Abstract
Impairment of cortical circuit function is increasingly believed to be central to the pathophysiology of schizophrenia (Sz). Such impairments are suggested to result in abnormal gamma band oscillatory activity observed in Sz patients, and likely underlie the psychosis and cognitive deficits linked to this disease. Development of improved therapeutic strategies to enhance functional outcome of Sz patients is contingent upon a detailed understanding of the mechanisms behind the cortical circuit development and maintenance. Convergent evidence from both Sz clinical and preclinical studies suggests impaired activity of a particular subclass of interneuron which expresses the calcium binding protein parvalbumin is central to the cortical circuit impairment observed. Here we review our current understanding of the Sz related cortical circuit dysfunction with a particular focus on the role of fast spiking parvalbumin interneurons in both normal cortical circuit activity and in NMDA receptor hypofunction models of the Sz disease state.
Keywords: Schizophrenia, Gamma oscillations, Interneurons, Parvalbumin, NMDAR, GAD67, Psychiatry
Introduction
Schizophrenia (Sz) is identified clinically by the appearance of positive symptoms (psychosis, hallucinations, paranoia) and negative symptoms (flat affect, impaired attention and motivation). However, deficits in fundamental cognitive processes (working memory, executive function) are currently believed to serve as the core feature of this disease. Cognitive deficits appear to be present for years prior to clinical diagnosis and are observed throughout the lifespan of Sz patients [1]. Due to the strong relationship between cognitive performance and functional outcome, these impairments represent the major determinant of the long-term disability associated with Sz [2]. Current Sz therapeutics, including both first and second generation antipsychotics, do not provide a cure for the disease, and fail to alleviate many of the symptoms [3]. Thus, improved therapies which better address all Sz symptoms are urgently required. Development of such novel treatments is contingent upon a detailed understanding of the cortical circuit abnormalities underlying the pathophysiology of this disease. Numerous genetic, developmental, and environmental factors are associated with this complex disorder [4, 5]. These factors can affect many aspects of cortical circuit development and function, as assessed by different neurophysiological paradigms. Here we focus specifically on gamma band oscillation (GBO) abnormalities observed clinically in Sz. We propose that GBO abnormalities serve as useful markers of cortical circuit dysfunction which can be used to derive novel treatments for executive function deficits in Sz. We discuss the convergent evidence from preclinical and clinical experiments which suggests that impaired inhibition mediated by fast spiking parvalbumin-positive interneurons is central to these abnormalities. GBO abnormalities can be modeled in animal studies using NMDA receptor blockade, allowing a translational model for the development of therapeutic agents targeting this aspect of the disease.
Gamma Band Oscillation Abnormalities are Associated with Sz Symptoms
Sz has been proposed to arise from a failure of the brain to properly integrate the activity of local and distributed neuronal circuits [6]. Neuronal oscillations, particularly those in the gamma frequency range (30–80 Hz) have been suggested to support the integration of such activity [7, 8]. Further, GBO activity has been suggested to be critical for a number of cognitive tasks, including selective attention, working memory, long term memory, and motor control [9–11]. Interestingly, clinical studies from our group [12–14] and others [10] have revealed impairments in GBO activity in Sz patients. Thus, these abnormalities have been suggested to underlie both the psychosis and impairments in higher cognitive function associated with this disease [9]. In fact, deficits in cognitive control observed in Sz patients are correlated with deficits in GBO activity [1, 15]. Sz patients display aberrant recruitment of cortical circuits and diminished GBO activity in response to cognitive and sensory tasks [16]. Higher demand for cognitive control is normally associated with increased induced GBO activity in the prefrontal cortex [15]. However, such demand-related modulation of GBO is absent in Sz-patients.
Convergent research suggests that the Sz-related GBO abnormalities arise from impairments of the cortical circuitry responsible for their generation and maintenance. As GBO activity is crucial for cognition, it is important to understand the mechanisms behind the generation and maintenance of this activity. As such, GBO activity offers an increasingly intriguing target for Sz research, representing a central aspect of the underlying pathophysiology of this disease, and may provide a sensitive biomarker for assessing the integrity of local circuit function [17].
A Cortical Circuit Consisting of Excitatory Pyramidal Neurons and Inhibitory Fast-Spiking Interneurons Underlies GBO Activity
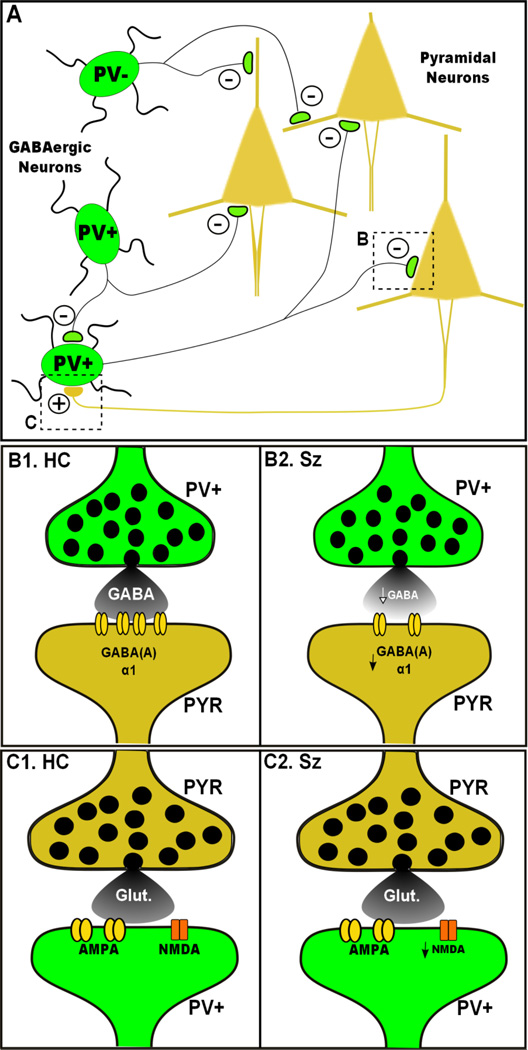
Cortical GBO activity is primarily governed by the interaction between excitatory pyramidal cells (PYR), and inhibitory GABAergic interneurons (INT) (see Fig. 1). Synaptic inhibition mediated by various inhibitory GABAergic INT is crucially important for the regulation of PYR activity, and is central to the generation and maintenance of neural oscillations [18, 19]. As described by Whittington and colleagues [20], inhibitory output from INT onto PYR defines a window of time in which the excitatory neurons are capable of firing, allowing entrainment/synchronization of their activity. Further, phasic recurrent excitatory output from PYR onto INT is believed to be necessary for the generation of GBO [21]. The rate of this synchronous activity is in large part determined by the time constants of the synaptic currents which mediate both the inhibitory (GABA) and excitatory (NMDA, AMPA) synaptic currents generated between the PYR and INT in this circuit [19, 22].
Fig. 1.
Simplified model of cortical circuitry involved in generation of GBO and Sz. a) The PrL cortical circuit consist principally of excitatory PYR (brown) and inhibitory GABAergic INT (green). Inhibitory drive generated by INT plays an important role in the generation of oscillatory output. Fast spiking PV-Pos INT (PV+) form an interconnected network which generates GBO activity through synchronized inhibition of PYR. Recurrent excitatory glutamatergic synapses onto GABAergic INT are also important for synchronous neuronal activity. b) Compared to healthy controls (B1), inhibitory synaptic connections between FS/PV INT and PYR are impaired in Sz patients (B2). These impairments include reduced expression of GAD67, leading to GABA release, as well as altered GABA(A) α1 receptor expression. c) Glutamatergic inputs onto FS/PV INT are also impaired in Sz. Compared to healthy controls (C1), these synaptic connections show reduced NMDAR mediated input in Sz patients (C2)
Increasingly, Sz research has implicated impaired GABAergic neurotransmission as a central component of the pathophysiology of this disease [23]. Numerous subtypes of GABAergic INT have been characterized throughout the brain, based upon their anatomical, physiological, and molecular features [24, 25]. Such heterogeneity has made it difficult to explicitly define the role of these INT in cortical circuit activity. However, numerous studies have shown that a particular INT subtype, which have fast action potential firing (fast spiking; FS) and express the calcium binding protein parvalbumin (PV), are of critical importance for the generation and maintenance of GBO [26•, 27•, 28•, 29, 30, 31•]. FS/PV-INT display remarkably fast synaptic activation [32], enabling them to fire at a rate capable of entraining GBO activity. Further, these INT synapse perisomatically onto PYR, providing an increased ability of these INT to control PYR output [33]. In contrast, non-FS varieties of INT generally form synaptic connections on the distal dendrites of PYR, and are suggested to regulate dendritic integration and synaptic plasticity of excitatory inputs. Additionally, FS/PV INT are interconnected via chemical and electrical synapses allowing them to entrain rhythmic firing across a large network of INT [34, 35]. Together these characteristics seemingly endow FS/PV INT with an innate ability to generate and maintain GBO activity in the cortical circuit.
Several recent studies have taken advantage of newly developed optogenetic techniques to directly asses the role of FS/PV INT in the generation of GBO activity. Through selective expression of channelrhodopsin-2 (ChR2) in either FS/PV INT or PYR in the somatosensory cortex, Cardin et al. [27•], showed that direct rhythmic stimulation of FS/PV INT increased in vivo LFP power at the frequency of stimulation, but only at frequencies in the gamma frequency range (20–60 Hz), while gamma frequency stimulation of PYR did not increase LFP power. Additional work by Sohal et al. [31•] showed that in vivo optogenetic inhibition of PV INT suppressed evoked GBO activity in the prefrontal cortex (PFC). Together these findings directly demonstrate that FS/PV-INT can powerfully drive GBO activity in vivo.
Further complexity in defining the role of FS/PV INT in the cortical circuit is derived from the fact that they are divided into two distinct subtypes: basket cells, which provide perisomatic inhibitory input to PYR, and chandelier cells, which synapse at the axon initial segment of PYR [25, 36]. Intriguingly, while basket cells are inhibitory, recent findings suggest that chandelier neurons may in fact have excitatory effects on PYR activity [37, 38], suggesting that these two INT subtypes play distinct roles in cortical circuit activity. In regards to Sz, convergent evidence (reviewed next) suggests that FS/PV INT are functionally impaired, potentially providing a neural basis for the abnormal generation of GBO activity.
FS/PV INT are functionally Impaired in Sz
Numerous lines of evidence support the hypothesis that FS/PV INT are impaired in Sz (see Fig. 1b). Postmortem studies of Sz patients have consistently observed reduced levels of the GABA synthesizing enzyme, glutamic acid decarboxylase 67 (GAD67) [39–41], particularly in FS/PV INT. Specifically in the PFC of Sz patients, postmortem findings show a ~45% decrease in GAD67 mRNA in PV expressing neurons [41].
Reduced GAD67 expression likely results in reduced GABA synthesis, and may lead to impaired activity of inhibitory inputs in the cortical circuit. Activity driven expression of GAD67 is critical for controlling the synthesis of GABA and thus the filling of secretory vesicles with transmitter. Illustrating this idea, a recent study where GAD67 expression was disrupted via insertion of GFP (42), showed reduced miniature inhibitory postsynaptic current amplitude. Additionally, reducing overall neuronal activity with tetrodotoxin reduced GFP expression in INT in these mice. Together these findings suggest that the expression of GAD67 is a key regulatory sensor of cortical circuit activity.
The putative reduction in activity of FS/PV INT associated with Sz may also lead to a number of downstream compensatory changes [23]. Expression for PV mRNA is also reduced in FS/PV INT in Sz patients. Decreased PV expression has been observed to facilitate GABA release [43]. Additionally, PV expression is essential for synchronizing GABA release to neuronal firing, and computational modeling studies suggest that decreased PV expression would impair GBO activity [44].
Downstream of GABA release, mounting genetic and molecular evidence suggests altered expression of GABA(A) receptor and GABAergic signaling in Sz [45, 46]. Deficits in GABAergic neurotransmission from FS/PV INT may lead to impaired activation of GABAergic receptors at the postsynaptic targets of these INT. Postmortem findings support this hypothesis, as the expression of GABA(A) receptor α1 subunits is decreased at FS/PV INT synapses onto PYR [47, 48]. As mentioned above, the frequency of cortical oscillations is largely determined by the decay kinetics of GABA(A) receptor mediated inhibitory currents [19]. The GABA(A) receptor α1 subunit displays fast kinetics capable of supporting GBO activity. Additionally, the expression of the GABA transporter, GAT-1, is reduced at FS/PV INT synapses onto PYR [48]. Thus, the reduced expression of these factors observed in Sz patients may also underlie altered GABAergic signaling and GBO abnormalities associated with this disorder.
NMDA Hypofunction may Result in Changes in Inhibition and Cortical Circuit Dysfunction
In addition to changes in GABAergic function, deficits in glutamatergic synaptic connectivity have been increasingly implicated as a core feature behind the pathophysiology of Sz [49]. This has resulted in the development and refinement of the NMDA receptor (NMDAR) hypofunction model of this disease [50]. This model is largely derived from the fact that NMDAR antagonists (Ketamine, PCP, etc.), are capable of reproducing the full range of symptoms associated with Sz, including positive symptoms, negative symptoms, as well as cognitive deficits [51, 52]. Such findings have led to widespread usage of these agents to model Sz in both humans and animal studies [53]. Further, recent clinical findings have shown reduced binding of an NMDAR probe in the hippocampus of medication-free Sz patients, providing some direct evidence for NMDAR hypofunction [54].
A number of convergent studies provide evidence connecting NMDAR hypofunction and inhibitory abnormalities (see Fig. 1c). Chronic NMDAR antagonist treatment in rodents reduces the expression of GAD67 and PV in FS/PV INT in a similar manner to that observed in Sz postmortem studies [55, 56]. However, such results remain somewhat controversial, as more recent attempts to replicate these findings have failed to reproduce this effect [57]. Additionally, clinical and experimental findings have shown that the expression of NMDAR and a number of proteins that interact with these receptors are altered in Sz [58]. For instance, postmortem studies of Sz patients have found evidence for altered NMDAR expression specific to FS/PV INT [17, 59•]. However, these findings require replication and show considerable variation from region to region within the brain [58].
Interestingly, in rodents, acute administration of NMDAR antagonists elicits a paradoxical increase in the activity of cortical PYR [60]. This effect was accompanied by a decrease in the activity of INT, suggesting that the observed increase in PYR activity was mediated by a decrease in INT-mediated tonic inhibition. This idea is supported by previous findings suggesting that INT are especially sensitive to NMDAR antagonists [61, 62]. Further, acute NMDAR antagonist administration in human studies also increases cortical excitability [63], suggesting that NMDAR hypofunction in Sz produces PYR disinhibition through reducing the activity specifically of FS/PV INT [64, 65].
The above findings suggest that a reduction in NMDAR-mediated signaling may represent a core component of the mechanism responsible for the development of Sz pathophysiology. As such, it has been suggested that NMDAR hypofunction is an upstream cause of the observed FS/PV-INT dysfunction in Sz [65]. NMDAR mediated input at glutamatergic synapses onto FS/PV INT may play an essential role in regulating the activity of these INT. Thus, impaired excitatory drive onto these INT could result in decreased activity of these neurons and decreased GABAergic inhibition.
Use of NMDA Antagonists to Model Sz Related Cortical Circuit Abnormalities
NMDAR antagonists alter GBO and induce Sz-like psychosis and cognitive impairments in both humans and animal models [3, 53]. Thus, they represent a useful tool to model the cortical circuit abnormalities observed in Sz. Imaging studies in healthy humans have revealed increased metabolic activity and glutamate release in medial PFC following acute ketamine treatment [66–70]. In rodent models, in vivo studies have shown that acute systemic administration of NMDAR antagonists leads to significant increases in the power of both baseline and stimulus-evoked GBO in the hippocampus and frontal cortex (71–73, 74•). Such findings have been largely confirmed in vitro by our lab and others (75•, 76) although see (77). Interestingly, in our study (75•) acute ketamine potentiated GBO power in the medial PFC, paralleling the effect of systemic ketamine observed in vivo, and this effect was mimicked by selective NMDAR antagonists MK-801 and AP-5. However, ketamine, unlike more specific NMDAR antagonists, also significantly reduced peak oscillatory frequency. This effect was mediated by a slowing of the kinetics of GABA(A) mediated currents in identified GABAergic interneurons, suggesting that acute ketamine alters GBO synchronization locally in the mouse prefrontal cortex by acting on both NMDA and GABA(A) receptors.
Chronic administration of ketamine, as well as other NMDAR antagonists (PCP, MK-801), has been used to mimic the longer term effects of NMDAR hypofunction. Such treatment paradigms likely lead to structural alterations in neocortical circuitry, and negative/cognitive symptoms of Sz [52, 78–80]. Recent, in vivo studies suggest that chronic ketamine, unlike acute administration, reduces both the power of hippocampal GBO activity and the number of detectable FS/PV INT [74•]. Similarly, in preliminary studies we have found that chronic ketamine reduces prefrontal GBO [81].
It is important to note that, over the years, studies employing NMDAR antagonists to model Sz have used numerous acute and (sub)chronic dosing regimens, as well as a number of different pharmacological agents with varying levels of specificity for the NMDAR. Further, several recent studies, including our own, suggest that the Sz-like behavioral and neurophysiological effects elicits by certain non-specific NMDAR antagonist (Ketamine and PCP) may be elicited at least in part through effects of these agents on targets beyond the NMDAR alone [75•, 82]. Finally, models that involve chronic NMDAR antagonist administration are most likely to correspond to the full complexity of the Sz related disease state, whereas acute models more closely model acute psychotic states [3].
Does Deficient NMDAR Mediated Excitatory Input to FS/PV INT Cause GBO Abnormalities Typical of Sz?
Although widespread NMDA blockade reliably results in abnormal GBO activity, the site where NMDAR antagonists act in the cortical circuit is unclear. One hypothesis is that impaired NMDAR input specifically in FS-PV INT gives rise to Sz-related neural network impairment/dysfunction. Supporting this idea, postmortem analysis of the prefrontal cortex of Sz patients has shown that the expression of NR2A mRNA is reduced by ~50% in PV INT [59•], suggesting altered NMDAR mediated signaling. Interestingly, the amount of NR2A in FS/PV INT has been found to be five-fold that observed in PYR, and antagonists specific for NR2A downregulate GAD67 and PV expression in INT in primary culture [83]. Additionally, genetic deletion of NMDAR selectively from FS/PV-INT increases the power of GBO in the cortex and hippocampus [28•, 30]. Taken together, these findings indicate that reduced glutamatergic input onto FS/PV INT via NMDAR is perhaps responsible for the reduced GAD67 and PV expression observed in SZ, and could lead to circuit impairments responsible for abnormal GBO activity.
In a recent study by Carlen and colleagues [28•] optogenetic techniques were employed to directly assess the role of NMDAR input onto FS/PV INT on cortical activity and cognition. Here they demonstrated that transgenic mice with impaired NMDAR expression, specifically on FS/PV INT, had enhanced baseline cortical GBO activity in vivo. However, GBO induction via optogenetic stimulation of FS-PV INT was impaired. Thus, both enhanced baseline GBO and reduced evoked GBO were observed in the same animals. Additionally, these mice showed reduced sensitivity to NMDAR antagonists mediated effects on GBO activity and behavior, as well as Sz-like cognitive impairments. These findings provide strong evidence linking impaired NMDAR function on FS/PV INT to abnormal GBO activity and cognition.
The above results notwithstanding, the role of NMDAR-mediated input into FS/PV INT remains somewhat controversial. Recent findings from the Gonzalez-Burgos lab, and others, show that excitatory input into FS/PV INT in the adult mouse PFC is mediated largely by AMPA receptors (AMPAR) with little to no contribution of NMDAR [84•, 85]. AMPAR mediated currents have much faster kinetics than those mediated NMDAR, which they suggest may be required for the fast and temporally precise activity of these neurons [86]. Supporting this idea, earlier studies showed that selective KO of AMPA mediated input into FS/PV INT led to a reduction in GBO activity [87]. Further, EM studies have observed a lower density of NMDAR in glutamatergic synapses onto FS/PV-INT vs those onto PYR [88]. Additionally, a recent study by Sarihi et al. [89] showed that LTP induction is NMDAR independent in cortical FS/PV-INT, but not PYR. Thus, it is possible that NMDAR antagonists may produce cortical disinhibition and GABA neuron alterations via NMDAR receptors at synaptic sites different from the glutamatergic synapses on FS/PV INT [86]. Alternatively, taking into account the studies described above which show that deletion of NMDAR on these neurons leads to altered GBO, it is possible that, while quite modest, the low levels of NMDAR mediated excitatory drive onto FS/PV-INT may be sufficient to alter the GAD67 level of these INT and thereby impact inhibition and cortical circuit function.
Many now consider Sz to be a neurodevelopmental disorder in which psychosis represents a late stage outcome of the disease [5]. Significant developmental changes in GABAergic neurotransmission occur during adolescence. NMDAR-mediated currents in FS/PV-INT are stronger early in development, and progressively weaken as these neurons mature [85]. Postnatal maturation of FS/PV INT in sensory cortical regions occurs during a period of experience dependent refinement of neural circuits [90], which also coincides with the period of maturation of GBO activity [91, 92]. Recent findings show that selective NMDAR deletion in cortical INT (including FS/PV-INT), only produces Sz-like behavioral alterations and enhanced GBO in mice if NMDAR deletion is induced early in development [26•]. This suggests increased complexity of the role of NMDAR hypofunction in cortical circuit development and activity, and that the developmental timing and nature of dysfunctional NMDAR function likely plays a key role the pathophysiology of Sz [86].
Relating NMDAR Antagonist Modeling of Sz with Clinical Findings
NMDAR antagonist treatment currently represents the gold-standard for preclinical, modeling of Sz since it causes behavioral and molecular changes reminiscent of the disease state. Use of NMDAR antagonists to study GBO deficits is a more recent development and it is important to evaluate to what extent the preclinical models recapitulate clinical findings. The clinical literature relating to GBO changes in Sz is quite complex. However, the majority of studies have found impaired GBO in response to stimuli or during the performance of cognitive tasks. However, acute NMDAR antagonism (e.g., with ketamine) causes enhanced GBO. How are these findings to be reconciled?
While GBO deficits observed with chronic NMDAR hypofunction may impair communication between and within brain regions and cause cognitive deficits, abnormally elevated GBO, as observed with acute NMDAR antagonism, may also lead to positive Sz symptoms such as hallucinations. Thus, signals that would normally be ignored may instead be misinterpreted. Recent clinical findings provide some support for this idea. Increased high-amplitude gamma band EEG oscillations observed during psychosis [93], and auditory hallucinations appear to be associated with increased power or synchrony of GBO activity [94–96]. Additionally, a number of studies by Spencer et al. [14, 95, 97] have shown positive correlations between the prevalence of psychotic symptoms and GBO power, further suggesting that increased GBO activity is linked to psychosis. Abnormally elevated GBO activity could cause a ceiling effect, preventing further gamma recruitment during cognitive tasks [98•]. Interestingly, the Carlen study described above confirmed that both increased basal GBO and reduced stimulus-evoked GBO can co-exist. Background GBO is difficult to evaluate in between-subject clinical studies. Thus, increases in background GBO activity may have been overlooked in previous clinical studies which focused exclusively on stimulus locked evoked GBO responses.
To test this hypothesis, the Spencer laboratory has recently reanalyzed data from one of their earlier studies which showed deficits in auditory-evoked GBO activity in Sz patients versus healthy controls. Looking specifically at the pre-stimulus baseline GBO power (40 Hz), a significant increase was observed in the left auditory cortex of chronic Sz patients compared to healthy controls [98•]. Further, computational modeling suggests that reducing the level of NMDA input to FS/PV INT would increase both cortical excitability and increase GBO level [95] in a manner similar to that observed in NMDAR antagonist modeling studies described above. Thus, Sz may not just be associated with deficits in GBO activity, but pathological increases as well. These findings provide a critical link between clinical studies and NMDAR hypofunction models of Sz.
As suggested above, Sz related dysfunction of NMDAR input into FS/PV INT may lead to an overall decrease in the inhibitory output of these neurons, resulting in increased excitation in the cortical circuitry. Such an elevation in the ratio of excitation to inhibition (E/I balance) has been theorized to give rise to GBO abnormalities, and certain Sz-related symptoms. Recently, Yizhar and colleagues [99•] have directly tested the E/I balance hypothesis through direct optogenetic manipulation of specific neuronal subtypes in the medial PFC of freely moving mice. In this study, the authors demonstrated that optogenetic upregulation of PFC excitatory neuronal activity results in a profound, yet reversible, impairment in both social function and cognition, suggesting elevated E/I balance impaired information transmission within cortical circuitry. Interestingly, these findings were not observed with upregulation of inhibitory neuronal (PV INT) activity and were specific for the PFC. The authors of this study also showed that elevated E/I ratio was associated with an increase in GBO activity. Their findings suggest that high background gamma activity may interfere with normal cortical processing, and contribute to certain neuropsychiatric symptom classes.
Conclusions
The studies reviewed above almost universally implicate impaired FS/PV INT function as a central component of the pathophysiology underlying a number of the symptoms associated with Sz. However, there is still much debate over how such dysfunction arises throughout the course of neuronal development, and how this dysfunction results in abnormal GBO activity observed in Sz patients. Recent advances (e.g., optogenetics, genome wide analysis, etc.), provide the opportunity to better model Sz, allowing better characterization of genetic and developmental mechanisms involved in the cortical circuit abnormalities observed in this disease. Beyond its role in Sz, GBO abnormalities are observed in a number of other neuropsychiatric disorders (autism, Alzheimer’s disease, epilepsy) [100]. Thus, elucidation of the mechanisms mediating these abnormalities holds the promise of improved therapeutic intervention for a number of devastating disorders.
Acknowledgments
This work was supported by Department of Veterans Affairs Medical Research Service Awards (R.W. McCarley), and grants from the National Institutes of Health: MH040799 (R.W. McCarley), MH039683 (R.W. McCarley), and MH094803 (R.E. Brown).
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
Contributor Information
James M. McNally, Email: james_mcnally@hms.harvard.edu.
Robert W. McCarley, Email: robert_mccarley@hms.harvard.edu.
Ritchie E. Brown, Email: ritchie_brown@hms.harvard.edu.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Lesh TA, Niendam TA, Minzenberg MJ, et al. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Pratt J, Winchester C, Dawson N, et al. Advancing schizophrenia drug discovery: optimizing rodent models to bridge the translational gap. Nat Rev Drug Discov. 2012;11:560–579. doi: 10.1038/nrd3649. [DOI] [PubMed] [Google Scholar]
- 4.Lewis DA. Cortical circuit dysfunction and cognitive deficits in schizophrenia--implications for preemptive interventions. Eur J Neurosci. 2012;35:1871–1878. doi: 10.1111/j.1460-9568.2012.08156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 6.Andreasen NC. Schizophrenia: the fundamental questions. Brain Res Brain Res Rev. 2000;31:106–112. doi: 10.1016/s0165-0173(99)00027-2. [DOI] [PubMed] [Google Scholar]
- 7.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Tallon-Baudry C. Attention and awareness in synchrony. Trends Cogn Sci. 2004;8:523–525. doi: 10.1016/j.tics.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Uhlhaas PJ, Haenschel C, Nikolic D, et al. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 11.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 12.Kwon JS, O'Donnell BF, Wallenstein GV, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spencer KM, Nestor PG, Niznikiewicz MA, et al. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spencer KM, Nestor PG, Perlmutter R, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basar-Eroglu C, Brand A, Hildebrandt H, et al. Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol. 2007;64:39–45. doi: 10.1016/j.ijpsycho.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Woo TU, Spencer K, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010;18:173–189. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fries P, Nikolic D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Traub RD, Bibbig A, LeBeau FE, et al. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci. 2004;27:247–278. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- 20.Whittington MA, Faulkner HJ, Doheny HC, et al. Neuronal fast oscillations as a target site for psychoactive drugs. Pharmacol Ther. 2000;86:171–190. doi: 10.1016/s0163-7258(00)00038-3. [DOI] [PubMed] [Google Scholar]
- 21.Hajos N, Paulsen O. Network mechanisms of gamma oscillations in the CA3 region of the hippocampus. Neural Netw. 2009;22:1113–1119. doi: 10.1016/j.neunet.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 23.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 24.Ascoli GA, Alonso-Nanclares L, Anderson SA, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somogyi P, Tamas G, Lujan R, et al. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 26. Belforte JE, Zsiros V, Sklar ER, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447.. In order to recapitulate Sz-like NMDAR hypofunction, this study used a transgenic line of mice where NMDAR were selectively eliminated in 40–50% of cortical and hippocampal interneurons, including FS/PV INT. When INT NMDAR expression was impaired early in development, but not post-adolescence, mice displayed a number of Sz-like physiological and behavioral symptoms. These findings suggest that impaired INT NMDAR activity early in development can result in pathophysiological circuit abnormalities which result in Sz-related symptoms.
- 27. Cardin JA, Carlen M, Meletis K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002.. This optogenetic study provides direct evidence of the central role FS/PV INT play in the generation of cortical GBO activity. Here the authors show that in vivo optical stimulation of cortical FS/PV INT at varying frequencies, selectively amplified the local LFP response in the gamma frequency range. In contrast, identical stimulation of PYR only amplified activity at lower frequencies.
- 28. Carlen M, Meletis K, Siegle JH, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012;17:537–548. doi: 10.1038/mp.2011.31.. This study combined the use of transgenic mice and optogenetics to directly examine the role of NMDAR signaling specifically in cortical FS/PV INT on neural network activity and behavior. Here they show that mice lacking NMDAR specifically on FS/PV cells exhibit enhanced baseline GBO activity, impaired evoked GBO, and cognitive impairment.
- 29.Gloveli T, Dugladze T, Saha S, et al. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol. 2005;562:131–147. doi: 10.1113/jphysiol.2004.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korotkova T, Fuchs EC, Ponomarenko A, et al. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 31. Sohal VS, Zhang F, Yizhar O, et al. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991.. This study provides direct evidence for the central role of neocortical FS/PV INT in the generation of GBO. Using targeted optogenetic modulation of neuronal activity, they show that inhibition of FS/PV INT impairs GBO, while enhancement of their activity potentiates GBO. Further, gamma-frequency modulation of excitatory input reduced circuit noise and amplified circuit signals leading to an enhancement of cortical signal transmission.
- 32.Hu H, Martina M, Jonas P. Dendritic mechanisms underlying rapid synaptic activation of fast-spiking hippocampal interneurons. Science. 2010;327:52–58. doi: 10.1126/science.1177876. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 35.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 36.Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 37.Woodruff AR, Anderson SA, Yuste R. The enigmatic function of chandelier cells. Front Neurosci. 2010;4:201. doi: 10.3389/fnins.2010.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szabadics J, Varga C, Molnar G, et al. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 39.Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 40.Volk DW, Austin MC, Pierri JN, et al. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto T, Volk DW, Eggan SM, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lau CG, Murthy VN. Activity-dependent regulation of inhibition via GAD67. J Neurosci. 2012;32:8521–8531. doi: 10.1523/JNEUROSCI.1245-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vreugdenhil M, Jefferys JG, Celio MR, et al. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol. 2003;89:1414–1422. doi: 10.1152/jn.00576.2002. [DOI] [PubMed] [Google Scholar]
- 44.Volman V, Behrens MM, Sejnowski TJ. Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci. 2011;31:18137–18148. doi: 10.1523/JNEUROSCI.3041-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charych EI, Liu F, Moss SJ, et al. GABA(A) receptors and their associated proteins: implications in the etiology and treatment of schizophrenia and related disorders. Neuropharmacology. 2009;57:481–495. doi: 10.1016/j.neuropharm.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JY, Liu CY, Zhang F, et al. Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell. 2012;148:1051–1064. doi: 10.1016/j.cell.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor alpha1 subunit messenger RNA expression in schizophrenia. Neuropsychopharmacology. 2011;36:2103–2110. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohnuma T, Augood SJ, Arai H, et al. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABA(A) receptor alpha-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neuroscience. 1999;93:441–448. doi: 10.1016/s0306-4522(99)00189-x. [DOI] [PubMed] [Google Scholar]
- 49.Mirnics K, Middleton FA, Lewis DA, et al. Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci. 2001;24:479–486. doi: 10.1016/s0166-2236(00)01862-2. [DOI] [PubMed] [Google Scholar]
- 50.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 51.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 52.Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 53.Bubenikova-Valesova V, Horacek J, Vrajova M, et al. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci Biobehav Rev. 2008;32:1014–1023. doi: 10.1016/j.neubiorev.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Pilowsky LS, Bressan RA, Stone JM, et al. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol Psychiatry. 2006;11:118–119. doi: 10.1038/sj.mp.4001751. [DOI] [PubMed] [Google Scholar]
- 55.Behrens MM, Ali SS, Dao DN, et al. Ketamine-induced loss of phenotype of fastspiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 56.Keilhoff G, Becker A, Grecksch G, et al. Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience. 2004;126:591–598. doi: 10.1016/j.neuroscience.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 57.Benneyworth MA, Roseman AS, Basu AC, et al. Failure of NMDA receptor hypofunction to induce a pathological reduction in PV-positive GABAergic cell markers. Neurosci Lett. 2011;488:267–271. doi: 10.1016/j.neulet.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kristiansen LV, Huerta I, Beneyto M, et al. NMDA receptors and schizophrenia. Curr Opin Pharmacol. 2007;7:48–55. doi: 10.1016/j.coph.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 59. Bitanihirwe BK, Lim MP, Kelley JF, et al. Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC Psychiatry. 2009;9:71. doi: 10.1186/1471-244X-9-71.. This postmortem Sz study examined expression of the NMDAR subunit NR2A (mRNA), in PFC FS/PV INT. The authors observed a ~50% reduction in the density of FS/PV INT with detectable levels of NR2A expression. This finding provides evidence for deficient NMDAR mediated neurotransmission in FS/PV INT in Sz.
- 60.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greene R, Bergeron R, McCarley R, et al. Short-term and long-term effects of N-methyl-D-aspartate receptor hypofunction. Arch Gen Psychiatry. 2000;57:1180–1181. doi: 10.1001/archpsyc.57.12.1180. author reply 1182–1183. [DOI] [PubMed] [Google Scholar]
- 62.Grunze HC, Rainnie DG, Hasselmo ME, et al. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Lazzaro V, Oliviero A, Profice P, et al. Ketamine increases human motor cortex excitability to transcranial magnetic stimulation. J Physiol. 2003;547:485–496. doi: 10.1113/jphysiol.2002.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lisman JE, Coyle JT, Green RW, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holcomb HH, Lahti AC, Medoff DR, et al. Sequential regional cerebral blood flow brain scans using PET with H2(15)O demonstrate ketamine actions in CNS dynamically. Neuropsychopharmacology. 2001;25:165–172. doi: 10.1016/S0893-133X(01)00229-9. [DOI] [PubMed] [Google Scholar]
- 67.Breier A, Malhotra AK, Pinals DA, et al. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154:805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- 68.Deakin JF, Lees J, McKie S, et al. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- 69.Honey RA, Honey GD, O'Loughlin C, et al. Acute ketamine administration alters the brain responses to executive demands in a verbal working memory task: an FMRI study. Neuropsychopharmacology. 2004;29:1203–1214. doi: 10.1038/sj.npp.1300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corlett PR, Honey GD, Aitken MR, et al. Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: linking cognition, brain activity, and psychosis. Arch Gen Psychiatry. 2006;63:611–621. doi: 10.1001/archpsyc.63.6.611. [DOI] [PubMed] [Google Scholar]
- 71.Hakami T, Jones NC, Tolmacheva EA, et al. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One. 2009;4:e6755. doi: 10.1371/journal.pone.0006755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 73.Lazarewicz MT, Ehrlichman RS, Maxwell CR, et al. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2010;22:1452–1464. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- 74. Kittelberger K, Hur EE, Sazegar S, et al. Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia. Brain Struct Funct. 2012;217:395–409. doi: 10.1007/s00429-011-0351-8.. The authors of this study examined the ability of both acute and chronic administration of NMDAR antagonists to recapitulate the oscillatory dysfunctions observed clinically in Sz. They show that in EEG recordings from freely moving rats, acute injection with NMDAR antagonists led to increased hippocampal GBO activity, while chronic NMDAR antagonist treatment (2–4 weeks) resulted in decreased GBO. These findings indicate that NMDAR antagonists can be employed to model both Sz-related pathological increases in GBO as well as GBO impairment.
- 75. McNally JM, McCarley RW, McKenna JT, et al. Complex receptor mediation of acute ketamine application on in vitro gamma oscillations in mouse prefrontal cortex: modeling gamma band oscillation abnormalities in schizophrenia. Neuroscience. 2011;199:51–63. doi: 10.1016/j.neuroscience.2011.10.015.. This study investigated the effects of several NMDAR antagonists on kainate evoked GBO activity, in vitro. Here the authors showed that acute application of such antagonists (ketamine, MK-801, and AP5) lead to a significant potentiation of evoked GBO power, providing further support for NMDAR hypofunction mediated GBO enhancement. Interestingly, ketamine also caused a significant decrease in the frequency of evoked oscillations, similar to that observed in clinical Sz studies (14).
- 76.Anver H, Ward PD, Magony A, et al. NMDA receptor hypofunction phase couples independent gamma-oscillations in the rat visual cortex. Neuropsychopharmacology. 2011;36(2):519–528. doi: 10.1038/npp.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roopun AK, Cunningham MO, Racca C, et al. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull. 2008;34:962–973. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 79.Paradiso S, Chemerinski E, Yazici KM, et al. Frontal lobe syndrome reassessed: comparison of patients with lateral or medial frontal brain damage. J Neurol Neurosurg Psychiatry. 1999;67:664–667. doi: 10.1136/jnnp.67.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corlett PR, Murray GK, Honey GD, et al. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain. 2007;130:2387–2400. doi: 10.1093/brain/awm173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McNally JM, Kim T, Yanagawa Y, et al. Acute and Chronic Effects of Ketamine on Gamma Oscillations in Mouse Prefrontal Cortex Soc. Neurosci Abs. 2011;661.07 [Google Scholar]
- 82.Seillier A, Giuffrida A. Evaluation of NMDA receptor models of schizophrenia: divergences in the behavioral effects of sub-chronic PCP and MK-801. Behav Brain Res. 2009;204:410–415. doi: 10.1016/j.bbr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 83.Kinney JW, Davis CN, Tabarean I, et al. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rotaru DC, Yoshino H, Lewis DA, et al. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31:142–156. doi: 10.1523/JNEUROSCI.1970-10.2011.. This study directly analyzed the contribution of NMDAR to excitatory synaptic activation of FS/PV INT in mouse PFC. The results of this work showed little to no NMDAR contribution, and that excitatory input in these INT is mainly mediated by AMPAR. These findings suggest that Sz-related NMDAR hypofunction is important at glutamatergic inputs separate from those present on FS/PV INT.
- 85.Wang HX, Gao WJ. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology. 2009;34:2028–2040. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rotaru DC, Lewis DA, Gonzalez-Burgos G. The role of glutamatergic inputs onto parvalbumin-positive interneurons: relevance for schizophrenia. Rev Neurosci. 2012;23:97–109. doi: 10.1515/revneuro-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fuchs EC, Zivkovic AR, Cunningham MO, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 88.Nyiri G, Stephenson FA, Freund TF, et al. Large variability in synaptic N-methyl-D-aspartate receptor density on interneurons and a comparison with pyramidal-cell spines in the rat hippocampus. Neuroscience. 2003;119:347–363. doi: 10.1016/s0306-4522(03)00157-x. [DOI] [PubMed] [Google Scholar]
- 89.Sarihi A, Jiang B, Komaki A, et al. Metabotropic glutamate receptor type 5-dependent long-term potentiation of excitatory synapses on fast-spiking GABAergic neurons in mouse visual cortex. J Neurosci. 2008;28:1224–1235. doi: 10.1523/JNEUROSCI.4928-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 91.Rojas DC, Maharajh K, Teale PD, et al. Development of the 40Hz steady state auditory evoked magnetic field from ages 5 to 52. Clin Neurophysiol. 2006;117:110–117. doi: 10.1016/j.clinph.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 92.Uhlhaas PJ, Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr Bull. 2011;37:514–523. doi: 10.1093/schbul/sbr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baldeweg T, Spence S, Hirsch SR, et al. Gamma-band electroencephalographic oscillations in a patient with somatic hallucinations. Lancet. 1998;352:620–621. doi: 10.1016/S0140-6736(05)79575-1. [DOI] [PubMed] [Google Scholar]
- 94.Lee SH, Wynn JK, Green MF, et al. Quantitative EEG and low resolution electromagnetic tomography (LORETA) imaging of patients with persistent auditory hallucinations. Schizophr Res. 2006;83:111–119. doi: 10.1016/j.schres.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 95.Spencer KM, Niznikiewicz MA, Nestor PG, et al. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mulert C, Kirsch V, Pascual-Marqui R, et al. Long-range synchrony of gamma oscillations and auditory hallucination symptoms in schizophrenia. Int J Psychophysiol. 2011;79:55–63. doi: 10.1016/j.ijpsycho.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spencer KM, Salisbury DF, Shenton ME, et al. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Spencer KM. Baseline gamma power during auditory steady-state stimulation in schizophrenia. Front Hum Neurosci. 2011;5:190. doi: 10.3389/fnhum.2011.00190.. The aim of this study was to examine the conflicting findings of clinical Sz studies, which generally show impaired GBO activity, to preclinical findings, which suggest that Sz related NMDAR hypofunction results in cortical hyperexcitabililty predicted to lead to increased GBO. Here the authors reexamined data from an earlier study showing an impairment in auditory evoke GBO in Sz patients, and observed that baseline GBO power (40 Hz) was higher in Sz patients than healthy controls in the left auditory cortex. These findings suggest that Sz-related GBO abnormalities can include pathological increases in GBO as well as impairment, and provides an important link between clinical and preclinical findings.
- 99. Yizhar O, Fenno LE, Prigge M, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360.. This study used a targeted optogenetic approach to examine how alteration of the balance of excitation and inhibition (E/I balance) within neural circuitry effects circuit physiology and behavior. Here they find that increased excitation, but not inhibition, results in GBO abnormalities and cognitive impairment. These results support the hypothesis that elevated E/I balance is central to a number of symptoms related to neuropsychiatric disorders such as Sz and autism.
- 100.Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]