 [http://www.fil.ion.ucl.ac.uk/spm/software/spm2/]) and applied to the PET data to generate time-activity data. Similarly defined control comparison regions included other striatal regions (putamen, caudate) and cortical regions (prefrontal and occipital cortices). Pixel-by-pixel analysis was done using a multilinear reference tissue model (Ichise et al. 2003) to produce images of BPND (Innis et al. 2007). The interpretation of BPND is fND × Bavail/Kd, where fND is the tracer-free fraction in a region without specific binding, Bavail is the unoccupied receptor concentration, and Kd is dissociation equilibrium constant of the tracer. The cerebellum was used as the reference region as it is virtually devoid of 5-HT 1BRs (Varnas et al. 2005) and to allow comparability across studies (Hu et al. 2010). Assuming there is no change in affinity or non-specific binding between subject groups, changes in BPND were interpreted as changes in receptor concentration. The BPND values from multilinear reference tissues models have yielded highly comparable results to those obtained with arterial input functions (Gallezot et al. 2010).
Statistical analyses
Between subject groups, unpaired two-tailed t-tests compared clinical and demographic variables and a Mann – Whitney U-test compared BPND values. Relationships between BPND values and SOGS scores were investigated using Spearman correlation coefficients. Analyses were not corrected for multiple comparisons given the specific hypotheses being tested.
Results
Demographic and smoking measures did not differ between the PG and CC groups (Table I). Individuals with PG had mean(SD) SOGS scores of 14.00(2.71). CC subjects had SOGS scores of zero. PET injection measures (injected dose, specific activity, and injected mass) did not differ between groups (each P > 0.05). Between-group [11C]P943 BPND values did not differ (each P > 0.05) in ventral striatum (CC: 1.64 ± 0.27; PG: 1.64 ± 0.43) and anterior cingulate (CC: 0.94 ± 0.14; PG: 0.90 ± 0.17) nor in comparison regions of putamen (CC: 0.87 ± 0.07; PG: 0.88 ± 0.20), caudate (CC: 0.50 ± 0.15; PG: 0.50 ± 0.23), frontal cortex (CC: 0.75 ± 0.16; PG: 0.73 ± 0.15) or occipital cortex (CC: 0.76 ± 0.08; PG: 0.81 ± 0.10). In PG subjects, SOGS scores correlated positively with BPND values in ventral striatum (r = 0.66; P < 0.05) and anterior cingulate (r = 0.78; P < 0.02) (Figure 1) as well as in putamen (r = 0.67; P < 0.05), but not in caudate (r = 0.45; P > 0.1) or frontal (r = 0.52; P > 0.1) or occipital (r = 0.57; P = 0.09) cortices.
Table I.
Demographic and clinical characteristics of PG and CC subjects.
| Group | PG (n = 10) | CC (n = 10) | P |
|---|---|---|---|
| Age, years (mean ± SD) | 36.3 ± 9.44 | 35.80 ± 9.875 | 0.91 |
| Gender, male (n; %) | 9; 90% | 9; 90% | 1 |
| Race, white (n; %) | 7; 70% | 8; 80% | 0.62 |
| Current tobacco smoker, yes (n;%) | 3; 30% | 2; 20% | 0.62 |
PG, pathological gambling; CC, control comparison.
Figure 1.
Correlations between 5-HT 1BR BPND values and SOGS scores in PG subjects in the ventral striatum (a) and anterior cingulate (b).
Discussion
Our a priori hypotheses in this investigation of 5-HT 1BRs in PG were partially supported. The hypothesis that 5-HT 1BR BPND values would differ in PG and CC subjects was not observed for either the ventral striatum or anterior cingulate. However, the hypothesis that 5-HT 1BR BPND values within these regions would correlate positively with SOGS scores, reflecting problem gambling severity, was observed in both regions. Additionally, SOGS scores also correlated with 5-HT 1BR BPND values in the putamen but not the caudate or occipital or frontal cortices, suggesting specificity to regions of the striatum and anterior cingulate. Interpretations and clinical implications are discussed below.
No significant between-group differences in 5-HT 1BR BPND values were observed in the PG and CC groups. This result contrasts with a previous finding in alcohol dependent subjects in whom elevated 5-HT 1BR BPND values were seen (Hu et al. 2010). Both similarities and differences between alcohol dependence and PG have been reported. For example, alcohol dependent and problem gambling subjects show similar impairments in risky decision-making but differences in cognitive function (Lawrence et al. 2009), and the extent to which 5-HT 1BRs might contribute to these processes in PG and alcohol dependence warrants additional investigation.
The observation of no between-group differences in 5-HT 1BR BPND values observed in the PG and CC groups in the ventral striatum and anterior cingulate cortex suggests that 5-HT 1BR availability in these regions is not a defining feature of the disorder. However, significant inter-individual variability was observed in the PG group, particularly with respect to the ventral striatal findings. These findings are consistent with the notion that, like many psychiatric conditions, PG is a heterogeneous disorder. Several subtypes of PG have been proposed, with different groups characterized by high impulsivity and sensation-seeking and others by emotional vulnerability who may be motivated to gamble to escape from distress or dysphoria (Blaszczynski and Nower 2002). Such typologies may explain PG ’ s frequent co-occurrence with both externalizing disorders like substance dependences and internalizing disorders like depression (Petry et al. 2005b). It is tempting to speculate that impulsive, sensation-seeking individuals with PG may show elevated 5-HT 1BR BPND values, akin to those with alcohol dependence (Hu et al. 2010), and emotionally vulnerable individuals with PG may show depressed 5-HT 1BR BPND values, akin to those with major depression (Murrough et al. 2011). The latter group may be particularly important to consider with respect to sex differences as women with PG are more likely than men with PG to acknowledge gambling for negative reinforcement motivations, consistent with sex differences in the relationships between problem/PG and depression (Blanco et al. 2006; Desai and Potenza 2008). Further study is needed to investigate these possibilities.
Although serotonergic differences have been observed in PG and CC groups (Potenza 2008), drugs that influence serotonergic function have shown in randomized clinical trials either negative results (e.g., antagonists like olanzapine) or mixed findings (e.g., for serotonin reuptake inhibitors like paroxetine and fluvoxamine) (Brewer et al. 2008). It is tempting to speculate that some variability in treatment response to SRIs in PG might relate to differences in 5-HT 1BR function, although direct investigation of this idea is needed. Such investigations seem timely given current interest in developing gene therapies that alter 5-HT 1BR function in the ventral striatum for depression based on findings that in the nucleus accumbens region of the ventral striatum, increasing levels of p11, a 5-HT 1BR-binding protein that enhances cell surface location of the 5-HT 1BR, have been associated with remission of depressive features (Alexander et al. 2010; Chen et al. 2010). It is possible that drugs that influence 5-HT 1BR function may operate to indirectly modulate activity in the ventral striatum. As indirect modulation of dopamine function in the striatum has been proposed to underlie the effects of opioid antagonists that have in multiple placebo-controlled trials shown efficacy in the treatment of PG (Wareham and Potenza 2010), the development of tolerable drugs targeting the 5-HT 1BR may represent a novel approach in the treatment of PG. However, variability in 5-HT 1BR function as might be related to different subtypes of PG warrant consideration in this process.
The significant inter-individual variation in 5-HT 1BR BPND values in the PG group provided a range that facilitated correlations with SOGS scores. The mean SOGS score in the PG group was similar to that reported in other PG samples and reflects significant gambling problems (Potenza et al. 2003). The positive correlation between 5-HT 1BR BPND values and SOGS scores in the ventral striatum differs from the inverse correlation observed between scores on a German analog of the SOGS and ventral striatal activation during simulated gambling in PG subjects (Reuter et al. 2005). Together, these findings raise the question whether the number of 5-HT 1BRs might correlate inversely with gambling-related activation of the ventral striatum, a supposition consistent with a role for 5-HT 1BRs in the nucleus accumbens in regulating mesolimbic pathways (Ferguson et al. 2009). Given the influence of stress on 5-HT 1BR modulation of mesolimbic function (Ferguson et al. 2009), future studies should investigate the extent to which 5-HT 1BR function relates to stress and trauma in PG (Petry et al. 2005a; Elman et al. 2010). Other intermediary phenotypes that are relevant to PG, such as those relating to impulsivity and mood state or tendencies, warrant investigation in future studies to determine their relationship with 5-HT 1BR function in PG.
As with the ventral striatum (Potenza 2008), relatively diminished activation of the anterior cingulate cortex has been observed in PG subjects when viewing gambling-related stimuli (Potenza et al. 2003). Thus, the similar patterns of positive correlations between 5-HT 1BR BPND values and SOGS scores in the ventral striatum and anterior cingulate are not surprising. The anterior cingulate cortex has been implicated in phenomena relevant to PG including emotional and motivational processing and cognitive control. Given a role for 5-HT 1BR expression in the anterior cingulate in depression (Svenningsson et al. 2006) and strong biological relationships between depression and PG (Potenza et al. 2005) and negative mood states and gambling urges (Thomsen et al. 2009), future research should investigate the relationship between 5-HT 1BRs in the anterior cingulate and affective dysregulation in PG. Also, given data associating p11 levels in the ventral striatum with remission of depressive features, studies should investigate the relationship between ventral striatal 5-HT 1BRs and depressive symptomatology in PG. However, the nature of the relationship between PG, major depression, 5-HT 1BRs, and ventral striatal function appears complex given that increased numbers of 5-HT 1BRs appear associated with greater problem gambling severity in the current study and improved mood in other studies (Alexander et al. 2010; Chen et al. 2010). Given the reduced 5-HT 1BR BPND values observed in major depression (Murrough et al. 2011), the frequent co-occurrence of PG and major depression (Potenza et al. 2005) and the clinical implications of the relationship, additional studies should clarify the nature of a potential role for 5-HT 1BRs in individuals dually diagnosed with PG and major depression. Additionally, therapies that target mood improvement through elevating ventral striatal 5-HT 1BRs should consider the potential influence on problem gambling and other addictive behaviours like alcohol abuse or dependence (Hu et al. 2010).
5-HT 1BRs influence GABAergic and dopaminergic neurotransmission in the mesolimbic pathway (Yan and Yan 2001a,b). For example, infusion of a 5-HT 1BR agonist into the nucleus accumbens increases local dopamine concentration in a dose-dependent fashion (Yan and Yan 2001a). Thus, in individuals with PG, increased availability of 5-HT 1BRs in the ventral striatum could increase levels of ventral striatal dopamine. Increased ventral striatal dopamine release has been reported during performance of gambling tasks both in PG as compared to CC subjects losing money and, amongst individuals with Parkinson ’ s disease, in those with PG as compared to those without PG (Steeves et al. 2009; Linnet et al. 2010). Future studies should investigate the interaction between serotonin and dopamine systems in PG, particularly as also data suggest complementary roles for the two neurotransmitters in gambling behaviours (Zeeb et al. 2009; Campbell-Meiklejohn et al. 2011).
The current study has limitations including small samples and co-occurring tobacco smoking. Given the small sample of PG subjects, statements relating to inter-individual variability should be considered cautiously. Although the groups did not differ on smoking status, future studies should investigate 5-HT 1BRs in tobacco smoking. Additionally, SOGS scores were not collected on most CC subjects as we had envisioned the SOGS scores and their neurobiological and clinical correlates to be mainly relevant to the PG group. The existing data and our prior experience with CC imaging subjects denying heavy gambling problems suggest that most if not all CC subjects would score zero. Nonetheless, future studies should assess gambling severity using the same structured scales in both PG and CC subjects. Despite these limitations, the current study represents the first to investigate with radiotracers 5-HTRs in PG and provides important initial data with respect to a role for 5-HT 1BR function in PG.
Acknowledgements
Support was provided by the following Grants: National Institutes of Health grants R21 AA018329, RL1 AA017539, RL1 AA017540, RC1 DA028279, P20 DA 027844, a Center of Excellence in Gambling Research Award from the National Center for Responsible Gaming, the Research Council of Norway Division for Science, the Department of Psychology at the University of Oslo in Norway, the VA National Center for Posttraumatic Stress Disorder at the West Haven VA Connecticut Clinical Neuroscience Division, and the Veterans Integrated Service Network 1 Mental Illness Research, Education, and Clinical Center (MIRECC). The authors alone are responsible for the writing and the content of this manuscript.
Footnotes
Statement of Interest
The authors report that they have no financial conflicts of interest with respect to the content of this manuscript. Dr Potenza has received financial support or compensation for the following: Dr Potenza has consulted for and advised Boehringer Ingelheim; has consulted for and has financial interests in Somaxon; has received research support from the National Institutes of Health, Veteran ' s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Forest Laboratories, Ortho-McNeil, Oy-Control/Biotie and Glaxo-SmithKline pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for law offices and the federal public defender ' s office in issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
References
- Alexander B, Warner-Schmidt J, Eriksson T, Tamminga C, Arango-Lievano M, Ghose S, et al. Reversal of depressed behaviors in mice by p11 gene therapy in the nucleus accumbens. Sci Transl Med. 2010;2(54):54ps51. doi: 10.1126/scitranslmed.3001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, et al. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Bergh C, Eklund T, Sodersten P, Nordin C. Altered dopamine function in pathological gambling. Psychol Med. 1997;27:473–475. doi: 10.1017/s0033291796003789. [DOI] [PubMed] [Google Scholar]
- Blanco C, Hasin DS, Petry N, Stinson FS, Grant BF. Sex differences in subclinical and DSM-IV pathological gambling: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2006;36:943–953. doi: 10.1017/S0033291706007410. [DOI] [PubMed] [Google Scholar]
- Blaszczynski A, Nower L. A pathways model of problem and pathological gambling. Addiction. 2002;97:487–499. doi: 10.1046/j.1360-0443.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Grant JE, Potenza MN. The treatment of pathologic gambling. Addict Disord Treat. 2008;7:1–14. [Google Scholar]
- Campbell-Meiklejohn DK, Wakeley J, Herbert V, Cook J, Scollo P, Kar Ray M, et al. Serotonin and dopamine play complementary roles in gambling to recover losses. Neuropsychopharmacology. 2011;36:402–410. doi: 10.1038/npp.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Twyman R, Manji HK. p11 and gene therapy for severe psychiatric disorders: a practical goal? Sci Transl Med. 2010;2(54):54–76. doi: 10.1126/scitranslmed.3001754. [DOI] [PubMed] [Google Scholar]
- Desai RA, Potenza MN. Gender differences in the associations between problem gambling and psychiatric disorders. Soc Psychol Psychiatr Epi. 2008;43:173–183. doi: 10.1007/s00127-007-0283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Tschibelu E, Borsook D. Psychosocial stress and its relationship to gambling urges in individuals with pathological gambling. Am J Addictions. 2010;19:332–339. doi: 10.1111/j.1521-0391.2010.00055.x. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Sandygren NA, Neumeier JF. Pairing mild stress with increased serotonin-1B receptor expression in the nucleus accumbens increases susceptability to amphetamine. Eur J Neurosci. 2009;30:1576–1584. doi: 10.1111/j.1460-9568.2009.06933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. Patient Edition. Washington, DC: American Psychiatric Press Inc.; 1995. [Google Scholar]
- Gallezot JD, Nabulsi N, Neumeister A, Planeta-Wilson B, Williams JW, Singhal T, et al. Kinetic modeling of the serotonin (5-HT1B) receptor radioligand [11C]P943 in humans. J Cereb Blood Flow Metab. 2010;30:196–210. doi: 10.1038/jcbfm.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Steinberg MA, Kim SW, Rounsaville BJ, Potenza MN. Preliminary validity and reliability testing of a structured clinical interview for pathological gambling (SCI-PG) Psychiatry Res. 2004;128:79–88. doi: 10.1016/j.psychres.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Hu J, Henry S, Gallezot J-D, Ropchan J, Neumaier JF, Potenza MN, et al. Serotonin 1B receptor imaging in alcohol dependence. Biol Psychiatry. 2010;63:300–303. doi: 10.1016/j.biopsych.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Problem gamblers share deficits in impulsive decision-making with alcohol dependent individuals. Addiction. 2009;104:1006–1015. doi: 10.1111/j.1360-0443.2009.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): a new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144:1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Linnet J, Peterson E, Doudet DJ, Gjedde A, Moller A. Dopamine release in ventral striatum of pathological gamblers losing money. Acta Psychiatrica Scand. 2010;122:326–333. doi: 10.1111/j.1600-0447.2010.01591.x. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Henry S, Hu J, Gallezot J-D, Planeta-Wilson B, Neumaier JF, et al. Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder. Psychopharmacology. 2011;213:547–553. doi: 10.1007/s00213-010-1881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Czermak C, Henry S, Nabulsi N, Gallezot J-D, Gueorguieva R, et al. Age at first trauma is associated with serotonin 1B receptor reductions in posttraumatic stress disorder. Arch Gen Psychiatry. in press. [Google Scholar]
- Nabulsi N, Huang Y, Weinzimmer D, Ropchan J, Frost JJ, Neumeister A, et al. High resolution imaging of brain 5 HT1B receptors in rhesus monkey using [11C]P943. Nuc Med Biol. 2010;37:205–214. doi: 10.1016/j.nucmedbio.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin C, Eklundh T. Altered CSF 5-HIAA disposition in pathologic male gamblers. CNS Spectrums. 1999;4:25–33. doi: 10.1017/s1092852900006799. [DOI] [PubMed] [Google Scholar]
- Petry NM, Steinberg KL Women’s Problem Gambling Research Center. Childhood maltreatment in male and female treatment-seeking pathological gamblers. Psychology Addict Behav. 2005a;19:226–229. doi: 10.1037/0893-164X.19.2.226. [DOI] [PubMed] [Google Scholar]
- Petry NM, Stinson FS, Grant BF. Co-morbidity of DSM-IV pathological gambling and other psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2005b;66:564–574. doi: 10.4088/jcp.v66n0504. [DOI] [PubMed] [Google Scholar]
- Potenza MN. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Phil Trans R Soc B. 2008;363:3181–3189. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN. The importance of animal models of decision-making, gambling and related behaviors: implications for translational research in addiction. Neuropsychopharmacology. 2009;34:2623–2624. doi: 10.1038/npp.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Steinberg MA, Skudlarski P, Fulbright RK, Lacadie CM, Wilber MK, et al. Gambling urges in pathological gamblers: An fMRI study. Arch Gen Psychiatry. 2003;60:828–836. doi: 10.1001/archpsyc.60.8.828. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Xian H, Shah K, Scherrer JF, Eisen SA. Shared genetic contributions to pathological gambling and major depression in men. Arch Gen Psychiatry. 2005;62:1015–1021. doi: 10.1001/archpsyc.62.9.1015. [DOI] [PubMed] [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Glascher J, Buchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Eisen S, True WR, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for pathological gambling and alcohol dependence in men. Arch Gen Psychiatry. 2000;57:666–674. doi: 10.1001/archpsyc.57.7.666. [DOI] [PubMed] [Google Scholar]
- Steeves TDL, Miyasaki J, Zurowski M, Lang AE, Pellecchia G, van Eimeren T, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132:1376–1385. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, et al. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- Thomsen KR, Callesen MB, Linnet J, Kringelbach ML, Moller A. Severity of gambling is associated with severity of depressive symptoms in pathological gamblers. Behav Pharmacol. 2009;20:527–536. doi: 10.1097/FBP.0b013e3283305e7a. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Varnas K, Hurd YL, Hall H. Regional expression of 5-HT1B receptor mRNA in the human brain. Synapse. 2005;56:21–28. doi: 10.1002/syn.20128. [DOI] [PubMed] [Google Scholar]
- Wareham JD, Potenza MN. Pathological gambling and substance use disorders. Am J Drug Alcohol Abuse. 2010;36:242–247. doi: 10.3109/00952991003721118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q-S, Yan S-E. Activation of 5-HT1B/1D receptors in the mesolimbic dopamine system increases dopamine release from the nucleus accumbens: a microdialysis study. Eur J Pharmacol. 2001a;418:55–64. doi: 10.1016/s0014-2999(01)00913-x. [DOI] [PubMed] [Google Scholar]
- Yan Q-S, Yan S-E. Serotonin-1B receptor-mediated inhibition of [3H]GABA release from rat ventral tegmental area slices. Eur J Neurochem. 2001b;79:914–922. doi: 10.1046/j.1471-4159.2001.00643.x. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacol. 2009;34:2329–2343. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]